Abstract
In vivo studies have demonstrated that the superior colliculus (SC) integrates sensory information and plays a role in controlling orienting motor output. However, how the complex microcircuitry within the SC, as documented by slice studies, subserves these functions is unclear. Optogenetics affords the potential to examine, in behaving animals, the functional roles of specific neuron types that comprise heterogeneous nuclei. As a first step toward understanding how SC microcircuitry underlies motor output, we applied optogenetics to mice performing an odor discrimination task in which sensory decisions are reported by either a leftward or rightward SC-dependent orienting movement. We unilaterally expressed either channelrhodopsin-2 or halorhodopsin in the SC and delivered light in order to excite or inhibit motor-related SC activity as the movement was planned. We found that manipulating SC activity predictably affected the direction of the selected movement in a manner that depended on the difficulty of the odor discrimination. This study demonstrates that the SC plays a similar role in directional orienting movements in mice as it does in other species, and provides a framework for future investigations into how specific SC cell types contribute to motor control.
Keywords: Decision making, midbrain, mouse behavior, motor planning, channelrhodopsin-2, halorhodopsin
1. Introduction1
Sensorimotor decision making is mediated by an interconnected network of cortical and subcortical brain regions [1]. A critical hub for the integration of sensory information and the control of orienting motor output is the superior colliculus, which contains spatially registered sensory and motor representations in its superficial and deeper layers, respectively [2–6]. Electrophysiological recording and manipulation of neural activity in behaving animals, across a range of species, have revealed the organization of motor maps: Activity of neurons in specific regions of the SC corresponds to initiating movement of the eyes, head, neck, and body in specific directions [2, 7–13], and even to spatially-specific shifts of attention without an overt motor component [14, 15]. Indeed, SC activity is also known to play a role in selecting and planning specific movements [12, 16–19]. In general, SC stimulation mediates contraversive (i.e., toward the contralateral side) movement [10, 20–25], although some studies have demonstrated a more complex relationship between stimulation location and movement direction [24, 26].
Electrophysiological examination of the SC in slices has elucidated the organization of microcircuits [27–33]. Most importantly, excitatory projection neurons originating in the intermediate and deep layers are thought to be responsible for initiating contraversive movements, while local and intercollicular inhibitory neurons, although less numerous, may play the key role of mediating the interactions between competing motor output plans [32, 34]. However, previous studies have not been able to target excitatory or inhibitory neurons for recording or manipulation in behaving animals, so the relationship between the functional microcircuitry described by slice studies and behavioral output remains speculative.
Optogenetics affords the possibility to interrogate the functional roles of targeted cell types in vivo [35]. As a first step toward utilizing optogenetics to examine the roles of excitatory and inhibitory SC neurons in motor control, we trained mice on a task requiring them to select and implement an SC-dependent leftward or rightward movement. Mice are an ideal model system for examining the behavioral roles of genetically-defined cell types due to the relative ease of obtaining specific expression of light-gated proteins [36, 37, 38]. We focused on whether unilateral optogenetic stimulation of SC neurons, as the mouse planned its movement, would affect motor output. We hypothesized that, because excitatory projection neurons are more numerous than inhibitory interneurons, channelrhodopsin-2- (ChR2-)mediated excitation of one SC would bias the mouse toward contraversive movement, while the analogous halorhodopsin-(NpHR-)mediated inhibition would bias the mouse ipsiversively (i.e., toward the ipsilateral side). As described below, our results support this hypothesis, consistent with previous data in other species demonstrating that the balance of activity between the two SCs plays a role in selecting and initiating the direction of motor output [16–19, 39–41]. This study provides a framework for future studies to utilize optogenetics in the behaving mouse system to examine the functional roles of excitatory and inhibitory SC neurons in selecting and implementing actions.
2. Material and methods
2.1 Animal subjects
Procedures using animals were approved by the University of Colorado Animal Care and Use Committee, and were in accordance with standards implemented by the National Institutes of Health. Data from five C57BL/6 mice (Jackson Laboratories) were included in this study. Three mice were used in the excitation experiments (Results 3.2), one mouse was used in the inhibition experiments (Results 3.3), and two mice (one of which was also used in the excitation experiments) were used in the control experiments (Results 3.4). Mice had free access to food, but were restricted to 1.5 mL of water per day (per mouse) in order to sufficiently motivate them to perform the task for a water reward while maintaining them above 85% of baseline body weight.
2.2 Surgery
Mice were prepared for cannula implantation and injections of viral constructs (Material and methods 2.5). Anesthesia was induced with 2% isoflurane and maintained between 1% and 1.5% isoflurane throughout the procedure. Depth of anesthesia was monitored by tail- and toe-pinch responses. Body temperature was maintained using a heating pad. The mouse was placed in a stereotaxic frame (Kopf Instruments), and a small incision was made in the skin with a stainless-steel surgical blade. The skull was cleaned and dried, and a craniotomy was performed using a dental drill. Viral injections (Material and methods 2.5) were administered at 250 nl/min with an infusion pump attached via a Hamilton syringe and tubing to a stainless steel cannula (Plastics One;) [42] and targeted to the intermediate and deep layers of the SC within the left hemisphere at 4.04 mm posterior to bregma, 1.1 mm lateral to the midline, and 1.3 mm ventral to the brain surface, an area of the SC consistent with eliciting contraversive orienting behaviors [24, 43, 44]. A steel guide/dummy cannula assembly (Plastics One) was targeted to 0.2 mm dorsal to the virus injection and affixed to the skull using two small screws, luting (3M), and dental acrylic (A-M Systems). Following surgery, mice were immediately rehydrated with sterile 0.9% saline (1 ml/kg) and administered the analgesics ketofen (Pfizer) (5 mg/kg) for 3 days and children’s ibuprofen (50 mg/kg/day) for 7 days following surgery. The incision site was treated with a topical antibiotic. Mice were allowed to recover for at least 7 days before water restriction and behavioral training began.
2.3 Delayed-response, odor-cued movement task
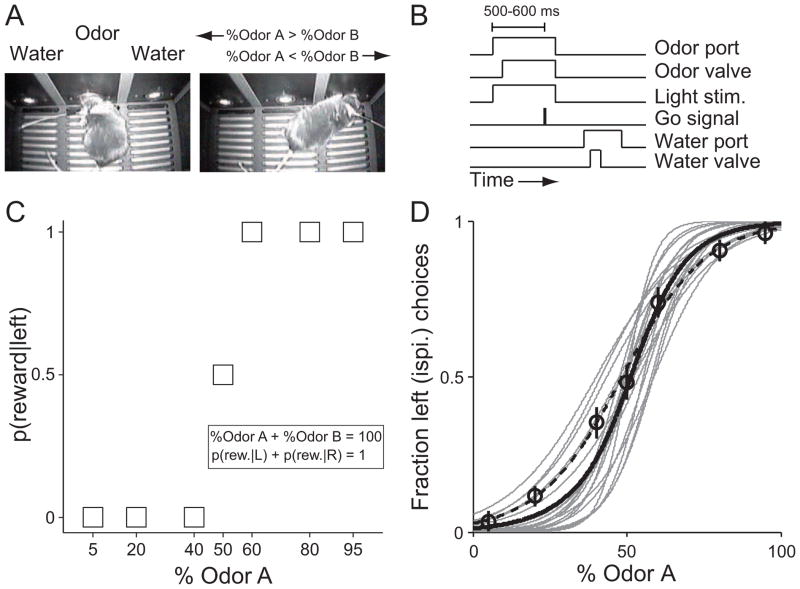
Adult mice were trained and tested on a delayed-response version of a two-alternative movement task in which odor identity was associated with the location of a water reward [12, 45, 46]. The behavioral apparatus was 24.5 cm long by 20 cm wide by 19 cm tall. Mice performed the task on a raised platform within the box and were trained to nose-poke into three ports along the front wall, all of which were raised 2 cm from the platform floor (Island Motion, Tappan, NY). In each trial of the task, the mouse first entered the centrally-located odor port, triggering the delivery of an odor, waited 500–600 ms for the presentation of a go signal, and then moved to one of the two reward ports (located 6.5 cm to the left and right of the central odor port) to receive water (Fig. 1A, B). On 50% of randomly interleaved trials, light was delivered to the left SC during the movement planning epoch (from odor port entry until odor port exit, Fig. 1B). Premature odor port exit resulted in the absence of reward at either reward port. Infrared photodiode/phototransistor pairs were housed within each port to determine the precise timing of port entry and exit.
Fig. 1. Behavioral task and performance.
(A) The task environment, showing the implanted mouse in the odor port (left image) and the right reward port (right image).
(B) Timing of task events. Light stimulation was delivered on 50% of randomly selected trials. For ChR2 sessions, blue light was pulsed at 25 Hz; for NpHR sessions, yellow-green light was delivered continuously.
(C) Probability of reward at the left port as a function of %Odor A in the binary mixture.
(D) Task performance. Circles show data from 1 session (mean ± SEM); dashed line shows corresponding best-fit psychometric function. Gray lines show best-fit psychometric functions for the final 3 training sessions (in which no light was delivered) preceding the experimental sessions for each of 5 mice. Solid black line shows best-fit psychometric function for data across all sessions.
Odors consisted of binary mixtures of (−)-carvone (Odor A) and (+)-carvone (Odor B). Mice were rewarded at the left port when %Odor A > %Odor B and the right reward port when %Odor B > %Odor A. When %Odor A = %Odor B, reward was available at both the left and right on 50% of randomly selected trials (Fig. 1C). Each odor mixture was diluted tenfold in light mineral oil (Fisher Scientific) and 20 μl of the dilution was pipetted onto a syringe filter. The olfactometer delivered air at 100 ml/min through individual polyethylene tubes connected to each filter. This odorized air was further diluted eightfold in carrier air provided by a standard air cylinder. Thus, 800 ml/min of odorized air was delivered to the odor port. The olfactometer, water valves, and go signals were controlled by custom software (Matlab, Mathworks).
2.4 Initial behavioral training
After full recovery from surgery, mice were restricted to 1.5 mL water/day for 5 days. Mice were then trained to perform the behavioral task using the following sequence of steps:
With the center port blocked, mice were water-rewarded for entering either the left or right reward ports. These sessions were repeated until the mice obtained 1.5 ml of water from the side ports within one session (2–3 days).
With only the right port blocked, the mice were required to enter the center port before receiving a reward at the left port. Once mice received 1.5 ml of water, the barrier was switched to the left port and the process was repeated with water delivery to the right port (3–5 days).
In alternating blocks of 30 rewarded trials, one reward port was blocked and 95/5 (%Odor A/%Odor B) (corresponding to open left port) or 5/95 (corresponding to open right port) mixtures were presented upon entry to the center port. A minimum required odor sampling time, indicated by a go signal (a light flash for some mice or a tone for others), began at 0.01 s and increased in 0.01 s increments upon completion (i.e., successfully waiting for the go signal and subsequently entering the reward port) of 80% of the previous 20 trials. This process was repeated until the mice achieved a required odor sampling time between 0.5 s and 0.6 s (14–28 days).
All ports were open and delivery of 95/5 and 5/95 mixtures was randomized trial by trial. Upon completing > 65% of trials in the session at > 80% correct, the 80/20 and 20/80 mixtures were introduced. This process was repeated for the 60/40 and 40/60, and then the 50/50, mixtures.
Mice performed approximately 200–300 trials/session during training, depending on motivation, performed 1 session/day for 6 days/week, and were fully trained within 6–8 weeks.
2.5 Optogenetic stimulation
In order to express ChR2 or NpHR in SC neurons, each adult mouse was injected with 600 nl of a 1:1 mixture of a virus containing Cre (AAV2-EF1a-mCherry-IRES-WGA-Cre) and either a double-floxed-inverted hChR2-EYFP construct [pAAV5-Ef1a-DIO-hChR2(H134R)-EYFP-WPRE-pA] or a double-floxed-inverted eNpHR3.0-EYFP construct (pAAV5-Ef1a-DIO-eNpHR-EYFP-WPRE-pA) [all viruses were obtained from the University of North Carolina Vector Core with permission from Dr. Karl Deisseroth (Stanford University)] [47, 48]. A steel cannula (Plastics One,) was permanently implanted in order to guide an optic fiber, inserted prior to each behavioral session, to deliver light to opsin-expressing neurons located ventral to the fiber tip.
Light was delivered via diode-pumped, solid-state lasers (473 nm or 532 nm; Shanghai Laser & Optics Century) coupled to the optic fiber. Lasers were calibrated weekly with an optic power meter (Melles Griot). The power range was measured to be 50–110 mW/mm2 for 473 nm light, a range previously demonstrated to depolarize ChR2-expressing neurons [49, 50]. Power output for the 532 nm laser was 140–160 mW/mm2, which is near a previously demonstrated range to hyperpolarize NpHR-expressing neurons [51, 52]. At these ranges of power output, we estimate that effective light stimulation was restricted to the intermediate and deep layers of the left superior colliculus, given precise histological confirmation of the ventral tip of optic fiber (cannula) location. Such light delivery did not elicit observable responses in freely-moving, non-behaving mice. Consistent with previous experiments blue light was pulsed at 25 Hz to activate ChR2, and yellow-green light was delivered continuously to activate NpHR [50, 53].
2.6 Histology
Opsin expression and cannula/optic fiber location in all mice were verified histologically after final behavioral training and testing. Mice were deeply anaesthetized with 250 mg/kg pentobarbital and transcardially perfused with 4% paraformaldehyde. The brain was then excised and placed into 4% paraformaldehyde for 24 hours at 4 degrees C. Coronal sections (100 μm) were prepared in 0.1% phosphate buffered saline (0.9% NaCl) using a Vibratome and Nissl stained at a dilution of 1:750, mounted on slides, and coverslipped for fluorescence microscopy. Images were captured at 4x using an inverted Olympus microscope. Only mice that met both of the following criteria were included in the experimental groups: 1) opsin-EYFP expression in the intermediate and deep SC layers and 2) cannulae and optic fiber targeted to the same location as in (1).
2.7 Statistics and quantification of directional bias
Psychometric functions were fitted to , where x is the proportion of the left odor (Odor A) in the mixture, p is the fraction of left choices, and a and b are the best-fit free parameters. The slope of the function is captured by b, while its directional bias was calculated as [11]. Positive bias values reflect a leftward (ipsiversive) shift relative to a perfectly left/right symmetric psychometric function, and negative values reflect a rightward (contraversive) shift. Separate psychometric functions were fitted to the data from light-on and light-off trials.
In order to determine whether the bias values associated with the psychometric functions for the light-off and light-on trials of a given session (BiasOFF and BiasON, respectively) were significantly different from each other, we performed a bootstrap test. We first calculated BiasDIFF = BiasON − BiasOFF. We then shuffled the identity of light-on and light-off trials, calculated the bias values for these shuffled groups as BiasON*and BiasOFF*, and calculated BiasDIFF*= BiasON*− BiasOFF*. We repeated this procedure 1000 times to calculate a set of 1000 BiasDIFF* values. The significance of BiasDIFF was then obtained by comparing it to the set of BiasDIFF* values. To test whether BiasDIFF < 0, our hypothesized effect of exciting the left SC (in ChR2-expressing mice), the p-value was calculated as the fraction of BiasDIFF* values that were less than BiasDIFF. Therefore, the p-value represents the probability of observing an effect at least as large as BiasDIFF if the light exerted no effect. SlopeOFF and SlopeON correspond to the slope of the psychometric functions for the light-off and light-on trials, respectively, of a given session. Significance was set to p < 0.05 for all statistical tests.
3. Results
3.1 Behavioral performance
We sought to examine the role of the SC in controlling orienting movements in freely moving animals. To that end, we trained mice on a delayed-response olfactory discrimination task that required a directional orienting movement in order to obtain a reward (Fig. 1; Material and methods 2.3 and 2.4). In each trial of the task, the reward side was determined by the dominant component in a binary odor mixture (Fig. 1C). Mice were able to achieve sufficient performance on the task, selecting the left reward port when %Odor A > 50 and the right reward port when %Odor A < 50 (Material and methods 2.4; Fig. 1D). Mice performed 338 ± 13.5 trials/session (mean ± SEM), and 1 session/day.
3.2 Effect of exciting SC intermediate and deep layer neurons
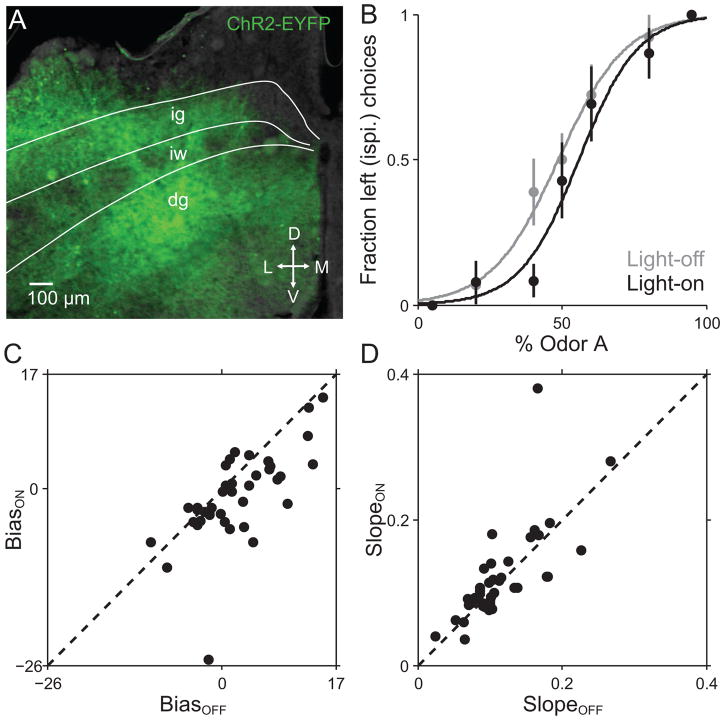
Upon achieving stable performance in one group of mice, we tested how SC activity affects movement direction by optogenetically exciting SC neurons immediately preceding movement initiation (Fig. 1B). Since SC activity has been shown to be correlated with contraversive movement [11, 44, 54, 55], we hypothesized that exciting left SC neurons would bias the mouse toward rightward movement. In order to test this hypothesis, prior to beginning training we expressed ChR2 in intermediate and deep layer SC neurons (Fig. 2A) and implanted a cannula to guide an optical fiber to this region (Material and methods 2.5). The behavior proceeded exactly as described above, now with blue light delivery on 50% of randomly selected trials. We then fitted separate psychometric functions to the choices on “light-on” and “light-off” trials (Material and methods 2.7; Fig. 2B), and estimated the bias exhibited during each of these groups of trials (Material and methods 2.7; Fig. 2C). Consistent with our hypothesis, we found that unilateral blue light delivery biased mice contraversively on the task. Figure 2B shows an example session in which the psychometric function corresponding to light-on trials was shifted to the right relative to the function corresponding to light-off trials. Figure 2C shows the bias exhibited during light-on trials in a given session (BiasON) against the bias exhibited during the light-off trials in that same session (BiasOFF). Across sessions, BiasON was lower (i.e., more rightward) than the BiasOFF (p = 0.00029, t-test; 10/39 sessions individually significant, bootstrap test Material and methods 2.7). In contrast, SlopeON was not significantly different from SlopeOFF, indicating that overall behavioral performance was not affected by light delivery (Fig. 2D, p = 0.49, Wilcoxon signed rank test). These results support our hypothesis that unilateral SC excitation biases the mouse toward contraversive movements in this task.
Fig. 2. Behavioral effect of ChR2-mediated unilateral SC excitation.
(A) Histological confirmation of ChR2-EYFP expression in the SC. Green, EYFP. Coronal section shown was estimated as 3.8 mm posterior from bregma (200 μm anterior to both viral injection and cannula implantation site). Layers of the SC: ig, intermediate gray layer; iw, intermediate white layer; dg, deep gray layer.
(B) Choices and best-fit psychometric functions shown separately for light-off trials (gray) and light-on trials (black) for 1 example session in a ChR2-expressing mouse.
(C) BiasON values plotted against corresponding BiasOFF values for each behavioral session. Across sessions, BiasON values were lower than BiasOFF values, indicating that light delivery resulted in a rightward (contraversive) shift, as exemplified in (B).
(D) SlopeON values plotted against corresponding SlopeOFF values for each session. Across sessions, SlopeON values were no different from SlopeOFF values, indicating that light did not degrade overall behavioral performance.
3.3 Effect of inhibiting SC intermediate and deep layer neurons
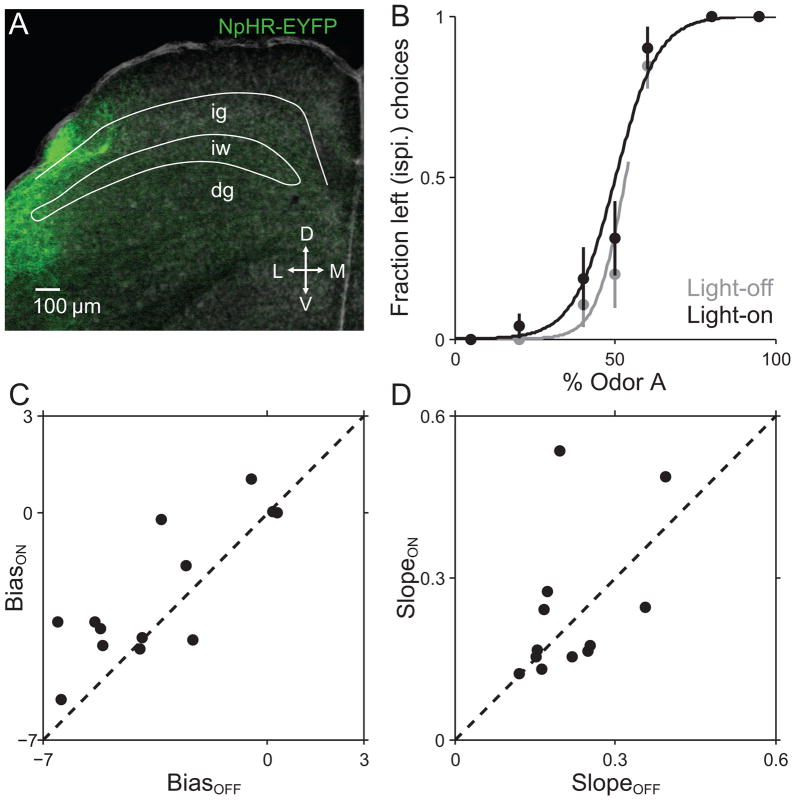
To complement the excitation experiments described above, in a separate set of mice we expressed NpHR in intermediate and deep layer SC neurons and repeated the stimulation experiments described above with 532 nm light to activate NpHR. We hypothesized that inhibiting SC activity just prior to movement onset would reduce the likelihood of contraversive movements, thus biasing the mouse ipsiversively. Indeed, psychometric functions fitted to light-on trials exhibited a leftward (ipsiversive) shift relative to light-off trials (Fig. 3B), therefore exhibiting larger BiasON than BiasOFF values, across sessions (Fig. 3C; p = 0.036, t-test). Again, overall behavioral performance was not affected by light delivery, as SlopeON did not differ from SlopeOFF (Fig. 3D, p = 0.50, Wilcoxon signed rank test).
Fig. 3. Behavioral effect of NPHR-mediated unilateral SC inhibition.
(A) Histological confirmation of NpHR-EYFP expression in the SC. Green, EYFP. Coronal section shown was estimated as 4.7 mm posterior from bregma (700 μm posterior to both viral injection and cannula implantation site). Layers of the SC: ig, intermediate gray layer; iw, intermediate white layer; dg, deep gray layer.
(B) Choices and best-fit psychometric functions shown separately for light-off trials (gray) and light-on trials (black) for 1 example session in an NpHR-expressing mouse.
(C) BiasON values plotted against corresponding BiasOFF values for each behavioral session. Across the population, BiasON values were higher than BiasOFF values, indicating that light delivery resulted in a leftward (ipsiversive) shift, as exemplified in (B).
(D) SlopeON values plotted against corresponding SlopeOFF values for each session. Across sessions, SlopeON values were no different from SlopeOFF values, indicating that light did not degrade overall behavioral performance.
3.4 Behavioral effect of opsin expression or light delivery alone
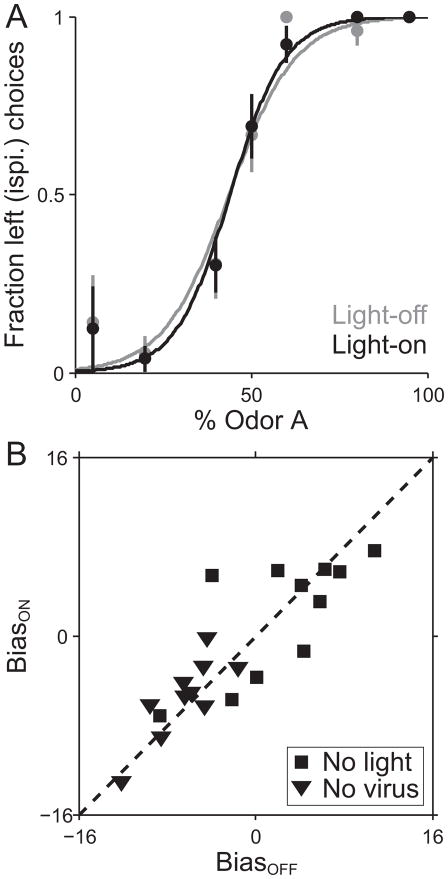
To ensure that the effects of in vivo light delivery on behavior were mediated via opsin activation of neural activity and not a potential secondary mechanism (e.g., the light activating neural activity via thermal transduction [56] or cueing to the light potentially visible through the fiber [57]) we repeated the above experiments in control mice. In one set of experiments, no opsin was virally delivered to SC neurons, but blue light was still delivered to the SC as described for the opsin-expressing mice. As expected, no directional bias resulted from these experiments, nor was there a change in behavioral performance (Fig 4A, B; BiasON compared to BiasOFF, p = 0.85, t-test; SlopeON compared to SlopeOFF, p = 0.63, Wilcoxon signed rank test). Thus, light alone is not sufficient for activation of SC neurons, nor are the mice visually cued by the light. Additionally, to determine that opsin expression alone was not sufficient to drive a bias in movement choice, ChR2 was virally expressed in the SC as described above, but light was blocked from exiting the implanted cannula. As expected, there was again no directional bias or change in performance observed in these sessions (Fig. 4B; BiasON compared to BiasOFF, p = 0.85, t-test; SlopeON compared to SlopeOFF, p = 0.97, Wilcoxon signed rank test). Taken together, these results demonstrate that optical activation of opsins was necessary to observe a directional bias (Fig. 4). Thus, our reported behavioral effect during light delivery to ChR2- and NpHR-expressing SC neurons is, in fact, due to exciting or inhibiting, respectively, activity of these neurons.
Fig. 4. Behavioral effect of opsin expression or light delivery alone.
(A) Choices and best-fit psychometric functions shown separately for light-off trials (gray) and light-on trials (black) for 1 example session, for a control animal with no virus injection and therefore no opsin expression.
(B) BiasON values plotted against corresponding BiasOFF values for each session, for all control sessions. In “No light” controls, light was delivered through the fiber but was blocked from reaching the SC. In “No virus” controls, light was delivered normally, but the mouse did not receive a viral injection and therefore no opsin was expressed in the SC. Across the population, BiasON values did not significantly differ from BiasOFF values.
3.5 Difficulty dependence of directional shifts induced by manipulation of activity
The above calculations of BiasON and BiasOFF values do not differentiate between trials in which odor discrimination is relatively easy, on which mice perform well, and trials in which the discrimination is hard, on which mice perform poorly (Fig. 1D). We now examine whether the difficulty of discrimination affects the degree to which unilateral manipulation alters movement direction. If the SC were involved in implementing actions that have previously been selected, we would expect the shift in movement to be independent of discrimination difficulty. However, if the SC were involved in selecting the movement, then we would expect to observe a larger effect of manipulating activity on movement direction during hard than easy trials [19].
For each session, we calculated the fractions of light-on trials in which the animal selected the left water port, separately for easy (%Odor A = 5, 20, 80, or 95) and hard (%Odor A = 40, 50, or 60) discrimination trials, as well as the corresponding fractions of light-off trials. In the excitation experiments, we found that light delivery had a greater effect (i.e., caused more contraversive choices) on hard trials than on easy trials (Fig. 5A; p = 0.000096, t-test). However, in the inhibition experiments, we found no difference in the effect of light delivery on hard or easy trials (Fig. 5B; p = 0.42, t-test). As expected, we observed no effect of discrimination difficulty in our control sessions (Fig. 5C, p = 0.90, t-test). These results suggest that in mice, as in primates, the SC plays a role in the process of selecting movements [9, 16–19, 41, 58–64].
Fig. 5. Difficulty dependence of directional bias.
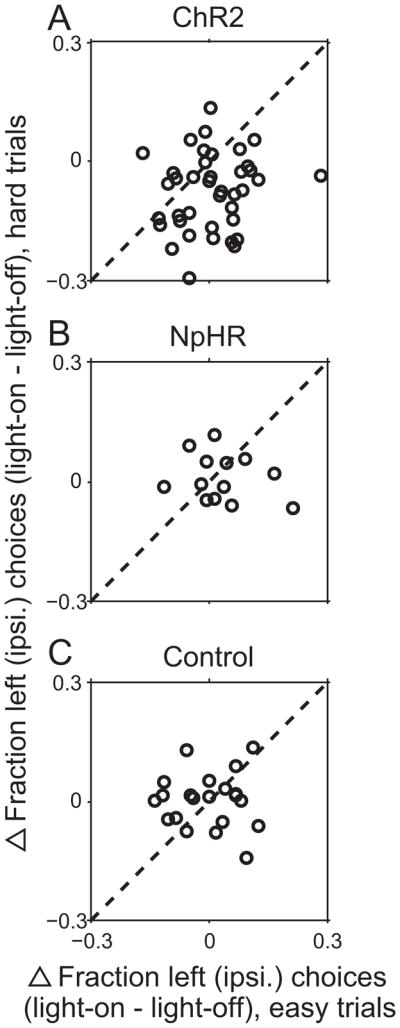
(A) Difference between the fraction of leftward choices on light-on and light-off trials in each session, separately for easy (%Odor A = 5, 20, 80, or 95) and hard (%Odor A = 40, 50, or 60) discrimination trials, in the ChR2 sessions (shown in Fig. 2). Each circle represents 1 session. Light delivery resulted in a larger rightward (contraversive) shift in the hard compared to easy trials.
(B) As in (A), for NpHR sessions (shown in Fig. 3).
(C) As in (A), for control sessions (shown in Fig. 4).
4. Discussion
We examined the role of the intermediate and deep layers of the mouse SC in orienting movements. Our results demonstrated that exciting these neurons biased movements contraversively (Fig. 2), while inhibiting them resulted in an ipsiversive bias (Fig. 3). Importantly, our results were not due to opsin expression alone or light delivery alone (Fig. 4), confirming that they were due to optogenetic manipulation. These results are consistent with those of previous studies in the SC of other mammalian species, thus establishing the behaving mouse as a model for future studies to further elucidate the functional role of SC microcircuitry.
4.1 Effects of optogenetic excitation and inhibition of SC neurons on movement direction
Previous studies have demonstrated that unilateral activation of intermediate and deep layer SC neurons elicits contraversive movements, across species [10, 20–25]. Unilateral pharmacological excitation of the rat SC similarly resulted in a preponderance of contraversive head movements [43]. Although inhibitory neurons also populate the intermediate layers of the SC [30, 34, 65, 66], the contraversive effects of unilateral SC activation are thought, in part, to be mediated by the more numerous excitatory neurons that project to brainstem and spinal cord motor nuclei [26, 32, 67].
In this study, we did not restrict opsin expression to any particular type of SC neuron. Thus, although we did not histologically quantify relative opsin expression in glutamatergic and GABAergic neurons, we speculated that, as was the case in these previous studies, the behavioral effect of unilateral optogenetic excitation would be mediated by the excitatory projection neurons. Indeed, we found that optical excitation biased the mice contraversively (Fig. 2). By the same reasoning, we speculated that the effects of our inhibition experiments (using NpHR) would be mediated by these same projection neurons. We therefore hypothesized that unilateral inhibition would result in an ipsiversive bias, as we and others using pharmacological methods have observed [11, 68–70]. Our results were again consistent with previous findings: unilateral inhibition resulted in an ipsiversive bias (Fig. 3).
We did observe a notable difference between our excitation and inhibition experiments: There was a difficulty-dependence of the behavioral shift only under excitation (Fig. 5A,B). These differences may be due to the relative expression levels of ChR2 and NpHR (Figs. 2A and 3A). In addition, previous studies have observed modest effects of NpHR-mediated inhibition compared to ChR2-mediated excitation [50, 53], possibly due to a transient excitatory effect following NpHR activation [71].
4.2 The behaving mouse as a model for examining SC function
As described above, the contraversive and ipsiversive biases that we have observed under conditions of unilateral excitation and inhibition, respectively, are consistent with previous findings in other species. Further, we found that unilateral excitation had a larger effect on difficult discrimination trials than on easy trials (Fig. 5), consistent with findings in the primate SC [19]. These results suggest that in mice, as in primates, the SC may be involved in selecting the action and not simply executing a previously selected action [9, 16–19, 41, 58–64]. Recently, optogenetic manipulation in the primate SC has yielded similar findings [72]. Together, the consistency of our results with those obtained in primates suggests that the mouse is a viable model for studying SC function.
The mouse model also offers some clear advantages over other species. Although some optogenetic tools can be applied to primates [73–76], transgenic mouse lines currently permit the restricted expression of Cre-dependent constructs, allowing for in vivo examination of the functional roles of particular cell types [37, 56, 77, 78]. For example, VGlut-Cre mice and GAD-Cre mice can be used to study glutamatergic and GABAergic neurons, respectively [77]. Given that many of the slice studies in the SC have utilized mice, examining the SC in behaving transgenic mice has the potential to unify findings across mechanistic and functional experiments, providing a valuable complement to the primate SC model.
4.3 Long-term in vivo effects of ChR2 excitation
The in vivo application of optogenetics has inspired a number of elegant approaches to examining the functional roles of heterogeneous neuronal networks [57, 79–81]. However, whether there exist long-term physiological effects of repeated activation of opsin proteins is unknown [56]. We found that one mouse that had demonstrated a robust contraversive bias in response to SC excitation during its initial 13 sessions (shown in Fig. 2C) ultimately then showed a significant, and more robust, ipsiversive bias in its final sessions, conducted 11–13 weeks later (initial contraversive effect: p = 0.041, 3/13 sessions individually significant, bootstrap test; final ipsiversive effect: p = 0.00021, 8/15 sessions individually significant, bootstrap test). A parsimonious explanation for these results is that frequent, long-term activation of ChR2 in vivo may cause calcium-mediated excitotoxic cell death in neurons that co-express ionotropic NMDA receptors, such as SC projection neurons [82–84]. ChR2 conductance is mainly mediated by calcium [85, 86]. Increased calcium accumulation via ChR2 activation could rapidly depolarize neurons to relieve the magnesium block of NMDA receptors, causing further calcium accumulation sufficient to induce excitotoxic cell death [87, 88]. Although the specific mechanisms are unclear, inhibitory neurons may remain viable under these excitotoxic conditions [89–91]. Thus, unilateral loss of SC excitatory neurons due to excitotoxicity could leave only the ChR2-expressing inhibitory interneurons to be activated by light. Although this subpopulation has not been the focus of in vivo study, a reasonable hypothesis based on the fact that they inhibit excitatory projection neurons is that activating inhibitory SC neurons would result in an ipsiversive bias. Indeed, some previous microstimulation experiments have elicited ipsiversive movements in rats [24, 44] and a significant minority of SC neurons in rats exhibit higher firing rates preceding ipsiversive movements [11, 12]; both findings may reflect the activity of the relatively small GABAergic population. Thus, we speculate that the strong ChR2-driven ipsiversive bias observed in later sessions may be due to selective activation of inhibitory neurons that were spared from ChR2-mediated excitotoxicity. These findings suggest that the long-term effects of opsin expression, which are particularly relevant to experiments utilizing optogenetics in a behavioral context, should be examined in future studies.
4.4 Summary and future directions
Our results demonstrate that unilateral ChR2-mediated excitation and NpHR-mediated inhibition of the intermediate and deep SC layers cause significant shifts in movement direction in behaving mice. Although in the present study we did not excite or inhibit specific classes of cells in order to determine their roles in motor control, our work provides a basis for future experiments that can make use of transgenic mice for the selective manipulation of excitatory and inhibitory neurons, and even further restricted neuronal subpopulations, optogenetically. Future experiments can build upon ours to examine the role of the SC in specific aspects of decisions by stimulating neural activity in restricted phases of the task (e.g., well before movement initiation); such temporal specificity has not been possible in previous inhibition experiments. Ultimately, the application of optogenetics to behaving mice, as utilized here, can be used to elucidate the roles of a range of cell types and brain regions involved in sensorimotor decision making.
Highlights.
We applied optogenetics to mice performing a sensorimotor decision task
Manipulating superior colliculus activity predictably biases movement direction
The mouse is a valuable model for studying superior colliculus function in vivo
Acknowledgments
We would like to thank John A. Thompson, Mario J. Lintz, and Theodore K. Doykos for helpful comments and discussions. Imaging experiments were performed in the University of Colorado Anschutz Medical Campus Advance Light Microscopy Core supported in part by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780. This work was supported by NIH grants 5T32NS007083 (E. A. S.) and 1R01NS079518 (G. F.), the Boettcher Foundation’s Webb-Waring Biomedical Research Award (G. F.), and the University of Colorado Anschutz Medical Campus Optogenetics Pilot Program.
Footnotes
Abbreviations. SC: superior colliculus; ChR2: channelrhodopsin-2; NpHR: halorhodopsin; mW/mm2 : milliwatts per millimeter squared (power output measured at optic fiber tip); contra.: contraversive; ipsi.: ipsiversive
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gold JI, Shadlen MN. The neural basis of decision making. Annual Review of Neuroscience. 2007;30:1535–74. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- 2.Sparks DL. Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiological Reviews. 1986;66:118–71. doi: 10.1152/physrev.1986.66.1.118. [DOI] [PubMed] [Google Scholar]
- 3.Jay MF, Sparks DL. Sensorimotor integration in the primate superior colliculus. I. Motor convergence. Journal of Neurophysiology. 1987;57:22–34. doi: 10.1152/jn.1987.57.1.22. [DOI] [PubMed] [Google Scholar]
- 4.Stein BE, Stanford TR. Multisensory integration: current issues from the perspective of the single neuron. Nature Reviews Neuroscience. 2008;9:255–66. doi: 10.1038/nrn2331. [DOI] [PubMed] [Google Scholar]
- 5.King AJ. The superior colliculus. Current Biology. 2004:R335–38. doi: 10.1016/j.cub.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi NJ, Katnani HA. Motor functions of the superior colliculus. Annual Review of Neuroscience. 2011;34:205–31. doi: 10.1146/annurev-neuro-061010-113728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wurtz RH, Goldberg ME. Superior colliculus cell responses related to eye movements in awake monkeys. Science. 1971;171:82–84. doi: 10.1126/science.171.3966.82. [DOI] [PubMed] [Google Scholar]
- 8.Redgrave P, Westby GW, Dean P. Functional architecture of rodent superior colliculus: relevance of multiple output channels. Progressive in Brain Research. 1993;95:69–77. doi: 10.1016/s0079-6123(08)60358-1. [DOI] [PubMed] [Google Scholar]
- 9.Horwitz GD, Newsome WT. Separate signals for target selection and movement specification in the superior colliculus. Science. 1999;284:1158–61. doi: 10.1126/science.284.5417.1158. [DOI] [PubMed] [Google Scholar]
- 10.Harris LR. The superior colliculus and movements of the head and eyes in cats. Journal of Physiology. 1980;300:67–91. doi: 10.1113/jphysiol.1980.sp013167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felsen G, Mainen ZF. Neural substrates of sensory-guided locomotor decisions in the rat superior colliculus. Neuron. 2008;60:137–48. doi: 10.1016/j.neuron.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felsen G, Mainen ZF. Midbrain contributions to sensorimotor decision making. Journal of Neurophysiology. 2012;108:135–47. doi: 10.1152/jn.01181.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walton MMG, Bechara B, Gandhi NJ. Role of the primate superior colliculus in the control of head movements. Journal of Neurophysiology. 2007;98:2022–37. doi: 10.1152/jn.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nature Neuroscience. 2003;7:56–64. doi: 10.1038/nn1169. [DOI] [PubMed] [Google Scholar]
- 15.Müller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proceedings of the National Academy of Sciences. 2005;102:524–29. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carello CJ, Krauzlis RJ. Manipulating intent: evidence for a causal role of the superior colliculus in target selection. Neuron. 2004;43:575–83. doi: 10.1016/j.neuron.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 17.Glimcher PW, Sparks DL. Movement selection in advance of action in the superior colliculus. Nature. 1992;355:542–45. doi: 10.1038/355542a0. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz GD, Batista AP, Newsome WT. Representation of an abstract perceptual decision in macaque superior colliculus. Journal of Neurophysiology. 2004;91:2281–96. doi: 10.1152/jn.00872.2003. [DOI] [PubMed] [Google Scholar]
- 19.McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nature Neuroscience. 2004;7:757–63. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- 20.Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Research. 1972;12:1795–1808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- 21.Corneil BD, Olivier E, Munoz DP. Neck muscle responses to stimulation of monkey superior colliculus. I. Topography and manipulation of stimulation parameters. Journal of Neurophysiology. 2002;88:1980–99. doi: 10.1152/jn.2002.88.4.1980. [DOI] [PubMed] [Google Scholar]
- 22.Freedman EG, Stanford TR, Sparks DL. Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. Journal of Neurophysiology. 1996;76:927–52. doi: 10.1152/jn.1996.76.2.927. [DOI] [PubMed] [Google Scholar]
- 23.Roucoux A, Guitton D, Crommelinck M. Stimulation of the superior colliculus in the alert cat. II. Eye and head movements evoked when the head is unrestrained. Experimental Brain Research. 1980;39:75–85. doi: 10.1007/BF00237071. [DOI] [PubMed] [Google Scholar]
- 24.Sahibzada N, Dean P, Redgrave P. Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. The Journal of Neuroscience. 1986;6:723–33. doi: 10.1523/JNEUROSCI.06-03-00723.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McHaffie JG, Stein BE. Eye movements evoked by electrical stimulation in the superior colliculus of rats and hamsters. Brain Research. 1982;247:243–53. doi: 10.1016/0006-8993(82)91249-5. [DOI] [PubMed] [Google Scholar]
- 26.Dean P, Redgrave P, Westby GW. Event or emergency? Two response systems in the mammalian superior colliculus. Trends in Neuroscience. 1989;12:137–47. doi: 10.1016/0166-2236(89)90052-0. [DOI] [PubMed] [Google Scholar]
- 27.Isa TT, Endo Y. Saito The visuo-motor pathway in the local circuit of the rat superior colliculus. The Journal of Neuroscience. 1998;18:8496–504. doi: 10.1523/JNEUROSCI.18-20-08496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettit DL, Helms MC, Lee P, Augustine GJ, Hall WC. Local excitatory circuits in the intermediate gray layer of the superior colliculus. Journal of Neurophysiology. 1999;81:1424–27. doi: 10.1152/jn.1999.81.3.1424. [DOI] [PubMed] [Google Scholar]
- 29.Helms MC, Özen G, Hall WC. Organization of the intermediate gray layer of the superior colliculus. I. Intrinsic vertical connections. Journal of Neurophysiology. 2004;91:1706–15. doi: 10.1152/jn.00705.2003. [DOI] [PubMed] [Google Scholar]
- 30.Li F, Endo T, Isa T. Presynaptic muscarinic acetylcholine receptors suppress GABAergic synaptic transmission in the intermediate grey layer of mouse superior colliculus. European Journal of Neuroscience. 2004;20:2079–88. doi: 10.1111/j.1460-9568.2004.03668.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee P, Hall WC. An in vitro study of horizontal connections in the intermediate layer of the superior colliculus. The Journal of Neuroscience. 2006;26:4763–68. doi: 10.1523/JNEUROSCI.0724-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isa T, Hall WC. Exploring the superior colliculus in vitro. Journal of Neurophysiology. 2009;102:2581–93. doi: 10.1152/jn.00498.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phongphanphanee P, Mizuno F, Lee PH, Yanagawa Y, Isa T, Hall WC. A circuit model for saccadic suppression in the superior colliculus. Jounal of Neuroscience. 2011;31:1949–54. doi: 10.1523/JNEUROSCI.2305-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sooksawate T, Isa K, Behan M, Yanagawa Y, Isa T. Organization of GABAergic inhibition in the motor output layer of the superior colliculus. European Journal of Neuroscience. 2011;33:421–32. doi: 10.1111/j.1460-9568.2010.07535.x. [DOI] [PubMed] [Google Scholar]
- 35.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;7:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Busse L, Ayaz A, Dhruv NT, Katzner S, Saleem AB, Schölvinck ML, et al. The detection of visual contrast in the behaving mouse. Journal of Neuroscience. 2011;31:11351–61. doi: 10.1523/JNEUROSCI.6689-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–8. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernstein JG, Boyden ES. Optogenetic tools for analyzing the neural circuits of behavior. Trends in Cognitive Sciences. 2011;15:592–600. doi: 10.1016/j.tics.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lo C-C, Wang X-J. Cortico–basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nature Neuroscience. 2006;9:956–63. doi: 10.1038/nn1722. [DOI] [PubMed] [Google Scholar]
- 40.Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. Journal of Neurophysiology. 2006;95:567–84. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- 41.Kim B, Basso MA. Saccade target selection in the superior colliculus: A signal detection theory approach. Journal of Neuroscience. 2008;28:2991–3007. doi: 10.1523/JNEUROSCI.5424-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nature Protocols. 2010;5:439–56. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dean P, Mitchell IJ, Redgrave P. Contralateral head movements produced by microinjection of glutamate in superior colliculus of rats: evidence for mediation by multiple output pathways. Neuroscience. 1988;24:491–500. doi: 10.1016/0306-4522(88)90344-2. [DOI] [PubMed] [Google Scholar]
- 44.Dean P, Redgrave P, Sahibzada N, Tsuji K. Head and body movements produced by electrical stimulation of superior colliculus in rats: effects of interruption of crossed tectoreticulospinal pathway. Neuroscience. 1986;19:367–80. doi: 10.1016/0306-4522(86)90267-8. [DOI] [PubMed] [Google Scholar]
- 45.Uchida, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nature Neuroscience. 2003;6:1224–29. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- 46.Rinberg DA, Koulakov A, Gelperin A. Speed-accuracy tradeoff in olfaction. Neuron. 2006;51:351–58. doi: 10.1016/j.neuron.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 47.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neuroscience. 2005;8:1263–68. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 48.Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen, et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–65. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aravanis AM, Wang L-P, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. Journal of Neural Enginering. 2007;4:143–56. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- 50.Anikeeva P, Andalman AS, Witten I, Warden M, Goshen I, Grosenick L, Gunaydin LA, Frank LM, Deisseroth K. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nature Neuroscience. 2012;15:163–70. doi: 10.1038/nn.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gradinaru V, Thompson KR, Deisseroth K. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biology. 2008;36:129–39. doi: 10.1007/s11068-008-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–62. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, et al. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–81. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wurtz RH, Goldberg ME. Activity of superior colliculus in behaving monkey. 3. Cells discharging before eye movements. Journal of Neurophysiology. 1972;35:575–86. doi: 10.1152/jn.1972.35.4.575. [DOI] [PubMed] [Google Scholar]
- 55.Horwitz GD, Newsome WT. Target selection for saccadic eye movements: direction-selective visual responses in the superior colliculus. Journal of Neurophysiology. 2011;86:2527–42. doi: 10.1152/jn.2001.86.5.2527. [DOI] [PubMed] [Google Scholar]
- 56.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nature Protocols. 2010;5:247–54. doi: 10.1038/nprot.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huber D, Petreanu L, Ghitani N, Ranade S, Hromadka T, Mainen Z, et al. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2007;451:61–4. doi: 10.1038/nature06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krauzlis RJ, Liston D, Carello CD. Target selection and the superior colliculus: goals, choices and hypotheses. Vision Research. 2004;44:1445–51. doi: 10.1016/j.visres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 59.Port NL, Wurtz RH. Target selection and saccade generation in monkey superior colliculus. Experimental Brain Research. 2008;192:465–77. doi: 10.1007/s00221-008-1609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Basso MA, Wurtz RH. Modulation of neuronal activity by target uncertainty. Nature. 1997;389:66–9. doi: 10.1038/37975. [DOI] [PubMed] [Google Scholar]
- 61.Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384:74–7. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- 62.McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual searcht task. Journal of Neurophysiology. 2002;88:2019–34. doi: 10.1152/jn.2002.88.4.2019. [DOI] [PubMed] [Google Scholar]
- 63.Nummela SU, Krauzlis RJ. Inactivation of primate superior colliculus biases target choice for smooth pursuit, saccades, and button press responses. Journal of Neurophysiology. 2012;104:1538–48. doi: 10.1152/jn.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thevarajah D, Mikulić A, Dorris MC. Role of the superior colliculus in choosing mixed- strategy saccades. Journal of Neuroscience. 2009;29:1998–2008. doi: 10.1523/JNEUROSCI.4764-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mize RR. Immunocytochemical localization of gamma-aminobutyric acid (GABA) in the cat superior colliculus. Journal of Comparative Neurology. 1988;276:169–87. doi: 10.1002/cne.902760203. [DOI] [PubMed] [Google Scholar]
- 66.Meredith MA, Ramoa RS. Intrinsic circuitry of the superior colliculus: pharmacophysiological identification of horizontally oriented inhibitory interneurons. Journal of Neurophysiology. 1998;79:1597–602. doi: 10.1152/jn.1998.79.3.1597. [DOI] [PubMed] [Google Scholar]
- 67.Lee PH, Helms MC, Augustine GJ, Hall WC. Role of intrinsic synaptic circuitry in collicular sensorimotor integration. Proceeding of the National Academy of Sciences. 1997;94:13299–304. doi: 10.1073/pnas.94.24.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. Journal of Neurophysiology. 1985;53:266–91. doi: 10.1152/jn.1985.53.1.266. [DOI] [PubMed] [Google Scholar]
- 69.Sinnamon HM, Garcia EJ. Lateral neglect in a head movement task: More impairment with unilateral than bilateral lesions of the superior colliculus in the rat. Behavioural Brain Research. 1988;27:131–43. doi: 10.1016/0166-4328(88)90039-3. [DOI] [PubMed] [Google Scholar]
- 70.Wang S, Redgrave P. Microinjections of muscimol into lateral superior colliculus disrupt orienting and oral movements in the formalin model of pain. Neuroscience. 1997;81:967–88. doi: 10.1016/s0306-4522(97)00191-7. [DOI] [PubMed] [Google Scholar]
- 71.Raimondo JV, Kay L, Ellender TJ, Akerman CJ. Optogenetic silencing strategies differ in their effects on inhibitory synaptic transmission. Nature Neuroscience. 2012;15:1102–04. doi: 10.1038/nn.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cavanaugh J, Monosov IE, McAlonan K, Berman R, Smith MK, Cao V, Wang KH, Boyden ES, Wurtz RH. Optogenetic Inactivation Modifies Monkey Visuomotor Behavior. Neuron. 2012;76:901–07. doi: 10.1016/j.neuron.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, Ramakrishnan C, Deisseroth K, Shenoy KV. An optogenetic toolbox designed for primates. Nature Neuroscience. 2013;14:387–397. doi: 10.1038/nn.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gerits A, Farivar R, Rosen BR, Wald LL, Boyden ES, Vanduffel W. Optogenetically induced behavioral and functional network changes in primates. Current Biology. 2012;22:1722–26. doi: 10.1016/j.cub.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han X, Qian X, Bernstein JG, Zhou HH, Franzesi GT, Stern P, Bronson RT, Graybiel AM, Desimone R, Boyden ES. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron. 2009;62:191–98. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jazayeri M, Lindbloom-Brown Z, Horwitz GD. Saccadic eye movements evoked by optogenetic activation of primate V1. Nature Neuroscience. 2012;15:1368–70. doi: 10.1038/nn.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuhlman SJ, Huang ZJ. High-resolution labeling and functional manipulation of specific neuron types in mouse brain by Cre-activated viral gene expression. PLoS ONE. 2008;3:e2005. doi: 10.1371/journal.pone.0002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Madisen L, Mao T, Koch H, Zhuo J, Berenyi A, Fujisawa, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nature Neuroscience. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang W, Ge W, Wang Z. A toolbox for light control of Drosophila behaviors through Channelrhodopsin 2 mediated photoactivation of targeted neurons. European Journal of Neuroscience. 2007;26:2405–16. doi: 10.1111/j.1460-9568.2007.05862.x. [DOI] [PubMed] [Google Scholar]
- 80.Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proceeding for the National Academy of Sciences. 2005;102:17816–21. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arenkiel BR, Klein ME, Davison IG, Katz LC, Ehlers MD. Genetic control of neuronal activity in mice conditionally expressing TRPV1. Nature Methods. 2008;5:299–302. doi: 10.1038/nmeth.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mize RR, Butler GD. The NMDAR1 subunit of the N-methyl-D-aspartate receptor is localized at postsynaptic sites opposite both retinal and cortical terminals in the cat superior colliculus. Visual Neuroscience. 2008;17:41–53. doi: 10.1017/s0952523800171044. [DOI] [PubMed] [Google Scholar]
- 83.Shi J, Aamodt SM, Constantine-Paton M. Temporal correlations between functional and molecular changes in NMDA receptors and GABA neurotransmission in the superior colliculus. Journal of Neuroscience. 1997;17:6264–76. doi: 10.1523/JNEUROSCI.17-16-06264.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Phongphanphanee P, Kaneda K, Isa T. Spatiotemporal profiles of field potentials in mouse superior colliculus analyzed by multichannel recording. Journal of Neuroscience. 2008;28:9309–18. doi: 10.1523/JNEUROSCI.1905-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proceedings of the National Academy of Sciences. 2003;100:13940–45. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang YP, Oertner TG. Optical induction of synaptic plasticity using a light-sensitive channel. Nature Methods. 2007;4:139–41. doi: 10.1038/nmeth988. [DOI] [PubMed] [Google Scholar]
- 87.Choi DW. Ionic dependence of glutamate neurotoxicity. Journal of Neuroscience. 1987;7:369–79. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abele AE, Scholz KP, Scholz WK, Miller RJ. Excitotoxicity induced by enhanced excitatory neurotransmission in cultured hippocampal pyramidal neurons. Neuron. 1990;4:413–19. doi: 10.1016/0896-6273(90)90053-i. [DOI] [PubMed] [Google Scholar]
- 89.Tecoma ES, Choi DW. GABAergic neocortical neurons are resistant to NMDA receptor- mediated injury. Neurology. 1989;39:676–82. doi: 10.1212/wnl.39.5.676. [DOI] [PubMed] [Google Scholar]
- 90.Monnerie H, Le Roux PD. Reduced dendrite growth and altered glutamic acid decarboxylase (GAD) 65- and 67-kDa isoform protein expression from mouse cortical GABAergic neurons following excitotoxic injury in vitro. Experimental Neurology. 2007;205:367–82. doi: 10.1016/j.expneurol.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 91.Gomes JR, Lobo AC, Melo CV, Inácio AR, Takano J, Iwata N, et al. Cleavage of the vesicular GABA transporter under excitotoxic conditions is followed by accumulation of the truncated transporter in nonsynaptic sites. Journal of Neuroscience. 2011;31:4622–35. doi: 10.1523/JNEUROSCI.3541-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]