Abstract
Background
The relationship between FGF23 and vitamin D production and catabolism post renal transplantation has not been characterized.
Methods
Circulating creatinine, calcium, phosphorus, albumin, PTH, FGF23, and 1,25(OH)2vitamin D values were obtained pre-transplantation, daily post-operatively for 5 days, and at 6 months post transplantation in 44 patients aged 16.4 ± 0.4 years undergoing renal transplantation at UCLA from 8/1/2005 through 4/30/2007. 25(OH)vitamin D and 24,25(OH)2vitamin D concentrations were obtained at baseline, day 5, and day 180, and urinary concentrations of creatinine, phosphorus and FGF23 were measured on post-operative days 1, 3, 5, and 180.
Results
Circulating phosphate concentrations declined more rapidly and the FEPO4 was higher in the first week post-transplantation in subjects with higher FGF23 values. Fractional excretion of FGF23 was low at all time-points. Circulating 1,25(OH)2vitamin D levels rose more rapidly and were consistently higher in patients with lower FGF23 values; however, 25(OH)vitamin D and 24,25(OH)2vitamin D values were unrelated to FGF23 concentrations.
Conclusions
Inhibition of renal 1α-hydroxylase, rather than stimulation of 24-hydroxylase, may primarily contribute to the relationship between FGF23 values and calcitriol. The rapid decline in FGF23 levels post transplantation is not mediated solely by filtration of intact FGF23 by the new kidney.
Keywords: FGF23, PTH, vitamin D, renal transplantation, phosphorus
Introduction
Fibroblast growth factor 23 (FGF23), a phosphaturic hormone that suppresses renal 1α-hydroxylase activity, has been implicated in the development of secondary hyperparathyroidism in CKD[1] and circulating FGF23 values are often markedly elevated in patients treated with maintenance dialysis [2]. These increased levels have been associated with systemic outcomes such as more rapid deterioration in renal function [3, 4], higher incidence of cardiovascular disease [5, 6] and increased mortality in patients with all stages of CKD [4, 7] and are a predictor of rejection and progressive renal dysfunction in renal transplant recipients [8, 9], making FGF23 of great interest both in the control of CKD mineral ion metabolism and as a mediator for end-organ damage.
Profound changes in mineral ion metabolism occur during the first few weeks post transplantation with marked declines in serum phosphorus concentrations often resulting in the need for oral phosphate supplementation in the first few post-operative months [10]. 1,25(OH)2vitamin D values are also decreased in the immediate post-renal transplant period, despite normal to elevated circulating PTH levels and low circulating phosphate concentrations, which should stimulate renal 1α-hydroxylase activity [11, 12]. Circulating FGF23 levels are markedly elevated in individuals with end-stage kidney disease [2, 13] and several studies have demonstrated that values decline after successful renal transplantation [14-16], approaching those of individuals with normal kidney function by 3 to 6 months post-transplantation [14-16]. Although FGF23 is both filtered by the kidneys [17] and enzymatically cleaved [18], the relative contributions of filtration and degradation to its rapid declines post-transplantation are unknown. Furthermore, while some studies have linked FGF23 values to diminished circulating calcitriol levels and to increased renal phosphate excretion in the first months post-renal transplantation [19, 20], this observation has not been confirmed by others [21]. FGF23 normally suppresses renal 1α-hydroxylase and stimulates 24-hydroxylase activity; whether FGF23 contributes to decreased production or increased metabolism of calcitriol in the early transplant period remains unknown. Thus, the current study was designed to assess the relative contribution of renal filtration to changing FGF23 values early post renal transplantation and to assess the contribution of FGF23 to changes in 1,25(OH)2 vitamin D metabolism during this timeframe.
Subjects and Methods
Clinical characterization
All pediatric patients aged 8-21 years undergoing renal transplantation at UCLA from 8/1/2005 through 4/30/2007 were potential study candidates. Exclusion criteria included: post-operative follow up at another institution, delayed graft function, and the use of active vitamin D sterols post transplantation. Post-operative phosphate supplementation was prescribed at the treating physician’s discretion. The study was approved by the UCLA Human Subject Protection Committee and informed consent and/or assent was obtained from all parents and/or patients.
Demographic data, including patient age, gender, ethnicity, cause of renal insufficiency, and total dialysis time were obtained at baseline and amount of phosphate supplementation was recorded daily during the initial period of hospitalization. Plasma, serum, and urine samples were obtained upon admission to the hospital for transplantation, daily during the first five post-operative days, and at 6 months post-transplantation. All samples other than the initial (pre-operative admission) sample were drawn between 6 and 8 AM. All samples were stored at −70C and non-routine measurements of serum, plasma, and urine biochemical parameters were performed after study completion.
Circulating levels of creatinine, calcium, phosphorus, albumin, PTH, FGF23, and 1,25(OH)2vitamin D were measured pre-operatively (“baseline” values), daily throughout the first 5 days of the post-operative period as well as on post-operative day 180. Circulating 25(OH)vitamin D and 24,25(OH)2vitamin D values were obtained at baseline, day 5, and day 180, and urinary concentrations of creatinine, phosphorus and FGF23 were measured on post-operative days 1, 3, 5, and 180. Biochemical determinations for serum creatinine, calcium, albumin, and phosphorus and for urinary creatinine and phosphorus were determined using an Olympus AU5400 (Olympus America Incorporated, Center Valley, PA). Serum calcium levels were adjusted based on serum albumin level by the formula: serum Ca = measured calcium + (0.8 ×(4-serum albumin). Serum 25(OH)vitamin D (the sum of circulating 25(OH)vitamin D2 and 25(OH)vitamin D3 concentrations) (Mayo Clinic, Rochester, MN) and 1,25(OH)2 vitamin D (Mayo Clinic) levels and 24,25(OH)2vitamin D (Heartland Assays, Charleston, SC) were assessed by radioimmunoassay. PTH levels were measured in plasma by a 1st generation assay (Immutopics, San Clemente, CA, normal range: 10 to 65 pg/ml) and FGF23 levels were determined in EDTA plasma and urine (13) using the 2nd generation C-terminal assay (Immutopics). The fractional excretion of phosphorus (FEPO4) was expressed as a percentage and calculated according to the formula: [(Uphos * Screat)/(Ucreat * Sphos)] * 100. Renal FGF23 excretion has not yet been formally characterized. We therefore calculated fractional excretion of the FGF23 molecule using method equivalent to that for the calculation of phosphate excretion. FGF23 (FEFGF23) was expressed as a percentage and calculated according to the formula: [(UFGF23 * Screat)/(Ucreat * SFGF23)] * 100.
Characterization of post-transplant FGF23 excretion and degradation
Two sets of 15% poly-acrylamide gels were loaded with 45 microliters of undiluted urine from 4 patients on day 0 and at days 0, 1, 3 and 5 post-transplantation for Western blot analysis. Pre-operative urinary FGF23 concentrations, as determined by ELISA, ranged from 6650 to 77500 RU/ml and had declined to between 12 and 24 RU/ml in the 4 subjects by day 5. Both sets of gels were run simultaneously and transferred to cellulose membranes. The first set of membranes were probed with goat anti-human FGF23(51-69) (Immutopics, Intl) (dilution 1:1000) while the second set of membranes were probed with FGF23(225-244) (Immutopics, Intl) (dilution 1:1000). Both sets of membranes were then simultaneously incubated with horseradish peroxidase-conjugated rabbit anti-goat secondary antibody (Sigma-Aldrich, St. Louis, MO) (dilution 1:5000). Bands were visualized by chemiluminescence (Cell Signaling, Danvers, MA).
Statistical Analysis
Data were expressed as mean ± standard deviation or median (IQ range). A restricted maximum likelihood (REML) mixed model with random intercept was used to examine the contribution of the following predictors: pre-operative FGF23 (log transformed), pre-operative PTH (log transformed), pre-operative creatinine, and time since transplantation (days) and their interactions to the following outcomes: post-transplantation measurements of circulating creatinine, calcium, phosphate, PTH, FGF23, 1,25(OH)2vitamin D, and 24,25(OH)2vitamin D and to the fractional excretion of phosphate (FEPO4), and the fractional excretion of FGF23 (FEFGF23). For graphic depictions of the relationship between FGF23 and post-transplant mineral metabolism, subjects were divided into two equal subgroups based on pre-operative FGF23 values above or below the median value of 759 RU/ml. SAS (version 9.2) was used for all statistical analyses.
Results
Study subjects and baseline biochemical values
Of 67 pediatric patients (37M, 30F) with an average age of 15.3 ± 3.2 years who underwent renal transplantation during the study period, 46 fulfilled pre-operative entry criteria and agreed to participate. Nine of the study subjects underwent pre-emptive renal transplantation; the rest were dialysis patients prior to transplantation. Two patients were excluded post-operatively—one due to delayed graft function and one due to the post-operative use of calcitriol. Thus, 44 (19 M, 25 F) subjects, aged 16.4 ± 2.9 years, who had been receiving maintenance dialysis treatment for 23.0 ± 25.6 months, completed the study. No patients in the study had an episode of acute rejection within the first 6 months. The majority of patients were Hispanic (52%) or Caucasian (39%); the rest (9%) were either African American or Asian. Baseline demographic and biochemical data, along with amount of phosphate supplementation prescribed at day five, in patients with baseline FGF23 values above the median as compared to those with values below the median are displayed in Table 1. The two groups did not differ based on gender, age, time on dialysis, the presence of residual renal function, cause of end-stage renal disease, or the amount of supplemental phosphate prescribed by post-operative day 5. Interestingly, however, all African American and Asian participants had pre-operative FGF23 values below the median value.
Table 1. Baseline demographic data by baseline FGF23 value.
| Patients with baseline FGF23 in the lower half of values (<759 RU/ml) (n=22) |
Patients with baseline FGF23 in the upper half of values (>759 RU/ml) (n=22) |
|
|---|---|---|
|
| ||
| Gender (M/F %) | 52/48 | 38/62 |
|
| ||
| Age (years) | 15.5 ± 2.8 | 17.4 ± 2.8 |
|
| ||
| Time on dialysis (months) | 24.1 ± 34.7 | 23.4 ± 16.4 |
|
| ||
| Race (%) | ||
| Caucasian | 29 | 48 |
| Hispanic | 52 | 52 |
| African American | 14 | |
| Asian | 5 | |
|
| ||
| Steroid immunosuppression (%) | 29 | 33 |
|
| ||
| Residual urine (%) | 52 | 42 |
|
| ||
| ESRD etiology (%) | ||
| Dysplasia/reflux | 48 | 24 |
| Glomerulonephritis | 29 | 29 |
| Genetic disease | 19 | |
| Unknown | 24 | 29 |
|
| ||
| Donor type | ||
| Living related | 24 | 38 |
| Deceased donor | 76 | 62 |
|
| ||
| Phosphate supplementation on post- operative day 5 |
||
| Percentage receiving (%) | 33 | 43 |
| Amount received | ||
| Mean ± SD (mg) | 350 ± 586 | 400 ± 586 |
| Median (mg) (IQ range) | 0 (0,500) | 0 (0,750) |
FGF23, fibroblast growth factor 23; ESRD, end stage renal disease
Immediately pre-transplantation, FGF23 and PTH levels were elevated at 759 (455, 2385) RU/ml and 213 (130, 410) pg/ml, respectively, while serum calcium levels and phosphorus levels were in the normal range (9.4 ± 0.9 and 4.8 ± 1.1 mg/dl, respectively). Serum calcitriol values were 19.3 (6.3, 32.5) pg/ml, 25(OH)vitamin D values were 25.2 ± 11.1 ng/ml and 24,25(OH)2vitamin D levels were 1.7 (0.3, 2.5) ng/ml at baseline. Pre-operative FGF23 levels correlated directly with serum creatinine concentrations (r=0.37, p<0.05) and inversely with both 1,25(OH)2vitamin D (r= −0.32, p<0.05) and PTH (r= −0.31, p<0.05) values. There was no relationship between pre-operative FGF23 values and serum calcium, phosphorus, or 24,25(OH)2vitamin D concentrations.
Changes in FGF23, creatinine, and fractional excretion of FGF23
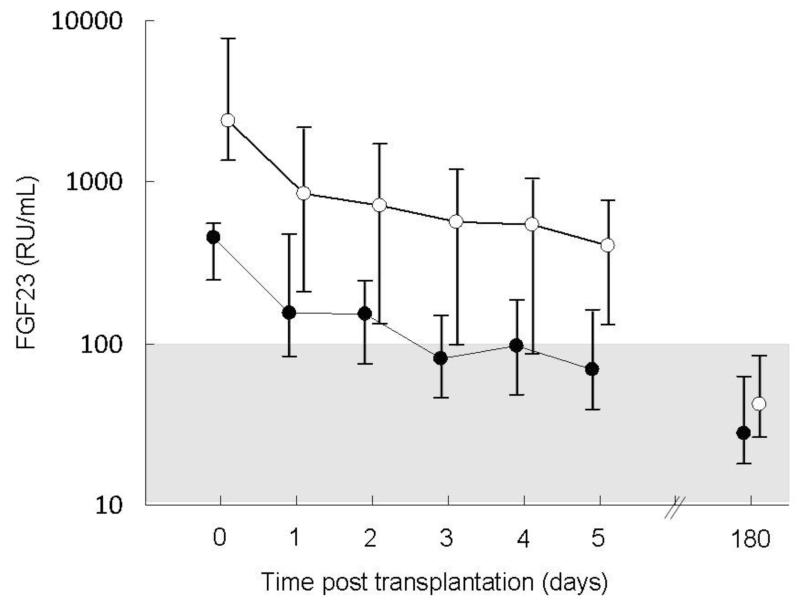
A decline in circulating FGF23 concentration was apparent by post-operative day 1 and values remained below pre-operative levels at all subsequent time-points (p<0.001). As expected, subjects with higher pre-operative FGF23 values had higher post-operative values (p<0.001) (Figure 1). Patients with higher pre-transplantation PTH values also had lower post-transplantation FGF23 values (p<0.05), independent of time and baseline FGF23 values. Pre-transplantation creatinine concentrations were unrelated to post-operative FGF23 values. The post-operative use of steroids had no effect on post-transplant circulating FGF23 levels; pre-transplantation values of FGF23 were similar in patients receiving steroid-based and steroid-free immunosuppressive regimens, values declined similarly in all subjects, and levels did not differ by steroid use either during the first 5 post-operative days or at post-operative day 180.
Figure 1.
Plasma fibroblast growth factor 23 (FGF23) values in patients with pre-transplantation FGF23 concentrations below the median value (closed circles) as compared to patients with pre-transplantation FGF23 values above the median (open circles). The shaded area represents the normal range. FGF23 concentrations values declined over time (p<0.001) and subjects with higher pre-transplantation FGF23 values had higher post-transplantation values (p<0.001).
Pre-operative serum creatinine concentrations did not differ in patients with lower versus those with higher pre-operative FGF23 values (7.1 ± 0.6 mg/dl and 8.8 ± 0.9 mg/dl in the two groups, respectively). Values declined in all patients post-transplantation; however, post-operative creatinine concentrations were higher in patients with higher pre-transplantation FGF23 values. Creatinine values at day 5 averaged 1.2 ± 0.2 mg/dl and 1.9 ± 0.3 mg/dl in those with lower versus those with higher FGF23 values, respectively (p<0.001 for both groups from baseline, p<0.05 between groups). Pre-transplantation PTH values were unrelated to post-operative creatinine concentrations.
Pre-operatively, slightly over half the population had residual urine output. In these individuals, the fractional excretion of FGF23 was 0.52 ± 0 .23% (subjects with higher baseline FGF23 values, n=11) and 0.17 ± 0.07% (subjects with lower values, n=12) (NS between groups). Post-transplantation, values were below 5% at all post-operative time-points, even in patients with the highest baseline circulating FGF23 values, despite markedly elevated circulating plasma concentrations and despite a rapid post-operative decline in circulating concentrations in all individuals. This decline did not depend on pre-transplantation circulating values of FGF23, PTH, or creatinine.
Changes in calcium, PTH, phosphorus, and the fractional excretion of phosphate
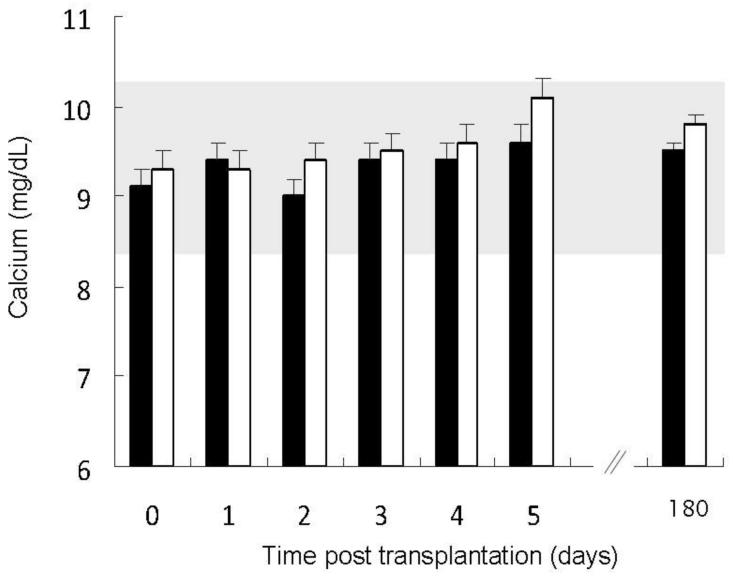
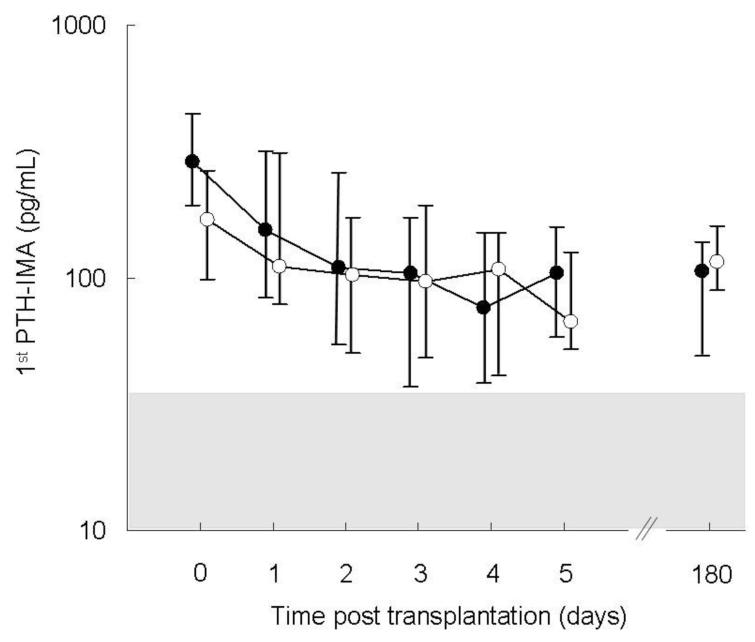
Serum calcium concentrations rose post operatively (p<0.01), although a greater increase was observed in subjects with higher pre-operative FGF23 values (p<0.001 for FGF23 and p<0.01 for the interaction between pre-operative FGF23 and time) (Figure 2). Preoperative PTH and creatinine were unrelated to changes in serum calcium. Post-transplantation, PTH values declined with time (p<0.05) and values were related to pre-transplantation PTH concentrations (p<0.001) but not to pre-operative FGF23 (Figure 3) or creatinine.
Figure 2.
Total serum calcium values in patients with pre-transplantation fibroblast growth factor 23 (FGF23) concentrations below the median value (closed bars) as compared to patients with pre-transplantation FGF23 values above the median (open bars). The shaded area represents the normal range. Serum calcium concentrations increased with time (p<0.01) with a greater increase in subjects with higher pre-transplantation FGF23 values (p<0.001 for the interaction between time and pre-transplantation FGF23).
Figure 3.
Circulating 1st PTH-IMA values in patients with pre-transplantation FGF23 concentrations below the median value (closed circles) as compared to patients with pre-transplantation FGF23 values above the median (open circles). The shaded area (value: 10-65 pg/ml) represents the normal range. Parathyroid hormone (PTH) levels declined in all subjects post-transplantation (p<0.05); no differences were observed based on pre-transplantation fibroblast growth factor (FGF23) values.
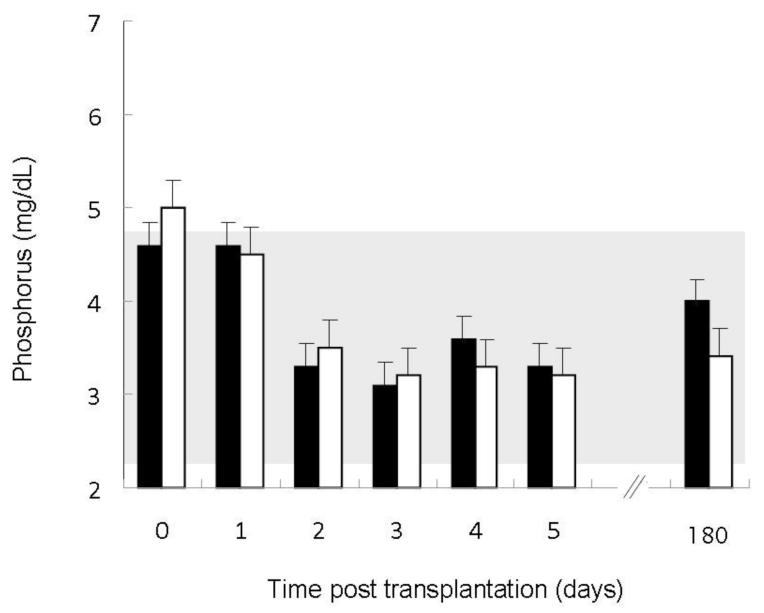
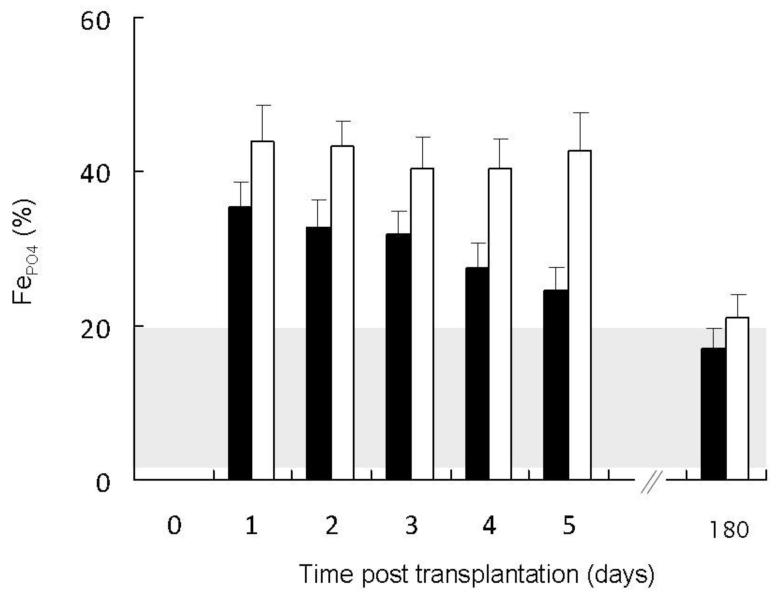
Serum phosphorus concentrations decreased over time (p<0.01) in all subjects, although a greater decrease was observed in individuals with higher baseline FGF23 (p<0.001 for the interaction between time and pre-operative FGF23 concentration) (Figure 4). No post-operative differences in serum phosphate were observed based on pre-operative PTH or creatinine. Similarly, post-operative renal phosphate excretion was related to both time (p<0.001) and the pre-operative FGF23 level (p<0.01) (Figure 5), but not to pre-operative PTH or creatinine.
Figure 4.
Serum phosphorus values in patients with pre-transplantation fibroblast growth factor 23 (FGF23) concentrations below the median value (closed bars) as compared to patients with pre-transplantation FGF23 values above the median (open bars). The shaded area represents the normal range. Serum phosphorus concentrations decreased over time (p<0.01) with a greater decline in patients with higher pre-transplantation FGF23 concentrations (p<0.001 for the interaction between time and pre-transplantation FGF23).
Figure 5.
Fractional excretion of phosphate (FEPO4) values in patients with pre-transplantation FGF23 concentrations below the median value (closed bars) as compared to patients with pre-transplantation fibroblast growth factor 23 (FGF23) values above the median (open bars). The shaded area represents the normal range. Post-operative renal phosphate excretion was related to both pre-operative FGF23 (p<0.01) and time (p<0.001).
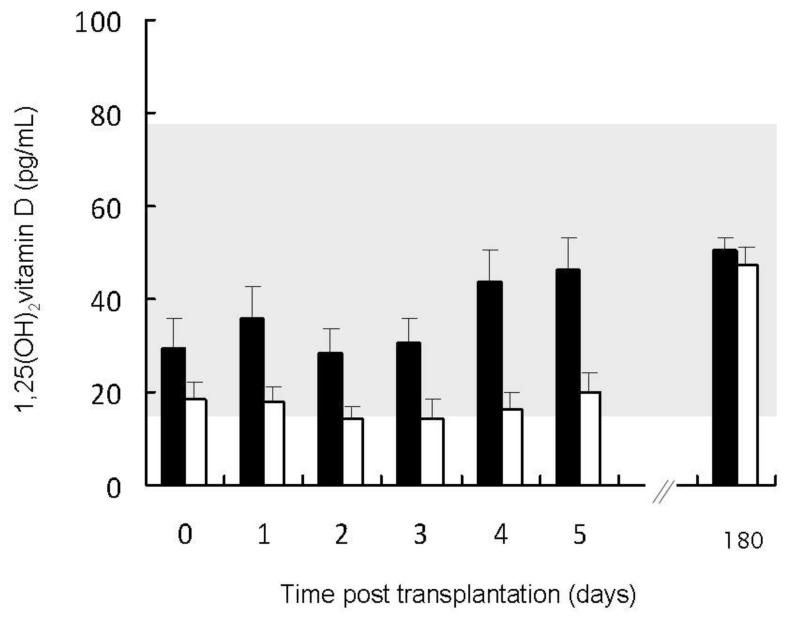
Changes in 1,25(OH)2vitamin D, 25(OH)vitamin D, and 24,25(OH)2vitamin D levels
Pre-operative circulating 1,25(OH)2vitamin D levels did not differ in patients with lower compared to those with higher pre-operative FGF23 concentrations; however, 1,25(OH)2vitamin D values rose more rapidly during the first 5 days post transplantation in patients with lower pre-transplantation FGF23 values (p<0.0001 for pre-operative FGF23 and p<0.001 for the interaction between time and pre-operative FGF23) (Figure 6). Higher pre-operative PTH (p<0.05) and lower pre-operative creatinine (p<0.01) concentrations were also independently associated with greater post-operative increases in 1,25(OH)2vitamin D values. By contrast, 25(OH)vitamin D and 24,25(OH)2vitamin D levels were similar in all subjects at baseline and no differences were observed in post-operative levels of either metabolite based on pre-operative FGF23, PTH, or creatinine concentrations. 25(OH)vitamin D values decreased by post-operative day 5 (from 25.2 ± 11.1 ng/ml to 15.8 ± 9.2 ng/ml, p<0.001) and returned to baseline (20.2 ± 9.2 ng/ml) by post-operative month 6; 24,25(OH)2vitamin D concentrations were 1.8 ± 1.6 ng.ml at baseline and values did not change over time.
Figure 6.
Circulating 1,25(OH)2vitamin D values in patients with pre-transplantation fibroblast growth factor 23 (FGF23) concentrations below the median value (closed bars) as compared to patients with pre-transplantation FGF23 values above the median (open bars). The shaded area represents the normal range. 1,25(OH)2vitamin D values increased with time (p<0.01) with a greater increase observed in subjects with higher baseline FGF23 concentrations (p<0.001 for the interaction between pre-operative FGF23 and time).
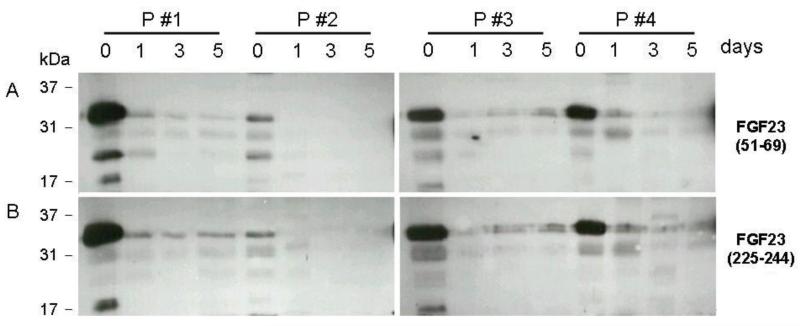
Western blot characterization of FGF23 in urine
Urine FGF23 was present predominantly as the full-length, presumably glycosylated 32 kD form. Much smaller quantities of 26 kDa and 17 kDa FGF23 fragments were also detected at baseline; the quantity of full-length FGF23 decreased and detectable fragments disappeared from the urine quickly after transplantation (Figure 7).
Figure 7.
Western blot analysis of unconcentrated urine samples from 4 patients with “high” baseline FGF23 values and residual urine production pre-operatively, and on days 1, 3 and 5 after successful renal transplantation. Primary antibodies were directed against fibroblast growth factor 23 (FGF23)(51-69) (Panel A) and FGF23(225-244) (Panel B); an HRP-labeled secondary antibody was used for detection. The location of different protein size markers is as indicated. Urinary FGF23 concentrations, as detected by ELISA, ranged from 6650 (pt 2) to 77500 (pt 1) RU/ml on day 0 in the 4 patients. By day 5, urinary FGF23 concentrations ranged from 12 to 24 RU/ml in the 4 subjects.
Discussion
The current study demonstrates that circulating FGF23 concentrations in the early transplant period are associated with changes in circulating phosphate and 1,25(OH)2vitamin D concentrations as well as with urinary phosphate excretion. As expected, patients with higher pre-transplantation FGF23 levels had higher post-operative values of FGF23. 1,25(OH)2vitamin D values were lower, serum phosphorus concentrations declined more rapidly, and the fractional excretion of phosphate was higher in patients with higher pre-operative FGF23 values. 25(OH)vitamin D and 24,25(OH)2vitamin D values were not related to FGF23 concentrations. The fractional excretion of FGF23 was less than 5% at all time points, even in patients with the highest pre-operative FGF23 values, despite markedly elevated circulating FGF23 levels and a rapid decline in values in the immediate post-transplantation period. FGF23 values did not differ whether or not steroid immune suppression was prescribed post-transplantation.
FGF23 has been implicated as a regulator of phosphate and vitamin D metabolism in individuals with normal kidney function and as a major factor in the development of secondary hyperparathyroidism in CKD [1, 22]. FGF23 values have also previously been related to circulating 1,25(OH)2vitamin D values early in the post-transplantation period [19, 20] although this finding has been variable between studies [21]. In the current study, FGF23 and vitamin D kinetics were assessed in the first 6 post-operative days and after 6 months as this latter time point marks a time at which many aspects of mineral metabolism post-transplantation has reached equilibrium and sufficient re-growth of the renal nerves has occurred [23]. Individuals with higher FGF23 levels pre-transplantation failed to increase 1,25(OH)2vitamin D levels post-operatively, as evidence for persistent suppression of 1a-hydroxylase expression. Although 25(OH)vitamin D values declined in all subjects by day 5 post-transplantation, 24,25(OH)2vitamin D values did not differ in patients with higher versus those with lower FGF23 concentrations and did not change after renal transplantation, suggesting that marked upregulation of 24-hydroxylase, the enzyme responsible for the degradation of both 1,25(OH)2vitamin D and 25(OH)vitamin D, may not be responsible for the lower 1,25(OH)2vitamin D values in patients with higher concentrations of FGF23 [24]. The return of 25(OH)vitamin D values to close to pre-operative concentrations by month 6 post-transplantation, despite increasing concentrations of 1,25(OH)2vitamin D and no change in 24,25(OH)2vitamin D during this timeframe, likely reflects improved nutritional intake post-transplantation.
Although pre-transplantation FGF23 values were unrelated to post-operative serum phosphate concentrations, patients with higher pre-operative FGF23 values demonstrated greater post-operative renal phosphate wasting. This finding suggests that circulating FGF23 values may be a better predictor of total-body phosphate burden and of post-operative phosphate wasting than is serum phosphorus itself. Indeed, 80-90% of total body phosphate resides in bone, bone is the major buffer for acute changes in phosphate load, as occur with meals or during hemodialysis, and circulating phosphate concentrations are a poor marker of total body phosphate stores [25]. Currently, at our institution, the need for phosphate supplementation is determined solely based on a serum phosphate concentration of less than 2.5 mg/dl. Since circulating phosphate concentrations in the first 5 days post-transplantation did not vary despite differences in circulating FGF23 values, it is not surprising that phosphate supplementation in this timeframe also did not differ between groups. Total body phosphate excretion, however, did differ markedly between groups; thus, it is likely that a difference in the need for phosphate supplementation may have manifested at a later date (i.e. weeks post transplantation). Since calcitriol levels showed significant differences between groups, elevated pre-transplant FGF23 values might help identify patients who will benefit most from calcitriol treatment post-transplantation to enhance intestinal phosphate absorption; however, calcitriol is likely to enhance FGF23 production and should thus be used with caution.
Circulating FGF23 values declined rapidly in all patients with a functioning allograft, suggesting that a difference in pre-operative kidney function may have contributed to differences in pre-operative FGF23 concentrations. A similar percentage of patients with pre-transplantation FGF23 values above the median value had residual renal function when compared to subjects with pre-transplantation FGF23 concentrations below the median value, however. Western blot analysis of urine demonstrated prior to surgery large amounts of FGF23 in patients with residual urine output, most of which appeared as a 36 kDa band; this size of urinary FGF23 is indistinguishable from the intact, glycosylated FGF23 observed in the circulation of patients with ESRD [26]. Only very smaller quantities of 17 kDa and 23 kDa FGF23 fragments were detected pre-operatively, and these disappeared rapidly from the urine post-transplantation. The antibodies used for the Western blot analysis were obtained from Immutopics and are the same antibodies as those used in their FGF23 assay kits; because this technique identified predominantly intact FGF23, it is very likely that the quantity of FGF23 identified in the urine by the C-terminal FGF23 ELISA represents both the intact molecule and FGF23 fragments. However, it is important to note that the proportion of FGF23 fragments in urine was tiny as compared to the amount of the intact molecule. Whether similar quantities of fragment are detectable in the plasma post-transplantation is currently unknown. Regardless, the rapid decline in circulating FGF23, in combination with the low fractional excretion (below 5% at all timepoints) suggests that declining FGF23 values post-operatively must be due in large part to degradation of FGF23, with or without suppressed FGF23 production/secretion by osteocytes, rather than due to filtration and/or secretion of the intact molecule into urine alone.
Previous data have suggested that steroid use is associated with higher FGF23 values in stable renal transplant recipients [27]. In the current study, however, similar percentages (approximately one-third) of patients with baseline circulating FGF23 levels above and below the median concentration were assigned to steroid-based regimens and no differences in FGF23 or in any other biochemical marker were observed between patients receiving steroids as compared to those on steroid-free protocols at any time-point. Although even patients maintained on “steroid-free” immunosuppressant regimens receive a large bolus of steroids intra-operatively which might be expected to influence FGF23 values in the first 5 days post transplantation, no differences were observed at day 180, by which time the stimulatory effect of intra-operative steroids would likely have disappeared. Although this study was not specifically designed to assess the clinical effects of steroids on circulating FGF23 concentrations, and although inflammation, which is suppressed by steroid therapy, was not assessed [28], the current data suggest that circulating FGF23 values may not be modified by steroid immunotherapy post-renal transplantation.
Although the current sample size is limited in its ability to detect major differences in FGF23 values based on ethnicity or on the etiology of end-stage renal disease, it is interesting to note that all African American and Asian participants had pre-operative FGF23 values that were below the median and thus consistent with findings in previous epidemiologic studies of African American patients [4, 7]. Similarly, although definitive studies linking etiology of renal disease with FGF23 values are lacking, all patients with genetic causes of their renal disease had baseline FGF23 values above the median. Further studies are warranted to assess whether these data represent a true phenomenon.
FGF23 has been identified as a potential regulator of PTH levels in animal models [29, 30]; however, the current study does not support a role for FGF23 levels in regulating PTH values in the early post-transplant period. Although an inverse relationship between FGF23 and PTH values existed pre-transplantation, PTH levels declined rapidly post-operatively and values were not related to pre- or post-operative FGF23 concentrations. The current study is unable to rule out a potential suppressive effect of FGF23 on PTH post-transplantation, however, as increasing values of 1,25(OH)2vitamin D in subjects with lower FGF23 values may have obscured the suppressive effect of FGF23 on PTH.
Although the study was limited by a short window of observation, a relatively small sample size, and the use of a convenience sample (with its potential for bias), the study highlights important physiological changes in mineral metabolism in the early post-transplantation period. Pre-transplant FGF23 values are significantly related to 1,25(OH)2vitamin D levels but not 24,25(OH)2vitamin D values in the early transplant period, suggesting that suppression of renal 1α-hydroxylase may play a greater role than stimulation of 24-hydroxylase activity in FGF23-mediated post-operative calcitriol deficiency. Filtration of the intact molecule or of large fragments does not appear to be a major contributor to the rapid post-transplantation decline in circulating FGF23. Although data have suggested that chronically elevated levels of FGF23 may have consequences on graft function [8, 9] and on overall survival [9], prospective studies are needed to determine whether control of FGF23 levels either pre- or post-transplantation can affect clinical outcomes.
Acknowledgements
This work was supported in part by USPHS grants DK-67563, DK-35423, DK-51081, DK-073039, DK-080984, UL1 RR-033176 and UL1TR000124, PO1 DK11794, and by funds from the Casey Lee Ball Foundation. The authors would like to thank Barbara Gales and Georgina Ramos for their invaluable help in sample collection and storage for the current study.
Footnotes
Disclosure: H Jüppner is named on a patent describing the FGF-23 assay that was used in this study. None of the other authors of this paper have any financial interest in the information contained in this manuscript.
References
- 1.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 2.Wesseling-Perry K, Pereira RC, Wang H, Elashoff RM, Sahney S, Gales B, Juppner H, Salusky IB. Relationship between plasma FGF-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J Clin Endocrinol Metab. 2009;94:511–517. doi: 10.1210/jc.2008-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, Konig P, Kraatz G, Mann JF, Muller GA, Kohler H, Riegler P. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 4.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutierrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jean G, Bresson E, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C. Peripheral vascular calcification in long-haemodialysis patients: associated factors and survival consequences. Nephrol Dial Transplant. 2009;24:948–955. doi: 10.1093/ndt/gfn571. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wesseling-Perry K, Tsai EW, Ettenger RB, Juppner H, Salusky IB. Mineral abnormalities and long-term graft function in pediatric renal transplant recipients: a role for FGF-23? Nephrol Dial Transplant. 2011;26:3779–3784. doi: 10.1093/ndt/gfr126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, Kiss I, Rosivall L, Kosa J, Lakatos P, Kovesdy CP, Mucsi I. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol. 2011;22:956–966. doi: 10.1681/ASN.2010080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garabedian M, Silve C, Levy-Bentolila D, Bourdeau A, Ulmann A, Nguyen TM, Lieberherr M, Broyer M, Balsan S. Changes in plasma 1,25 and 24,25-dihydroxyvitamin D after renal transplantation in children. Kidney Int. 1981;20:403–410. doi: 10.1038/ki.1981.153. [DOI] [PubMed] [Google Scholar]
- 11.Reinhardt W, Bartelworth H, Jockenhovel F, Schmidt-Gayk H, Witzke O, Wagner K, Heemann UW, Reinwein D, Philipp T, Mann K. Sequential changes of biochemical bone parameters after kidney transplantation. Nephrol Dial Transplant. 1998;13:436–442. doi: 10.1093/oxfordjournals.ndt.a027843. [DOI] [PubMed] [Google Scholar]
- 12.Nordal KP, Dahl E, Halse J, Aksnes L, Thomassen Y, Flatmark A. Aluminum metabolism and bone histology after kidney transplantation: a one-year follow-up study. J Clin Endocrinol Metab. 1992;74:1140–1145. doi: 10.1210/jcem.74.5.1569161. [DOI] [PubMed] [Google Scholar]
- 13.Larsson T, Nisbeth U, Ljunggren O, Jüppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 14.Bhan I, Shah A, Holmes J, Isakova T, Gutierrez O, Burnett SA, Jüppner H, Wolf M. Post-transplant hypophosphatemia: Tertiary ‘Hyper-Phosphatoninism’? Kidney Int. 2006;70:1486–1494. doi: 10.1038/sj.ki.5001788. [DOI] [PubMed] [Google Scholar]
- 15.Evenepoel P, Naesens M, Claes K, Kuypers D, Vanrenterghem Y. Tertiary ‘hyperphosphatoninism’ accentuates hypophosphatemia and suppresses calcitriol levels in renal transplant recipients. Am J Transplant. 2007;7:1193–1200. doi: 10.1111/j.1600-6143.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- 16.Pande S, Ritter CS, Rothstein M, Wiesen K, Vassiliadis J, Kumar R, Schiavi SC, Slatapolsky E, Brown AJ. FGF-23 and sFRP-4 in chronic kidney disease and post-renal transplantation. Nephron Physiol. 2006;104:23–32. doi: 10.1159/000093277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isakova T, Xie H, Barchi-Chung A, Vargas G, Sowden N, Houston J, Wahl P, Lundquist A, Epstein M, Smith K, Contreras G, Ortega L, Lenz O, Briones P, Egbert P, Ikizler TA, Jueppner H, Wolf M. Fibroblast growth factor 23 in patients undergoing peritoneal dialysis. Clin J Am Soc Nephrol. 2011;6:2688–2695. doi: 10.2215/CJN.04290511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharyya N, Wiench M, Dumitrescu C, Connolly BM, Bugge TH, Patel HV, Gafni RI, Cherman N, Cho M, Hager GL, Collins MT. Mechanism of FGF23 processing in fibrous dysplasia. J Bone Miner Res. 2012;27:1132–1141. doi: 10.1002/jbmr.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato T, Fukagawa M, Uchida K, Katayama A, Nagasaka T, Matsuoka S, Goto N, Tominaga Y, Kobayashi T, Nakao A. 1,25-dihydroxyvitamin D synthesis after renal transplantation: the role of fibroblast growth factor 23 and cyclosporine. Clin Transplant. 2009;23:368–374. doi: 10.1111/j.1399-0012.2009.00995.x. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez Fructuoso AI, Maestro ML, Calvo N, De La Orden V, Perez Flores I, Vidaurreta M, Valero R, Fernandez-Perez C, Barrientos A. Role of fibroblast growth factor 23 (FGF23) in the metabolism of phosphorus and calcium immediately after kidney transplantation. Transplant Proc. 2012;44:2551–2554. doi: 10.1016/j.transproceed.2012.09.070. [DOI] [PubMed] [Google Scholar]
- 21.Kawarazaki H, Shibagaki Y, Fukumoto S, Kido R, Nakajima I, Fuchinoue S, Fujita T, Fukagawa M, Teraoka S. The relative role of fibroblast growth factor 23 and parathyroid hormone in predicting future hypophosphatemia and hypercalcemia after living donor kidney transplantation: a 1-year prospective observational study. Nephrol Dial Transplant. 2011;26:2691–2695. doi: 10.1093/ndt/gfq777. [DOI] [PubMed] [Google Scholar]
- 22.Nakanishi S, Kazama JJ, Nii-Kono T, Omori K, Yamashita T, Fukumoto S, Gejyo F, Shigematsu T, Fukagawa M. Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients. Kidney Int. 2005;67:1171–1178. doi: 10.1111/j.1523-1755.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- 23.Gazdar AF, Dammin GJ. Neural degeneration and regeneration in human renal transplants. N Engl J Med. 1970;283:222–224. doi: 10.1056/NEJM197007302830502. [DOI] [PubMed] [Google Scholar]
- 24.Dai B, David V, Alshayeb HM, Showkat A, Gyamlani G, Horst RL, Wall BM, Quarles LD. Assessment of 24,25(OH)2D levels does not support FGF23-mediated catabolism of vitamin D metabolites. Kidney Int. 2012;82:1061–1070. doi: 10.1038/ki.2012.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spalding EM, Chamney PW, Farrington K. Phosphate kinetics during hemodialysis: Evidence for biphasic regulation. Kidney Int. 2002;61:655–667. doi: 10.1046/j.1523-1755.2002.00146.x. [DOI] [PubMed] [Google Scholar]
- 26.Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, Wolf M, Salusky IB, Jüppner H. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010;95:578–585. doi: 10.1210/jc.2009-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bacchetta J, Dubourg L, Harambat J, Ranchin B, bou-Jaoude P, Arnaud S, Carlier MC, Richard M, Cochat P. The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J Clin Endocrinol Metab. 2010;95:1741–1748. doi: 10.1210/jc.2009-1576. [DOI] [PubMed] [Google Scholar]
- 28.Mendoza JM, Isakova T, Ricardo AC, Xie H, Navaneethan SD, Anderson AH, Bazzano LA, Xie D, Kretzler M, Nessel L, Hamm LL, Negrea L, Leonard MB, Raj D, Wolf M. Fibroblast Growth Factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol. 2012;7:1155–1162. doi: 10.2215/CJN.13281211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, Westin G, Larsson TE. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro O, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]