Abstract
Background
Intestinal transplant recipients are at risk for micronutrient deficiency due to the slow process of post-transplant adaptation. Another contributing factor is calcineurin inhibitor-induced renal tubular dysfunction. Patients are typically supplemented with micronutrients during parenteral nutrition; however the risk of deficiency may persist even after a successful transition to full enteral nutrition.
Objective
To determine the prevalence of, and associated risk factors for, iron, zinc, magnesium, phosphorus, selenium, copper, folate, vitamins A, D, E and B12 deficiency in pediatric intestinal transplant recipients after successful transition to full enteral nutrition.
Method
A retrospective review of prospectively collected data from children who underwent intestinal transplantation at Cincinnati Children's Hospital Medical Center. Deficiencies of various micronutrients were defined using the hospital reference values.
Results
Twenty-one intestinal transplant recipients, aged one to 23 years that were successfully transitioned to full enteral nutrition were included in the study. The prevalence of micronutrient deficiency was 95.2%. The common deficient micronutrients were iron (94.7%) and magnesium (90.5%). Age ≤10 years (P=0.002) and tube feeding (P= 0.02) were significant risk factors for micronutrient deficiencies.
Conclusion
Pediatric intestinal transplant recipients have a high risk of micronutrient and mineral deficiencies. These deficiencies were more common among younger patients and those who received jejunal feeding.
Keywords: Intestinal transplant, micronutrient deficiency, enteral nutrition
Introduction
Advances in neonatology and transplant surgery have resulted in an increased number of pediatric patients requiring and receiving combined liver-intestine, isolated intestine, or multivisceral transplant allografts [1 - 4]. Most experienced centers now report 70-90% one year patient and graft survival rates for pediatric intestinal transplant recipients [5]. In spite of the improved survival, there are still concerns for nutrient deficiencies and other morbidities in recipients of intestinal transplants. Studies on pediatric intestinal transplant patients have reported difficulties in attaining normal or catch up somatic growth after successful transition to enteral nutrition [6 -8]. This is due in part to the suboptimal absorption of fat by the intestinal graft [9]. Limited published data have also shown that intestinal transplant recipients do have micronutrient deficiencies after they have been successfully transitioned to enteral nutrition [8]. We hypothesize that micronutrient deficiency is common after successful transition to full enteral nutrition (FEN) in pediatric intestinal transplant patients. This study assesses the prevalence of various micronutrient deficiencies and the associated risk factors for deficiencies in pediatric intestinal transplant recipients who have successfully transitioned to FEN.
Materials and Methods
Setting
This study was conducted at the Cincinnati Children Hospital Medical Center (CCHMC), Cincinnati, Ohio, USA.
Study design
This was a single-center retrospective review of data from 21 patients who underwent intestinal or combined liver-intestine transplant between January 2003 and August 2012.
Participants
Twenty-one of 39 transplanted patients who were successfully transitioned to FEN for at least three months were included in the data analysis. Those more than 25 years of age were excluded. Patients were undergoing follow-up in the Comprehensive Nutrition Clinic of CCHMC, Cincinnati, Ohio, during the review period. Recipients in our program are supplemented with multiple or specific micronutrients if they show a trend toward deficiency during follow-up visits. Those who develop deficiency are given therapeutic doses of deficient micronutrients until deficiency is corrected and are then continued on micronutrient supplementations. All of our patients received proton pump inhibitors and /or H2 blockers. Micronutrient levels of our patients are monitored as shown in table 1. Our immunosuppression induction regimen for all recipients of combined liver-intestine transplant consisted of pre-operative and intra-operative intravenous doses of methylprednisolone and Thymoglobulin®, as well as an additional dose of Thymoglobulin® on post-operative day one. All of our patients received Tacrolimus as maintenance immunosuppression The target level for Tacrolimus was < 20 ng/ml for post-operative days 1-3; 8-18 ng/ml for post op days 4-7; 12-18 ng/ml post op week 2 to week 12, 8-12 ng/ml for week 13 to week 26, then 5-8 ng/ml from week 26 thereafter. They underwent steroid taper over six weeks if there were no episodes of acute cellular rejection. In the setting of early acute rejection, this steroid taper was prolonged to 12 weeks. Trimethoprim-sulfamethoxazole for life was our preferred choice for prophylaxis against pneumocystis jiroveci pneumonia (PCP), but occasional patients were transitioned to intravenous pentamidine or oral atovaquone due to adverse side effects related to trimethroprim-sulfamethoxazole.
Table 1.
Laboratory Monitoring of micronutrient status of post intestinal transplant patients
| a. Recommend schedule for patients on parenteral nutrition | |||
|---|---|---|---|
| Labs (deficiency status) | Initial frequency | Long term /home | Indications |
| TPN profile : Renal, Calcium, Phoshorus, Magnesium, Liver panel, Triglyceride, Bile acid, Prealbumin, glucose | With every TPN change, then weekly until stable then per MD discretion | Every 2 weeks then monthly Per MD discretion | |
| CBC with diff | Weekly initially then per MD discretion | Monthly/As indicated | Monitor anemia |
| 25 (OH) Vitamin D (< 20 ng/ml) | First level should occur after 3 months on TPN | Every 6 months; If indicated q 1 month | Deficiency |
| RBC folate (<1120 nmol/L) | Every 6 months | ||
| Iron/TIBC/Ferritin | Q 3 months; If indicated Q 1 month | Anemia with microcytosis and patient on enteral or parenteral iron | |
| Ceruloplasmin (<1 μmol/L) Copper (<80 mcg/dL) | After 30 days of TPN | Every 2 months if indicated | Cholestasis, persistent anemia, metabolic bone disease |
| Zinc (< 60 mcg/dL) Selenium | If indicated, first level should occur after 6 mo on TPN or if high ostomy output, after 30 days, and then q 3-6 months | Patients with chronic diarrhea, high ostomy output | |
| Vitamin A (<200 mcg/L) Vitamin E (<5.0 mcg/mL) Vitamin K | First level should occur after 6 months on TPN and then every 6 months | Cholestasis | |
| Chromium | If indicated | Glycemic control difficult despite GIR<15 | |
| Urine Methylmalonic acid (> | If indicated, first level at a year then Q 6 months | Ileal resection, Macrocytic anemia | |
| Serum B12 (<211 pg/mL) | If indicated, first level at a year then Q 6 months | Ileal resection, Macrocytic anemia | |
| b. Recommended schedule for patients weaned off parenteral nutrition | |||
| Labs | Frequency | Indications | |
| 25 OH Vitamin D | Q 6 months If indicated Q 3 months | On Vit D supplementation, chronic liver disease, cholestasis | |
| TPN profile | Q 6 months | ||
| CBC w/ Diff | Q 6 months | ||
| *Fe/TIBC/Ferritin | If indicated Q 6-12 months | If anemic and on supplements | |
| Urine Methylmalonic acid | If indicated, first level at a year then Q 12 months | Ileal resection, Macrocytic anemia | |
| Serum B12 | If indicated, first level at a year then Q 12 months | Ileal resection, Macrocytic anemia | |
| RBC folate | Q 6 months | Extensive resection | |
| Zinc Level (<60 mcg/dL) | Q6 months | Extensive resection | |
| Ceruloplasmin | as indicated | Persistent Neutropenia and Microcytic Anemia | |
| Serum copper | as indicated | Persistent Neutropenia and Microcytic Anemia, cholestasis | |
Data
Relevant electronic data from eligible patients were retrieved. Information obtained included date of birth, gestational age, birth weight, gender, ethnicity, anthropometric centiles (weight, height), age at transplantation, parenteral nutrition (PN) duration, duration off PN, post-transplant duration, history of cholestasis, disease leading to intestinal failure, and type of transplant and number of grade 2 or higher rejection episodes [10]. Serum levels of iron, TIBC, ferritin, zinc, magnesium, phosphorus, selenium, copper, folate, vitamins A, D, E and B12 after successful transition to FEN were retrieved. The frequency of monitoring and the definition for deficiency are shown in table 1. Data were obtained longitudinally for every visit throughout the follow-up period. Full enteral nutrition (FEN) was defined as the patient tolerating all of the estimated required nutrition (100%) enterally for > 3 months.
Data Analysis
The data were analyzed using SPSS version 19 (Chicago IL). Covariates examined as potential predictors of micronutrient deficiencies were race, gender, PN duration, transplant duration, history and number of grade 2 or higher rejection episodes, type of tube feeding, type of health insurance, weight and height percentile. Type of insurance was used as a marker for socioeconomic status in this population. Fisher's exact test was used to test significant association of the categorical variables. The distributions of all continuous variables were checked for normality using Shapiro Wilk's test. The t-test was used to compare the means of normally distributed continuous variables, while the Mann-Whitney U test was used if the data were not normally distributed. Age, gender and P-values <0.15 were fitted into a binary logistic model. A P-value of less than 0.05 was regarded as significant and all reported P-values are 2-sided.
Ethical Approval
Ethical approval was obtained from the Institutional Review Board (IRB) of the Cincinnati Children's Hospital Medical Center. Parenteral consent and patient assent were waived by the IRB in view of the fact that patient identifiers were anonymized and that this was merely a retrospective analysis of data that had been collected as part of the patients’ routine nutritional assessment.
Results
Subjects
A total of 39 patients underwent intestinal or combined liver-intestine transplants during the period under review. The 1 year patient survival rate in this cohort of patients was 67 % with 3 years survival rate of 54% measured from 2003 onward. Outcomes have improved dramatically since 2009 with one and two year survival rates both of 100%. . There were 21 intestinal transplant recipients (13 males and 8 females) who met the inclusion criteria of successfully transitioning to full enteral nutrition for 3 months and were included in the data analysis. The etiologies of intestinal failure were as follows: 6 patients (28.6%) had necrotizing enterocolitis and 5 patients (23.8%) had gastroschisis. The other five had Hirschsprung disease, anorectal malformation with congenital short bowel syndrome, malrotation/volvulus, jejunoileal atresia and megacystitis-micro-colon intestinal hypo-peristalsis syndrome (MMIH), respectively. There were 14 (66.7%) Caucasians, 5 (23.8%) African-Americans, and 2 (9.5%) Hispanics. Their ages at time of transplant ranged from 3 to 72 months (median, 12.8 months). Prior to intestinal transplantation, all of the patients were receiving 80-100% of required calories from PN. The median PN duration post-transplant was 2 months (range 1-14.3 months) and the median duration off PN post-transplant was 37.3 months. Patients with multiple rejection episodes and post lymphoproliferative disorder required longer duration of PN with four of these patients being on PN for more than 6 months after transplant. The median duration of post-transplant follow up was 42.7 months. After weaning off PN, 66.7% (14) were on IV fluids but only 2/21 (9.5%) required IV fluids after ostomy closure. The detailed characteristics of the patients are reported in table 2.
Table 2.
Demographic and clinical characteristics of the patients
| Sex | Age (years) | Caucasian | GA (weeks) | BW (g) | Primary GI disease (cause of intestinal failure) | PN duration (months) | Duration off PN (months) | Post-transplant (months) | Post-transplant PN (months) |
|---|---|---|---|---|---|---|---|---|---|
| M | 12 | Yes | NA | NA | Hirschsprung's disease (dysmotility) | 10 | 147 | 148 | 1 |
| F | 12 | No | 26 | NA | NEC (SBS) | 18 | 126 | 132 | 6 |
| M | 8 | Yes | 40 | 4,820 | Malrotation with volvulus (SBS) | 40.8 | 90 | 92 | 2 |
| M | 4 | No | 28 | 855 | NEC (SBS) | 18.2 | 35.5 | 38.8 | 3.3 |
| M | 2 | Yes | 36 | 2,655 | Gastroschisis (SBS) | 12 | 11.5 | 13.1 | 1.6 |
| M | 1 | Yes | 35 | 2,355 | Gastroschisis (SBS) | 17 | 4.1 | 6.6 | 2.5 |
| F | 5 | Yes | NA | NA | Gastroschisis (SBS) | 24.3 | 43 | 57.3 | 14.3 |
| F | 6 | Yes | 37 | NA | NEC (SBS) | 19.6 | 54.4 | 55.5 | 1.1 |
| F | 6 | Yes | 35 | 2,220 | ARM (SBS) | 20.2 | 53.1 | 54.1 | 1 |
| M | 5 | Yes | 36 | 3,090 | Gastroschisis (SBS) | 12.4 | 48.8 | 52.8 | 4 |
| M | 4 | No | 32 | NA | Small intestinal atresia (SBS) | 12.4 | 43.4 | 53.7 | 10.3 |
| M | 5 | Yes | 38 | 4,400 | Hirschsprung's disease (dysmotility) | 30 | 30.3 | 42.7 | 12.4 |
| M | 4 | Yes | 31 | 1,570 | NEC (SBS) | 14.5 | 36 | 41.7 | 5.7 |
| F | 4 | Yes | 35 | NA | Meconium peritonitis (SBS) | 16.1 | 37.3 | 40.6 | 3.3 |
| F | 3 | No | 31 | 1,630 | Gastroschisis (SBS) | 6.6 | 31.3 | 32.4 | 1.1 |
| F | 5 | No | 33 | 3,530 | MMIHS (dysmotility) | 41.3 | 18.2 | 19.8 | 1.6 |
| F | 3 | No | 36 | NA | MMIHS (dysmotility) | 21.3 | 18 | 18.8 | 0.8 |
| M | 1 | Yes | 37 | 2,920 | Small intestinal atresia (SBS) | 1.7 | 1.7 | 3.3 | 1.6 |
| M | 10 | Yes | 40 | 3,490 | Malrotation with midgut volvulus (SBS) | 12 | 113.6 | 115.8 | 2.2 |
| M | 23 | Yes | NA | NA | NEC (SBS) | 60 | 218 | 219 | 1 |
| M | 8 | No | 25 | NA | NEC (SBS) | 67 | 26.5 | 27.5 | 1 |
PN parenteral nutrition, EN=enteral nutrition, GA gestational age, BW birth weight, M male, F female, SBS short bowel syndrome, NEC necrotizing enterocolitis, MMIHS megacysitis microcolon intestinal hypoperistalsis syndrome, ARM=anorectal malformation, NA not available.
Micronutrient deficiencies
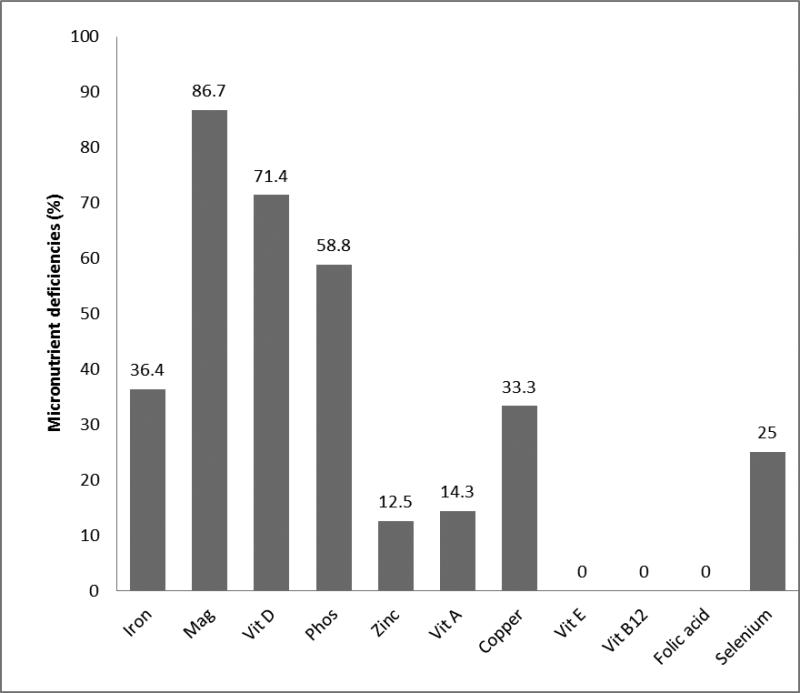
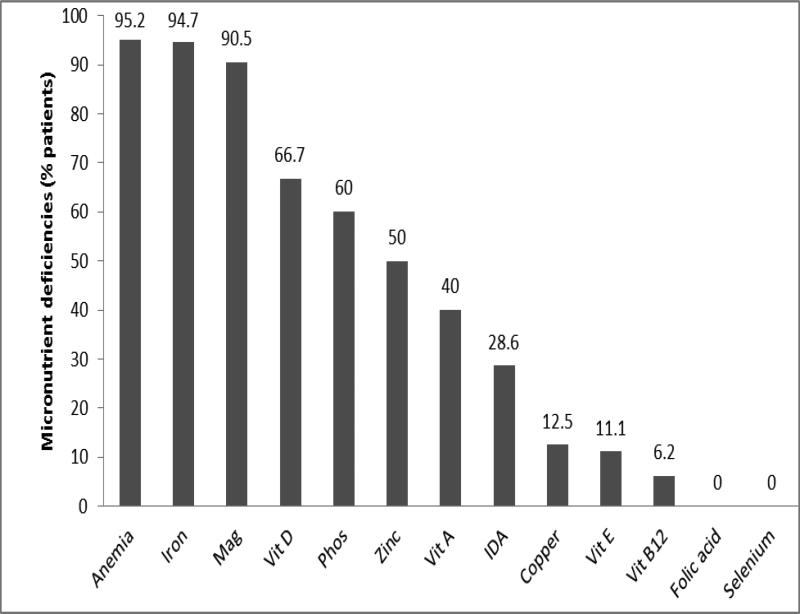
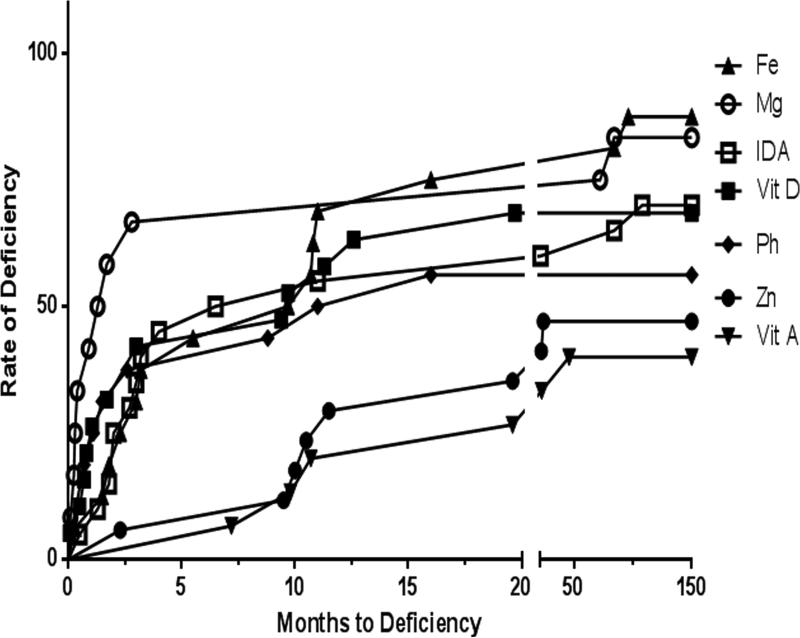
The overall prevalence of micronutrient deficiency after successful transition to EN was 95.2%. Multiple vitamin deficiencies were identified in 19%, while multiple mineral deficiencies were seen in 81% of patients. The prevalence of deficiencies of various micronutrients before successful transition to EN is shown in Figure 1. The most common micronutrient deficiencies after successful transition to EN were: iron (94.7%), magnesium (90.5%), zinc (50%), vitamin D (66.7%) and vitamin A (40%). Anemia was present in 95.2% of the patients. The least commonly deficient micronutrients were folate (0%), selenium (0%) and vitamin B12 (6.2%), as shown in Figure 2., The prevalence rate of micronutrient deficiencies of all micronutrients (except vitamin D, copper, and selenium) increased after successful transition to EN. The median times to developing vitamin A, zinc and iron deficiencies were 15.2, 11.5 and 5.5 months, respectively. Deficiencies of vitamin D, phosphorus and magnesium occurred at a median time of 2.4, 1.5 and 0.3 months, respectively. The time to developing deficiency of the most commonly deficient micronutrients is shown in Figure 3. There was no relationship between frequency of grade 2 or higher rejection episodes in the graft and detection of deficiency in the patient. All patients (100%) with documented grade 2 or higher rejection episodes compared to only 67% without a history of grade 2 or higher rejection had micronutrient deficiencies reported (P=0.2). The prevalence of micronutrient deficiencies was higher in the patients with ostomy (95.5%) compared to after ostomy takedown (50%) (p=0.004). When specific micronutrient or trace mineral deficiencies were identified; they were successfully managed by the team using therapeutic doses of the deficient micronutrient/trace mineral.
Figure 1.
Prevalence of deficiency of various micronutrients before successful transition to full EN. Bar showing percentage of patients with specific micronutrient deficiency. Mag=magnesium, Phos=phosphorus, Vit=vitamin.
Figure 2.
Prevalence of deficiency of various micronutrients after successful transition to full EN. Bar showing percentage of patients with specific micronutrient deficiency. Mag=magnesium, Phos=phosphorus, Vit=vitamin, IDA=iron deficiency anemia.
Figure 3.
Most commonly deficient micronutrients and months to their deficiencies after successful transition to enteral nutrition. A=vitamin A, D=vitamin D, Fe=iron, Mg=Magnesium, Ph=phosphorus, Zn=zinc, IDA=iron deficiency anemia.
Risk factors for micronutrient deficiency
All children aged 1-10 years (100%) at study entry had a documented micronutrient deficiency compared to 33.3% of those above 10 years (P=0.002). Of 18 patients with available data on vitamin D, all six African Americans (AA) developed vitamin D deficiency compared to 50% (6/12) of the non-AA (P=0.054) patients. The mean post-transplant duration for patients with vitamin D deficiency was 7.7 months compared to 13.2 months among the non-deficient group (P=0.04). Female gender showed a trend towards zinc deficiency (P=0.05). The mean duration off PN were 10.1 months and 19.5 months for patients with and without magnesium deficiency, respectively (P=0.04). The mean duration since transplant was also significantly lower (10.1 months) in patients deficient in magnesium compared to the non-deficient group (19.5 months) (P=0.04). Patients that received jejunal feeds (P=0.02) and those still on formula supplementation (P=0.049) significantly had more phosphorus deficiency. The risk factors for vitamin D, zinc, phosphorus and magnesium deficiency are shown in Table 3. With respect to multiple micronutrients deficiencies, weight below the 5th percentile showed a trend toward having multiple vitamin deficiency (P=0.06) while height below the 5th percentile showed a trend toward having multiple mineral deficiencies (P=0.053). A significant majority (92.9%) of patients with vitamin D deficiency were on government health insurance (P=0.014). Higher mean rank time since transplant (P=0.04) and lower mean rank duration off PN (P=0.03) were significantly associated with combined mineral-vitamin deficiencies. None of the risk factors remained statistically significant for deficiency of specific or multiple micronutrients after multivariate adjustments.
Table 3.
Risk factors for specific micronutrient deficiency
| McN Risk factors | Vitamin D N=18 | Zinc N=18 | Magnesium N=21 | Phosphorus N=20 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D n=12 (%) | ND n=6 (%) | P-value | D n=9 (%) | ND n=9 (%) | P-value | D n=19 (%) | ND n=2 (%) | P-value | D n=12 (%) | ND n=8 (%) | P-value | |
| F | 50 | 16.7 | 0.32 | 66.7 | 11.1 | 0.05 | 42.1 | 0 | 0.505 | 41.7 | 25 | 0.642 |
| Age† | 8.1 | 12.3 | 0.13 | 10.8 | 8.2 | 0.3 | 10.1 | 19.5 | 0.04* | 8.8 | 13.1 | 0.12 |
| C | 50 | 100 | 0.054 | 77.8 | 66.7 | 1 | 63.2 | 100 | 0.533 | 66.7 | 75 | 1 |
| NJ-feeds | 83.3 | 83.3 | 1 | 77.8 | 88.9 | 1 | 52.6 | 0 | 0.476 | 75 | 12.5 | 0.02* |
| HT<5th | 83.3 | 66.7 | 0.57 | 77.8 | 77.8 | 1 | 78.9 | 0 | 0.071 | 83.3 | 62.5 | 0.347 |
| WT<5th | 66.7 | 33.3 | 1 | 44.4 | 22.2 | 0.62 | 68.4 | 100 | 1 | 25 | 42.9 | 0.617 |
| No McN | 33.3 | 0 | 0.22 | 25 | 22.2 | 1 | 27.8 | 50 | 0.521 | 25 | 37.5 | 0.642 |
| PND† | 10.3 | 7.8 | 0.39 | 10.6 | 8.4 | 0.3 | 10.9 | 12.3 | 0.77 | 10.9 | 9.9 | 0.73 |
| off PN† | 7.3 | 13.8 | 0.13 | 11.4 | 7.6 | 0.14 | 10.1 | 19.5 | 0.04* | 9 | 12.8 | 0.18 |
| TST† | 7.7 | 13.2 | 0.04* | 11.7 | 7.3 | 0.09 | 10.1 | 19.5 | 0.04* | 9.3 | 12.4 | 0.27 |
No McN= No micronutrient supplementation, F=female, HT<5th = height less than 5th percentile, WT<5th =weight less than 5th percentile, D=deficient, ND=non-deficient, PND=parenteral nutrition duration, off PN= duration off parenteral nutrition, TST=time since transplant
mean rank, C=Caucasians, NJ=naso-jejenal, P=private, G=government.
Discussion
We report a very high prevalence of micronutrient deficiency (95.2%) among pediatric intestinal transplant recipients on enteral nutrition. Most of these deficiencies occurred in the early months after transitioning to full EN. The proportion of patients with at least one vitamin and one mineral deficiency were 77.8 and 100%, respectively. Multiple vitamin and mineral deficiencies occurred in 19% and 81% of the patients, respectively. This paper shows that intestinal transplant recipients have a higher risk for mineral deficiencies than for vitamin deficiencies. More than 90% of our patients had iron and magnesium deficiencies, as well as anemia. Other commonly deficient micronutrients noted were zinc, copper, vitamin D and vitamin A, while no deficiency was observed for selenium and folate. Interestingly isolated liver transplant patients are also frequently deficient in iron and magnesium [11, 12]. Iron deficiency may be due to blood loss in both isolated liver and small bowel transplant [12]. Furthermore, acid suppression employed for both types of transplant may impede iron oxidation in the stomach. As for magnesium deficiency, both isolated liver and small bowel transplant patients receive calcineurin inhibitors which impair renal tubular function causing tubular magnesium loss.
Venick and colleagues have previously reported their experience with 33 pediatric intestinal transplant recipients and revealed that zinc (44%), iron (41%) and copper (25%) were the most commonly deficient micronutrients at 6 months post-transplantation [13]. Their group had also reported in another study involving 19 children with history of intestinal transplant that zinc deficiency was common (documented in 17 of 19 (89.5%) children). It is not clear from these reports whether the patients had been transitioned to full enteral nutrition at the time of the study. The findings from previous studies that iron, zinc and copper are the most commonly deficient micronutrients in children who underwent intestinal transplantation concur with our results. However, our study showed a higher prevalence rate of deficiencies for most of these micronutrients.
There are a number of reasons for the high prevalence of zinc, copper and vitamin D deficiencies in intestinal transplant patients. Zinc deficiency has been related to high stoma output after transitioning to EN post-transplant [14] and to an increased rate of intestinal mucosal turnover that requires large amounts of zinc [13]. Multiple factors are responsible for iron deficiency including impaired absorption, bleeding from the allograft, frequent blood draws, and the use of acid suppression medication [13]. All of our patients received proton pump inhibitors and H2 blockers during post-transplant management. Cotrimoxazole used for PCP prophylaxis can also contribute to anemia through bone marrow suppression. Furthermore, 10 (47.6%) of our 21 patients underwent prolonged jejunal feedings, thereby bypassing the duodenum where under normal circumstances, most (75%) iron absorption occurs [15].
The finding of micronutrient deficiencies among intestinal transplant recipients contrasts with the course of patients who undergo isolated liver transplantation. Pescovitz et al [16] reported 94% prevalence of subnormal zinc levels in pre-transplant liver patients. The mean serum zinc level however significantly increased to normal values post-transplant in these patients. This suggests that micronutrient deficiencies in intestinal transplant patients may primarily be due to intestinal malabsorption. Suboptimal intestinal graft absorption which necessitates energy intake of at least twice the resting energy expenditure has been documented in intestinal transplant patients [9]. Graft absorptive capacity is likely suboptimal in these patients even in the absence of obvious rejection or malfunction [9]. The sex difference in the zinc status in our patients could not be explained by the data collected. This needs to be evaluated in a prospective study. The prevalence of vitamin B12 deficiency was low and there was no folate or selenium deficiency documented in our patients. This may be explained by the fact that folate and vitamin B12 are water soluble and their absorption and metabolism are minimally affected by any associated liver disease or fat malabsorption. The median times for developing vitamin A, vitamin D, zinc and iron deficiencies were longer than for developing magnesium and phosphorus deficiencies.
In the current study, we documented a high prevalence of magnesium and phosphorus deficiencies. The high rate of deficiency of magnesium and phosphorus early after transplant and prior to transition to full EN is related in large part to renal tubular dysfunction due to the use of tacrolimus immunosuppression. A second contributing factor may be the high stoma output observed in a recently transplanted bowel.
Younger children (≤10 years) had significantly more deficiencies than older children (P=0.002). Studies have shown that younger children generally have increased risk of micronutrient deficiencies [17, 18]. We theorize therefore, that older children have greater stores of micronutrients going into transplant, so that they do not become deficient as easily.
Conclusions
Children who have undergone intestinal transplantation are at significant risk for micronutrient deficiencies (especially minerals) in the early post-transplant period. Deficiencies were more common among younger patients and those who received jejunal feeding. Monitoring and multi-mineral supplementation in the first few months after intestinal transplantation is essential.
Acknowledgements
Our profound gratitude to Misty Troutt, Project Manager, Intestinal Rehabilitation Program and Justina Dunigan, Administrative Assistant, Intestinal Care Center for their support. We are also grateful to Dr MB Rao of the Department of Environmental Health, University of Cincinnati for his assistance.
Funding for this study was provided by the Global Health Center, Cincinnati Children's Hospital Medical Center OH. The project described was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8 UL1 TR000077-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Authors’ contributions: CRC, SAK, AGM, JDN, GMT, MHA conceptualized the study. CRC and SAK designed the study. ACU and CJH collected the data. CRC, ACU and CJH did the statistical analysis. CRC, SAK and ACU drafted the manuscript. All the authors participated in revising it the paper critically and approved the final manuscript.
Competing interest: The authors declare that they have no competing interests.
References
- 1.Soden JS. Clinical assessment of the child with intestinal failure. Seminars in Pediatric Surgery. 2010;19:10–19. doi: 10.1053/j.sempedsurg.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Kocoshis SA. Medical management of pediatric intestinal failure. Seminars in Pediatric Surgery. 2010;19:20–26. doi: 10.1053/j.sempedsurg.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Wessel JJ, Kocoshis SA. Nutritional management of Infants with Short Bowel Syndrome. Semin Perinatol. 2007;31:104–111. doi: 10.1053/j.semperi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Kato T, Mittal N, Nishida S, et al. The role of intestinal transplantation in the management of babies with extensive gut resections. Journal of Pediatric Surgery. 2003;38:145–149. doi: 10.1053/jpsu.2003.50033. [DOI] [PubMed] [Google Scholar]
- 5.Pirenne J, Koshiba T, Coosemans W, Herman J, Lombaerts R. Recent advances and future prospects in intestinal and multi-visceral transplantation. Pediatr Transplantation. 2001;5:452–456. doi: 10.1034/j.1399-3046.2001.t01-2-00025.x. [DOI] [PubMed] [Google Scholar]
- 6.Porubsky M, Testa G, John E, Holterman M, Tsou M, Benedetti E. Pattern of growth after pediatric living-donor small bowel transplantation. Pediatr Transplantation. 2006;10:701–706. doi: 10.1111/j.1399-3046.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 7.Nucci AM, Barksdale EM, Beserock N, et al. Long-term nutritional outcome after pediatric intestinal transplantation. Journal of Pediatric Surgery. 2002;37:460–463. doi: 10.1053/jpsu.2002.30863. [DOI] [PubMed] [Google Scholar]
- 8.Venick RS, Farmer DG, Saikali D, et al. Nutritional Outcomes Following Pediatric Intestinal Transplantation. Transplantation Proceedings. 2006;38:1718–1719. doi: 10.1016/j.transproceed.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 9.Ordonez F, Barbot-Trystram L, Lacaille F, et al. Intestinal absorption rate in children after small intestinal transplantation. Am J Clin Nutr. 2013;97:743–749. doi: 10.3945/ajcn.112.050799. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz P, Takahashi H, Delacruz V, et al. International grading scheme for acute cellular rejection in small-bowel transplantation: single-center experience. Transplantation proceedings. 2010;42:47–53. doi: 10.1016/j.transproceed.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Elgend HM, El Moghazy WM, Uemoto S, Fukuda K. Pre transplant serum magnesium level predicts outcome after pediatric living donor liver transplantation. Ann Transplant. 2012;17:29–37. doi: 10.12659/aot.883220. [DOI] [PubMed] [Google Scholar]
- 12.Maheshwari A, Mishra R, Thuluvath PJ. Post-liver-transplant anemia: etiology and management. Liver Transpl. 2004;10:165–73. doi: 10.1002/lt.20031. [DOI] [PubMed] [Google Scholar]
- 13.Venick RS, Wozniak LJ, Colangelo J, et al. Long-term nutrition and predictors of growth and weight gain following pediatric intestinal transplantation. Transplantation. 2011;92:1058–1062. doi: 10.1097/TP.0b013e31822f2b1b. [DOI] [PubMed] [Google Scholar]
- 14.Strohm S, Koehler A, Mazariegos G, Reyes J. Nutrition management in pediatric small bowel transplant. Nutr Clin Pract. 1999;14:58–63. [Google Scholar]
- 15.Zhu A, Kaneshiro M, Kaunitz JD. Evaluation and treatment of iron deficiency anemia: a gastroenterological perspective. Dig Dis Sci. 2010;55:548–559. doi: 10.1007/s10620-009-1108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pescovitz MD, Mehta PL, Jindal RM, Milgrom ML, Leapman SB, Filo RS. Zinc deficiency and its repletion following liver transplantation in humans. Clin Transplant. 1996;10:256–260. [PubMed] [Google Scholar]
- 17.Laillou A, Pham TV, Tran NT, Le HT, Wieringa F, et al. Micronutrient Deficits Are Still Public Health Issues among Women and Young Children in Vietnam. PLoS ONE. 2012;7:e34906. doi: 10.1371/journal.pone.0034906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thurlow RA, Winichagoon P, Pongcharoen T, et al. Risk of zinc, iodine and other micronutrient deficiencies among school children in North East Thailand. European Journal of Clinical Nutrition. 2006;60:623–632. doi: 10.1038/sj.ejcn.1602361. [DOI] [PubMed] [Google Scholar]