SUMMARY
Experience shapes neural circuits during critical periods in early life. The timing of critical periods is regulated by both genetics and the environment. Here we study the functional significance of such temporal regulations in the mouse primary visual cortex, where critical period plasticity drives binocular matching of orientation preference. We find that the binocular matching is permanently disrupted in mice that have a precocious critical period due to genetically enhanced inhibition. The disruption is specific to one type of neurons, the complex cells, which, as we reveal, normally match after the simple cells. Early environmental enrichment completely rescues the deficit by inducing histone acetylation and consequently advancing the matching process to coincide with the precocious plasticity. Our experiments thus demonstrate that the proper timing of the critical period is essential for establishing normal binocularity and the detrimental impact of its genetic misregulation can be ameliorated by environmental manipulations via epigenetic mechanisms.
INTRODUCTION
Neuronal connections in the brain are first established by genetic programs, and then shaped during postnatal development by an individual’s sensory and motor experience when interacting with the environment. The experience-induced changes occur during “critical periods” in early life, and failing to receive appropriate experience during these time windows leads to abnormal circuit formation that is difficult to repair later in life (Lewis and Maurer, 2009; Nelson et al., 2007; Popescu and Polley, 2010; Wiesel and Hubel, 1965).
A classic example in critical period studies is ocular dominance (OD) plasticity in the visual cortex. During the critical period of OD plasticity, monocular visual deprivation leads to a loss of cortical response to the deprived eye, and an increase to the non-deprived eye (Emerson et al., 1982; Fagiolini et al., 1994; Gordon and Stryker, 1996; Hubel et al., 1977; Issa et al., 1999; Van Sluyters and Stewart, 1974; Wiesel and Hubel, 1963). The critical period of OD plasticity does not start immediately after eye opening (Fagiolini et al., 1994; Gordon and Stryker, 1996; Hubel and Wiesel, 1970; Issa et al., 1999; Wiesel and Hubel, 1963), and its opening and closure are regulated by both environmental and genetic factors (Hensch, 2005; Majdan and Shatz, 2006). For example, complete visual deprivation from birth delays the onset of the critical period (Cynader et al., 1976; Fagiolini et al., 1994; Mower, 1991). On the other hand, the critical period can be reactivated in adulthood by enriched sensory, motor, and social interactions with the environment (Sale et al., 2007). In addition, the critical period of OD plasticity can also be advanced or delayed genetically by enhancing or reducing synaptic inhibition in the cortex (Fagiolini et al., 2004; Fagiolini and Hensch, 2000; Hanover et al., 1999; Hensch et al., 1998; Huang et al., 1999; Iwai et al., 2003).
Although the above studies have identified methods to alter critical period timing experimentally, it is unknown whether and how these alterations influence normal visual development. On the one hand, cortical functions could still achieve their normal levels as long as visual stimulation is present during the critical period, regardless of whether it is earlier or later. On the other hand, if the timing of the critical period is important, its alteration would result in abnormal visual development despite the normal visual experience. However, which of the two scenarios is true cannot be determined with OD plasticity because it is a deprivation-induced plasticity that does not normally occur. We recently discovered that visual experience during the critical period drives the binocular matching of orientation preference in the mouse primary visual cortex (Wang et al., 2010a), thus making it possible to study the functional significance of the proper timing of the critical period in normal development.
In this study, we have first examined binocular matching of orientation preference in mice that have a precocious critical period due to genetically enhanced inhibition (Huang et al., 1999). We find that binocular matching in these mice is permanently disrupted and the disruption is due to a temporal discrepancy between the genetically-induced precocious critical period and normal binocular development. The matching deficit in these mice is fully rescued by environmental enrichment during early postnatal development, which induces acetylation of histone H4 via Insulin-like Growth Factor 1 (IGF-1) and consequently advances the matching process to coincide with the precocious plasticity. Together, our experiments demonstrate for the first time that a properly-timed critical period is essential for establishing normal binocularity in the visual cortex and the detrimental impact of its genetic misregulation can be rescued by environmental manipulations via epigenetic mechanisms.
RESULTS
Binocular matching of orientation preference is disrupted in mice with precocious cortical plasticity
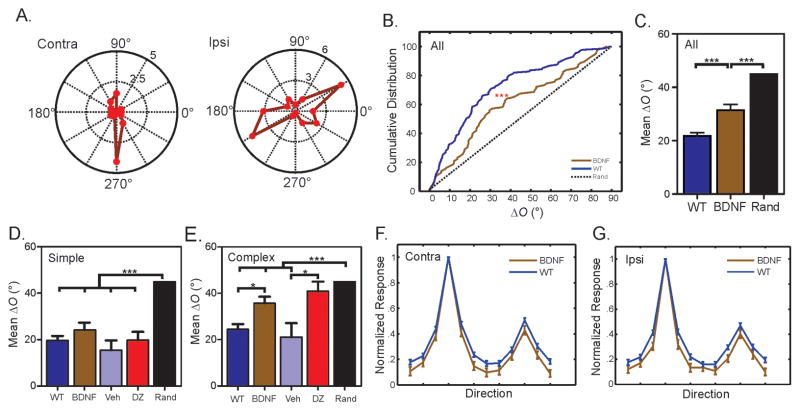
To study the functional significance of a properly-timed critical period, we examined binocular matching of orientation preference in a line of transgenic mice that overexpress brain-derived neurotropic factor (BDNF) in their forebrains (Huang et al., 1999). These BDNF overexpression (BDNF-OE) mice display accelerated maturation of GABAergic innervation and inhibition in the visual cortex, which leads to precocious OD plasticity (Hanover et al., 1999; Huang et al., 1999). We made single-unit recordings in the binocular zone of the primary visual cortex (V1) in these mice and determined individual neurons’ orientation tuning separately for each eye. The monocularly preferred orientations were then compared between the two eyes, and their difference (referred to as ΔO) was used to quantify the degree of binocular matching (Wang et al., 2010a).
We first studied the BDNF-OE mice after postnatal day 30 (P30), when binocular matching has normally reached adult level in WT mice (Wang et al., 2010a). Remarkably, we found that many cortical neurons in these mice were still tuned to very different orientations through the two eyes (Figure 1A–B), and the deficit persisted well into adulthood (P31–36: mean ΔO = 29.1° ± 3.3°, n = 44; P60–90: mean ΔO = 33.4° ± 2.9°, n = 93; P = 0.58). Across the population, ΔO in the BDNF-OE mice was significantly greater than the age-matched WT controls (BDNF-OE: mean ΔO = 32.0 ± 2.2°, n = 137; WT: mean ΔO = 21.8° ± 1.2°, n = 297; P < 0.001; Figure 1B–C), indicating a mismatch of orientation preference through the two eyes. The disrupted binocular matching in BDNF-OE mice was not due to a possible change in orientation selectivity. In fact, both orientation selectivity index (OSI, BDNF-OE: contra = 0.70 ± 0.03, ipsi = 0.64 ± 0.03, n = 137; WT: contra = 0.67 ± 0.02, ipsi = 0.64 ± 0.02, n = 297; P = 0.25 & 0.73, respectively) and tuning width (BDNF-OE: contra = 26.0 ± 1.2, ipsi = 24.2 ± 1.2, n = 92; WT: contra = 27.2 ± 0.8, ipsi = 26.6 ± 0.8, n = 197; P = 0.31 & 0.13, respectively) were similar to WT controls (Figure 1F–G). In other words, BDNF overexpression specifically disrupts the matching of the two streams of eye-specific inputs in the cortex while keeping monocular tuning properties intact.
Figure 1. Disrupted binocular matching in mice with precocious critical period plasticity.
(A) Polar plots of orientation tuning curves of a V1 neuron in BDNF-OE mice. The neuron prefers different orientations through the contralateral (“Contra”) and ipsilateral (“Ipsi”) eye. (B) Cumulative distribution of ΔO for WT and BDNF-OE mice. The dotted line represents a uniform distribution if the matching were completely random. (C) Mean ΔO of WT, BDNF-OE mice and random matching (45°). (D) Normal binocular matching in simple cells of BDNF-OE and diazepam-treated WT mice, compared to WT and vehicle controls. (E) Disrupted binocular matching in complex cells of BDNF-OE and diazepam-treated WT mice. (F–G). Mean monocular tuning curves through contralateral (F) and ipsilateral (G) eyes for BDNF-OE and WT mice. Error bars represent mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001). See also Figure S1 and Table S1.
Although disrupted, the binocular matching of orientation preference in the BDNF-OE mice was not completely random, which would result in a uniform distribution of ΔO between 0 and 90 degree (Figure 1B–C; P < 0.001). We thus studied whether some cells were better matched than others in the BDNF-OE mice. Neurons in the visual cortex can be classified into simple and complex cells, with the two classes of cells representing successive stages in visual information processing (Hubel and Wiesel, 1962; Martinez and Alonso, 2001). We divided the recorded neurons into these two groups based on the linearity of their responses to drifting sinusoidal gratings (see Methods for details), and then determined their degree of matching. Remarkably, simple and complex cells in the BDNF-OE mice showed different phenotypes in binocular matching. While simple cells in the BDNF-OE mice had normal and well matched orientation preference (BDNF-OE: mean ΔO = 24.2° ± 3.2°, n = 51; WT: mean ΔO = 19.1° ± 1.5°, n = 170; P = 0.46, Figure 1D), the binocular matching of complex cells was severely disrupted (BDNF-OE: ΔO = 35.7° ± 2.9°, n = 86; WT: ΔO = 25.4° ± 2.0°, n = 127; P < 0.05, Figure 1E; also see Figure S1A and C for examples). Again, the monocular orientation tuning properties of both simple and complex cells in BDNF–OE mice were normal (See Figure S1 and Table S1), consistent with the observation across the whole population.
We next sought to confirm that the cell type specific disruption of binocular matching in the BDNF-OE mice is indeed due to the precocious cortical plasticity induced by accelerated inhibition maturation. It was shown that brief enhancement of inhibition with the GABAA receptor agonist diazepam after eye opening can induce precocious critical period for OD plasticity (Iwai et al., 2003; Kanold et al., 2009). We followed the published protocol (Kanold et al., 2009) and injected diazepam into WT mice daily at P16 and P17, and then studied their binocular matching at P31–36. The pharmacological manipulation of inhibition indeed phenocopied the matching defect of the BDNF-OE mice (Figure 1D–E). While simple cells in the diazepam-treated mice showed normal binocular matching (mean ΔO = 19.8° ± 3.6°, n = 39; P = 0.52, Figure 1D), the matching of complex cells were completely disrupted (mean ΔO = 40.9° ± 4.1°, n = 48; P < 0.01 compared to WT; P = 0.02 compared to vehicle-treated controls; P = 0.26 compared to random; Figure 1E). Just like in the BDNF-OE mice, the development of monocular orientation tuning was not affected by the diazepam injection (See Table S1).
Simple cells match binocularly before complex cells during normal development
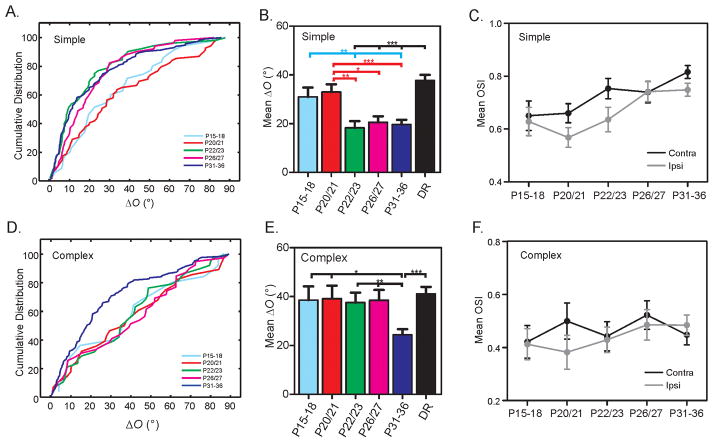
To understand the cause of the complex cell specific disruption in mice with precocious cortical plasticity, we determined the normal time course of binocular matching in WT mice. At P15–18, a few days after eye opening, most cells had mismatched orientation preference (mean ΔO = 34.2° ± 3.3°, n = 60), and the mismatch did not improve until P20/21 (mean ΔO = 34.8° ± 2.7°, n = 97; P = 0.99 between the two groups). Furthermore, the level of matching at P20/21 was similarly poor as in mice that had never experienced any visual stimulation (reared in complete darkness from P11 to P31–36: mean ΔO = 39.2° ± 1.8°, n = 234, P = 0.26). At this age, the orientation preferences were mismatched in both simple (mean ΔO = 33.0° ± 3.1°, n = 69, Figure 2A–B) and complex cells (mean ΔO = 39.2° ± 5.3°, n = 28, Figure 2D–E), comparable to their dark-reared counterparts (simple: mean ΔO = 37.7° ± 2.3°, n = 135, P = 0.16; complex: mean ΔO = 41.2° ± 2.8°, n = 99, P = 0.96; Figure 2B and E). In other words, even with 6–7 days of visual experience after eye opening, the level of binocular matching did not significantly improve before P20. Just a couple days later, by P22/23, however, the matching in simple cells had already reached the adult level (mean ΔO = 18.3° ± 2.7°, n = 52, p= 0.99, Figure 2A–B). In contrast, the complex cells were still mismatched at this age (mean ΔO = 37.6° ± 4.1°, n = 38), at a similar level as in the dark-reared animals (P = 0.26). The complex cells remained mismatched until P26/27 (mean ΔO = 38.5° ± 4.2°, n = 39, p = 0.60 compared to dark-reared group; Figure 2D–E), and their matching reached the mature level by P31 (mean ΔO = 24.4° ± 2.3°, n = 84, Figure 2D–E, p = 0.37 compared to P60–P90 adults). These results thus demonstrate that complex cells normally match orientation preference binocularly after simple cells.
Figure 2. Simple cells match binocular orientation preference before complex cells.
(A) Cumulative distribution of ΔO for simple cells in five age groups during development: P15–18, P20/21, P22/23, P26/27, P31–36. (B) Mean ΔO of simple cells is high at P15–18 and P20/21, similar to the dark-reared (DR) mice, then decreases and reaches the mature level by P22/23. (C) Mean OSI of simple cells increases during development for both contralateral (P15–18 vs. P31–36, P < 0.05) and ipsilateral (P15–18 vs. P31–36, P = 0.06; P20/21 vs. P31–36, P < 0.001) eyes. (D) Cumulative distribution of ΔO for complex cells in all five age groups during development. (E) Mean ΔO of complex cells is high from P15 to P27, very similar to the DR group. It decreases after P27 and reaches mature level by P31–36. (F) Mean OSI of complex cells remains similar during development for both contralateral (P15–18 vs. P31–36, P = 0.79) and ipsilateral (P15–18 vs. P31–36, P = 0.50) eyes. Error bars represent mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001). See also Table S1.
In addition to the sequential process of binocular matching in simple and complex cells, the development of monocular orientation selectivity also differs in these two cell types. As a whole population, V1 neurons increased their contralateral OSI after eye opening (P15–18: mean OSI = 0.55 ± 0.04, P31–36: mean OSI = 0.66 ± 0.02; P < 0.05), consistent with previous studies (Kuhlman et al., 2011; Li et al., 2012; Rochefort et al., 2011). We also revealed for the first time an increase in V1 orientation selectivity through the ipsilateral eye (P15–18: mean OSI = 0.54 ± 0.04, P31–36: mean OSI = 0.64 ± 0.02, P = 0.06). Interestingly, such orientation selectivity improvement was restricted to simple cells (Figure 2C, also see table S1), but not in complex cells (Figure 2F).
Taken together, these results revealed a detailed temporal profile of visual cortical development. While acquiring their orientation selectivity during the first two weeks after eye opening, the simple cells match their orientation preference binocularly and reach adult level by P23. In contrast, the complex cells match a few days after the simple cells, without any obvious improvement of orientation selectivity. Importantly, the discovery that the binocular matching of simple and complex cells occurs at two distinct stages, together with the complex cell specific disruption in mice with a precocious critical period, indicate that the matching in the complex cells is not a trivial or automatic consequence of simple cells being matched. Instead, the intracortical connections from simple to complex cells, after simple cells have already matched, must still undergo synaptic changes in order to match the complex cells.
Simple cells do not match earlier in BDNF-OE mice despite precocious cortical plasticity
Although previous studies of the BDNF-OE mice showed that OD plasticity in these mice peaked earlier than in WT mice (Hanover et al., 1999; Huang et al., 1999), they did not test cortical plasticity before P20, i.e., the period before the normal binocular matching process as we just discovered. We therefore initiated monocular deprivation at P15 in both WT and BDNF-OE mice and studied the degree of OD plasticity 5–6 days later, using optical imaging of intrinsic signals to measure the visually-evoked responses in V1 (Cang et al., 2005, and Figure 3A). The response amplitude through the contralateral eye (C) and that through the ipsilateral eye (I) were determined separately and used to calculate an ocular dominance index (ODI = (C−I)/(C+I)). Monocular deprivation of the contralateral eye between P15 and P20 did not cause any ODI change in the WT mice (Figure 3A–B). In contrast, the same manipulation in the BDNF-OE mice induced a significant ODI shift (Figure 3A–B, P = 0.007), demonstrating a precocious onset of cortical plasticity in these mice.
Figure 3. Precocious cortical plasticity does not advance binocular matching in BDNF-OE mice.
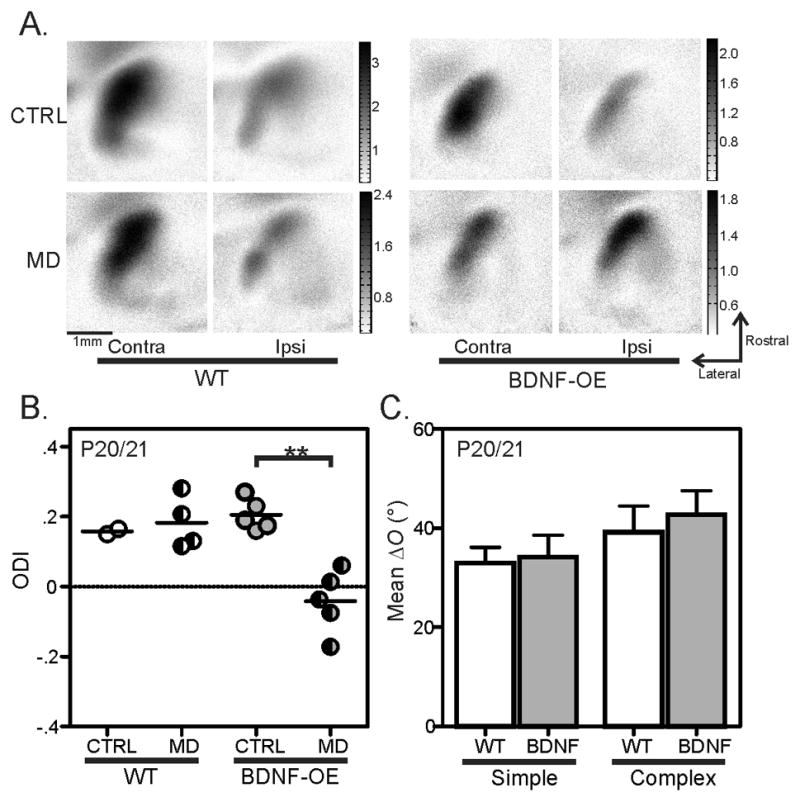
(A) Examples of response magnitude maps from WT (left) and BDNF-OE mice (right), without MD (top row, imaged at P20), or with 5 days of MD from P15 to P20 (bottom row). (B) A shift in ocular dominance is seen in BDNF-OE mice with 5–6 days of MD starting at P15 (Mann-Whitney rank sum test: p < 0.01), but not in WT mice. (C) Mean ΔO of both simple and complex cells in P20/21 BDNF-OE mice is similarly high as in age-matched WT controls. Error bars represent mean ± SEM (**p < 0.01).
Despite the precocious plasticity before P20, however, binocular matching of simple cells in the BDNF-OE mice does not occur earlier. At P20/21, simple cells in the BDNF-OE mice still remained mismatched, with ΔO comparable to the WT age-matched controls (mean ΔO = 34.1° ± 4.5°, n = 29; Figure 3C, P = 0.82). The OSI was also similar between these cells (See Table S1), showing that advancing critical period plasticity has no impact on the development of monocular orientation tuning. These results thus revealed a genetically-induced temporal “mismatch” between the critical period and binocular matching in the BDNF-OE mice: the early onset of cortical plasticity does not advance binocular matching and the precocious closure of the critical period (Hanover et al., 1999; Huang et al., 1999) prevents the second phase of the matching process, namely, the matching of complex cells.
Environmental enrichment rescues binocular matching in BDNF-OE mice
The above findings suggest that the matching deficits in the BDNF-OE mice could potentially be rescued if the timing of the binocular matching process is shifted earlier to coincide with the precocious cortical plasticity. To test this, we reared mice in an enriched environment, which is known to accelerate many aspects of visual system development (Cancedda et al., 2004; Landi et al., 2007).
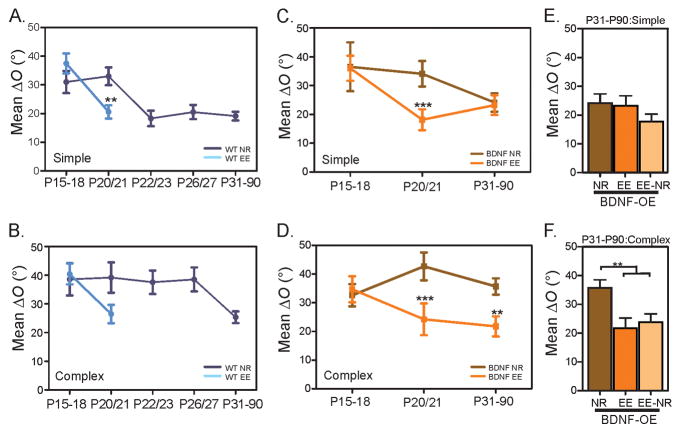
First, we examined whether environmental enrichment (EE) accelerates binocular matching in WT mice. We recorded mice that were born and reared in an environment with enhanced sensory, motor and social experiences (see Supplemental Video and Methods for details). At P20/21, when the binocular matching process had not initiated under normal rearing (NR) conditions, the matching in the enriched mice was significantly improved (mean ΔO = 22.9° ± 1.9°, n = 129, P < 0.001 compared to NR). In fact, the degree of binocular matching in both simple and complex cells had already reached the adult level (Simple cells: ΔO = 20.6° ± 2.3°, n = 78, P < 0.01 compared to the age-matched control, and P = 0.43 compared to NR adult; Complex cells: ΔO = 26.5° ± 3.2°, n = 51, P = 0.09 compared to the age-matched control, and P = 0.83 compared to NR adult; Figure 4A and B). The enrichment also accelerated the maturation of monocular orientation selectivity in simple cells, with their OSI significantly greater than in the age-matched NR animals for both contralateral (EE: mean OSI = 0.78 ± 0.03, n = 78; NR: mean OSI = 0.66 ± 0.04, n = 69; P < 0.05) and ipsilateral eyes (EE: mean OSI = 0.72 ± 0.03, n = 78; NR: mean OSI = 0.57 ± 0.04, n = 69, P < 0.05; Figures S2 and S3). These results thus demonstrate that environmental enrichment can indeed advance the time course of orientation selectivity maturation and binocular matching.
Figure 4. Environmental enrichment advances binocular matching and rescues the deficit in BDNF-OE mice.
(A–B) Mean ΔO of simple (A) and complex (B) cells in WT mice reared in normal (NR) and enriched environment (EE). Binocular matching improves significantly and reaches adult level by P20/21. (C–D) Mean ΔO of simple (C) and complex (D) cells in BDNF-OE mice reared in normal and enriched environment. (E–F) Mean ΔO of simple (E) and complex (F) in mice reared in EE from birth to P17, and then followed by NR (EE-NR). Error bars represent mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001). See also Figure S2, Table S1 and Movie S1.
We next tested whether shifting the timing of binocular matching earlier can rescue the matching deficits in BDNF-OE mice. In one set of experiments, we reared these mice in EE from birth to P31–36, and then studied the matching in both simple and complex cells. As expected, the simple cells were well matched, similar to those in adult NR mice (mean ΔO = 23.2° ± 3.4°, n = 48, P = 0.70; Figure 4C). Importantly, the binocular matching of complex cells was significantly improved by the enrichment (mean ΔO = 21.8° ± 3.5°, n = 41, P < 0.01 compared to NR; Figure 4D). In fact, the degree of matching was similar to the adult level in WT mice (mean ΔO = 25.4° ± 2.0°, n = 127, P = 0.42), indicating a complete rescue of the binocular matching deficit by EE. Moreover, the rescue was not due to a potential reactivation of cortical plasticity by the EE (Baroncelli et al., 2010; Scali et al., 2012), as the matching in the enriched BDNF-OE mice was already complete by P20/21 (Simple: mean ΔO = 18.2° ± 3.6°, n = 29, P < 0.001 compared to NR; Complex: mean ΔO = 24.2° ± 5.5°, n = 23, P < 0.001 compared to NR; Figure 4C & 4D).
Furthermore, to determine whether the rescue was due to the shift in the timing of visual system development or due to the enriched experience itself, we reared the BDNF-OE pups in EE from birth to only P17, when the matching has not yet started in either standard or enriched conditions (Figure 4C and D). The pups were then placed to standard rearing conditions until the time of recording between P31–P36. The “enriched followed by normal” rearing (EE-NR) was indeed able to rescue the binocular matching deficits in the BDNF-OE mice (complex cells in EE-NR: mean ΔO = 23.8° ± 2.9°, n = 63, P < 0.01 compared to NR; Figure 4F), indicating that the enriched experience itself is not required during binocular matching. Together, our results demonstrate that environmental enrichment during early postnatal development is able to advance the binocular matching process to coincide with the precocious cortical plasticity, thereby rescuing the detrimental impact of genetic misregulation of critical period timing.
IGF-1 advances binocular matching and rescues the matching deficit in BDNF-OE mice
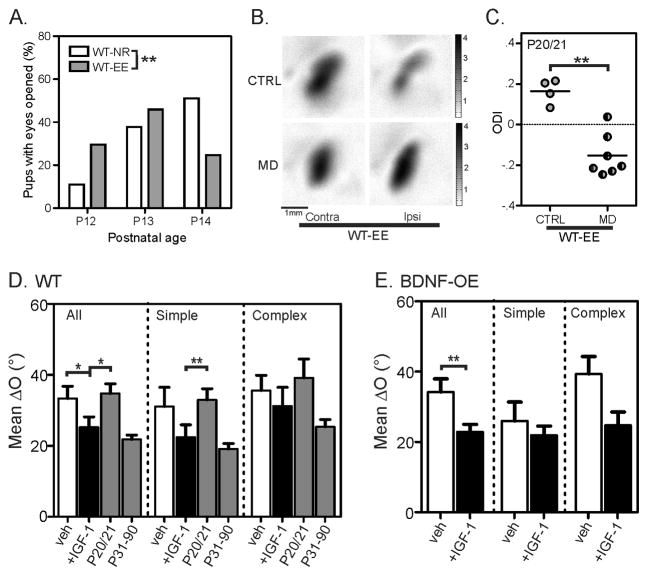
EE was known to induce a precocious eye-opening (Cancedda et al., 2004), but the enriched mice only opened their eyes less than 1 day earlier than the normal-reared ones under our conditions (NR: mean = 13.4 ± 0.1 days, n = 53 mice; EE: mean = 12.9 ± 0.1 days, n = 61; Mann-Whitney rank sum test: P < 0.01; Figure 5A). Given that the complex cells, which normally matches between P27–P31 (Figure 2D–E), already shows improved matching by P20/21 in EE (Figure 4B&D), the extra 1 day of light exposure alone cannot account for the ~7 day acceleration in binocular matching.
Figure 5. IGF-1 advances binocular matching and rescues the matching deficit in BDNF-OE mice.
(A) EE induces slightly earlier eye opening (WT-NR: mean = 13.4 ± 0.1 days, n = 53 mice; WT-EE: mean = 12.9 ± 0.1 days, n = 61; Mann-Whitney rank sum test: p < 0.01). (B) Examples of response magnitude maps from enriched WT mice (WT-EE), with strong contralateral bias in control (top row, imaged at P20) and more balanced responses after depriving the contralateral eye from P15 to P20 (bottom row). (C) A significant shift in ocular dominance index (ODI) is seen in the WT-EE mice after 5–6 days of MD starting at P15 (Mann-Whitney rank sum test: p < 0.01). (D) Mean ΔO of all (left), simple (center), and complex (right) cells in vehicle (0.1% BSA, open bars) and IGF-1 (black bars) treated P20/21 WT. The unmanipulated P20/21 and P31–90 groups are shown in the gray bars to illustrate the normal level of binocular matching at the two ages for comparison. (E) Mean ΔO of all (left), simple (center), and complex (right) cells in vehicle (0.1% BSA, open bars) and IGF-1 (black bars) treated P31–38 BDNF-OE mice. Error bars represent mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001). See also Figure S4 and Table S1.
We next examined EE’s effect on OD plasticity. As expected, monocular deprivation from P15 to P20/21, before the normal critical period of OD plasticity, induced a significant shift in ODI in the enriched WT mice (Figure 5B–C), unlike in the normally-reared WT mice as described above (Figure 3A–B). Although this resembles the precocious OD plasticity in the BDNF-OE mice (compare Figure 3A–B and Figure 5B–C), a fundamental difference exists between the two conditions: the onset of binocular matching is also advanced in the EE mice, but not in BDNF-OE mice (compare Figure 3C and Figure 4A–B). In other words, environmental enrichment during early postnatal development must trigger other factors that are required for binocular matching.
Insulin-like Growth Factor 1 (IGF-1) is a likely candidate to mediate EE’s effect on binocular matching. It was shown that physical exercise, an important component of EE, increases IGF-1 uptake in adult brain (Carro et al., 2000). More recently, EE was shown to increase IGF-1 levels in the visual cortex at P18 (Ciucci et al., 2007), around the time of binocular matching under EE. We thus followed an established protocol (Tropea et al., 2006) and administered IGF-1 daily into normally-reared WT mice daily starting from P14/15. This treatment was indeed able to advance the matching process. By P20/21, when matching has not yet started in vehicle-treated WT controls, the degree of matching in the IGF-1-treated mice had already reached the mature level for simple cells, just like in the EE mice (mean ΔO = 22.5° ± 3.5°, n = 45, P < 0.05 compared to vehicle-treated controls, and P = 0.60 compared to Adults; Figure 5D). Notably, the monocular orientation selectivity remained unchanged in the IGF-1 injected mice (Figure S4 and Table S1).
Since IGF-1 treatment advances the timing of binocular matching in WT mice, we next tested whether IGF-1 can rescue the matching deficit in BDNF-OE mice. We injected BDNF-OE mice with IGF-1 from P14/15 to P21 and then recorded about 10 days later. Indeed, these mice had normal level of binocular matching in both simple, and more importantly, complex cells (Figure 5E, P < 0.01 for all cells compared to vehicle-treated controls. For complex cells: mean ΔO = 24.7° ± 3.8°, n = 38; P = 0.06 compared to vehicle-treated controls: ΔO = 39.4° ± 4.9°, n = 24; and P = 0.82 compared to adult WTs). Together, these results suggest that IGF-1 is a key factor in mediating the EE’s effect on binocular matching.
Environmental enrichment induces histone acetylation to advance binocular matching
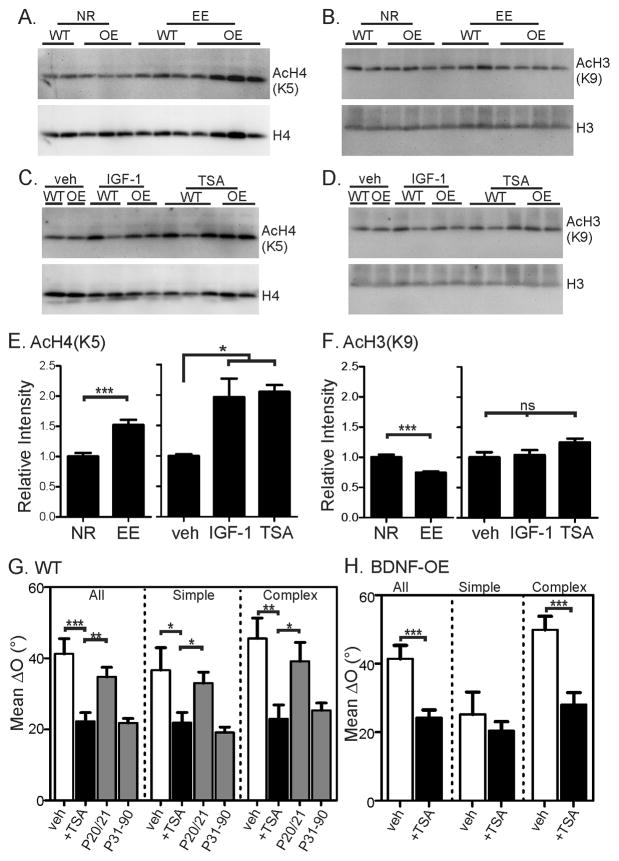
The genetic programs that initiate binocular matching are entirely unknown, but the above results indicate that they are likely regulated by environmental factors. Histone acetylation is an epigenetic mechanism that regulates gene expression through chromatin modification, and previous studies have shown that EE increase histone acetylation in the hippocampus of adult mice (Fischer et al., 2007). We therefore examined whether EE affect histone acetylation in the developing mice. WT and BDNF-OE littermates were kept in standard or enriched conditions from birth and their visual cortices were collected at P17 for western blot analyses. Antibodies against acetylated H3 (at Lysine 9, H3K9) or acetylated H4 (H4K5) were used to assess their acetylation levels. Compared to the normally-reared (NR) mice, the EE group displayed a pronounced increase in H4K5 acetylation in the visual cortex (Figure 6A and E, P <0.001, t-test). In contrast, H3K9 acetylation showed a trend of decrease in the EE mice (Figure 6B and F, P <0.001). These results demonstrate for the first time that EE induces histone modifications in the visual cortex of developing mice.
Figure 6. Environmental enrichment induces acetylation of histone H4 to advance binocular matching.
(A) Representative images of Western blots for acetylated H4K5 (top) and total H4 (bottom) in the visual cortex of normal-reared (NR) and EE mice. (B) Representative Western blots for acetylated H3K9 (top) and total H3 (bottom) in NR and EE mice. (C–D) Representative Western blots for acetylated and total H4 (C) and H3 (D) in IGF-1 and TSA treated mice. (E–F) Quantification of relative levels of acetylated H4K5 (E) and H3K9 (F). Each group included both WT and BDNF-OE (OE) mice and was normalized by the mean of the unmanipulated samples on the same gel. T-test was used to compare NR vs. EE; 1-way ANOVA and Tukey post test was used to compare vehicle (veh), IGF-1, and TSA-treated mice. In E, NR: n = 16 (8 mice); EE: n = 20 (8); veh, n = 4 (2); IGF-1, n = 8 (4); and TSA, n = 10 (5). In F, NR: n = 15 (8); EE: n = 19 (8); veh, n = 4 (2); IGF-1, n = 8 (4); and TSA, n = 10 (5). (G) Mean ΔO of all (left), simple (center), and complex (right) cells in vehicle (0.5% DMSO, open bars) and TSA injected (black bars) WT mice. The unmanipulated P20/21 and P31–90 groups (grey bars) are shown to illustrate the normal level of binocular matching at the two ages for comparison. (H) Mean ΔO of all (left), simple (center), and complex (right) cells in vehicle (0.5% DMSO, open bars) and TSA treated (black bars) P31–38 BDNF-OE mice. Error bars represent mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001). See also Figure S4 and Table S1.
We next tested whether histone modifications is involved in binocular matching. Because IGF-1 application mimics EE’s effect on binocular matching, we first examined whether IGF-1 also affects histone modifications in the developing visual cortex. Indeed, daily IGF-1 injections from P15 to 17 induced a marked elevation of H4K5 acetylation in the visual cortex of normally-reared mice (P <0.05), but had no effect on H3K9 (Figure 6C–F). Given that both EE and IGF-1 advance binocular matching, the elevated H4 acetylation seen in both conditions indicates that it could be a key factor in this process. We then tested this hypothesis directly using a pharmacological method to elevate histone acetylation during development.
Trichostatin A (TSA) is a histone deacetylase (HDAC) inhibitor that has been used to elevate histone acetylation in the brain (Korzus et al., 2004; Levenson et al., 2004; Putignano et al., 2007). We injected normally-reared mice with TSA daily from P15 to P17 and found that it resulted in increased acetylation of H4K5 (P < 0.05), but not H3K9 in the visual cortex (Figure 6C–F). Importantly, TSA treatment from P15 to P20/21 was able to completely mimic EE’s effects on binocular matching in both WT and BDNF-OE mice. In the TSA-treated WT mice, the matching had already reached the adult level by P20/21, demonstrating an advanced time course (simple cells: mean ΔO = 21.8° ± 2.9°, n = 45, P < 0.05 compared to vehicle-treated controls, and P = 0.30 compared to adults; complex cells: ΔO = 22.9° ± 4.0°, n = 34, P < 0.01 compared to control, and P = 0.74 compared to adults; Figure 6G). Consistently, the same TSA treatment rescued the matching deficits in the BDNF-OE mice (complex cells: ΔO = 28.0° ± 3.5°, n = 61, P < 0.001 compared to vehicle-treated controls; Figure 6H), just like EE.
Taken together, these results indicate that the EE’s effect on binocular matching is likely mediated by the increased H4 acetylation via IGF-1. Our studies therefore reveal an epigenetic mechanism linking environmental manipulations and the timing of visual cortical development.
DISCUSSION
Detrimental effect of a precocious critical period
In this study, we examined whether and how altering critical period timing affects normal visual development. In particular, we studied binocular matching of orientation preference in mice that have a precocious critical period due to accelerated inhibition maturation. We found that the binocular matching process does not start earlier in these mice even though the cortex is precociously plastic, suggesting that additional factors are needed to initiate the matching process. Instead, binocular matching in these mice fails to reach adult level, resulting in permanent mismatch in complex cells, presumably due to the precocious closure of cortical plasticity. We further showed that the matching deficit is rescued by brief EE during early postnatal development, which shifts the binocular matching process earlier and allows it to complete. Our results therefore demonstrate that the precocious cortical plasticity induced by genetic misregulation is detrimental to the development of binocular vision, thus illustrating the importance of properly-timed critical periods in neural development.
In addition to genetic regulation, critical period plasticity is also under the control of experience. For example, the critical period of OD plasticity is delayed by complete visual deprivation from birth (Mower, 1991), possibly through reduced inhibition (Chen et al., 2001; Morales et al., 2002). On the other hand, EE can extend or reactivate plasticity for both OD plasticity (Sale et al., 2007) and visual-auditory map alignment in barn owls (Brainard and Knudsen, 1998). Our current study adds to these observations by showing that EE from birth advances the onset of binocular matching. It was shown that EE elevates BDNF and GAD65 levels in the visual cortex during early postnatal development (Cancedda et al., 2004), but it must exert additional effects since binocular matching does not initiate earlier in the BDNF-OE mice. Furthermore, the BDNF-OE matching deficits can be rescued even in normal rearing condition following EE from birth to P17, indicating that EE during early postnatal development must trigger certain factors to initiate binocular matching. It was shown that EE increases IGF-1 levels (Ciucci et al., 2007) and we show here for the first time that EE and IGF-1 induces histone modifications in the developing visual cortex. Administration of an HDAC inhibitor mimics EE’s effects on binocular matching in both WT and BDNF-OE mice. Although the effect of blocking IGF-1 has not been tested, our results strongly suggest that histone acetylation via IGF-1 is likely a key factor that EE induces to affect visual cortical development.
Histone acetylation is an epigenetic mechanism of gene regulation and has been implicated in synaptic plasticity in adult learning and memory (Peixoto and Abel, 2013; Sweatt, 2009). There is accumulating evidence that histone acetylation promotes the expression of specific genes instead of global increase in transcription (Fass et al., 2003; Shafaati et al., 2009; Vecsey et al., 2007; Weaver et al., 2006). For example, histone acetylation is important for the expression of CREB-regulated genes (Fass et al., 2003; Vecsey et al., 2007). Because some of these genes are regulated by visual experience during the critical period (Cancedda et al., 2003; Putignano et al., 2007), EE-induced acetylation of histone H4 would activate potential “plasticity genes” responsible for binocular matching. Although the identity of such genes remains to be explored, our study has revealed a direct epigenetic link between genetic (“nature”) and environmental (“nurture”) factors in neural system development, thus contributing to the “nature vs. nurture” debate at a mechanistic level.
Critical period plasticity and neurodevelopmental disorders
Critical periods are developmental time windows during which neuronal connections and neural functions are shaped by sensory experience, motor use and social interactions. Such experience-dependent development are especially important for wiring up the circuits that integrate different streams of information, such as in multimodal sensory integration and language development, where setting up the underlying neural circuits entirely by genetic programs is difficult or even impossible. Consequently, deficits in critical period plasticity and its timing regulation could lead to problems in language, cognitive and social development, as seen in autism spectrum disorders (LeBlanc and Fagiolini, 2011). In support of this idea, many mouse models of human neurodevelopmental disorders display abnormal levels of excitatory-inhibitory balance (Baroncelli et al., 2011; Begenisic et al., 2011; Gogolla et al., 2009), which is known to control the timing and expression of critical period plasticity (Hensch, 2005). Indeed, several studies have reported altered OD plasticity in these mouse models, including the ones for Angelman syndrome (Sato and Stryker, 2010; Yashiro et al., 2009), Fragile X syndrome (Dolen et al., 2007), and Rett Syndrome (Tropea et al., 2009). Here we show for the first time that precocious cortical plasticity leads to a detrimental consequence in binocular matching, thus suggesting that these animal models may also display deficits in binocular development due to the altered timing of critical period plasticity. Importantly, binocular matching and development of higher neural functions may share similar mechanisms as they all require the proper integration of multiple channels of inputs. Our discoveries thus establish binocular matching as a more functionally relevant model in the study of critical period plasticity and experience-dependent neural development in normal and diseased conditions. Furthermore, our discoveries regarding environmental enrichment may also provide insights on using behavioral manipulations to treat certain neurodevelopmental disorders.
Sequential matching of simple and complex cells
Another intriguing finding of our study is that the binocular matching of simple and complex cells occurs in a sequence, rather than simultaneously. Because the orientation preference of a complex cell is presumably determined by the converging inputs from simple cells (Alonso and Martinez, 1998; Gilbert and Kelly, 1975; Hubel and Wiesel, 1962; Martinez and Alonso, 2001), one might expect that the matching of complex cells would be an automatic consequence of simple cells being matched (Figure S5). We show that this is not the case. In fact, a time window exists when the matching in simple cells has reached maturity, while the complex cells are still completely mismatched. This observation is further reinforced by the complex cell specific deficit in mice with a precocious critical period. Our discovery thus indicates that synaptic changes must still occur after simple cells have already matched, in order to match the complex cells.
With the inputs from individual simple cells already binocular and preferring similar orientations through the two eyes, how do they give rise to the mismatched orientation preference in complex cells and how can they change to mediate the subsequent matching? Recent studies of functional circuits in mouse V1 have revealed an important clue to these questions. It was shown that layer 2/3 cells in the monocular region of adult mice receive inputs that are individually tuned to a wide range of orientations (Jia et al., 2010; Ko et al., 2011). The orientation preference in a complex cell is thus determined by the summation of the heterogeneously-tuned synaptic inputs (Figure S5). For a binocular complex cell, its contralateral and ipsilateral orientation preference is largely determined by the same group of neurons as the vast majority of cells in its vicinity are binocular (Gordon and Stryker, 1996; Mrsic-Flogel et al., 2007). Importantly, although the simple cells are already binocularly matched, their response magnitudes through the two eyes are different and the OD level varies for individual cells. Consequently, pooling the inputs of diverse OD and heterogeneous orientation preference would result in a binocular mismatch in the complex cells (Figure S5). Therefore, the matching of complex cells would involve synaptic fine-tuning that may depend on the OD of individual inputs, or even changes of OD itself of each input neuron. Our observations thus suggest an exciting link between OD plasticity and binocular matching of orientation preference, and the plasticity rules that govern these processes are yet to be explored. Interestingly, there appears to be a “gap” period between the two phases of binocular matching (between P23 and P26). This, however, does not necessarily mean a lack of plasticity in this period. Connections to complex cells could be undergoing changes to match their orientation preferences during this period, but the effects are not yet visible at the population level. Future experiments that can follow the same cells during this process will be extremely informative.
The binocular matching of simple cells are better understood. Individual simple cells receive two streams of thalamic inputs that separately signal light increment (On) and decrement (Off), and the precise spatial layout of the On and Off subregions in the receptive fields renders the simple cells selective for stimulus orientation (Ferster and Miller, 2000; Hubel and Freeman, 1977). Our lab have recently shown that the same-sign subregions (On-On and Off-Off) preferentially overlap between the two monocular receptive fields of individual neurons and that such subregion correspondence is disrupted, though not completely, in dark-reared mice (Sarnaik et al., 2013). The experience-dependent subregion correspondence may be due to Hebbian plasticity as a result of correlated activity between the same-sign inputs that represent identical retinotopic locations (Erwin and Miller, 1999).
Finally, our discoveries lend additional supports to the notion that there are multiple critical periods in visual system development (Daw et al., 1978; Fagiolini et al., 2003; Jones et al., 1984; Lewis and Maurer, 2005). These periods are temporally organized in a hierarchical manner, wherein the neural circuits in the earlier stages of visual processing mature sooner (Daw, 1997; Knudsen, 2004; Lewis and Maurer, 2005). For example, it was shown in monkeys that the critical period for monocular acuity development ends earlier than that for binocular summation (Harwerth et al., 1990; Harwerth et al., 1986). We show here that such a sequence even exists for the binocular matching of two successive stages of cortical neurons. Our results also demonstrate that the different developmental processes must be regulated in a coordinated fashion. In the case of BDNF-OE mice, even though the manipulation of one factor alone leads to accelerated development of monocular acuity (Huang et al., 1999), it disrupts the binocular matching of complex cells, thus compromising the function of the entire visual system.
In conclusion, our studies reveal two distinct stages of binocular matching, one for simple cells and the other for complex cells. The second stage, i.e., the matching of complex cells, is disrupted in mice with precocious cortical plasticity. This deficit is rescued by environmental enrichment which induces histone acetylation to shift the binocular matching process earlier so that it can finish before the precocious closure of the critical period. These findings have important implications for studying the synaptic mechanisms underlying binocular matching of orientation preference and for understanding and treating certain neurodevelopmental disorders.
EXPERIMENTAL PROCEDURES
Mice, Rearing conditions, and Drug injections
Wildtype C57BL/6 mice and BDNF-OE mice (Huang et al., 1999) of different ages and both genders were used in this study. All animals were used in accordance with protocols approved by Northwestern University Institutional Animal Care and Use Committee.
Mice were reared either under standard condition (NR) or in enriched environment (EE). The enriched environment consisted of a larger cage with toys that were altered periodically following an established protocol (Cancedda et al., 2004) (See supplemental procedure for details). To determine the age of eye-opening, pups were checked every day (WT-EE: n = 61, 15 litters; WT-NR: n = 53, 12 litters). Eye opening was defined as any opening in the lids of either eye.
In pharmacological experiments to advance inhibition maturation, diazepam (Sigma) was dissolved in 50% saline: 50% propylene glycol (Wako) at a concentration of 2 mg/ml. Its solution or the same volume of vehicle was injected intraperitoneally at a dose of 30 mg/kg (Hensch et al., 1998; Kanold et al., 2009) daily at P16 and P17. The animals were then reared in standard condition until recording at age of P31–36 (n = 4 for vehicle and n = 9 for diazepam treated). For IGF-1 treatments, its functional peptide, GPE (Bachem), was dissolved in 0.1% BSA solution. WT and BDNF-OE mice were administered with 300 μg of GPE daily (i.p.) from P14/15 to P20/21 for recording or from P15 to P17 for western blotting (WT: n = 23; BDNF-OE: n = 22). To inhibit histone deacetylase, TSA (5 mg/ml in 0.5% DMSO; Sigma) was administered daily (i.p.) at a dose of 2.0 μg/g (Korzus et al., 2004; Putignano et al., 2007) from P15 to P20/21 for recording or P15 to P17 for western blotting (WT: n = 13; BDNF-OE: n = 11).
Western Blot Analysis
Mice were deeply anesthetized with Euthasol (Virbac) and decapitated, and the skull was opened down the midline to expose the cortex. Visual cortices of both hemispheres were dissected (~2mm×2mm in size and the center was ~2.5mm lateral from the midline and ~1 mm anterior from the lambda), and total proteins were then isolated (Nuclear & Cytoplasmic Extraction Kit, G-Biosciences, MO; Liu et al., 2007). For histone analysis, total histone proteins were further extracted from the nuclear fraction with five volumes of 0.2 M HCl, centrifuged at 18,000 × g; 30 min at 4°C, and the supernatants were collected. Protein concentration was determined by BCA assay Kit (Life Technologies, NY). For comparing EE and NR, samples were collected during the night, ~5 hrs after lights off. IGF-1, TSA, and vehicle-treated samples were collected during the daytime, ~2 hrs after the last injection on P17. Western blots were performed and data quantified using NIH ImageJ as described before (Johnson et al., 2004; Liu et al., 2007). The used antibodies are listed in the Supplemental Information. For statistical analysis, WT and BDNF-OE mice were combined by condition (NR, EE, vehicle-, IGF-1-, or TSA-treated), and the signal of each sample was normalized by the controls on the same gel (either NR or vehicle-treated).
In vivo Physiology and Data Analysis
We followed our published procedures to perform in vivo single unit physiology in urethane-anesthetized mice (Cang et al., 2008; Wang et al., 2010a; Wang et al., 2009; Wang et al., 2010b). The details are described in Supplemental Experimental Procedure.
Drifting sinusoidal gratings were delivered through either eye separately to determine V1 neurons’ monocular orientation selectivity. The drifting direction (θ) and spatial frequency of the gratings (full contrast and temporal frequency of 2 Hz) were varied in a pseudorandom order between 0° – 360° (12 steps at 30° spacing), and 0.01 – 0.32 cycle/degree (6 logarithmic steps, or in some experiments, 4 logarithmic steps from 0.01 to 0.08 cycle/degree). The preferred direction was determined as the one that gave maximum response (Rpref), averaging across all spatial frequencies. The preferred spatial frequency (pref_SF) was the one that gave peak response at this direction. Responses across all directions at the preferred spatial frequency, R(θ), were used to calculate the preferred orientation, orientation selectivity index (OSI), and tuning width.
Half of the complex phase of ΣR(θ)*e2i*θ/ΣR(θ) was calculated (Niell and Stryker, 2008) and then converted to the preferred orientation (pref_O) by subtracting 90°. The difference in preferred orientation between the two eyes was calculated by subtracting ipsilateral pref_O from contralateral pref_O along the 180° cycle (−90° to 90°). The absolute values of these differences (ΔO) were used in all quantifications. All responsive cells were included in the analysis of ΔO.
Orientation Selectivity Index (OSI) was calculated as the ratio of (R′pref − Rorth)/(R′pref + Rorth), where R′pref was the mean response of Rpref at θpref and θpref+π as the two angles have the same orientation, and Rorth was the mean response of the two directions orthogonal to the preferred direction. For the mean tuning curves analysis, each tuning curve was normalized to the peak response and then shifted to the direction that elicited the maximum response.
Linearity of response was calculated from the responses at the preferred direction and spatial frequency. The responses were binned at 100ms intervals and then a discrete Fourier transform was used to compute F1/F0, the ratio of the first harmonic (response at the drift frequency) to the 0th harmonic (mean response). Cells that showed a temporal modulation at the stimulus frequency (an F1/F0 ratio ≥ 1) through both eyes were classified as simple and the rest, which showed an F1/F0 ratio < 1 through at least one eye were considered complex.
Optical imaging of ocular dominance plasticity
Monocular deprivation of the right eye was performed in WT and BDNF-OE mice at P15 under isofluorane anesthesia (1.5–2% in O2) following published procedures (Cang et al., 2005). The OD plasticity of these mice was determined 5–6 days later by optical imaging of intrinsic signals (Cang et al., 2005; and see supplemental procedure).
Statistical analysis
All values were presented as mean ± SEM. Differences between different groups were tested for significance using the Kolmogorov-Smirnov test (K-S test), unless otherwise indicated. Statistic analyses and graphing were done with Prism (GraphPad Software Inc) and Matlab (Mathworks). In the figures, *P < 0.05, **P < 0.01, and ***P < 0.001.
Supplementary Material
Acknowledgments
We thank Yong-Chao Ma and Rashmi Sarnaik for help with experiments; David Ferster, Josh Huang, Hermann Riecke, and members of the Cang laboratory for discussions and comments on early versions of the manuscript. This work was supported by US National Institutes of Health (NIH) grants (EY020950 to J.C. and EY019034 to X.L.), a Sloan Research Fellowship and a Klingenstein Fellowship Award in Neurosciences to J.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso JM, Martinez LM. Functional connectivity between simple cells and complex cells in cat striate cortex. Nat Neurosci. 1998;1:395–403. doi: 10.1038/1609. [DOI] [PubMed] [Google Scholar]
- Baroncelli L, Braschi C, Spolidoro M, Begenisic T, Maffei L, Sale A. Brain plasticity and disease: a matter of inhibition. Neural Plast. 2011;2011:286073. doi: 10.1155/2011/286073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroncelli L, Sale A, Viegi A, Maya Vetencourt JF, De Pasquale R, Baldini S, Maffei L. Experience-dependent reactivation of ocular dominance plasticity in the adult visual cortex. Exp Neurol. 2010;226:100–109. doi: 10.1016/j.expneurol.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Begenisic T, Spolidoro M, Braschi C, Baroncelli L, Milanese M, Pietra G, Fabbri ME, Bonanno G, Cioni G, Maffei L, Sale A. Environmental enrichment decreases GABAergic inhibition and improves cognitive abilities, synaptic plasticity, and visual functions in a mouse model of Down syndrome. Front Cell Neurosci. 2011;5:29. doi: 10.3389/fncel.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard MS, Knudsen EI. Sensitive periods for visual calibration of the auditory space map in the barn owl optic tectum. J Neurosci. 1998;18:3929–3942. doi: 10.1523/JNEUROSCI.18-10-03929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda L, Putignano E, Impey S, Maffei L, Ratto GM, Pizzorusso T. Patterned vision causes CRE-mediated gene expression in the visual cortex through PKA and ERK. J Neurosci. 2003;23:7012–7020. doi: 10.1523/JNEUROSCI.23-18-07012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda L, Putignano E, Sale A, Viegi A, Berardi N, Maffei L. Acceleration of visual system development by environmental enrichment. J Neurosci. 2004;24:4840–4848. doi: 10.1523/JNEUROSCI.0845-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Kalatsky VA, Lowel S, Stryker MP. Optical imaging of the intrinsic signal as a measure of cortical plasticity in the mouse. Vis Neurosci. 2005;22:685–691. doi: 10.1017/S0952523805225178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Niell CM, Liu X, Pfeiffenberger C, Feldheim DA, Stryker MP. Selective disruption of one Cartesian axis of cortical maps and receptive fields by deficiency in ephrin-As and structured activity. Neuron. 2008;57:511–523. doi: 10.1016/j.neuron.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yang C, Mower GD. Developmental changes in the expression of GABA(A) receptor subunits (alpha(1), alpha(2), alpha(3)) in the cat visual cortex and the effects of dark rearing. Brain Res Mol Brain Res. 2001;88:135–143. doi: 10.1016/s0169-328x(01)00042-0. [DOI] [PubMed] [Google Scholar]
- Ciucci F, Putignano E, Baroncelli L, Landi S, Berardi N, Maffei L. Insulin-like growth factor 1 (IGF-1) mediates the effects of enriched environment (EE) on visual cortical development. PLoS One. 2007;2:e475. doi: 10.1371/journal.pone.0000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M, Berman N, Hein A. Recovery of function in cat visual cortex following prolonged deprivation. Exp Brain Res. 1976;25:139–156. doi: 10.1007/BF00234899. [DOI] [PubMed] [Google Scholar]
- Daw NW. Critical periods and strabismus: what questions remain? Optom Vis Sci. 1997;74:690–694. doi: 10.1097/00006324-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Daw NW, Berman NE, Ariel M. Interaction of critical periods in the visual cortex of kittens. Science. 1978;199:565–567. doi: 10.1126/science.622560. [DOI] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson VF, Chalupa LM, Thompson ID, Talbot RJ. Behavioural, physiological, and anatomical consequences of monocular deprivation in the golden hamster (Mesocricetus auratus) Exp Brain Res. 1982;45:168–178. doi: 10.1007/BF00235776. [DOI] [PubMed] [Google Scholar]
- Erwin E, Miller KD. The subregion correspondence model of binocular simple cells. J Neurosci. 1999;19:7212–7229. doi: 10.1523/JNEUROSCI.19-16-07212.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Katagiri H, Miyamoto H, Mori H, Grant SG, Mishina M, Hensch TK. Separable features of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling. Proc Natl Acad Sci U S A. 2003;100:2854–2859. doi: 10.1073/pnas.0536089100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Fass DM, Butler JE, Goodman RH. Deacetylase activity is required for cAMP activation of a subset of CREB target genes. J Biol Chem. 2003;278:43014–43019. doi: 10.1074/jbc.M305905200. [DOI] [PubMed] [Google Scholar]
- Ferster D, Miller KD. Neural mechanisms of orientation selectivity in the visual cortex. Annu Rev Neurosci. 2000;23:441–471. doi: 10.1146/annurev.neuro.23.1.441. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Kelly JP. The projections of cells in different layers of the cat’s visual cortex. J Comp Neurol. 1975;163:81–105. doi: 10.1002/cne.901630106. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Leblanc JJ, Quast KB, Sudhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord. 2009;1:172–181. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci. 1999;19:RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwerth RS, Smith EL, 3rd, Crawford ML, von Noorden GK. Behavioral studies of the sensitive periods of development of visual functions in monkeys. Behav Brain Res. 1990;41:179–198. doi: 10.1016/0166-4328(90)90107-p. [DOI] [PubMed] [Google Scholar]
- Harwerth RS, Smith EL, 3rd, Duncan GC, Crawford ML, von Noorden GK. Multiple sensitive periods in the development of the primate visual system. Science. 1986;232:235–238. doi: 10.1126/science.3952507. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Freeman DC. Projection into the visual field of ocular dominance columns in macaque monkey. Brain Res. 1977;122:336–343. doi: 10.1016/0006-8993(77)90299-2. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Issa NP, Trachtenberg JT, Chapman B, Zahs KR, Stryker MP. The critical period for ocular dominance plasticity in the Ferret’s visual cortex. J Neurosci. 1999;19:6965–6978. doi: 10.1523/JNEUROSCI.19-16-06965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai Y, Fagiolini M, Obata K, Hensch TK. Rapid critical period induction by tonic inhibition in visual cortex. J Neurosci. 2003;23:6695–6702. doi: 10.1523/JNEUROSCI.23-17-06695.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Rochefort NL, Chen X, Konnerth A. Dendritic organization of sensory input to cortical neurons in vivo. Nature. 2010;464:1307–1312. doi: 10.1038/nature08947. [DOI] [PubMed] [Google Scholar]
- Johnson J, Sherry DM, Liu X, Fremeau RT, Jr, Seal RP, Edwards RH, Copenhagen DR. Vesicular glutamate transporter 3 expression identifies glutamatergic amacrine cells in the rodent retina. J Comp Neurol. 2004;477:386–398. doi: 10.1002/cne.20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KR, Spear PD, Tong L. Critical periods for effects of monocular deprivation: differences between striate and extrastriate cortex. J Neurosci. 1984;4:2543–2552. doi: 10.1523/JNEUROSCI.04-10-02543.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Kim YA, GrandPre T, Shatz CJ. Co-regulation of ocular dominance plasticity and NMDA receptor subunit expression in glutamic acid decarboxylase-65 knock-out mice. J Physiol. 2009;587:2857–2867. doi: 10.1113/jphysiol.2009.171215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Ko H, Hofer SB, Pichler B, Buchanan KA, Sjostrom PJ, Mrsic-Flogel TD. Functional specificity of local synaptic connections in neocortical networks. Nature. 2011;473:87–91. doi: 10.1038/nature09880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Tring E, Trachtenberg JT. Fast-spiking interneurons have an initial orientation bias that is lost with vision. Nat Neurosci. 2011;14:1121–1123. doi: 10.1038/nn.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi S, Cenni MC, Maffei L, Berardi N. Environmental enrichment effects on development of retinal ganglion cell dendritic stratification require retinal BDNF. PLoS One. 2007;2:e346. doi: 10.1371/journal.pone.0000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc JJ, Fagiolini M. Autism: a “critical period” disorder? Neural Plast. 2011;2011:921680. doi: 10.1155/2011/921680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Lewis TL, Maurer D. Multiple sensitive periods in human visual development: evidence from visually deprived children. Dev Psychobiol. 2005;46:163–183. doi: 10.1002/dev.20055. [DOI] [PubMed] [Google Scholar]
- Lewis TL, Maurer D. Effects of early pattern deprivation on visual development. Optom Vis Sci. 2009;86:640–646. doi: 10.1097/OPX.0b013e3181a7296b. [DOI] [PubMed] [Google Scholar]
- Li YT, Ma WP, Pan CJ, Zhang LI, Tao HW. Broadening of cortical inhibition mediates developmental sharpening of orientation selectivity. J Neurosci. 2012;32:3981–3991. doi: 10.1523/JNEUROSCI.5514-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Grishanin RN, Tolwani RJ, Renteria RC, Xu B, Reichardt LF, Copenhagen DR. Brain-derived neurotrophic factor and TrkB modulate visual experience-dependent refinement of neuronal pathways in retina. J Neurosci. 2007;27:7256–7267. doi: 10.1523/JNEUROSCI.0779-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdan M, Shatz CJ. Effects of visual experience on activity-dependent gene regulation in cortex. Nat Neurosci. 2006;9:650–659. doi: 10.1038/nn1674. [DOI] [PubMed] [Google Scholar]
- Martinez LM, Alonso JM. Construction of complex receptive fields in cat primary visual cortex. Neuron. 2001;32:515–525. doi: 10.1016/s0896-6273(01)00489-5. [DOI] [PubMed] [Google Scholar]
- Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci. 2002;22:8084–8090. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mower GD. The effect of dark rearing on the time course of the critical period in cat visual cortex. Brain Res Dev Brain Res. 1991;58:151–158. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Nelson CA, 3rd, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J Neurosci. 2008;28:7520–7536. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto L, Abel T. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology. 2013;38:62–76. doi: 10.1038/npp.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu MV, Polley DB. Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron. 2010;65:718–731. doi: 10.1016/j.neuron.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putignano E, Lonetti G, Cancedda L, Ratto G, Costa M, Maffei L, Pizzorusso T. Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron. 2007;53:747–759. doi: 10.1016/j.neuron.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Rochefort NL, Narushima M, Grienberger C, Marandi N, Hill DN, Konnerth A. Development of direction selectivity in mouse cortical neurons. Neuron. 2011;71:425–432. doi: 10.1016/j.neuron.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Sale A, Maya Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R, Maffei L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10:679–681. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- Sarnaik R, Wang BS, Cang J. Experience-Dependent and Independent Binocular Correspondence of Receptive Field Subregions in Mouse Visual Cortex. Cereb Cortex 2013. 2013 Feb 6; doi: 10.1093/cercor/bht027. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Stryker MP. Genomic imprinting of experience-dependent cortical plasticity by the ubiquitin ligase gene Ube3a. Proc Natl Acad Sci U S A. 2010;107:5611–5616. doi: 10.1073/pnas.1001281107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scali M, Baroncelli L, Cenni MC, Sale A, Maffei L. A rich environmental experience reactivates visual cortex plasticity in aged rats. Exp Gerontol. 2012;47:337–341. doi: 10.1016/j.exger.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Shafaati M, O’Driscoll R, Bjorkhem I, Meaney S. Transcriptional regulation of cholesterol 24-hydroxylase by histone deacetylase inhibitors. Biochem Biophys Res Commun. 2009;378:689–694. doi: 10.1016/j.bbrc.2008.11.103. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65:191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Giacometti E, Wilson NR, Beard C, McCurry C, Fu DD, Flannery R, Jaenisch R, Sur M. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc Natl Acad Sci U S A. 2009;106:2029–2034. doi: 10.1073/pnas.0812394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Kreiman G, Lyckman A, Mukherjee S, Yu H, Horng S, Sur M. Gene expression changes and molecular pathways mediating activity-dependent plasticity in visual cortex. Nat Neurosci. 2006;9:660–668. doi: 10.1038/nn1689. [DOI] [PubMed] [Google Scholar]
- Van Sluyters RC, Stewart DL. Binocular neurons of the rabbit’s visual cortex: effects of monocular sensory deprivation. Exp Brain Res. 1974;19:196–204. doi: 10.1007/BF00238534. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, Wood MA. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BS, Sarnaik R, Cang J. Critical period plasticity matches binocular orientation preference in the visual cortex. Neuron. 2010a;65:246–256. doi: 10.1016/j.neuron.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Rangarajan KV, Lawhn-Heath CA, Sarnaik R, Wang BS, Liu X, Cang J. Direction-specific disruption of subcortical visual behavior and receptive fields in mice lacking the beta2 subunit of nicotinic acetylcholine receptor. J Neurosci. 2009;29:12909–12918. doi: 10.1523/JNEUROSCI.2128-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Sarnaik R, Rangarajan K, Liu X, Cang J. Visual receptive field properties of neurons in the superficial superior colliculus of the mouse. J Neurosci. 2010b;30:16573–16584. doi: 10.1523/JNEUROSCI.3305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci U S A. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cat’s lateral geniculate body. J Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol. 1965;28:1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Riday TT, Condon KH, Roberts AC, Bernardo DR, Prakash R, Weinberg RJ, Ehlers MD, Philpot BD. Ube3a is required for experience-dependent maturation of the neocortex. Nat Neurosci. 2009;12:777–783. doi: 10.1038/nn.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.