Abstract
Recent published studies have highlighted the complexity of the immune response to allergens, and the various asthma phenotypes that arise as a result. While the interplay of regulatory and effector immune cells responding to allergen would seem to dictate the nature of the asthmatic response, little is known as to how tolerance versus reactivity to allergen occurs in the lung. The vast majority of mouse models study allergen encounter in naïve animals, and therefore exclude the possibility that previous encounters with allergen may influence future sensitization. To address this, we studied sensitization to the model allergen OVA in mice in the context of pre-existing tolerance to OVA. Allergen sensitization by either systemic administration of OVA with aluminum hydroxide or mucosal administration of OVA with low-dose lipopolysaccharide (LPS) was suppressed in tolerized animals. However, higher doses of LPS induced a mixed Th2 and Th17 response to OVA in both naïve and tolerized mice. Interestingly, tolerized mice had more pronounced Th17 type inflammation than naïve mice receiving the same sensitization, suggesting pre-existing tolerance altered the inflammatory phenotype. These data show that a pre-existing tolerogenic immune response to allergen can impact subsequent sensitization in the lung. These findings have potential significance in understanding late-onset disease in severe asthmatics.
Introduction
Asthma is a chronic and sometimes debilitating human disease that is initiated by inappropriate effector immune responses to normally innocuous inhaled allergens. Most of the allergens that induce asthma are ubiquitous in the environment, and therefore it is likely that many humans have a rich history of allergen encounter. This is supported by published work that suggests both asthmatic and healthy non-asthmatic adults have made adaptive immune responses to allergen (1, 2). Given the fact that development of asthma is not restricted to young children and occurs at all ages, it is important to better understand whether and how exposure history impacts sensitizing responses to allergen.
Animal models of asthma have typically relied on artificial routes of sensitization (e.g. intraperitoneal injection) and studied synthetic adjuvants (e.g. alum) that induce Th2-dominant, eosinophilic airway inflammation after allergen re-exposure. More recent approaches using airway (mucosal) exposure models have found that allergic sensitization occurs when allergens are co-administered with an adjuvant such as lipopolysaccharide (LPS), while administration of protein alone causes a suppressive, tolerogenic response to allergen (3–5). In naïve hosts, subtle differences in the dose and timing of adjuvant exposure can translate into significant differences in the quantity and quality of mucosal effector responses induced following allergen re-exposure, typically involving Th2 or Th2/Th17-dependent airway inflammation (6). In contrast, the tolerogenic response involves IL-10 producing dendritic cells, regulatory T cells, and limited inflammation (7–11). However, most studies to-date have focused on the events following mucosal sensitization in naïve hosts, and very little is known about how tolerance shapes the development of subsequent immunity.
There is an emerging body of literature implicating the importance of Th17 responses in asthma pathogenesis. In one animal model of mucosal allergen sensitization and challenge, Th17 cells are induced along with Th2 cells and they promote a neutrophilic lung inflammatory response that contributes to airway hyperreactivity (AHR) (5). IL-17A produced by Th17 cells has been shown to enhance smooth muscle contraction in the lung and therefore contribute to airway narrowing during disease (12). The serum level of IL-23, a Th17-related cytokine, has also been shown to inversely correlate with airway obstruction in asthmatics (13). These data and others (14) seem to correlate well with severe asthmatics, whose disease manifestation often includes a prominent lung neutrophilia (15, 16).
While much has been learned through the study of asthma in the context of systemic administration of allergen in mice, there is an increased need to study sensitization in the context of mucosal allergen exposure. There is now a prominent body of literature suggesting innate and epithelial derived cytokines, as well as innate lymphoid cells, can significantly influence the nature of the mucosal immune response (17–20). Previous studies using mouse models of inhaled tolerance have studied the role of tolerance in inhibiting systemic allergen sensitization (7, 8). It is currently unclear how allergen sensitization via the mucosal route would impact pre-existing tolerance to a model allergen.
In an attempt to better understand mucosal sensitization in a host with a history of allergen exposure, we developed a mouse model to study mechanisms of allergic sensitization in the context of pre-existing inhaled tolerance. Our initial studies characterizing this system using chicken OVA as a model allergen suggest three major conclusions. The first is that typical Th2-promoting stimuli that induce robust inflammation in naïve mice are completely ineffective in the setting of previous tolerance, even when given via the mucosal route. Second is that breakdown of tolerance and initiation of allergic inflammation after administration of LPS is associated with a mixed Th2/Th17 response in mice. Thirdly, history of OVA exposure impacted the immune response following sensitization, resulting in an enhanced Th17 response. These studies indicate that pre-existing tolerance has a previously overlooked role in shaping the development of deleterious immune responses in the lung.
Methods
Mice
Male C57Bl/6 mice (6–12 weeks of age) were purchased from NCI. T cell receptor transgenic OT-II mice (21)(gift from Dr. David Topham) were crossed to the B6. PL (CD90.1) and B6. SJL (CD45.1) backgrounds and bred in the University of Rochester animal facility. All mice were housed in the University of Rochester animal facility. Animal protocols were approved through the University Committee on Animal Resources and were conducted according to safety guidelines.
Allergen sensitization models
On day 0–2, naïve mice were exposed daily to either PBS as control or 100ug Grade V OVA (Sigma) in 30ul via intranasal inoculation (using an IL-6 bioassay in RAW macrophages, we estimated that the OVA contained 10ng endotoxin per 100ug OVA). For the mucosal sensitization model, mice were sensitized day 12–14 daily by oropharyngeal administration of 100ug OVA mixed with 100ng LPS (E. Coli strain O55:B5 from Sigma), 1ug LPS, or nothing in 50ul. For the systemic model, a mixture of 10ug OVA and 2mg Alum (Thermo Scientific) was administered by intraperitoneal injection on day 12. For all sensitization groups, twice-daily challenges (1hr. each) were done day 26–28 in an inhalation chamber with 1% Grade V OVA aerosolized via jet nebulizer (Salter) at 10psi. Mice were sacrificed on day 30. Before intranasal and oropharyngeal inoculation, all mice were first sedated with Avertin (2,2,2-tribromoethanol).
CD4+ T cell adoptive transfer
Spleens were removed from OT-II mice and single cell suspensions were made. Following red blood cell lysis (eBioscience), cells were stained with antibodies and sorted by FACS (BD FACS Aria) based on CD3+CD4+CD25-CD44lo. Sorted cells were washed and intravenously injected into C57Bl/6 recipient mice as indicated in Supplementary Figure 1 and Figure 6A.
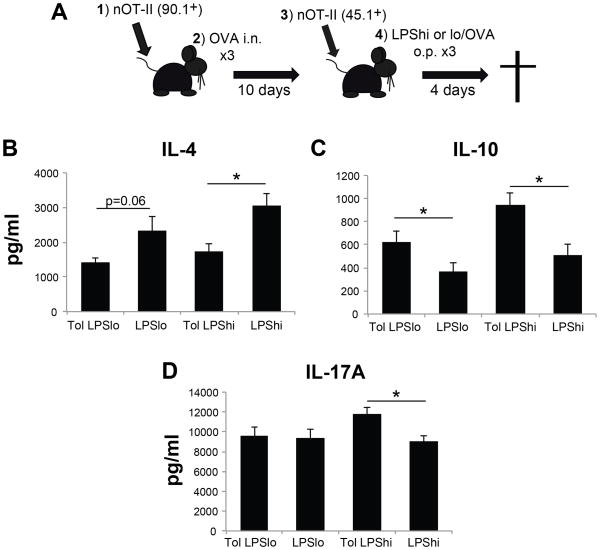
Figure 6. Pre-existing tolerance alters the effector cytokine response in MLN following mucosal sensitization.
A) Schematic of the dual adoptive transfer protocol. 1: Adoptive transfer of FACS-sorted naïve CD90.1+ OT-II cells (4×104); 2: 24hr after cell transfer, mice were given three consecutive daily doses of PBS or OVA intranasal; 3: ten days later, FACS-sorted naïve CD45.1+ OT-II cells (4×104) were adoptively transferred; 4: 24hr after cell transfer, mice were sensitized with LPSlo/OVA or LPShi/OVA o.p. Cytokine analyses were done four days after the last sensitization. B–D) 2×106 MLN cells from immunized mice were cultured for 4hr in PMA and A23187 in a 96-well round bottom plate. Culture supernatants were assayed via cytokine multiplex for IL-4 (B), IL-10 (C), and IL-17A (D). n = 9–11 mice per group, * = p<0.05.
Sample acquisition and analysis
After sacrifice, bronchoalveolar lavage (BAL) was obtained by washing the airways twice with 750ul PBS from a 1ml syringe connected to a Teflon cannula. Cell counts were performed using Trypan blue exclusion, and differential counts were done as previously described (22). For cytokine measurements, Bioplex (BioRad) and Milliplex (Millipore) cytokine bead arrays were performed on BAL supernatants and run on a Bioplex 200 analyzer. Lungs were processed for histological techniques as previously described (23). Scoring of H&E stained lung sections was done as previously described (23). Periodic acid Schiff (PAS) section scoring: airways that contained more than one PAS+ cell were considered positive. Mediastinal lymph nodes (MLN) were also removed and single cell suspensions made. After counting, cell populations were identified by surface and intracellular FACS staining. Fluorescent antibodies for CD3-FITC and PE, CD4-PerCP/Cy5.5, CD44-Pacific Blue, CD25-PE/Cy5, CD90.1-Biotin, CD45.1-Alexa780, RORγt-PE, and Foxp3-APC were purchased from BD, eBioscience, and Biolegend. Streptavidin-Pacific Orange was purchased from Invitrogen. For cytokine analysis, MLN cells were plated ex vivo at 2×106 cells per well in a 96-well round bottom plate and incubated for 5hrs with PMA (10ng/ml) and A23187 ionophore (1uM). Culture supernatants were analyzed using Bioplex bead array.
Measurement of airway hyperreactivity
Following sensitization and challenge, mice were sedated with Avertin, paralyzed with 0.5mg/kg succinylcholine, and tracheostomy was performed. Airway challenges with increasing doses of methacholine and measurements via plethysmography were done as previously described (24).
Statistical analysis
Statistical analyses were performed using student T test. A p value less than 0.05 was considered significant.
Results
Tolerized animals are protected from allergic inflammation under typical Th2 promoting conditions
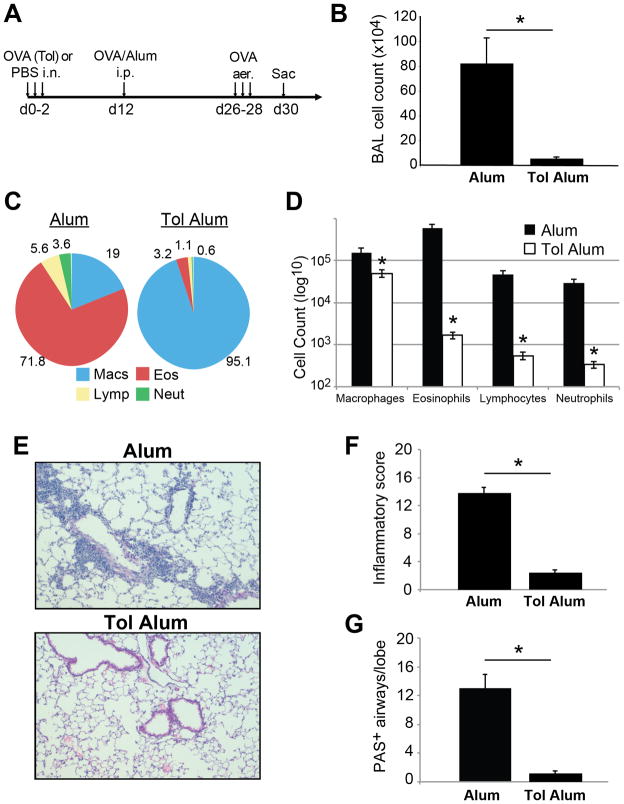
In order to study how pre-existing tolerance influenced the subsequent immune response to allergen exposure, we used the model allergen OVA and induced inhalation tolerance by repeated intranasal inoculation with OVA in the absence of adjuvant (4, 7). Using adoptive transfer, we found that this protocol caused the expansion and differentiation of antigen-specific Foxp3+ T cells (referred to as induced Tregs, or iTregs, from here on), as well as accumulation of these cells in the lung (Supplemental Fig. 1 and data not shown). Ten days after induction of tolerance, mice were given intraperitoneal OVA in Alum as a sensitizing agent, and then were challenged with aerosolized OVA to promote Th2-type pulmonary inflammation (Fig. 1A). In control mice, robust pulmonary inflammation and eosinophilia was observed following OVA challenge. However, mice with established tolerance had little to no pulmonary inflammation in response to OVA challenge, with the majority of recovered BAL cells identified as macrophages (Fig. 1B–D). There was a >90% reduction in both total BAL cell recovery and eosinophils comparing naïve and tolerized mice. This was also reflected in the extent of inflammation and number of mucus-secreting cells observed in lung sections from these mice taken post-challenge (Fig. 1E–G). Therefore similar to a previous report (7), we found that established inhalation tolerance prevents allergic sensitization by OVA/Alum in mice.
Figure 1. Prevention of OVA/Alum induced allergic inflammation in tolerized mice.
A) Immunization regimen for systemic OVA/Alum sensitization and challenge. i.p. = intraperitoneal; aer. = aerosol challenge. B–C) Tolerized and control mice were sensitized according to the OVA/Alum protocol. Total BAL cell recovery (B), differential counts (C) and absolute number of macrophages, lymphocytes, neutrophils and eosinophils at day 30 (D) are shown. E–G) Analysis of lung sections from OVA/Alum treated mice. E) Representative H&E lung sections from tolerized and control mice. F) Scoring of H&E lung sections for severity of inflammation; 0–4 score per lobe, maximum score of 20 per lung. G) Analysis of periodic acid schiff (PAS) stained lung sections from same mice, scored as the number of airways per lung lobe that contained >1 PAS+ epithelial cell. n=4–8 mice per group, * = p<0.05.
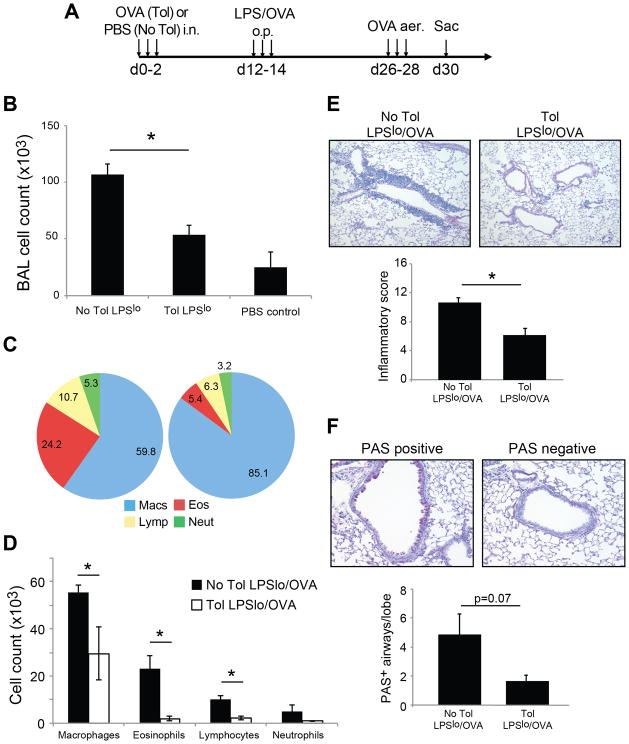
These data raised the question as to whether Th2-type OVA sensitization could be induced in tolerized mice using other sensitization strategies. To address this, we used a more physiologically relevant model of administering low-dose LPS (0.1ug) with OVA by oropharyngeal aspiration as a means of inducing Th2-type inflammation via the respiratory mucosa (25)(Fig. 2A). We first tested the accumulation of iTreg in this model by transfer of naïve OT-II cells. As expected, iTreg were induced in this model similar to OVA/Alum immunization, and were present in the draining MLN three days after sensitization (708+/− 256 OT-II iTreg in tolerance group vs. 334 +/−110 in no tolerance group). After antigen challenge, tolerized mice were also completely protected from the effects of sensitization with low-dose LPS plus OVA, whereas control non-tolerized animals exhibited increased BAL cell recovery and eosinophilia after OVA challenge using this immunization regimen (Fig. 2). Therefore, using both systemic and mucosal models of sensitization for Th2-type allergic inflammation, we were unable to induce pulmonary inflammation to OVA in tolerized mice.
Figure 2. Tolerized mice are protected from mucosal LPSlo/OVA induced allergic inflammation.
A) Immunization regimen for LPSlo/OVA mucosal sensitization and challenge. o.p. = oropharyngeal. Total BAL cell recovery in tolerance, no tolerance, and PBS control groups (B), BAL differential cell frequency (C) and absolute differential cell number (D) were determined on day 30. E) Scoring of inflammation from H&E stained lung sections with representative sections from each group shown. F) Quantification of PAS+ airways from PAS stained lung sections post-challenge. A representative ‘positive’ and ‘negative’ PAS stained airway are shown for comparison. n=5–19 mice per group, * = p<0.05.
Higher doses of LPS given during sensitization break established tolerance
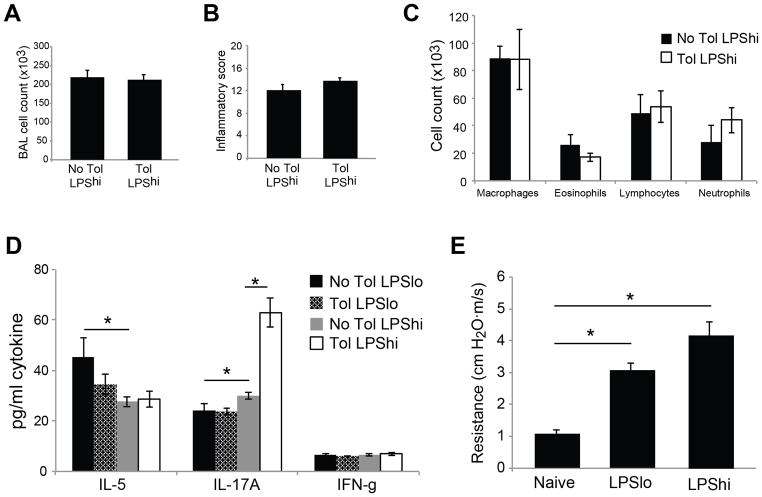
Published data has shown that administration of different doses of LPS can result in the generation of qualitatively distinct adaptive immune responses (25). Since low-dose LPS was insufficient to break tolerance when used as an inhaled adjuvant, we tested whether higher inhaled doses of LPS would act as an effective sensitizing agent. To test this hypothesis, mice were given a ten-fold higher dose of LPS (1ug LPS, LPShi) during mucosal sensitization with OVA. In contrast to the LPSlo/OVA group, LPShi/OVA sensitization induced robust pulmonary inflammation in both naïve and tolerized groups after subsequent OVA challenge (Fig. 3A–B). Sensitization with LPS in the absence of OVA was not sufficient to induce a response after challenge (Supplemental Fig. 2). As expected, LPShi/OVA sensitization resulted in greater overall BAL cell recovery than LPSlo/OVA (comparing Figs. 2B and 3A; p<0.05). Interestingly, increased numbers of neutrophils were also recovered compared to LPSlo, suggesting the two doses of LPS may be inducing qualitatively distinct lung inflammatory responses (comparing Figs. 2D and 3C; p<0.05). Cytokine analysis of BAL supernatants after OVA challenge supported this idea, and showed a reduction in IL-5 with a concomitant increase in IL-17A in LPShi/OVA sensitized animals compared to LPSlo/OVA (Fig. 3D). Interestingly, tolerized animals sensitized with LPShi/OVA had the most IL-17A recovered from BAL, suggesting the immunization history in tolerized mice influenced the subsequent effector response during the breakdown of tolerance. Despite the differences in response with LPS dose, both groups of mice developed pulmonary responses characteristic of allergic inflammation. Besides pulmonary eosinophilia, LPS/OVA sensitized mice had increased airway resistance and prominent airway mucus production after challenge, regardless of the dose used (Fig. 3E and data not shown). Taken together, these data suggest that LPShi/OVA sensitized mice induce a mixed Th2 and Th17 immune response that results in the breakdown of established tolerance and induction of allergic inflammation.
Figure 3. High-dose LPS/OVA sensitization breaks established tolerance and induces allergic inflammation.
Tolerized and control mice were sensitized with LPSlo/OVA or LPShi/OVA. All mice were challenged with OVA aerosol and assayed on day 30. A–C) BAL inflammatory cell recovery (A), scoring of lung inflammation from H&E stained sections (B) and absolute cell number from BAL differential counts (C) after LPShi/OVA sensitization and challenge. D) BAL supernatants from LPSlo/OVA or LPShi/OVA sensitized and challenged mice were assayed by cytokine multiplex for IL-5, IL-17A, and IFN-γ.E)Analysis of airway resistance to methacholine challenge using plethysmography. Resistance to 0.3mg/ml methacholine is reported in cm H2O•m/s. n = 5–11 mice per group, * = p<0.05.
Enhanced lung inflammatory response following LPShi/OVA sensitization
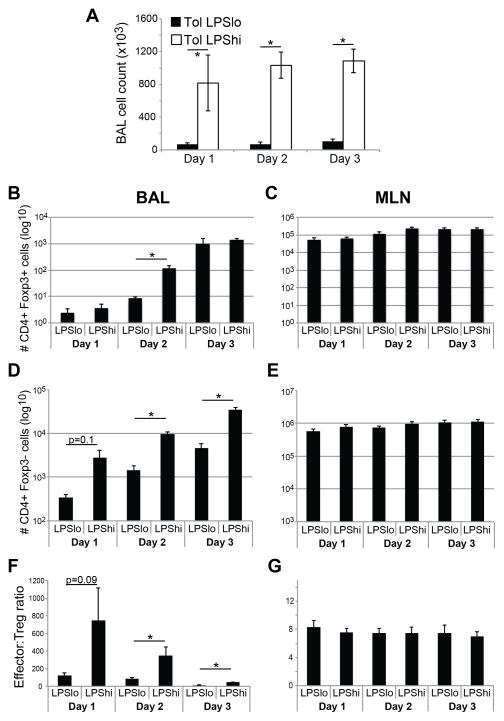
By using LPS as an adjuvant for mucosal sensitization, we observed a dose-dependent breakdown of established inhalation tolerance to OVA. This model, which uses the same adjuvant but at different doses, enabled us to study the mechanisms of endotoxin-induced tolerance breakdown in the lung. Since the immune response in this model is initiated during the sensitization phase (see Fig. 2A), we compared inflammatory responses in tolerized animals during sensitization with LPSlo/OVA or LPShi/OVA. There was an LPS dose-dependent increase in innate cytokines IL-1, IL-6, IL-17A and TNF-αin BAL during mucosal sensitization, particularly following the first two days of treatment (Fig. 4). Despite the increase in innate cytokines after LPShi/OVA sensitization, there was no difference in IL-10 recovery between groups (Fig. 4D). Consistent with the cytokine data, a higher dose of adjuvant yielded a more robust inflammatory response after each of the three sensitizations, as evidenced by total BAL cell recovery (Fig. 5A). Interestingly, while total BAL cellularity and Foxp3 negative CD4+ cells were increased in LPShi/OVA-sensitized mice (Fig. 5D), the recovery of CD4+ CD25+ Foxp3+ Treg cells was not markedly different between groups, with a significant difference only observed after the second sensitization (Fig. 5B). This prompted us to compare the recoveries of CD44hi CD4+ Foxp3- effector and CD4+ Foxp3+ Treg cells in BAL and MLN during sensitization. Interestingly, the effector:Treg ratio in BAL was higher during LPShi/OVA sensitization compared to LPSlo/OVA (Fig. 5F), suggesting many more targets per suppressor cell were present during an immunization regimen that broke tolerance. In contrast, the CD4+:Treg ratio in the draining MLN was the same in both groups (Fig. 5G).
Figure 4. Breakdown of tolerance is associated with an increased airway innate inflammatory response following mucosal sensitization.

BAL supernatants were taken from tolerized mice 18hr after each of three sensitizations with LPSlo/OVA (triangles) or LPShi/OVA (squares). Amount of IL-1α(A)IL-1β(B), IL-6 (C), IL-10 (D), IL-17A (E), and TNFα(F) were determined by cytokine multiplex. n = 5 per mice per group, * = p<0.05.
Figure 5. Analysis of cellular dynamics in BAL and draining MLN of tolerized mice following mucosal sensitization.
A) Total BAL cell recovery 18hr after each of three sensitizations with either LPSlo/OVA or LPShi/OVA. B–E) Flow cytometric analysis of BAL (B, D) and MLN (C, E) cells after sensitization. Absolute numbers of CD3+ CD4+ Foxp3+ cells (B–C) and CD3+ CD4+ CD44hi Foxp3- cells (D–E) are reported. F–G) Recovery of CD3+ CD4+ Foxp3+ and Foxp3- subsets were determined by flow cytometry. The ratio of CD4+ non-Treg to CD4+ Treg cells in BAL (F) and MLN (G) was determined by dividing the number of non-Treg by the number of Treg cells. n = 6–15 per group, * = p<0.05.
Capacity of iTregs in the MLN to suppress the effector response during breakdown of tolerance
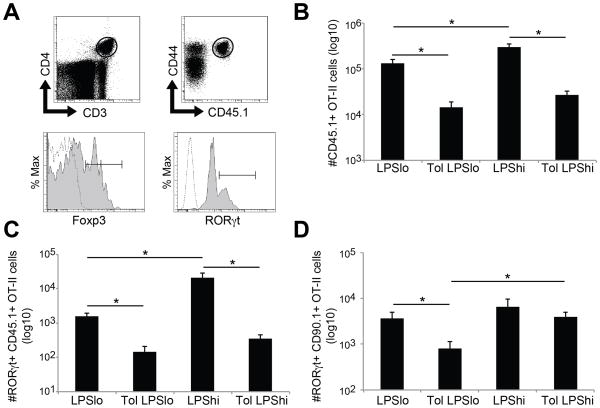
In contrast to the robust inflammation observed in BAL following LPShi/OVA, there were no significant differences observed in the cellularity or CD4+:Treg ratio of the MLN comparing low- and high-dose LPS sensitization (Fig. 5 and data not shown). Since this lymph node is a site of initiation of pulmonary immune responses, we hypothesized that antigen-specific iTregs in the MLN must be functionally impaired in tolerized mice in order to allow the development of effector T cell responses following LPShi/OVA sensitization. To address this, we adoptively transferred OT-II T cell receptor transgenic T cells to test the suppressive capacity of Tregs in the MLN after sensitization with low- or high-dose LPS and OVA (see Fig. 6A for protocol). OT-II cells used for adoptive transfer were derived from transgenic mice on the congenic B6. PL (CD90.1) and B6. SJL (CD45.1) backgrounds so they could be identified by FACS analysis in the same recipient animal. In preparation for adoptive transfer, all OT-II cells were FACS sorted for CD3+ CD4+ CD44lo CD25- naïve cells. This ensured that any activation or expansion of OT-II cells observed after sensitization was the result of the in vivo immunization. Re-stimulation of MLN cells from immunized mice ex vivo showed increased IL-10 in tolerized groups, and increased IL-4 in non-tolerized groups (Fig. 6B–C). Further experiments to determine the cellular source of IL-10 in the MLN suggested Treg as IL-10 producers (data not shown). Similar to BAL measurement after OVA challenge, increased IL-17A was recovered from tolerized mice sensitized with LPShi/OVA (Fig. 6D). In the absence of established tolerance, CD45.1+ OT-II cells expanded following both low- and high-dose LPS/OVA sensitization, with increased expansion observed following LPShi/OVA (Fig. 7B). Surprisingly, in tolerized animals, CD45.1+ OT-II cell recovery from MLN was reduced after both low- and high-dose LPS/OVA sensitization (Fig. 7B). Development of effector cells was also suppressed, as shown by reduced recovery of RORγt+ CD45.1+ OT-II cells from MLN (Fig. 7C). These data suggest that iTregs present in the draining lymph node function to suppress the lymph node response, even in a situation where tolerance to a model aeroallergen was being broken.
Figure 7. Unique populations of effector T cells develop during tolerance breakdown.
Adoptive cell transfer and immunization schedule are the same as in Figure 6A. All data is from MLN cells recovered four days after the last sensitization. A) Representative FACS plots showing the gating scheme for OT-II cell analysis. After gating based on cell scatter (not shown), CD3+ CD4+ cells were further gated into CD44+ CD45.1+ or CD44+ CD90.1+ populations to track the adoptively transferred OT-II cells. Transcription factor gating for RORγt or Foxp3 was done using fluorescence minus one (FMO) controls for each. Dotted line: FMO control. Solid line: antibody staining. B) Total recovery of CD45.1+ OT-II cells from MLN of tolerized or control animals after LPS/OVA sensitization. C–D) Total recovery of RORγt+ CD45.1+ (C) or CD90.1+ (D) OT-II cells from all immunization groups. n = 12–14 per group, * = p<0.05.
Given the seeming contradiction between the intense inflammation and paucity of CD45.1+ OT-II cell expansion in MLN during breakdown of tolerance, we further analyzed the CD90.1+ OT-II cells for phenotypic changes after sensitization. Similar to CD45.1+ OT-II cells, we found that RORγt+ CD90.1+ OT-II cells also developed after sensitization. Interestingly, RORγt+ CD90.1+ OT-II cell recovery was significantly increased in tolerized mice sensitized with LPShi/OVA, but not following LPSlo/OVA sensitization (Fig. 7D). Since only a fraction of the CD90.1+ OT-II cells became iTregs after inducing tolerance (Supplemental Figure 1), the RORγt+ cells may be developing from either Foxp3- precursor cells or Foxp3+ cells. However, there were few CD90.1+ OT-II cells that expressed both Foxp3 and RORγt after sensitization, and there was no observed difference in the frequency of Foxp3 and RORγt double positive cells comparing LPSlo/OVA and LPShi/OVA sensitization (data not shown). Taken together, these data suggest the stimuli resulting from LPShi/OVA sensitization causes intense local innate inflammation that results in the conversion of distinct populations of CD4+ T cells into effector cells that promote allergic inflammation.
Discussion
Mouse models of asthma have yielded important insights into disease pathogenesis, especially during the effector phase of the immune response (26–30). However, less is known about the initiation of maladaptive allergen-specific immunity in the airway, in part because most studies to-date have used non-physiologic routes of allergen sensitization (e.g. intraperitoneal injection of OVA/alum). The few studies that have investigated initiation of allergen-specific immune responses in the airway have used naïve hosts, therefore not taking into account the potential impact of exposure history on the immune response. In this report we describe a model of asthma dependent on the breakdown of established tolerance that provides new insights into the initiation of mucosal allergic immunity. Interestingly, the inflammatory phenotype after the breakdown of tolerance in mice showed some resemblance to human severe asthma, with increased inflammatory response in the lung, neutrophilia accompanying eosinophilia, increased airway hyper-responsiveness, and increased IL-17A production. We also found that a small difference in the dose of inhaled adjuvant has a powerful effect on the ability to breakdown tolerance, and influences the subsequent memory response both quantitatively and qualitatively. Using adoptive transfer experiments, we show that a fine balance of effector and regulatory T cells is required to maintain tolerance, and that development of distinct populations of effector cells may be a hallmark of tolerance breakdown. Characterization of this model suggests it will be useful to study the mechanisms of allergic sensitization in previously tolerized animals.
Using this model of OVA tolerance via the respiratory route, we found that tolerized animals are resistant to systemic immunization with OVA/Alum, as previously reported (7). Given the importance of innate and epithelial derived cytokines in influencing T cell priming and polarization (17, 19), our hypothesis was that mucosal sensitization with LPS would engage the lung innate response and induce Th2 inflammation in tolerized mice. The data show that only high doses of LPS are able to promote pulmonary inflammation in previously tolerized mice. In this inflammatory setting, increased innate lung inflammation induced a Th2/Th17 response that resulted in a mixed inflammatory infiltrate. A plausible explanation from these data is that tolerance increases the threshold of inflammation required to prime an effector response. If this is the case, low-level exposure to endotoxin will not be sufficient to induce allergic pulmonary inflammation, unlike in naïve mice (25). The clinical implication is that if an individual is tolerized through allergen exposure history, allergen re-exposure in the context of robust pulmonary inflammation is required in order to break tolerance. This may favor non-classical clinical presentations such as neutrophilic Th17-type inflammation.
Given the strong accumulation of CD44hi CD4+ cells in BAL of high-dose LPS sensitized mice, it is striking that recovery of Tregs was not as robust in comparison. One possibility is that different chemokine pathways regulate entry and retention of CD44hi non-Treg vs. CD25+Foxp3+Tregs (31). Alternatively, Tregs may be recruited to the lung, but have a short half-life due to a high rate of cell death in an inflammatory environment. In support of this notion, we found that the concentration of free ATP is increased in BAL after high-dose LPS sensitization (data not shown). Given the potentially deleterious effects of ATP signaling on Treg survival and function (32) as well as a potential role in Th17 cell development (33), this may be an important pathway leading to the breakdown of tolerance. Further work is needed to define the importance of this pathway in limiting suppression in the lung.
We noted consistent differences in the effector response when comparing high-dose LPS sensitization in the presence or absence of tolerance. One was increased IL-17A recovery from MLN and BAL after OVA sensitization and challenge, respectively. We also measured increased recovery of RORγt+ CD90.1+ OT-II cells when tolerance was broken. The difference in IL-17A recovery may be related to the populations of Th17 cells generated in the presence or absence of tolerance. Recent published data has shown that Tregs can promote Th17 responses in certain conditions (34, 35). In our model, we suspect that the process of inducing tolerance via intranasal OVA generates a population of precursor cells capable of becoming Th17 cells following high-dose LPS sensitization.
Studies that attempt to sensitize naïve mice to allergens have shown that a multitude of infectious, inflammatory and environmental stimuli are capable of acting as adjuvants for the induction of allergic inflammation. Given the rapidly increasing number of adjuvants being identified as potential risk factors for the induction of asthma, many of which are highly prevalent, it is unclear why there are not more asthmatic humans. The studies reported here, as well as ongoing studies in our lab, suggest that only a subset of adjuvants are capable of inducing allergic inflammation in tolerized mice. It is important to assess whether typical models of allergic inflammation in naïve mice are accurately reflecting allergic susceptibility in the human population. In order to properly identify critical risk factors for asthma, there needs to be a greater understanding of allergen responsiveness in humans both pre- and post-sensitization.
Little is currently known about the relationship between allergen exposure history and the inflammatory response present in asthmatics. The model used in this paper suggests an association between Th17 response to allergen and breakdown of tolerance. It will be important in future studies to determine whether the findings reported here can be generalized to other inhaled adjuvants. If regulatory T cells are raising the threshold of inflammation needed to develop pulmonary symptoms, strong stimuli such as viral or bacterial infection may be needed in order to break allergen tolerance for the eventual development of asthma. We conclude that initiation of allergic inflammation in previously tolerized individuals occurs through distinct mechanisms than in otherwise allergen naïve hosts. Furthermore, subtle differences in mucosal inflammation during allergen initiation can translate into qualitatively distinct effector responses. This observation may help explain the development of heterogeneous immune phenotypes in asthma despite similar environmental exposures.
Supplementary Material
Acknowledgments
Funding sources: NIH R01 HL071933, ES01247, and P30 ES001247 to SNG; NIH F32 HL110718-01 and T32 HL66988-09 to TJC; NIH K12 HD068373 to FR; and T32 AI007285 to SAK
We thank the Flow Core at the University of Rochester Medical Center for technical expertise on cell sorting experiments.
References
- 1.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, Kegel C, Disch R, Schmidt-Weber CB, Blaser K, Akdis CA. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palomares O, Yaman G, Azkur AK, Akkoc T, Akdis M, Akdis CA. Role of Treg in immune regulation of allergic diseases. European journal of immunology. 2010;40:1232–1240. doi: 10.1002/eji.200940045. [DOI] [PubMed] [Google Scholar]
- 3.Piggott DA, Eisenbarth SC, Xu L, Constant SL, Huleatt JW, Herrick CA, Bottomly K. MyD88-dependent induction of allergic Th2 responses to intranasal antigen. J Clin Invest. 2005;115:459–467. doi: 10.1172/JCI22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsitoura DC, Blumenthal RL, Berry G, Dekruyff RH, Umetsu DT. Mechanisms preventing allergen-induced airways hyperreactivity: role of tolerance and immune deviation. J Allergy Clin Immunol. 2000;106:239–246. doi: 10.1067/mai.2000.108429. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. American journal of respiratory and critical care medicine. 2009;180:720–730. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nature reviews Immunology. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 8.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 9.Campbell JD, Buckland KF, McMillan SJ, Kearley J, Oldfield WL, Stern LJ, Gronlund H, van Hage M, Reynolds CJ, Boyton RJ, Cobbold SP, Kay AB, Altmann DM, Lloyd CM, Larche M. Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. J Exp Med. 2009;206:1535–1547. doi: 10.1084/jem.20082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Kohl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strickland DH, Stumbles PA, Zosky GR, Subrata LS, Thomas JA, Turner DJ, Sly PD, Holt PG. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4+CD25+ regulatory T cells. J Exp Med. 2006;203:2649–2660. doi: 10.1084/jem.20060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, Huang X, Sheppard D. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med. 2012;18:547–554. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciprandi G, Cuppari C, Salpietro AM, Tosca MA, Rigoli L, Grasso L, La Rosa M, Marseglia GL, Del Giudice MM, Salpietro C. Serum IL-23 Strongly and Inversely Correlates with FEV(1) in Asthmatic Children. Int Arch Allergy Immunol. 2012;159:183–186. doi: 10.1159/000336418. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Lloyd CM, Noble A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance and contribute to airway remodeling. Mucosal immunology. 2012 doi: 10.1038/mi.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Just J, Gouvis-Echraghi R, Rouve S, Wanin S, Moreau D, Annesi-Maesano I. Two novel, severe asthma phenotypes identified during childhood using a clustering approach. Eur Respir J. 2012;40:55–60. doi: 10.1183/09031936.00123411. [DOI] [PubMed] [Google Scholar]
- 16.Siroux V, Basagana X, Boudier A, Pin I, Garcia-Aymerich J, Vesin A, Slama R, Jarvis D, Anto JM, Kauffmann F, Sunyer J. Identifying adult asthma phenotypes using a clustering approach. Eur Respir J. 2011;38:310–317. doi: 10.1183/09031936.00120810. [DOI] [PubMed] [Google Scholar]
- 17.Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the ‘epimmunome’. Nat Immunol. 2010;11:656–665. doi: 10.1038/ni.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker JA, McKenzie A. Innate lymphoid cells in the airways. European journal of immunology. 2012;42:1368–1374. doi: 10.1002/eji.201242425. [DOI] [PubMed] [Google Scholar]
- 19.Ather JL, Hodgkins SR, Janssen-Heininger YM, Poynter ME. Airway epithelial NF-kappaB activation promotes allergic sensitization to an innocuous inhaled antigen. Am J Respir Cell Mol Biol. 2011;44:631–638. doi: 10.1165/rcmb.2010-0106OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, Leikauf GD, Lee NA. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 22.Guo J, Lin X, Williams MA, Hamid Q, Georas SN. Yin-Yang 1 regulates effector cytokine gene expression and T(H)2 immune responses. J Allergy Clin Immunol. 2008;122:195–201. 201e191–195. doi: 10.1016/j.jaci.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emo J, Meednu N, Chapman TJ, Rezaee F, Balys M, Randall T, Rangasamy T, Georas SN. Lpa2 is a negative regulator of both dendritic cell activation and murine models of allergic lung inflammation. J Immunol. 2012;188:3784–3790. doi: 10.4049/jimmunol.1102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng G, Arima M, Honda K, Hirata H, Eda F, Yoshida N, Fukushima F, Ishii Y, Fukuda T. Anti-interleukin-9 antibody treatment inhibits airway inflammation and hyperreactivity in mouse asthma model. American journal of respiratory and critical care medicine. 2002;166:409–416. doi: 10.1164/rccm.2105079. [DOI] [PubMed] [Google Scholar]
- 28.Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, Ottoson P, Persson P, Delaney T, Lehar S, Lin S, Poisson L, Meisel C, Kamradt T, Bjerke T, Levinson D, Gutierrez-Ramos JC. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 31.Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D’Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aswad F, Kawamura H, Dennert G. High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J Immunol. 2005;175:3075–3083. doi: 10.4049/jimmunol.175.5.3075. [DOI] [PubMed] [Google Scholar]
- 33.Besnard AG, Togbe D, Couillin I, Tan Z, Zheng SG, Erard F, Le Bert M, Quesniaux V, Ryffel B. Inflammasome-IL-1-Th17 response in allergic lung inflammation. Journal of molecular cell biology. 2012;4:3–10. doi: 10.1093/jmcb/mjr042. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Haines CJ, Gutcher I, Hochweller K, Blumenschein WM, McClanahan T, Hammerling G, Li MO, Cua DJ, McGeachy MJ. Foxp3(+) regulatory T cells promote T helper 17 cell development in vivo through regulation of interleukin-2. Immunity. 2011;34:409–421. doi: 10.1016/j.immuni.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernandez-Santos N, Edgerton M, Gaffen SL, Lenardo MJ. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity. 2011;34:422–434. doi: 10.1016/j.immuni.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.