Abstract
Context
Dyspnea is one of the most distressing symptoms for cancer patients. The role of high flow oxygen (HFO) and bilevel positive airway pressure (BiPAP) in the palliation of dyspnea has not been well characterized.
Objectives
To determine the feasibility of conducting a randomized trial of HFO and BiPAP in cancer patients, and to examine the changes in dyspnea, physiologic parameters and adverse effects with these modalities.
Methods
In this randomized study (ClinicalTrials.gov Identifier: NCT01518140), we assigned hospitalized patients with advanced cancer and persistent dyspnea to either HFO or BiPAP for two hours. We assessed dyspnea with a numeric rating scale (NRS) and modified Borg scale (MBS) before and after the intervention. We also documented vital signs, transcutaneous carbon dioxide and adverse effects.
Results
Thirty patients were enrolled (1:1 ratio) and 23 (77%) completed the assigned intervention. HFO was associated with improvements in both NRS (mean 1.9, 95% confidence interval [CI] 0.4, 3.4; P=0.02) and MBS (mean 2.1, 95% CI 0.6, 3.5; P=0.007). BiPAP also was associated with improvements in NRS (mean 3.2; 95% CI 1.3, 5.1; P=0.004) and MBS (mean 1.5, 95% CI −0.3, 3.2; P=0.13). There were no significant differences between HFO and BiPAP in dyspnea NRS (P=0.14) and MBS (P=0.47). Oxygen saturation improved with HFO (93% vs. 99%, P=0.003), and respiratory rate had a non-statistically significant decrease with both interventions (HFO -3; P=0.11; BiPAP -2, P=0.11 ). No significant adverse effects were observed.
Conclusion
HFO and BiPAP alleviated dyspnea, improved physiologic parameters and were safe. Our results justify larger randomized controlled trials to confirm these findings.
Keywords: dyspnea, positive-pressure respiration, neoplasms, oxygen, randomized controlled trial, high flow oxygen, bilevel positive airway pressure
Introduction
Dyspnea is one of the most common and distressing symptoms among cancer patients (1). Dyspnea is associated with fatigue, anxiety, decreased function and quality of life, and increased mortality (2–4). The current management of dyspnea involves treatment of any reversible causes and supportive measures to minimize the sensation of dyspnea (5, 6). Low flow oxygen (5 L/min or less) has been shown to be efficacious for dyspnea only in hypoxemic patients (5, 7, 8). Many patients experience persistent dyspnea despite these interventions.
High flow oxygen (HFO) and bilevel positive airway pressure (BiPAP) represent two oxygen delivery modalities that may offer significant advantages compared with traditional low flow oxygen. HFO delivers up to 40L/min of humidified heated oxygen through a nasal cannula. In addition to high flow oxygenation, it also provides nasopharyngeal washout and positive distending pressure, and decreases airway resistance and the metabolic cost of breathing, all of which may alleviate dyspnea (9). BiPAP not only delivers supplemental oxygen but also assists ventilation and unloads respiratory muscles (10). These interventions may further stimulate the trigeminal nerve, similar to the mechanism of the fan (11). Although both HFO and BiPAP significantly improve oxygenation, neither has an established role in the palliation of persistent dyspnea in patients with advanced cancer. Evidence to support their efficacy may allow us to improve patients’ quality of life. In this Phase II “pick the winner” trial, we determined the feasibility of conducting a randomized trial of HFO and BiPAP in patients with advanced cancer and persistent dyspnea despite standard supplemental oxygen therapy. We also examined the effects of these interventions on the intensity of dyspnea, physiologic changes, and side effects as compared with baseline and with each other.
Methods
Patients
Eligible patients were recruited between February 5, 2007 and April 16, 2011. Hospitalized patients at M. D. Anderson Cancer Center, age 18 years or older, and with a diagnosis of advanced cancer (i.e., locally advanced, recurrent or metastatic disease) were eligible if they had an average intensity of dyspnea at rest over the past week ≥3/10 on a numeric rating scale (NRS) despite supplemental oxygen; the dyspnea was judged clinically to be predominantly the result of underlying malignancy; had a life expectancy of more than one week; and were English-speaking. Patients were excluded if they had hemodynamic instability, acute respiratory distress with impending intubation, delirium (Memorial Delirium Assessment Scale >13/30) (12), Glasglow coma scale score <8/15, contraindications to BiPAP, or noncancer-related dyspnea requiring supplemental home oxygen prior to hospitalization. The Institutional Review Board at M. D. Anderson Cancer Center approved this study. All patients provided written informed consent.
Study Design
This randomized, open-label study was initially designed as a crossover trial, and later amended to a parallel design with an optional second intervention because four of seven patients who completed BiPAP and two of ten patients who completed HFO experienced significant improvement for greater than one hour after the first intervention.
Patients were screened and approached for this study while admitted at M. D. Anderson Cancer Center. Enrolled patients were assigned by our study coordinator using a computer-generated simple randomization scheme created by our biostatistician in a 1:1 ratio to receive either 1) two hours of HFO followed by a variable washout period and then two hours of BiPAP or 2) two hours of BiPAP followed by a variable washout period and then two hours of HFO. The two-hour duration was chosen because other studies have shown that oxygenation and physiologic parameters improved within 30 minutes to two hours, and we wanted to ensure participants had the opportunity to benefit from the study interventions (13–15).
We introduced a variable washout/follow-up period after the first intervention to determine the optimal duration required for patients to return to baseline dyspnea level, which has not been examined previously. Patients were asked about their level of dyspnea every 10 minutes after they have completed the first intervention for up to one hour. They were able to proceed to the second intervention if 1) their dyspnea level was at the baseline dyspnea level-1 or greater; or 2) their dyspnea level was ≥3/10 after one hour. In this study, we defined “extended relief” as dyspnea ≤2/10 at the end of one hour off study intervention.
Study Interventions
All study interventions were delivered in acute care units outside of the intensive care setting. Our respiratory therapists worked with patients to titrate the device settings to minimize dyspnea while maximizing comfort. Fraction of inspired oxygen (FiO2) was set at 100% throughout the intervention period for both devices.
Patients on HFO received high flow, heated and humidified oxygen via nasal prongs using the Vapotherm® 2000i Respiratory Therapy Device (Vapotherm, Stevensville, MD, USA). The level of heat (35–37°C) and oxygen flow (10–40 L/min) were titrated to comfort.
Non-invasive ventilation was delivered using the BiPAP Vision® Ventilatory Support System (Respironics Inc. Murrysville, PA, USA) in pressure support mode (S/T). Patients received BiPAP through a leak-tolerant ResMed Latex Free Hospital Full Face Mask R143-340/5 (ResMed Ltd., Bella Vista, NSW, Australia). The level of support was started at an inspiratory pressure of 8 cm of H2O and expiratory pressure of 5cm of H2O, then the settings were titrated, with a target inspiratory pressure between 8–18cm of H2O, and target expiratory pressure of 3–10cm of H2O.
Study Assessments and Endpoints
At baseline, we collected information on patient characteristics (age, sex, cancer diagnosis, comorbidities and medication use) and the Cancer Dyspnoea Scale (CDS), a validated 12-item questionnaire that assesses the quality of dyspnea over the past few days (16, 17). Each item has a score between 1 and 5, with a total score of up to 60, and subscores for sense of effort, anxiety, and discomfort.
Our primary outcome was retention rate, defined as the percentage of subjects able to complete the first phase of this study. Secondary outcomes included changes in dyspnea, physiologic changes, and adverse effects immediately before starting and stopping each intervention. The intensity of dyspnea “now” was examined using both the NRS and the modified Borg scale (MBS). The NRS is a validated 11-point scale ranging from 0 (“no shortness of breath”) to 10 (“worst possible shortness of breath”) (16, 18). The MBS is a validated 0 to 10 ratio scale for rating the severity of dyspnea (16, 19). We employed two different measures to assess the convergent validity of dyspnea outcome. The NRS also was used to assess dyspnea every 10 minutes during the washout period.
We also examined physiologic variables such as heart rate, respiratory rate, blood pressure, transcutaneous carbon dioxide level (Sentec Digital Monitoring System TCO2M® Transcutaneous Monitor, Novametrix Medical Systems Inc., Wallingford, CT, USA) and oxygen saturation (Alaris® SpO2 module-8200 series oximeter, Alaris Medical Systems, San Diego, CA, USA) immediately before starting and stopping each intervention. Adverse effects related to the study interventions were assessed using an 11-point NRS, with 0 being absent and 10 denoting worst possible.
After completion of each intervention, we asked patients about their change in dyspnea (better/same/worse) using the Global Symptom Evaluation (20, 21).
Sample Size Calculation
Our primary objective was to determine the feasibility of conducting a randomized trial of HFO and BIPAP based on the retention rate for the first intervention. We hypothesized that more than 65% of the enrolled patients would complete the process of randomization, the first intervention, and the dyspnea NRS measurement.
We initially planned to enroll 25 patients per arm, which was powered to detect an effect size difference between the two study arms of 0.60 standard deviation (SD) units, with a two-tailed α of 0.05 and an 80% power assuming 35% attrition. As a result of funding limitations, this study was terminated after 30 patients. Fifteen patients per arm provided us with an 80% power to detect an effect size difference between the two study arms of 0.80 SD units, with a two-tailed α of 0.05.
Statistical Analysis
We summarized the baseline demographics using descriptive statistics, including medians, means, SDs, ranges, interquartile ranges (IQRs) and frequencies. This study was analyzed as a parallel study. We conducted a before-after analysis for the first intervention using the sign test and the signed-rank test for all continuous variables (e.g., dyspnea, physiologic changes, adverse effects). Intention-to-treat analysis was conducted to compare the change between the two interventions using the Wilcoxon rank sum test for continuous variables, and Fisher’s exact test for categorical variables. A two-sided P-value of <0.05 was considered to be statistically significant. The Statistical Analysis System (SAS version 9.2, SAS Institute, Cary, NC, USA) was used for statistical analysis.
Results
Patient Characteristics
The two study arms had similar baseline characteristics (Table 1). Thirteen (43%) patients had lung cancer. All patients had poor performance status. The average intensity of dyspnea was high, despite concurrent use of opioids (93%) and steroids (59%). A vast majority of participants (93%) were on supplemental oxygen at the time of enrollment, with a median of 3 L/min (IQR 2.4–5 L/min) and an average oxygen saturation of 95% (SD 4%).
Table 1.
Baseline Patient Characteristicsa
| BiPAP (n=14) n (%)b |
High Flow Oxygen (n=16) n (%)b |
All patients (N=30) n (%)b |
|
|---|---|---|---|
| Average age (range) | 63 (47–79) | 59 (29–79) | 61 (29–79) |
| Female sex | 6 (43) | 10 (63) | 16 (53) |
| Race | |||
| Caucasian | 11 (79) | 12 (75) | 23 (77) |
| Black | 3 (21) | 3 (19) | 6 (20) |
| Hispanic | 0 | 1 (6) | 1 (3) |
| Cancer type | |||
| Breast | 2 (14) | 3 (19) | 5 (17) |
| Gastrointestinal | 1 (7) | 2 (13) | 3 (10) |
| Genitourinary | 1 (7) | 0 | 1 (3) |
| Head and neck | 0 | 1 (6) | 1 (3) |
| Lung | 6 (43) | 7 (44) | 13 (43) |
| Other | 4 (29) | 3 (19) | 7 (23) |
| Cancer stage | |||
| Metastatic | 12 (86) | 14 (88) | 26 (87) |
| Locally advanced | 2 (14) | 2 (12) | 4 (13) |
| ECOG Performance Status | |||
| 3 | 13 (93) | 14 (88) | 27 (90) |
| 4 | 1 (7) | 2 (12) | 3 (10) |
| Cancer Dyspnea Scale, median (IQR) | |||
| Effort | 8 (5–10) | 8 (7–13) | 8 (5–12) |
| Anxiety | 5 (3–8) | 7 (2–10) | 7 (3–9) |
| Discomfort | 6 (5–7) | 5 (4–9) | 5 (4–8) |
| Total | 31 (27–37) | 37 (27–42) | 34 (27–40) |
| Dyspnea NRS over the last 2 weeks, median (IQR) | 8 (7–9) | 8 (6–8) | 8 (6–8) |
| Dyspnea NRS at the time of enrollment, median (IQR) | 7 (5–8) | 6 (4–8) | 7 (5–8) |
| Causes of dyspnea | |||
| Pulmonary parenchymal lesions | 10 (71) | 11 (69) | 21 (70) |
| Pleural effusion | 5 (36) | 10 (63) | 15 (50) |
| Lymphangitic carcinomatosis | 1 (7) | 1 (6) | 2 (7) |
| Other non-cancer causes, not already on home supplemental oxygen | 8 (57) | 13 (81) | 21 (70) |
| Comorbidities | |||
| COPD | 5 (36) | 5 (31) | 10 (33) |
| Heart failure | 0 (0) | 1 (6) | 1 (3) |
| Asthma | 1 (7) | 0 | 1 (3) |
| Bronchiectasis | 0 | 0 | 0 |
| Concurrent therapies | |||
| Opioids | 12 (92) | 15 (94) | 27 (93) |
| Steroids | 7 (54) | 10 (63) | 17 (59) |
| Supplemental oxygen | 14 (100) | 12 (86) | 26 (93) |
| Previous experience with devices | |||
| BiPAP | 1 (7) | 2 (13) | 3 (10) |
| CPAP | 1 (7) | 2 (13) | 3 (10) |
| None | 12 (86) | 12 (86) | 24 (80) |
BiPAP = bilevel positive airway pressure; IQR = interquartile range; COPD = chronic obstructive pulmonary disease; CPAP = continuous positive airway pressure; ECOG = Eastern Cooperative Oncology Group; NRS = numeric rating scale.
One patient assigned to receive BiPAP was mistakenly started on HFO, and was reported here in the HFO group
Unless otherwise specified.
One patient assigned to BiPAP as the first intervention was erroneously given HFO instead. This individual was analyzed as not having completed in the assigned intervention in our retention rate calculation, received BiPAP in intention-to-treat comparison between the two interventions, and received HFO in the before-and-after comparison.
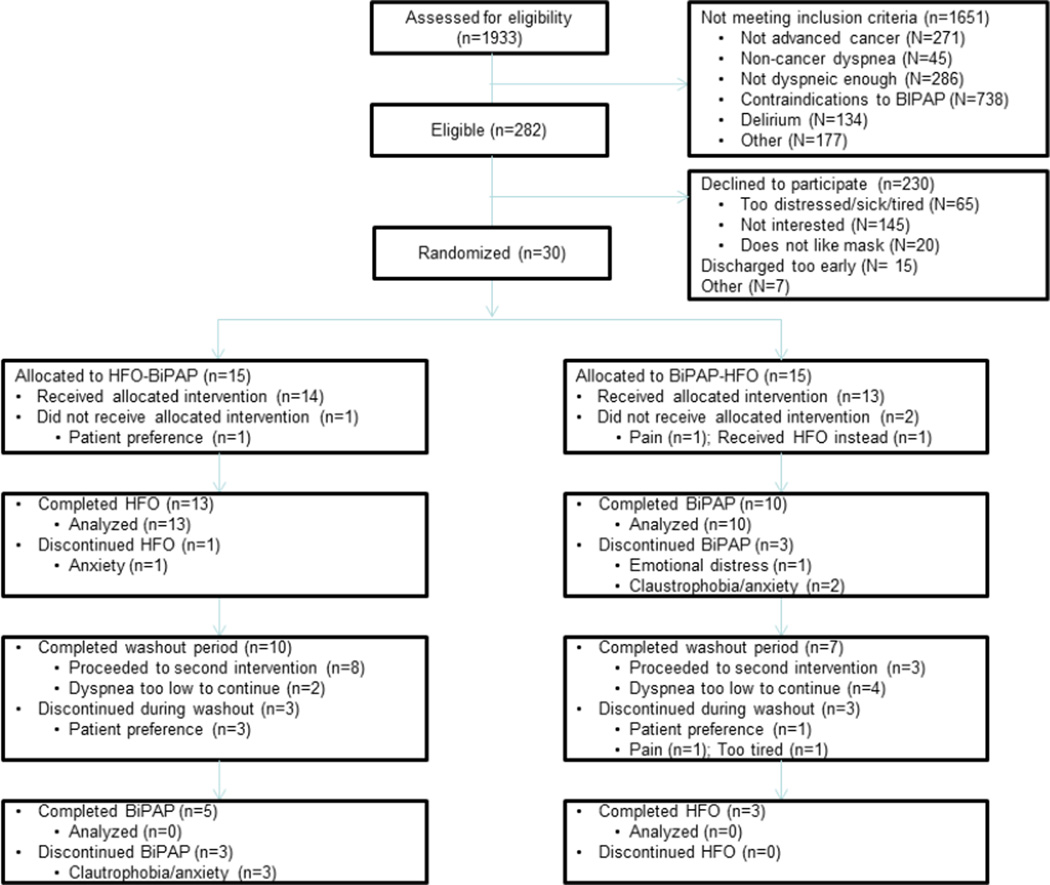
Study Feasibility
Among the 1933 patients screened, 282 (15%) were eligible, and 30 (11%) participated (Fig. 1). Thirteen of 15 (87%) patients assigned to HFO and 10 of 15 (67%) patients assigned to BiPAP completed the study intervention, for a total retention rate of 23 of 30 (77%) and no statistical difference between the two interventions (P=0.39).
Fig. 1. Study flow chart.
Changes in Dyspnea
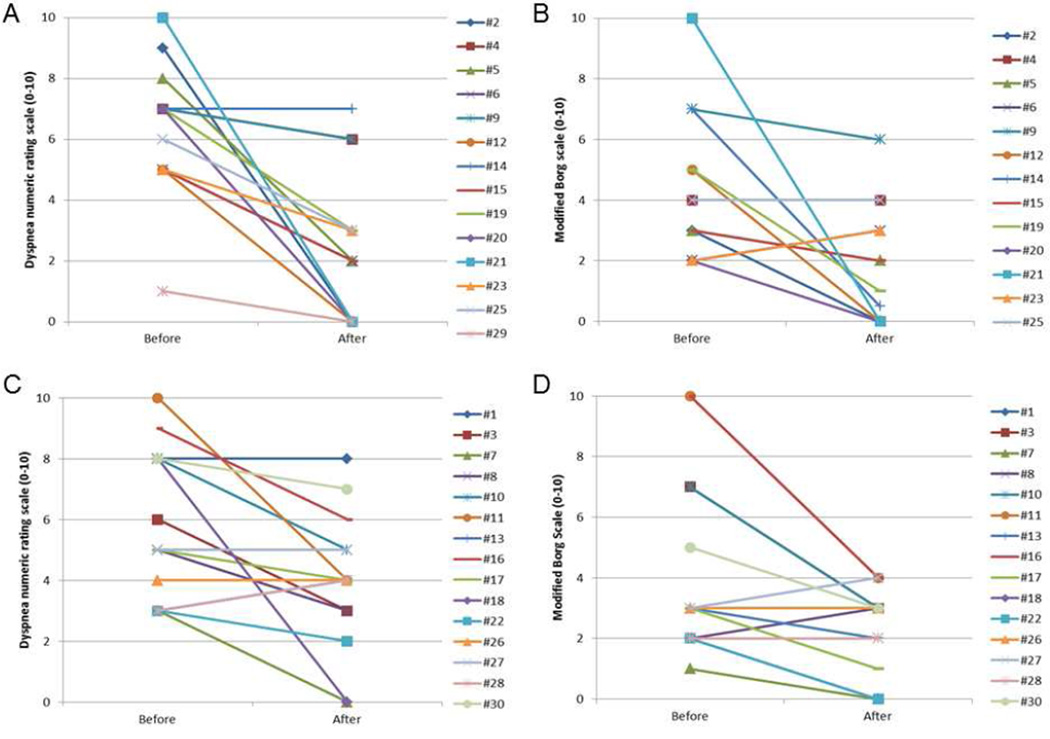
Fig. 2 shows that dyspnea improved with both devices in a before-and-after comparison. Specifically, BiPAP was associated with an average dyspnea improvement of 3.2 by NRS and 1.5 by MBS, whereas HFO was associated with an average improvement of 1.9 by NRS and 2.1 by MBS (Table 2). No significant differences in dyspnea relief were detected between the two devices (Table 2).
Fig. 2. Change in dyspnea scores with high flow oxygen and BiPAP.
As compared with baseline, BiPAP was associated with an average improvement in (A) the dyspnea numeric rating scale of 3.2 (P=0.004) and (B) the modified Borg scale of 1.5 (P=0.13). High flow oxygen was also associated with an average improvement in (C) the numeric rating scale of 1.9 (P=0.02) and (D) the modified Borg scale of 2.1 (P=0.007). Intention-to-treat analysis revealed no significant differences between the two devices with the dyspnea numeric rating scale (P=0.32) and the modified Borg scale (P=0.29).
Table 2.
Change in Dyspnea Scores, Physiologic Variables, and Adverse Effects Before and After BiPAP and High Flow Oxygen Administrationa
| BiPAP | High Flow Oxygen | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Beforeb Mean (95% CI) |
Afterb Mean (95% CI) |
Change Mean (95% CI) |
P- valuec |
Beforeb Mean (95% CI) |
Afterb Mean (95% CI) |
Change Mean (95% CI) |
P- valuec |
P- valued |
|
| Dyspnea scores | |||||||||
| Numeric rating scale (0–10) | 6.4 (5.1, 7.6) | 3.4 (1.8, 5.0) | −3.2 (−5.1, −1.3) | 0.004 | 5.9 (4.5, 7.2) | 4.2 (3.1, 5.4) | −1.9 (−3.4, −0.4) | 0.02 | 0.32 |
| Modified Borg scale (0–10) | 4.4 (2.9, 5.8) | 2.6 (1.2, 3.9) | −1.5 (−3.2, 0.3) | 0.13 | 4.3 (2.5, 6.0) | 2.5 (1.6, 3.3) | −2.1 (−3.5, −0.6) | 0.007 | 0.29 |
| Physiologic variables | |||||||||
| Heart rate (pr minute) | 95.6 (17.4) | 85.4 (10.5) | −5.0 (5.1) | 0.02 | 101.2 (17.2) | 97.7 (17.3) | −3.6 (7.8) | 0.42 | 0.43 |
| Respiratory rate (per minute) | 22.1 (6.4) | 20.1 (4.8) | −2.0 (4.0) | 0.11 | 22.1 (6.8) | 19.2 (5.2) | −3.0 (5.2) | 0.11 | 0.97 |
| Systolic blood pressure (mmHg) | 125 (18) | 124 (17) | −3.4 (14.5) | 0.73 | 135 (18) | 122 (16) | −12.6 (14.8) | 0.02 | 0.10 |
| Diastolic blood pressure (mmHg) | 73 (9) | 72 (14) | −0.2 (7.8) | >0.99 | 79 (12) | 76 (11) | −2.1 (9.4) | 0.79 | 0.23 |
| Oxygen saturation (%) | 98.8 (13.4) | 97.9 (4.0) | 3.3 (5.3) | 0.11 | 93.1 (5.4) | 98.5 (2.1) | 5.3 (5.2) | 0.003 | 0.62 |
| Transcutaneous carbon dioxide level | 35.5 (7.4) | 36.5 (9.2) | 1.9 (2.7) | 0.04 | 37.8 (6.3) | 36.9 (9.2) | −0.9 (5.5) | 0.06 | 0.02 |
|
Adverse Effects |
Beforeb Median (IQR) |
Afterb Median (IQR) |
Change Median (IQR) |
P- valuec |
Beforeb Median (IQR) |
Afterb Median (IQR) |
Change Median (IQR) |
P- valuec |
P- valuec |
| Dry eyes | 1.5 (0.0, 4.0) | 0.0 (0.0, 4.0) | 0.0 (−1.0, 0.0) | 0.63 | 3.0 (0.0, 4.0) | 0.0 (0.0, 3.0) | 0.0 (−3.0, 0.0) | 0.22 | 0.10 |
| Eye irritation | 1.0 (0.0, 3.0) | 0.0 (0.0, 3.0) | 0.0 (−1.0, 0.0) | 0.22 | 3.0 (0.0, 4.0) | 0.0 (0.0, 1.0) | 0.0 (−3.0, 0.0) | 0.13 | 0.47 |
| Feel anxious | 2.0 (1.5, 4.0) | 2.0 (0.0, 5.0) | 0.0 (−1.0, 0.0) | 0.69 | 1.0 (0.0, 5.0) | 1.0 (0.0, 2.0) | 0.0 (−4.0, 0.0) | 0.69 | 0.12 |
| Mask painful | 0.0 (0.0, 2.0) | 1.0 (0.0, 3.0) | 1.5 (0.0, 3.0) | >0.99 | NA | NA | NA | NA | 0.30 |
| Moist nose | 2.0 (1.5, 2.0) | 0.0 (0.0, 2.0) | −1.5 (−2.0, 0.0) | 0.13 | 2.0 (0.0, 5.0) | 5.0 (1.0, 7.0) | 0.0 (−1.0, 2.0) | 0.73 | 0.32 |
| Prong uncomfortable | 2.0 (0.0, 5.0) | 0.0 (0.0, 0.0) | −2.5 (−5.0, 0.0) | >0.99 | 2.0 (0.0, 5.5) | 2.0 (0.0, 4.0) | 0.0 (−1.0, 2.0) | 0.73 | 0.12 |
| Stomach bloating | 1.0 (0.0, 3.0) | 0.0 (0.0, 1.0) | −1.0 (−2.0, 0.0) | 0.13 | 3.0 (0.0, 6.0) | 2.0 (0.0, 4.0) | −0.5 (−1.0, 0.0) | 0.22 | 0.82 |
| Suffocating | 1.5 (0.0, 5.0) | 0.0 (0.0, 3.0) | −1.0 (−3.0, 0.0) | 0.22 | 1.5 (0.0, 7.0) | 1.5 (0.0, 4.0) | 0.0 (−1.0, 0.0) | >0.99 | 0.58 |
| Trouble drinking | 1.5 (0.0, 2.5) | 3.0 (0.0, 7.0) | 1.0 (−2.0, 5.0) | 0.51 | 0.0 (0.0, 5.0) | 0.0 (0.0, 3.0) | 0.0 (0.0, 1.0) | >0.99 | 0.46 |
| Trouble eating | 2.5 (1.0, 5.5) | 3.0 (0.0, 5.0) | −1.0 (−3.0, 3.0) | 0.75 | 5.0 (0.0, 7.0) | 2.0 (0.0, 6.0) | 0.0 (−1.0, 0.0) | 0.69 | 0.87 |
| Trouble sleeping | 2.0 (0.5, 4.5) | 2.0 (0.0, 7.0) | 0.0 (−1.0, 3.0) | >0.99 | 7.0 (6.0, 8.0) | 1.0 (0.0, 6.0) | −6.0 (−8.0, 0.0) | 0.04 | 0.02 |
| Trouble talking | 2.0 (0.5, 3.5) | 5.0 (2.0, 7.0) | 0.0 (0.0, 5.0) | 0.45 | 6.0 (2.0, 8.0) | 2.0 (0.0, 6.0) | 0.0 (−6.0, 2.0) | 0.73 | 0.004 |
BiPAP = bilevel positive airway pressure; CI = confidence interval; IQR = interquartile range; NA = not applicable; SD = standard deviation.
One patient assigned to receive BiPAP was erroneously started on HFO and completed the first intervention. This individual was analyzed as having received BiPAP in the comparison between the two interventions, and analyzed as having received HFO in the before-and-after comparison.
Before measurements were done while patients were on baseline oxygen; after measurements were conducted after two hours of study intervention.
The signed-rank test was used for before-after comparison.
The Wilcoxon rank sum test was used to compare the change in values between HFO and BiPAP.
Device Settings and Physiologic Changes
The average level of oxygen flow on HFO was 21 (SD 7) L/min. The average BiPAP inspiratory pressure was 9 (SD 2.6) cm of H2O, and the expiratory pressure was set at 5 (SD 1) cm of H2O. We identified a non-statistically significant decrease in respiratory rate with both HFO and BiPAP (Table 2). BiPAP was associated with a decrease in heart rate. HFO also was associated with a significant decrease in systolic blood pressure and improvement in oxygen saturation (Table 2).
Adverse Effects
We did not identify any significant adverse effects with BiPAP and HFO (Table 2). Patients reported less trouble sleeping (0–10 NRS) while on HFO as compared with BiPAP.
Follow-up Period
Seventeen of 23 (74%) patients completed the follow-up period. Among these 17 patients, two of 10 patients who completed HFO and four of seven patients who completed BiPAP experienced improvement in dyspnea level for more than one hour while on baseline supplemental oxygen (Table 3), and were ineligible to continue to the next intervention.
Table 3.
Dyspnea Numeric Rating Scores at Baseline and During Washout Period in Six Patients Who Experienced Extended and Significant Improvement in Dyspneaa
| Arm | Baseline | Post First Intervention |
10 min |
20 min |
30 min |
40 min |
50 min |
60 min |
|---|---|---|---|---|---|---|---|---|
| HFO | 10 | 4 | 2 | 1 | 1 | 4 | 2 | 2 |
| HFO | 8 | 0 | 0 | 0 | 2 | 2 | 2 | 0 |
| BiPAP | 8 | 2 | 3 | 1 | 1 | 2 | 2 | 2 |
| BiPAP | 7 | 7 | 0 | 0 | 0 | 0 | 1 | 1 |
| BiPAP | 5 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| BiPAP | 7 | 3 | 3 | 1 | 2 | 2 | 2 | 2 |
BiPAP = bilevel positive airway pressure; HFO = high flow oxygen.
These patients experienced extended (one hour) and significant improvement (less than baseline dyspnea level-1 and <3/10 at the end of one hour), and thus did not continue onto the next study intervention.
Global Symptom Evaluation
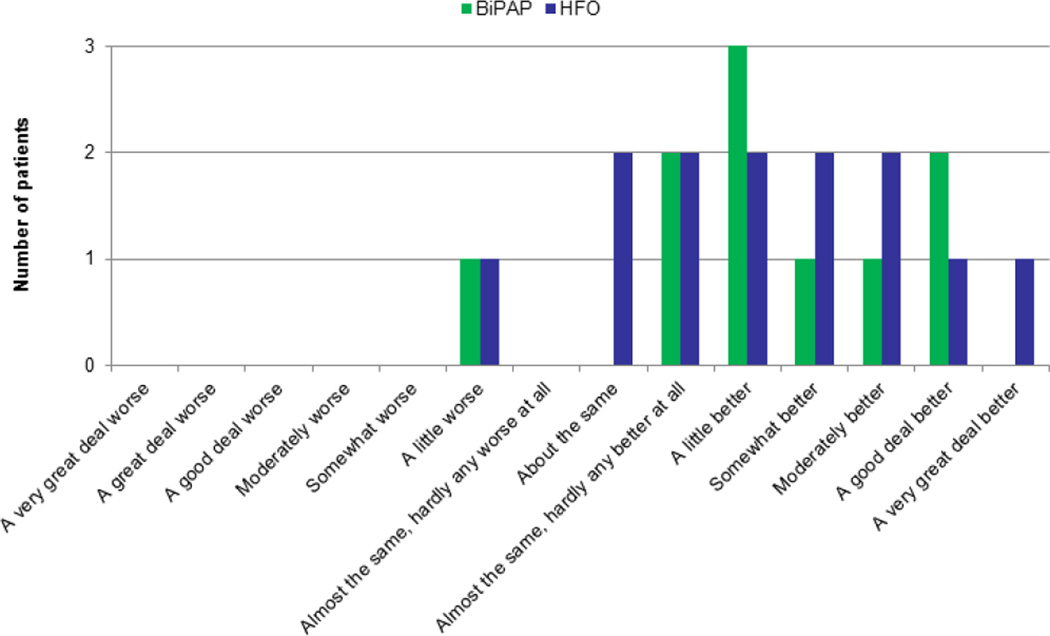
A vast majority of patients reported that HFO (10 of 13, 77%) and BiPAP (9 of 10) improved their dyspnea (Fig. 3).
Fig. 3. Global Symptom Evaluation for high flow oxygen and BiPAP.
Patients were asked to provide their overall impression about the change in dyspnea level (“worse,” “about the same,” or “better”) using the Global Symptom Evaluation scale. Consistent with the dyspnea scores, a majority of patients reported that high flow oxygen and BiPAP were efficacious in relieving their dyspnea.
Discussion
Our study showed that it is feasible to conduct a parallel randomized study of HFO and BiPAP in hospitalized cancer patients with persistent dyspnea. This study also provided preliminary evidence that both devices are associated with significant and extended relief of dyspnea, and enhanced physiologic parameters without significant adverse effects. Because this study was unblinded, placebo effect, clinician effect and obsequiousness bias may have contributed to the observed improvement in the patient-reported outcomes. Our study justifies the need for larger, blinded, randomized, controlled trials to confirm the efficacy of these interventions.
We were able to enroll acutely ill hospitalized patients with advanced cancer into this clinical trial and assess a multitude of patient-reported outcomes. This is a technically challenging clinical trial for multiple reasons. First, our patients had a poor performance status and were often in severe distress. This is in contrast to a majority of other dyspnea studies that enrolled ambulatory patients who are generally more functional and able to engage in studies of longer duration (22, 23). Second, high levels of dyspnea are a predictor of attrition among symptom control clinical trials (24). Third, our study was interrupted by clinical investigations and sometimes abrupt discharges. Fourth, not all patients were able to tolerate BiPAP. Because of the difficulty in enrollment and retention, a majority of dyspnea clinical trials have small sample sizes (5).
Although low flow oxygen has only been shown to relieve dyspnea in hypoxemic patients (25), we hypothesize that HFO and BiPAP could offer symptomatic benefit to a wider patient population because of their high oxygen delivery and varied mechanisms of action. Thus, our study did not specifically exclude non-hypoxemic patients. Rather, all patients had significant dyspnea despite having tried supplemental oxygen and a vast majority were already on opioids and steroids.
To our knowledge, this study is the first randomized trial to examine the role of HFO for dyspnea in patients with advanced cancer. This novel device is postulated to relieve dyspnea by 1) maintaining a high level of PaO2, 2) improving ventilation by nasopharyngeal washout, 3) decreasing the work of breathing and 4) increasing airway compliance and positive distending pressure (9). Recently, investigators have reported improvement in dyspnea with HFO in chronic obstructive pulmonary disease (13) and heart failure patients (26), and in the critical care (27) and emergency room settings (28). However, the effect of HFO on dyspnea in cancer patients has not been examined. Only one retrospective case series reported the usage pattern of HFO in cancer patients (29). In our study, HFO offered significant improvement in dyspnea assessed by the NRS, MBS and Global Symptom Evaluation. This was accompanied by an improvement in oxygen saturation, a non-significant decrease in respiratory rate, and no detectable adverse effects. Interestingly, a majority of patients in our study did not require the maximal oxygen flow. Further studies are needed to confirm our findings, and to determine the optimal duration, settings and population for this device.
BiPAP represents another attractive option for relief of dyspnea (30). We found significant improvement in the dyspnea level after two hours of BiPAP. Our results are consistent with the only other study examining dyspnea in cancer patients, in which the MBS decreased from 5.5 to 2.3 after one hour of BiPAP (15). BiPAP not only assists ventilation but also corrects hypoventilation, increases the inspiratory flow rate, resets the central respiratory drive, and reduces the work of respiratory muscles (10, 31). This last mechanism is of particular interest for patients with advanced cancer who have dyspnea secondary to respiratory muscle weakness.
We were pleasantly surprised by the extended relief of dyspnea during the follow-up period for some patients. We found that many patients continued to report dyspnea relief after discontinuation of over one hour. This may be explained by the fact that BiPAP augments respiratory muscles, giving them time to rest. Another plausible explanation is that dramatic improvement in SaO2 has long-lasting effects on dyspnea. However, we could not rule out trial or placebo effect as contributing factors. If our findings were confirmed, BiPAP and HFO may be highly effective even if used intermittently. Further studies are needed to test this hypothesis, assess respiratory muscle function, and examine the optimal duration for use.
Importantly, only 30 of 282 eligible patients enrolled in the study, raising concerns about selection bias and generalizability. Many patients declined participation because they were not interested or in too much distress (Fig. 1). Specifically, BiPAP has multiple exclusion criteria, and was not tolerated by some patients because of claustrophobia, anxiety or distress. However, individuals who have persistent dyspnea despite standard treatments and are able to tolerate this device may derive significant symptom benefit. Further studies are needed to examine if newer interfaces (i.e., mouthpieces) and delivery devices designed for home use could increase BiPAP’s applicability.
Our study has several limitations. First, this was an unblinded study. Future studies may compare different levels of FiO2 in a blinded design. Second, we had to screen a large number of patients to find eligible and willing participants. Multicenter studies may be helpful to maximize accrual. Third, we compared two active interventions and did not incorporate a control group. Non-rebreather mask delivering oxygen may be considered as a comparison group for future design. Fourth, the extended and unexpected improvement in dyspnea made it challenging to conduct a crossover study design. Fifth, we had to close the study early because of funding limitations, resulting in a reduction in statistical power. Even with the reduced sample size, we were able to demonstrate study feasibility and an improvement in dyspnea scores. Sixth, we have conducted multiple statistical comparisons. Although these were all pre-planned secondary endpoints, readers should interpret the significance of the study findings with caution. Seventh, we did not conduct baseline arterial blood gas nor measure the oxygen saturation while on room air. Future studies would need to collect these baseline data routinely. Finally, this study was conducted in a single academic institution with a unique patient population and health professional team. Further studies are needed to determine the efficacy of these devices in other settings.
Based on the findings from this study, we propose that HFO and BiPAP be examined separately in the future because of their differential eligibility criteria, patient tolerance and retention. We are in the process of developing a double-blind, randomized, controlled trial comparing high flow oxygen and high flow air over multiple days in non-hypoxemic ambulatory patients with refractory dyspnea (i.e., dyspnea despite regular opioids). We also propose a separate double-blind, randomized, controlled trial examining as needed use of BiPAP compared with oxygen delivered with minimal positive end-expiratory pressure in hypoxemic cancer patients, with particular attention paid to the duration of dyspnea relief while off BiPAP. Importantly, future studies need to be adequately powered to examine dyspnea as the primary outcome, which means that multicenter involvement is essential.
Conclusions
We completed a randomized trial of HFO and BiPAP in hospitalized cancer patients with persistent dyspnea. Patients reported that these devices were safe and efficacious in relieving dyspnea. Our study supports the need for larger, randomized, controlled clinical trials to confirm the therapeutic role of these novel devices.
Acknowledgments
Dr. Bruera is supported in part by National Institutes of Health grants RO1NR010162-01A1, RO1CA122292-01, and RO1CA124481-01. Dr. Hui is supported in part by an institutional startup grant (#18075582). This study also was supported by the M. D. Anderson Cancer Center Support Grant (CA 016672). The funding sources were not involved in the conduct of the study or development of the submission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no conflicts of interest
References
- 1.Tishelman C, Petersson LM, Degner LF, Sprangers MA. Symptom prevalence, intensity, and distress in patients with inoperable lung cancer in relation to time of death. J Clin Oncol. 2007;25:5381–5389. doi: 10.1200/JCO.2006.08.7874. [DOI] [PubMed] [Google Scholar]
- 2.Maltoni M, Caraceni A, Brunelli C, et al. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations--a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23:6240–6248. doi: 10.1200/JCO.2005.06.866. [DOI] [PubMed] [Google Scholar]
- 3.Hauser CA, Stockler MR, Tattersall MH. Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Support Care Cancer. 2006;14:999–1011. doi: 10.1007/s00520-006-0079-9. [DOI] [PubMed] [Google Scholar]
- 4.Reddy SK, Parsons HA, Elsayem A, Palmer JL, Bruera E. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med. 2009;12:29–36. doi: 10.1089/jpm.2008.0158. [DOI] [PubMed] [Google Scholar]
- 5.Bruera E, de Stoutz N, Velasco-Leiva A, Schoeller T, Hanson J. Effects of oxygen on dyspnoea in hypoxaemic terminal-cancer patients. Lancet. 1993;342:13–14. doi: 10.1016/0140-6736(93)91880-u. [DOI] [PubMed] [Google Scholar]
- 6.Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnoea. Thorax. 2002;57:939–944. doi: 10.1136/thorax.57.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cranston JM, Crockett A, Currow D. Oxygen therapy for dyspnoea in adults. Cochrane Database Syst Rev. 2008;(3):CD004769. doi: 10.1002/14651858.CD004769.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Abernethy AP, McDonald CF, Frith PA, et al. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind randomised controlled trial. Lancet. 2010;376:784–793. doi: 10.1016/S0140-6736(10)61115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103:1400–1405. doi: 10.1016/j.rmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Nava S, Cuomo AM. Acute respiratory failure in the cancer patient: the role of noninvasive mechanical ventilation. Crit Rev Oncol Hematol. 2004;51:91–103. doi: 10.1016/j.critrevonc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Galbraith S, Fagan P, Perkins P, Lynch A, Booth S. Does the use of a handheld fan improve chronic dyspnea? A randomized, controlled, crossover trial. J Pain Symptom Manage. 2010;39:831–838. doi: 10.1016/j.jpainsymman.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Assessment Scale. J Pain Symptom Manage. 1997;13:128–137. doi: 10.1016/s0885-3924(96)00316-8. [DOI] [PubMed] [Google Scholar]
- 13.Patel NB, Criner GJ, Chatila W. Effect of vapotherm on dyspnea in patients hospitalized for acute exacerbation of COPD [abstract] Am J Respir Crit Care Med. 2003;167:A232. [Google Scholar]
- 14.Guerrero ML, Cuneo BM, Hnatiuk OW, Shorr A. Vapotherm: a novel high-flow oxygen delivery system. Chest. 2003;124:93S. [Google Scholar]
- 15.Cuomo A, Delmastro M, Ceriana P, et al. Noninvasive mechanical ventilation as a palliative treatment of acute respiratory failure in patients with end-stage solid cancer. Palliat Med. 2004;18:602–610. doi: 10.1191/0269216304pm933oa. [DOI] [PubMed] [Google Scholar]
- 16.Dorman S, Byrne A, Edwards A. Which measurement scales should we use to measure breathlessness in palliative care? A systematic review. Palliat Med. 2007;21:177–191. doi: 10.1177/0269216307076398. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Development and validation of the Cancer Dyspnoea Scale: a multidimensional, brief, self-rating scale. Br J Cancer. 2000;82:800–805. doi: 10.1054/bjoc.1999.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gift AG, Narsavage G. Validity of the numeric rating scale as a measure of dyspnea. Am J Crit Care. 1998;7:200–204. [PubMed] [Google Scholar]
- 19.Kendrick KR, Baxi SC, Smith RM. Usefulness of the modified 0–10 Borg scale in assessing the degree of dyspnea in patients with COPD and asthma. J Emerg Nurs. 2000;26:216–222. doi: 10.1016/s0099-1767(00)90093-x. [DOI] [PubMed] [Google Scholar]
- 20.Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. J Clin Epidemiol. 1996;49:1215–1219. doi: 10.1016/s0895-4356(96)00206-5. [DOI] [PubMed] [Google Scholar]
- 21.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118:622–629. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 22.Bruera E, Sweeney C, Willey J, et al. A randomized controlled trial of supplemental oxygen versus air in cancer patients with dyspnea. Palliat Med. 2003;17:659–663. doi: 10.1191/0269216303pm826oa. [DOI] [PubMed] [Google Scholar]
- 23.Abernethy AP, McDonald CF, Frith PA, et al. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind, randomised controlled trial. Lancet. 2010;376:784–793. doi: 10.1016/S0140-6736(10)61115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui D, Glitza IC, Chisholm GB, Yennu S, Bruera E. Attrition rates, reasons, and predictive factors in supportive/palliative oncology clinical trials. Cancer. 2013;119:1098–1105. doi: 10.1002/cncr.27854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben-Aharon I, Gafter-Gvili A, Paul M, Leibovici L, Stemmer SM. Interventions for alleviating cancer-related dyspnea: a systematic review. J Clin Oncol. 2008;26:2396–2404. doi: 10.1200/JCO.2007.15.5796. [DOI] [PubMed] [Google Scholar]
- 26.Carratala Perales JM, Llorens P, Brouzet B, et al. High-flow therapy via nasal cannula in acute heart failure. Rev Esp Cardiol. 2011;64:723–725. doi: 10.1016/j.recesp.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 27.Sztrymf B, Messika J, Bertrand F, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med. 2011;37:1780–1786. doi: 10.1007/s00134-011-2354-6. [DOI] [PubMed] [Google Scholar]
- 28.Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55:408–413. [PubMed] [Google Scholar]
- 29.Epstein AS, Hartridge-Lambert SK, Ramaker JS, Voigt LP, Portlock CS. Humidified high-flow nasal oxygen utilization in patients with cancer at Memorial Sloan-Kettering Cancer Center. J Palliat Med. 2011;14:835–839. doi: 10.1089/jpm.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hillberg RE, Johnson DC. Noninvasive ventilation. N Engl J Med. 1997;337:1746–1752. doi: 10.1056/NEJM199712113372407. [DOI] [PubMed] [Google Scholar]
- 31.Cropp A, DiMarco AF. Effects of intermittent negative pressure ventilation on respiratory muscle function in patients with severe chronic obstructive pulmonary disease. Am Rev Respir Dis. 1987;135:1056–1061. doi: 10.1164/arrd.1987.135.5.1056. [DOI] [PubMed] [Google Scholar]