Abstract
Acute coronavirus encephalomyelitis is controlled by T cells while humoral responses suppress virus persistence. This study defines the contribution of interleukin (IL)-21, a regulator of T and B cell function, to central nervous system (CNS) immunity. IL-21 receptor deficiency did not affect peripheral T cell activation or trafficking, but dampened granzyme B, gamma interferon and IL-10 expression by CNS T cells and reduced serum and intrathecal humoral responses. Viral control was already lost prior to humoral CNS responses, but demyelination remained comparable. These data demonstrate a critical role of IL-21 in regulating CNS immunity, sustaining viral persistence and preventing mortality.
Keywords: Central nervous system, Neuroimmunology, CD4+ and CD8+ T cells, Inflammation, Interleukin
Highlights
-
•
IL-21 optimizes CNS CD4 and CD8 T cell responses during viral encephalomyelitis.
-
•
IL-21 promotes peripheral and CNS humoral immunity.
-
•
IL-21 promotes CNS viral control and prevents mortality.
1. Introduction
Interleukin (IL)-21 is a pleiotropic type I cytokine secreted by multiple CD4 T cell subsets, including follicular helper T cells (TFH), T helper 17 (TH17) and natural killer (NK) T cells (Yi et al., 2010a). It acts as a co-mitogen to drive CD8 T cell activation, expansion, differentiation and/or survival (Zeng et al., 2005, Casey and Mescher, 2007, Ostiguy et al., 2007, Elsaesser et al., 2009, Frohlich et al., 2009, Yi et al., 2009, Barker et al., 2010, Novy et al., 2011) and is a critical regulator of humoral immunity (Ozaki et al., 2002, Linterman et al., 2010, Zotos et al., 2010, Rankin et al., 2011). The IL-21 receptor (IL-21R) is expressed on several cell types, including T, B, NK cells, and NK T lymphocytes, as well as dendritic cells (DC) and macrophages (Yi et al., 2010a) and consists of an IL-21 binding protein and the common cytokine receptor γ-chain, which is shared by the receptors for IL-2, IL-4, IL-7, IL-9 and IL-15 (Rochman et al., 2009). During peripheral viral infections IL-21 plays a beneficial role by supporting multiple CD8 T cell functions, survival and generation of long-lived memory (Barker et al., 2010, Novy et al., 2011, Pallikkuth et al., 2012). IL-21 deficiency during chronic infection induced by lymphocytic choriomeningitis virus (LCMV) results in impaired long-term CD8 T cell functionality, enhanced CD8 T cell exhaustion, and viral persistence (Elsaesser et al., 2009, Frohlich et al., 2009, Yi et al., 2009). IL-21 also influences long-lived humoral responses during peripheral viral infections including LCMV, vesicular stomatitis virus and influenza virus infection (Elsaesser et al., 2009, Yi et al., 2009, Rasheed et al., 2013).
The role of IL-21 during neuroinflammatory diseases is relatively unexplored. In multiple sclerosis (MS) and neuromyelitis optica elevated cerebrospinal fluid IL-21 levels and polymorphisms in the IL-21R gene implicate IL-21 in autoimmune pathogenesis (Nohra et al., 2010, Wu et al., 2012). In white matter MS lesions IL-21 expression is primarily restricted to CD4 T cells, while IL-21R is predominantly detected on T and B lymphocytes, and sparsely on cortical neurons (Tzartos et al., 2011). Administration of IL-21 before induction of experimental allergic encephalomyelitis (EAE) enhances encephalitogenic T cell and NK function in the periphery and increases disease severity supporting IL-21 as a factor promoting pathology (Vollmer et al., 2005). By contrast, severe neurological impairment during EAE in the absence of IL-21 signaling, associated with a defect in peripheral CD4 T regulatory cells, implicates a protective role for IL-21 (Liu et al., 2008, Piao et al., 2008). More recently, IL-21 was shown to be essential for maintenance of local T cell responses in the central nervous system (CNS) during chronic parasitic infection with Toxoplasma gondii (Stumhofer et al., 2013). However, the influence of IL-21 on CNS immunity during viral encephalitis has not been studied.
Infection with the sub-lethal glia-tropic JHM strain of mouse hepatitis virus (JHMV) induces an acute encephalomyelitis that resolves into a persistent infection restricted primarily to oligodendroglia (Parra et al., 1999, Bergmann et al., 2006). CD8 T cells are the primary effectors reducing virus replication using both perforin and gamma interferon (IFN-γ)-mediated mechanisms (Lin et al., 1997, Bergmann et al., 2004, Gonzalez et al., 2006). CD4 T cells play a vital supportive role by enhancing peripheral CD8 T cell priming/expansion and promoting local effector function within the CNS (Phares et al., 2012b). By contrast, humoral immunity is essential to control the persistent phase of infection (Lin et al., 1999, Tschen et al., 2002, Ramakrishna et al., 2003, Tschen et al., 2006). As CD4 T cells express IL-21 within the CNS during JHMV infection (Phares et al., 2011), we explored a potential role of IL-21 as a prominent factor providing local help for CD8 T cells as well as B cells. Infection of IL-21R−/− mice revealed that expansion and activity of antiviral CD8 T cells in draining cervical lymph nodes (CLN) as well as their accumulation within the CNS was independent of IL-21 signaling. However granzyme B, IFN-γ and most prominently IL-10 expression were diminished in CNS-derived IL-21R−/− CD8 T cells. IFN-γ and IL-10 expression was also reduced in CNS-derived IL-21R−/− CD4 T cells. The absence of IL-21R further delayed peripheral B cell activation and significantly impaired CNS humoral responses. While altered T cell activity in IL-21R−/− mice did not impede early viral control, infectious virus persisted prior to and subsequent to emergence of CNS humoral responses. Nevertheless, clinical scores and the extent of myelin loss were comparable throughout the early persisting phase. Overall, these data support IL-21 as a cytokine optimizing both CNS T cell antiviral activity and humoral responses, thus lowering the set point of viral persistence and ultimately preventing mortality.
2. Materials and methods
2.1. Mice and virus infection
C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD). IL-21R−/− mice on the C57BL/6 background were previously described (Yi et al., 2010b). All mice were housed under pathogen free conditions at an accredited facility at the Cleveland Clinic Lerner Research Institute. Mice were infected at 6–7 wks of age by intracranial injection with 1000 plaque forming units (PFU) of the J.2.2v-1 monoclonal antibody (mAb)-derived gliatropic JHMV variant (Fleming et al., 1986). Animals were scored for clinical signs of disease with: 0, healthy; 1, ruffled fur and hunched back; 2, hind limb paralysis or inability to turn to upright position; 3, complete hind limb paralysis and wasting; and 4, moribund or dead. All animal experiments were performed in compliance with guidelines approved by the Cleveland Clinic Lerner Research Institute Institutional Animal Care and Use Committee.
2.2. Virus titers and cytokine determination
Virus titers within the CNS were determined in clarified supernatants by plaque assay using the murine delayed brain tumor (DBT) astrocytoma as detailed (Fleming et al., 1986). Plaques were counted after 48 h incubation at 37 °C. Clarified supernatants were also used to measure IFN-γ by ELISA as described (Phares et al., 2009). Briefly, 96 well plates were coated overnight at 4 °C with 100 μl of 1 μg/ml of anti-IFN-γ (R4-6A2; BD Bioscience). Non-specific binding was blocked with 10% fetal calf serum in phosphate buffered saline (PBS) overnight before the addition of IFN-γ recombinant cytokine standard (BD Bioscience) and samples. After a 2 h incubation at room temperature bound IFN-γ was detected using biotinylated anti-IFN-γ (XMG1.2, BD Bioscience) and avidin peroxidase followed by 3,3′,5,5′ Tetramethylbenzidine (TMB Reagent Set; BD Bioscience) 1 h later. Optical densities were read at 450 nm in a Bio-Rad Model 680 microplate reader and analyzed using Microplate Manager 5.2 software (Bio-Rad Laboratories, Hercules, CA).
2.3. Mononuclear cell isolation and fluorescence activated cell sorting
CNS-derived cells were isolated as described (Bergmann et al., 1999). Briefly, brains from PBS-perfused mice (n = 3–8) were homogenized in ice-cold Tenbroeck grinders in Dulbecco's PBS. Homogenates were clarified by centrifugation at 450 ×g for 7 min, and supernatants stored at − 80 °C for further analysis. Cell pellets were resuspended in RPMI medium, adjusted to 30% Percoll (Pharmacia, Piscataway, NJ), and underlaid with 1 ml of 70% Percoll. After centrifugation at 850 ×g for 30 min at 4 °C, cells were recovered from the 30%/70% interface, washed once, and resuspended in fluorescence-activated cell sorter (FACS) buffer (PBS with 0.5% bovine serum albumin). CNS-derived CD4 and CD8 T cells were purified from pooled brains (n = 6–8) using a BD FACS Aria (BD). The vast majority of CNS-derived T cells have an activated effector phenotype (CD44hiCD62LloCD69+PD-1+) (Bergmann et al., 1999, Phares et al., 2009). CD44hiCD62Llo CD4 or CD8 (effector) and CD44loCD62Lhi CD4 or CD8 (naive) cells were also purified from pooled CLNs. A minimum of 1 × 105 cells were collected per pooled sample and frozen in 400 μl TRIzol (Invitrogen, Carlsbad, CA) at − 80 °C for subsequent RNA extraction and PCR analysis as described (Ireland et al., 2009). Cell suspensions from CLN were prepared from identical animals as described (Bergmann et al., 1999).
2.4. Flow cytometric analysis
Cells were incubated with mixed serum (mouse, goat and horse) and rat anti-mouse FcγIII/II mAb (2.4G2; BD Bioscience, San Diego, CA) for 20 min on ice prior to staining. Expression of cell surface markers was determined by incubation of cells with mAb specific for CD45 (30-F11), CD4 (L3T4), CD8 (53-6.7), CD11b (M1/70), CD19 (1D3), CD25 (PC61), CD44 (IM7), CD62L (MEL-14), CD69 (H1.2F3), CD138 (281-2) (BD Bioscience, San Jose, CA), PD-1 (RMP1-30) (eBioScience) and F4/80 (CI:A3-1; Serotec, Raleigh, NC) for 30 min on ice. Virus-specific CD8 T cells were identified using Db/S510 major histocompatibility complex (MHC) class I tetramers (Beckman Coulter Inc., Fullerton, CA) as described previously (Bergmann et al., 1999). Stained cells were washed with FACS buffer and fixed in 2% paraformaldehyde. For intracellular detection of granzyme B and Foxp3 cells were stained for cell surface markers prior to permeabilization with either Cytofix/Cytoperm Reagent (BD PharMingen) or Fixation/Permeabilization Reagent (eBioScience) and staining with allophycocyanin-labeled anti-granzyme B (GB12, Caltag Laboratories Burlingame, CA) or FITC-labeled anti-Foxp3 (FJK-16s; eBioScience San Diego, CA), respectively. For detection of Annexin-V, cells were stained with anti-CD4, anti-CD8 and anti-CD45 Ab, washed, resuspended in 1 × Annexin-V binding buffer containing Annexin-V (BD Bioscience) and incubated for 15 min. Granzyme B, Foxp3 and Annexin-V were all measured directly ex vivo without stimulation. For detection of CXCR5, cells were stained with biotin rat anti-mouse CXCR5 Ab (BD Bioscience) and streptavidin phycoerythrin (BD Bioscience). A minimum of 2 × 105 viable cells were stained and analyzed on a FACSCalibur flow cytometer (BD, Mountain View, CA). Data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
Virus specific IFN-γ production by CLN-derived CD8 T cells was evaluated after peptide stimulation. Briefly, 2 × 106 CLN cells were cultured in the absence or presence of 1 μM S510 peptide encompassing the H-2Db restricted CD8 T cell epitope in RPMI supplemented with 10% fetal calf serum for 5 h at 37 °C with 1 μl Golgi Stop (BD Bioscience)/ml. After stimulation, cells were stained for surface expression of CD8 and CD62L, fixed, and then permeabilized to detect intracellular IFN-γ as recommended by the supplier (BD Bioscience).
2.5. Gene expression analysis
Snap frozen brains, spinal cords or CLN from PBS-perfused individual mice (n = 3–7) were placed into Trizol (Invitrogen, Grand Island, NY) and homogenized using a TissueLyser with stainless steel beads (Qiagen, Valencia, CA). RNA was extracted according to the manufacturer's instructions. DNA contamination was removed by treatment with DNase I for 30 min at 37 °C (DNA-free kit; Ambion, Austin, TX) and cDNA synthesized using M-MLV Reverse Transcriptase (Invitrogen), oligo-dT primers (20 μM) (Promega Madison, WI) and random primers (20 μM) (Promega). Quantitative real-time PCR was performed using 4 μl of cDNA and SYBR Green Master Mix (Applied Biosystems, Foster City, CA) in duplicate on a 7500 Fast Real-Time PCR System (Applied Biosystems). PCR conditions were 10 min at 95 °C followed by 40 cycles at 95 °C for 15 s, 60 °C for 30 s and 72 °C for 30 s. Primers used for transcripts encoding glyceraldehyde 3-phosphate dehydrogenase (GAPDH), CXCL9, CXCL10, CCL2, CCL3, CCL5, CCL7, IL-10, TNF and TRAIL were previously described (Phares et al., 2009, Phares et al., 2010, Savarin et al., 2010, Phares et al., 2012b). GAPDH, IFN-γ, IL-2, perforin, Tbet, IL-21, IgG, κ-light chain and IL-21R mRNA levels were determined using Applied Biosystems Gene Expression Arrays with Universal Taqman Fast Master Mix and Taqman primers (Applied Biosystems). PCR conditions were 20 s at 95 °C followed by 40 cycles at 95 °C for 3 s and 60 °C for 30 s. Transcript levels were calculated relative to the housekeeping gene GAPDH using the following formula: 2[CT(GAPDH) − CT(Target Gene)] × 1000, where CT represents the threshold cycle at which the fluorescent signal becomes significantly higher than that of the background.
2.6. Histology
Brains from PBS-perfused mice were snap-frozen in Tissue-Tek OCT compound (Sakura Finetex, Torrance, CA) and sectioned at 10 μm using a Thermo Shandon cryostat. Sections were fixed with 4% paraformaldehyde for 20 min, blocked with 5% bovine serum albumin and 10% goat serum for 30 min and then stained with rabbit anti-mouse laminin Ab (Cedarlane Laboratories, Ontario, Canada) and rat anti-mouse CD3 MAb (eBioscience) overnight at 4 °C. Alexa Fluor 594 goat anti-rabbit (Invitrogen) and Alexa Flour 488 goat anti-rat (Invitrogen) Ab were added, and the samples were incubated for 1 h at room temperature. Sections were mounted with ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen) and examined using a Leica DM4000B fluorescence microscope.
For distribution of viral antigen and myelin loss, spinal cords from PBS perfused mice were fixed in 10% Zinc formalin and embedded in paraffin. Spinal cords were divided into 6 sections prior to embedding, corresponding to cervical, thoracic and lumbar levels. Viral nucleocapsid protein was detected on 5 μm cross sections by immunoperoxidase staining using the anti-JHMV mAb J.3.3 as the primary Ab, horse anti-mouse as secondary Ab and 3,3′-diaminobenzidine substrate (Vectastain-ABC kit; Vector Laboratories, Burlingame, CA). Sections were scored in a blind manner for viral antigen. Demyelination was determined by staining 5 μm sections with Luxol fast blue (LFB). Stained spinal cord sections of all 6 levels on individual slides were scanned with an Aperio ScanScope (Vista, CA) at 40 × and digitally imaged at high resolution. Aperio software was used to quantify areas of demyelination within the white matter tracks of each of the sections.
2.7. Serum Ab
Neutralizing Ab was measured as described (Tschen et al., 2002). Briefly, duplicates of serial 2-fold dilutions of heat inactivated serum from individual mice (n ≥ 5) were incubated with 50 PFU of JHMV in 96-well plates for 90 min at 37 °C. DBT cells (8 × 104 cells/well) were then added, and plates were incubated at 37 °C for 48 h. Neutralization titers represent the log10 of the highest average serum dilution that inhibited cytopathic effect.
2.8. Statistical analysis
Results are expressed as the means ± standard errors of the means (SEM) for each group of mice. In all cases, a P value of < 0.05 was considered significant. Graphs were plotted and statistics assessed using GraphPad Prism 4.0 software.
3. Results
3.1. JHMV infection increases IL-21R on T cells within the CNS
Following JHMV infection virus-specific T cell responses are initiated in the draining CLN (Marten et al., 2003). T cell accumulation in the CNS peaks between days 7 and 10 post-infection (p.i.), however, effector function as monitored by IFN-γ secretion is maximal at day 7 p.i. and declines thereafter, coincident with a prominent decline in viral replication (Bergmann et al., 2006, Savarin et al., 2010, Phares et al., 2012b). This potent antiviral activity elicited by antigen re-encounter within the CNS is enhanced by CD4 T cells (Phares et al., 2012b). Similar to increased IL-21 in CLN, IL-21 expression is sustained throughout T cell-mediated clearance in the CNS (Phares et al., 2011). To assess whether IL-21R is upregulated upon prolonged antigen exposure in the CNS, we monitored the kinetics of IL-21R expression in activated WT CD4 and CD8 T cells in the CLN and CNS following JHMV infection. For this purpose, IL-21R transcripts were assessed in FACS purified populations of CLN-derived naïve CD44loCD62Lhi and activated CD44hiCD62Llo T cells, as well as CNS-derived T cells, which have an activated CD44hiCD62Llo phenotype (Bergmann et al., 1999). IL-21R mRNA levels were higher in CLN-derived naïve relative to activated T cells at both day 5 and 7 p.i., with no differences comparing the respective CD4 and CD8 T cell subsets or time points (Fig. 1A). In the CNS, IL-21R mRNA levels in CD4 T cells were similarly low to activated counterparts in CLN at day 7 p.i., but increased ~ 2.5 fold by day 10 p.i. (Fig. 1B). Transcript levels in CNS-derived CD8 T cells also increased ~ 3-fold between days 7 and 10 p.i. (Fig. 1B). Comparison between CNS-derived T cell subsets revealed that IL-21R mRNA levels were ~ 30 and ~ 50% higher in CD8 relative to CD4 T cells at 7 and 10 days p.i., respectively (Fig. 1B). The overall ~ 3-4 fold increase in IL-21R transcripts in both T cell subsets from day 7 to 10 p.i. suggested an increased IL-21 signaling capacity in the CNS, possibly to sustain T cell effector function.
Fig. 1.
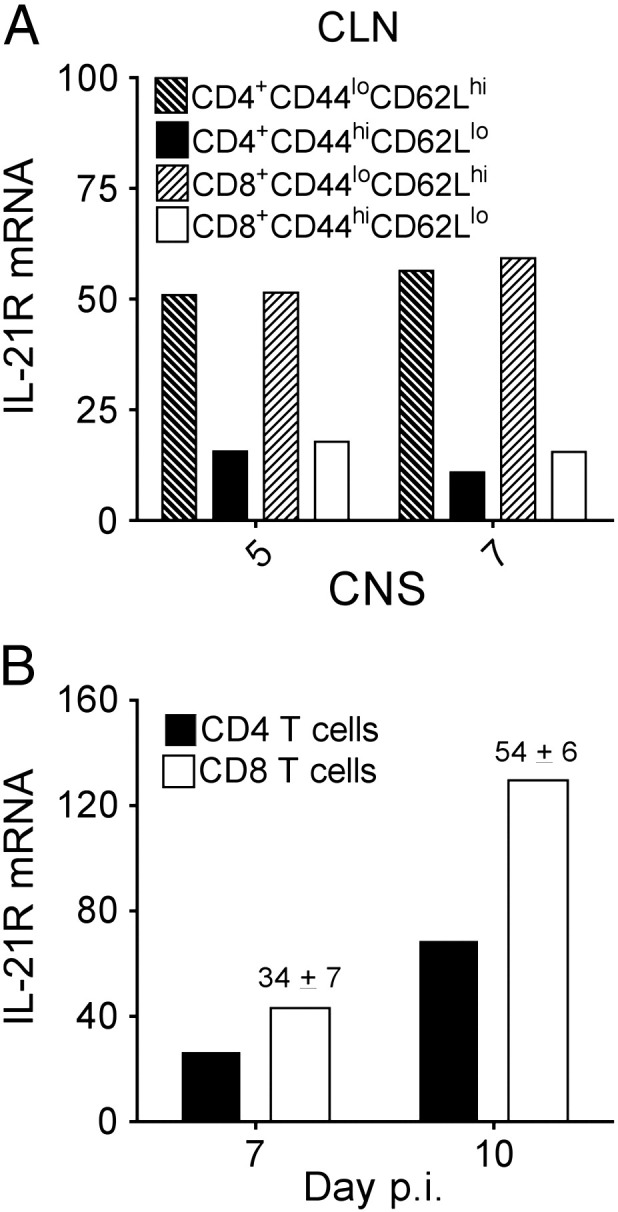
CNS T cells upregulate IL-21R expression during the effector phase. Relative transcript levels of IL-21R in FACS-purified activated CD44hiCD62Llo or naïve CD44loCD62Lhi WT CD4 or CD8 T cells from pooled CLN (A) or activated CD44hiCD62Llo T cells from brains (B) of 6 to 8 mice collected at either day 5, 7 or 10 p.i. were assessed by real-time PCR. Transcript levels are relative to GAPDH. Data are representative of two independent experiments with similar trends in each experiment. Numbers above bars in (B) represent the mean percent change ± SEM in transcript expression in sorted CD4 relative to CD8 T cells at the indicated time point from the two independent experiments.
3.2. Expansion and CNS recruitment of virus-specific CD8 T cells is independent of IL-21 signaling
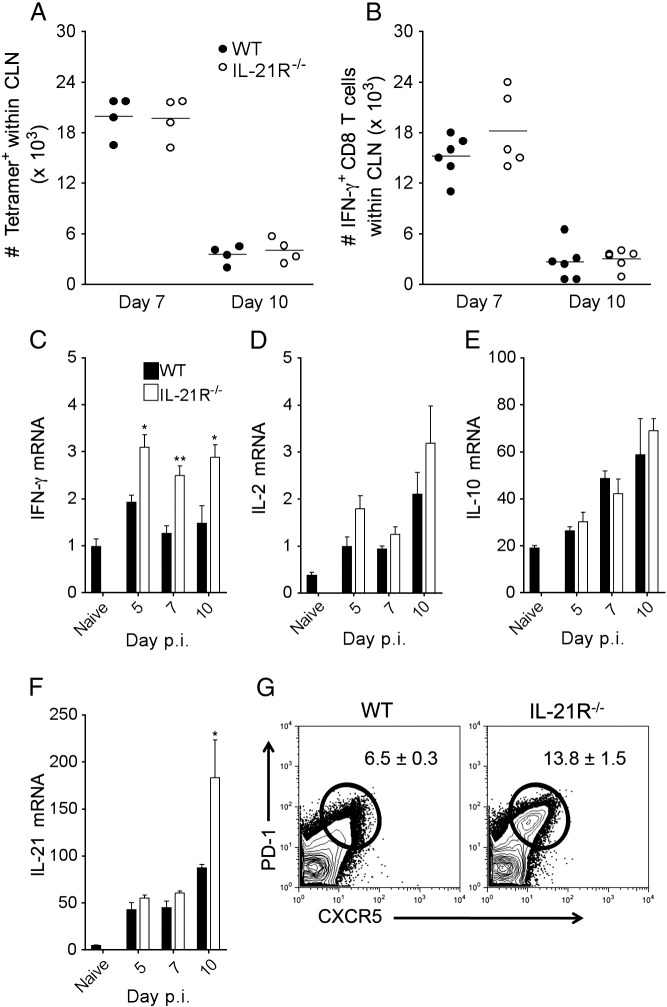
IL-21 can promote CD8 T cell expansion (Zeng et al., 2005) and is expressed in CLN during initial T cell priming following JHMV infection (Phares et al., 2011). We therefore monitored a potential influence of IL-21 on peripheral virus-specific CD8 T cell activation in the CLN prior to analysis of CNS T cells by comparing responses in infected WT and IL-21R−/− mice. Total numbers of T and B lymphocytes were similar in CLN by flow cytometric analysis in both groups (data not shown). There were also no differences in the expression of the activation markers CD69 or CD44 (data not shown), or numbers of virus-specific CD8 T cells by tetramer analysis (Fig. 2A) or IFN-γ secretion (Fig. 2B) in the CLN of IL-21R−/− compared to WT mice at days 7 and 10 p.i. The apparent IL-21R independent T cell activation in CLN was verified by analysis of IFN-γ, IL-2 and IL-10 mRNA, produced by both CD4 and CD8 T cells, and IL-21 mRNA, expressed mainly by CD4 T cells, during JHMV infection (Phares et al., 2011, Puntambekar et al., 2011, Phares et al., 2012a). Transcript levels of IFN-γ, IL-2, IL-10 and IL-21 were all comparable or slightly increased in total CLN mRNA of IL-21R−/− relative to WT mice (Fig. 2C – F). Consistent with enhanced IL-21 mRNA at day 10 p.i., TFH cells, identified as PD-1hiCXCR5+ CD4 T cells, were also elevated in the absence of IL-21R (Fig. 2G). The absence of IL-21 signaling thus did not impair T cell activation at the priming site following CNS infection.
Fig. 2.
T cell activation in CLN is independent of IL-21 signaling. Numbers of tetramer+ (A) or IFN-γ+ (B) CLN-derived CD8 T cells were determined by flow cytometry at day 7 and 10 p.i. Data are expressed as numbers of tetramer+ (A) or IFN-γ+ (B) CD8 T cells within total CLN and represent two independent experiments. Horizontal bars indicate mean number. Relative transcript levels of IFN-γ (C), IL-2 (D), IL-10 (E) and IL-21 (F) in total CLN of naive and infected mice assessed by real-time PCR. Data are expressed as the mean ± SEM transcript level relative to GAPDH mRNA from 3 to 4 individual mice. Significant differences between infected WT and IL-21R−/− mice denoted by * (P < 0.05) and ** (P < 0.01). (G) Flow cytometric analysis of TFH cells in the CLN at day 10 p.i. identified by PD-1hiCXCR5+ CD4 T cells. Data are expressed as the mean ± SEM percentage of PD-1hiCXCR5+ within total CD4 T cells and represent two independent experiments.
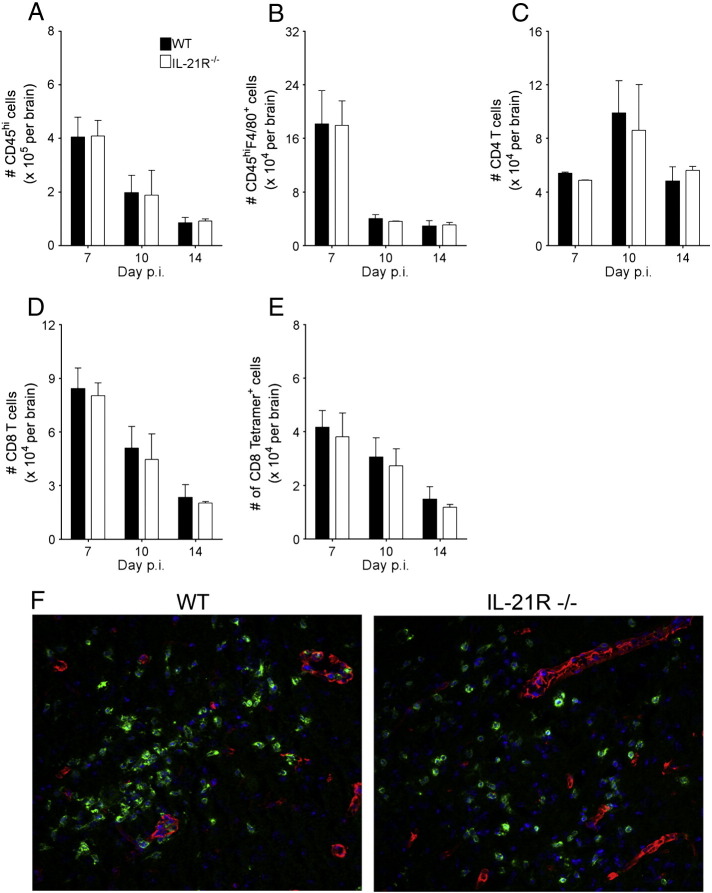
We subsequently evaluated whether IL-21 affects accumulation and function of T cells within the infected CNS. Total numbers of CD45hi CNS-infiltrating cells were similar in infected WT and IL-21R−/− mice (Fig. 3A). Moreover numbers of CD45hi F4/80+ macrophages, CD4, CD8 and virus-specific tetramer+ CD8 T cells were also comparable between both groups (Fig. 3B–E). Similar anatomic distribution of CD3+ T cells in the perivascular space versus parenchyma further suggested the absence of IL-21 signaling did not impair T cell access to the CNS parenchyma (Fig. 3F). These data were consistent with no significant differences in transcript levels encoding chemokines in the CNS, including CXCL9, CXCL10, CCL2, CCL3, CCL5 and CCL7 (data not shown). Taken together these results indicate that neither T cell expansion, accumulation, nor total inflammatory cell composition in the CNS was modified by the absence of IL-21R.
Fig. 3.
IL-21R deficiency does not alter CNS immune cell infiltration. CNS inflammation in WT and IL-21R−/− infected mice was analyzed by flow cytometry from pooled brains of ≥ 3 mice and immunohistochemistry at the indicated time points. Numbers of total CD45hi CNS-infiltrating cells (A), CD45hiF4/80+ macrophages (B), CD4 T cells (C), total CD8 T cells (D) and Db/S510 tetramer+ virus-specific CD8 T cells (E) are shown. The data are expressed as the means ± SEM from two independent experiments. T cell localization in the brain of WT or IL-21R−/− (F) mice at day 10 p.i. was analyzed using anti-CD3 (green) and anti-laminin (red) Ab.
3.3. Expression of T cell effector molecules is compromised in the CNS in absence of IL-21 signaling
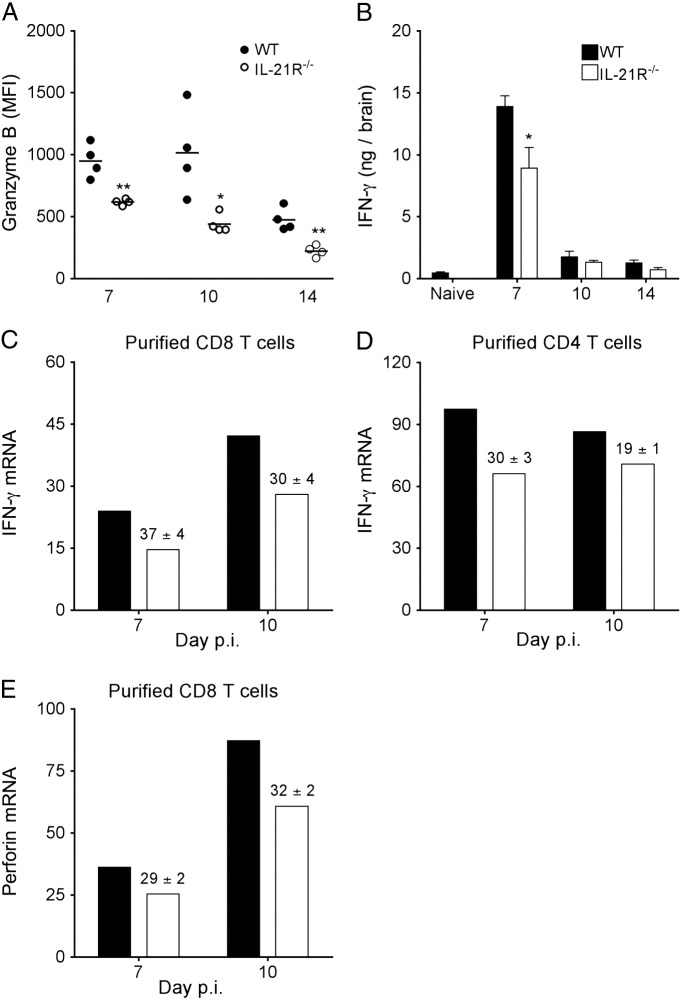
Cytolytic potential and IFN-γ were monitored to assess whether deficiency in IL-21 signaling altered antiviral T cell effector function within the CNS. Ex vivo analysis of the cytolytic effector molecule granzyme B demonstrated similar percentages of granzyme B+ CNS-derived CD8 T cells in WT and IL-21R−/− mice at all time-points (data not shown). However, the levels of granzyme B per cell were significantly reduced in IL-21R−/− CD8 T cells relative to WT counterparts at all time-points analyzed (Fig. 4A). Overall granzyme B expression was highest in WT mice throughout days 7 and 10 and declined by day 14 p.i. By contrast, IL-21R−/− granzyme B levels declined progressively after day 7 and only reached ~ 45% of WT values by day 14 p.i. (Fig. 4A). Reduced granzyme B protein suggests impaired cytolytic capacity by IL-21R−/− CD8 T cells which also correlated with a ~ 30% reduction of perforin transcripts in CNS-derived IL-21R−/− CD8 T cells relative to WT counterparts (Fig. 4E).
Fig. 4.
IL-21R deficiency reduces expression of antiviral T cell effector molecules in the CNS. (A) Brain cells from individual mice (n ≥ 4/group) isolated at the indicated time points were stained for CD45, CD8 and intracellular granzyme B. Mean fluorescence intensity (MFI) of granzyme B staining in CNS-derived CD8 T cells were determined by flow cytometry directly ex vivo. Data are the results of two independent experiments and expressed as MFI of granzyme B in CD8 T cells. Horizontal bars indicate mean MFI. (B) Brain IFN-γ levels from individual mice were assessed by ELISA at the indicated days p.i. Data are expressed as the mean ± SEM (n ≥ 6/group) from two independent experiments. Significant differences between infected WT and IL-21R−/− mice denoted by * (P < 0.05) and ** (P < 0.01). (C–E) Relative transcript levels of IFN-γ (C and D) or perforin (E) in FACS-purified CD8 or CD4 T cells as indicated from pooled brains of 6 to 8 mice collected at 7 and 10 days p.i. were assessed by real-time PCR without ex vivo peptide stimulation. Transcript levels are relative to GAPDH. Data are representative of two independent experiments with similar trends in each experiment. Numbers above bars represent the mean percent change ± SEM in transcript expression in IL-21R−/− relative to WT sorted cells from the two independent experiments.
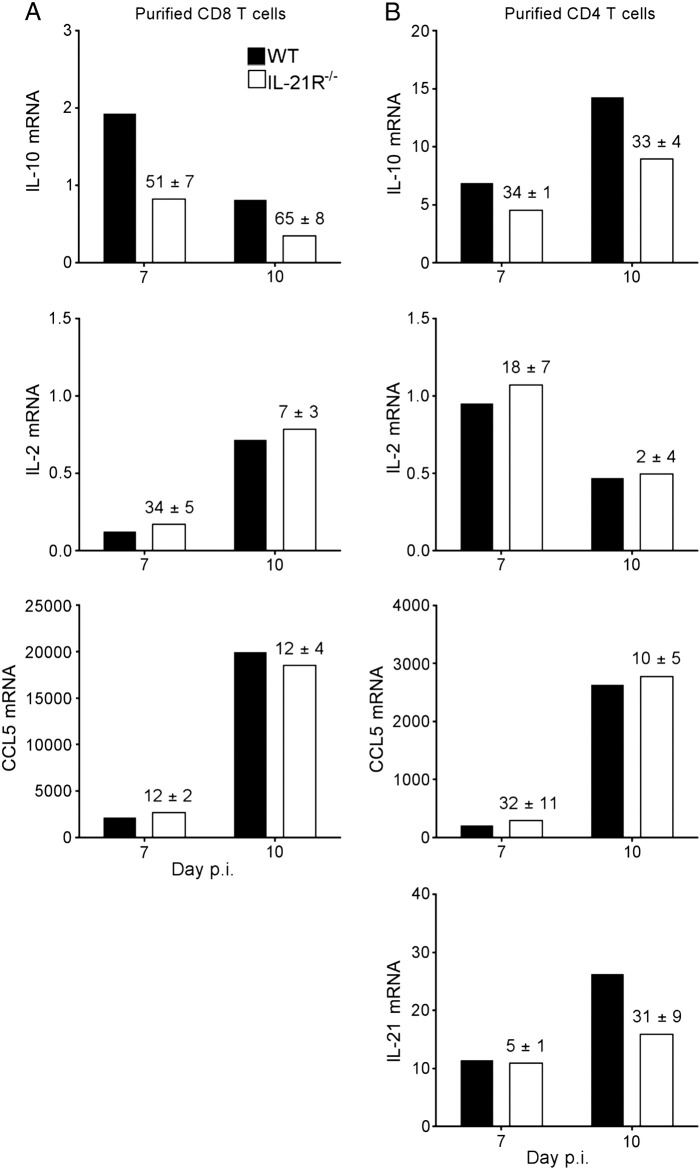
IFN-γ, produced by both CD4 and CD8 T cells within the CNS of JHMV-infected mice (Phares et al., 2010), is critical for viral control in oligodendroglia (Gonzalez et al., 2006) and upregulation of MHC molecules on CNS resident cells (Hamo et al., 2007, Malone et al., 2008). IFN-γ protein levels were significantly reduced in the CNS of IL-21R−/− mice at day 7 p.i., and dropped to the same low levels as in WT mice by day 10 and 14 p.i. (Fig. 4B). To assess whether decreased IFN-γ in the CNS of infected IL-21R−/− mice was attributed to diminished IFN-γ production by CD4 or CD8 T cells, T cell subsets were purified from the infected CNS at 7 and 10 days p.i. to directly assess transcriptional T cell activity in vivo. IFN-γ transcripts were reduced by ~ 35% in IL-21R−/− CD8 T cells compared to WT counterparts at day 7 and 10 days p.i., (Fig. 4C). IFN-γ transcripts were also ~ 30% lower in IL-21R−/− CD4 T cells relative to WT CD4 T cells at day 7 p.i. (Fig. 4B), suggesting IL-21 acts on both CD8 as well as CD4 T cells to promote IFN-γ production. The overall greater IFN-γ mRNA expression in CD4 relative to CD8 T cells (Fig. 4C), yet higher numbers of CD8 T cells in the CNS at day 7 p.i. (Fig. 3), argue the reduction in total CNS IFN-γ protein was attributed to both T cell populations. To determine whether other T cell effector molecules were also diminished in absence of IL-21 signaling, expression of IL-2, IL-10, IL-21 and CCL5 transcripts was assessed. IL-17 analysis was excluded, as there is no evidence for involvement of this cytokine during JHMV infection (Kapil et al., 2009). Recent findings demonstrated that highly lytic CNS-derived CD8 T cells during JHMV infection are marked by IL-10 expression (Trandem et al., 2011). Surprisingly, IL-10 transcripts were decreased by > 50% in IL-21R−/− relative to WT CD8 T cells at day 7 p.i., and ~ 65% by day 10 p.i. (Fig. 5A) further suggesting impaired cytolytic capacity by IL-21R−/− CD8 T cells. IL-10 mRNA was also lower in IL-21R−/− relative to WT CD4 T cells, however the decline was less severe (Fig. 5B). In EAE, IL-21 deficiency impaired the generation of CD25+CD4 T regulatory cells (Liu et al., 2008, Piao et al., 2008), a prominent source of IL-10 in the CNS of JHMV-infected mice (Puntambekar et al., 2011). However, frequencies of CD25+Foxp3+ CD4 T cells within total CD4 T cells were similar in the CNS of JHMV infected IL-21R−/− compared to WT mice (Day 7: WT 22 ± 2% vs IL-21R−/− 26 ± 2%; Day 10: WT 19 ± 1% vs IL-21R−/− 16 ± 1%). IL-21 independent CNS accumulation of CD25+Foxp3+ CD4 T cells, yet reduced IL-10 mRNA in CNS-derived IL-21R−/− CD4 T cells supports diminished IL-10 production on a cellular basis. In contrast to lower IFN-γ and IL-10 mRNA levels in IL-21R−/− CD4 T cells at day 7 p.i., IL-21 mRNA was only diminished at day 10 p.i. (Fig. 5B). Importantly, no reductions in IL-2 or CCL5 transcripts in either CD8 or CD4 T cells demonstrated that IL-21 signaling selectively affects distinct T cell responses (Fig. 5A and B).
Fig. 5.
T cell-derived IL-10 expression in the CNS is diminished in absence of IL-21 signaling. Relative transcript levels of IL-2, IL-21, IL-10 and CCL5 in FACS-purified CD8 (A) or CD4 (B) T cells from pooled brains of 6 to 8 mice collected at 7 and 10 days p.i. were assessed by real-time PCR. Transcript levels are relative to GAPDH. Data are representative of two independent experiments with similar trends in each experiment. Numbers above bars represent the mean percent change ± SEM in transcript expression in IL-21R−/− relative to WT sorted cells from the two independent experiments.
To account for a potential role of IL-21 in promoting CD8 T cell survival by regulating TNF-related apoptosis-inducing ligand (TRAIL) expression (Barker et al., 2010, Melief and Schoenberger, 2010), TRAIL transcripts were also assessed. However, purified CNS-derived WT and IL-21R−/− CD8 T cells expressed similar TRAIL mRNA levels (data not shown). There were also no detectable differences at day 10 p.i. in the frequencies of Annexin-V+ CNS-derived CD8 T cells (WT 8.3 ± 1.1%; IL-21R−/− 7.2 ± 1.5%) or CD4 T cells (WT 23.0 ± 3.3%; IL-21R−/− 22.5 ± 3.0%) analyzed directly ex vivo without peptide stimulation. Similar T cell recruitment (Fig. 3) and apoptosis thus supported IL-21 independent T cell survival within the CNS. Nevertheless, both transcriptional and flow cytometric analyses demonstrate a role for IL-21 in selectively optimizing both CD4 and CD8 T cell functions at the effector site.
3.4. IL-21 signaling is required to clear infectious virus from the CNS
Viral control and disease severity were compared between WT and IL-21R−/− mice to determine whether diminished expression of T cell effector molecules within the CNS altered JHMV pathogenesis. Infectious virus was similar in both brain and spinal cord of both groups at day 7 and reduced to the same levels by day 10 p.i. (Fig. 6A and B). However, IL-21R−/− mice harbored 10-fold higher viral titers at day 14 p.i. relative to WT mice in both brain and spinal cord (Fig. 6A and B). Furthermore in contrast to WT mice, in which infectious virus was undetectable by day 21 p.i., IL-21R−/− mice were unable to clear infectious virus. Notably, viral antigen and RNA persists in spinal cords of WT mice despite absence of infectious virus (Gonzalez et al., 2006). Consistent with increased infectious virus at day 14 and 21 p.i, the number of viral antigen positive cells in the spinal cord was also elevated (Fig. 6C). Nevertheless, the morphology of viral antigen positive cells in both IL-21R−/− and WT mice was consistent with predominant infection of oligodendroglia, suggesting absence of IL-21 signaling did not alter viral tropism (Fig. 6C).
Fig. 6.
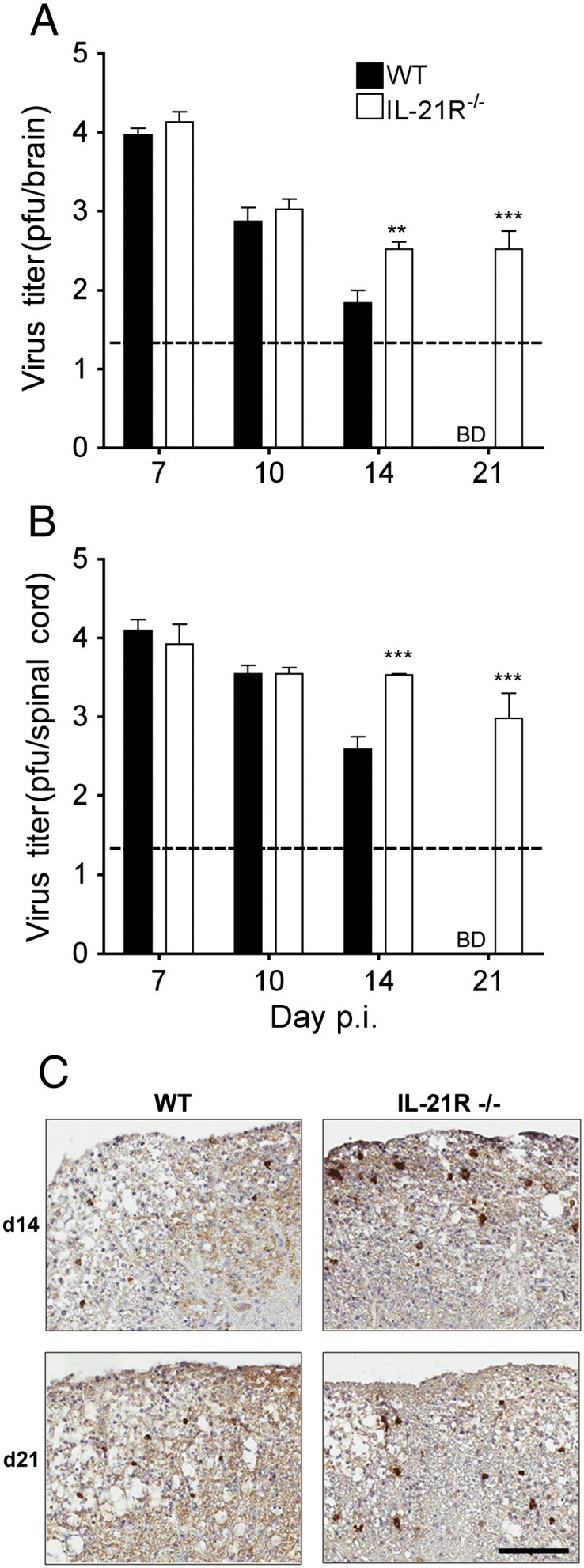
IL-21 signaling is required to clear infectious virus from the CNS. Virus titers in brain (A) and spinal cord (B) supernatants of WT and IL-21R−/− mice determined by plaque assay and expressed as the means ± SEM. The dashed line marks the limit of detection. Data are on a log10 scale. BD = Below Detection. Significant differences between infected WT and IL-21R−/− mice denoted by ** (P < 0.05) and *** (P < 0.001). (C) Virus-infected cells in spinal cord cross sections of WT and IL-21R−/− infected mice at 14 and 21 days p.i. Immunoperoxidase stain using anti-nucleocapsid mAb (brown) with hematoxylin counterstain. Scale bar, 100 μm.
3.5. B cell responses are impaired in absence of IL-21 signaling
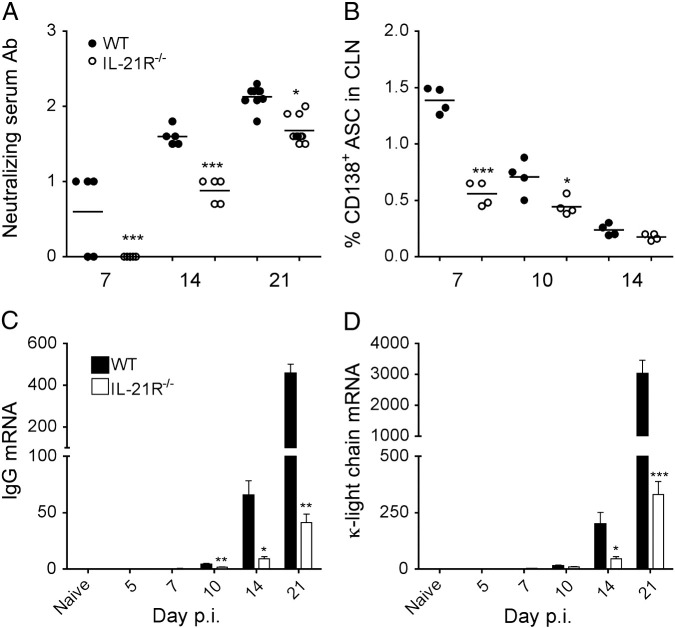
JHMV replication was initially controlled effectively in IL-21R−/− mice, despite altered T cell activity. Moreover, although JHMV infection is controlled by T cells through day 14 p.i., independent of humoral responses, Ab-secreting cells (ASC) prevent reemergence of persisting virus (Lin et al., 1999, Tschen et al., 2002, Ramakrishna et al., 2003, Tschen et al., 2006). Humoral responses were therefore analyzed in the periphery and CNS to monitor whether impaired Ab production contributes to sustained virus replication in IL-21R−/− mice. Serum neutralizing Ab were both delayed and reduced in IL-21R−/− compared to WT mice, but nevertheless increased by 10-fold between days 14 and 21 p.i. in both groups (Fig. 7A). Decreased circulating Ab correlated with lower frequencies of CD138+ ASC in the CLN of IL-21R−/− relative to WT mice at days 7 and 10 p.i. (Fig. 7B). These data support that IL-21R deficiency diminished, but did not abrogate peripheral ASC activation following JHMV infection, similar to peripheral infections (Elsaesser et al., 2009, Yi et al., 2009, Rasheed et al., 2013). To assess whether Ab production in the CNS was also altered, IgG and κ-light chain transcripts were measured as a reliable readout for ASC (Marques et al., 2011). IgG and κ-light chain mRNA levels were barely detectable in the CNS at day 7 p.i. and increased progressively throughout day 21 p.i. in both groups (Fig. 7C and D). However, the relative increase was very modest in IL-21R−/− mice, only reaching 10% of WT values by day 21 p.i. Taken together the results indicate that impaired local Ab production contributes to the inability to clear infectious virus in IL-21R−/− mice subsequent to day 14 p.i.
Fig. 7.
Ab production is impaired in the absence of IL-21R. (A) Kinetics of neutralizing Ab in sera of WT and IL-21R−/− infected mice. Data are expressed as the mean ± SEM (n ≥ 5/group). Data are on a log10 scale. (B) Frequencies of CLN-derived CD138+ CD19+ ASC at the indicated days p.i. were determined by flow cytometry. Data are the results of two independent experiments and expressed as percentages of CD138+ cells within total CLN. Horizontal bars indicate mean percentage. Relative transcript levels of IgG (C) and κ-light chain (D) in spinal cords of naïve and infected WT and IL-21R−/− mice assessed by real-time PCR. Data depict the mean ± SEM relative to GAPDH mRNA of two independent experiments with at least 3 mice per time point per experiment. Significant differences between WT and IL-21R−/− mice are denoted by *, P < 0.05 **, P < 0.005 and ***, P < 0.001. Day 7 p.i. transcript levels were as follows: IgG, WT 0.19 ± 0.03, IL-21R−/− 0.30 ± 0.11 (C); κ-light chain, WT 1.3 ± 0.3, IL-21R−/− 2.4 ± 0.7 (D).
3.6. Persisting infectious virus in absence of IL-21R is not associated with increased pathology
Demyelination is a predominant pathological hallmark of JHMV infection in immune competent mice (Bergmann et al., 2006, Weiss and Leibowitz, 2011). Both infection of oligodendrocytes and T cell effector activity contributes to the severity of demyelination, which is evident by day 10 p.i. and peaks between days 14 and 21 p.i., consistent with paralytic clinical disease. We therefore assessed whether increased viral load at day 14 p.i. in IL-21 R−/− mice correlated with enhanced demyelination. Surprisingly histological analysis indicated that demyelination was similar in both WT and IL-21R−/− mice (Fig. 8A). Furthermore, overall onset and progression of clinical symptoms were comparable in WT and IL-21R−/− mice (Fig. 8B) out to 21 days p.i. despite higher CNS viral loads in the IL-21R−/− mice after day 10 p.i. (Fig. 6). However, morbidity increased thereafter with mortality beginning 4–5 weeks p.i. and the majority of IL-21R−/− mice ultimately succumbing to infection (Fig. 8C). The inability to clear infectious virus was thus not associated with an immediate increase in demyelination or clinical disease.
Fig. 8.
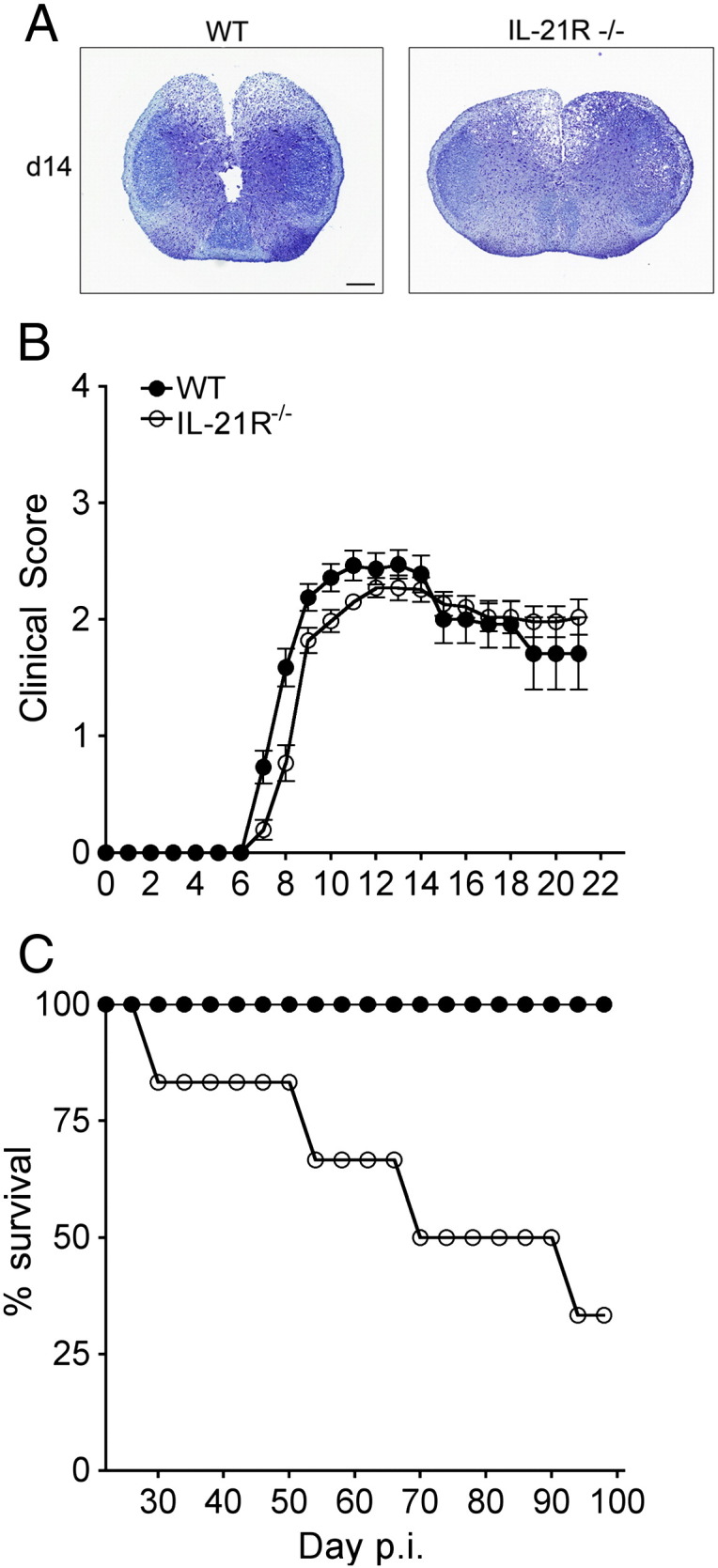
Sustained CNS viral replication in IL-21R−/− mice is not associated with accelerated demyelination, but increases mortality. (A) Representative demyelination in cross sections of spinal cords at day 14 p.i. identified with LFB. WT and IL-21R−/− mice show similar extent of demyelination. Magnification bar = 200 μm. (B) WT and IL-21R−/− infected mice were monitored daily for clinical symptoms. (C) Survival of infected WT and IL-21R−/− mice.
4. Discussion
The mechanisms by which IL-21 regulates CNS inflammation have only been explored in a limited number of experimental models including EAE and parasite infection (Vollmer et al., 2005, Nurieva et al., 2007, Liu et al., 2008, Piao et al., 2008, Stumhofer et al., 2013). IL-21 signaling appears protective in EAE by promoting peripheral CD4 T regulatory cell function, thus limiting expansion of auto reactive T cells (Liu et al., 2008, Piao et al., 2008). However, impaired TH17 differentiation and protection against EAE in IL-21−/− mice suggest a detrimental role (Nurieva et al., 2007). Following T. gondii infection, IL-21 acts to promote both peripheral humoral immunity and T cell responses in the CNS, thus controlling persisting CNS infection and preventing mortality (Stumhofer et al., 2013). Following sub-lethal JHMV induced encephalomyelitis CD4 T cells are the predominant cell type expressing IL-21 (Phares et al., 2012a), and unlike EAE, there is no evidence for a contribution of IL-17 to pathogenesis (Kapil et al., 2009). As CD4 T cells are vital in promoting antiviral CD8 T cell function within the CNS (Phares et al., 2012b), and IL-2 is dispensable for antiviral CD8 T cell function during JHMV infection (Zhou et al., 2005), IL-21 was tested as the likely candidate providing T cell help. While the present study supports a role for IL-21 signaling in enhancing IFN-γ and cytolytic effector molecules by CD8 T cells within the CNS, a more prominent effect was observed in aiding IL-10 transcript expression by both CNS-derived CD4 and CD8 T cell subsets; moreover, the major effect of IL-21R deficiency was significantly impaired humoral responses. These combined deficiencies resulted in loss of initial viral control, albeit without a concomitant increase in demyelination and clinical disease. Mortality was gradually observed after 3 weeks p.i., similar to B cell deficient mice (Ramakrishna et al., 2002).
Overall, the effects of IL-21R deficiency on CD8 T cells during JHMV infection were modest compared to CD4 T cell ablation, which impairs both peripheral CD8 T cell activation and function within the CNS (Phares et al., 2012b). IL-21 can directly regulate T cells at numerous levels (Zeng et al., 2005, Casey and Mescher, 2007, Ostiguy et al., 2007, Elsaesser et al., 2009, Frohlich et al., 2009, Yi et al., 2009, Barker et al., 2010, Novy et al., 2011), but can also modulate DC function and antigen presentation to T cells (Brandt et al., 2003a, Brandt et al., 2003b, Strengell et al., 2006). No differences in JHMV-specific CD8 T cell expansion and expression of T cell-derived cytokines in the CLN of IL-21R−/− and WT mice suggest IL-21 independent DC licensing and initial T cell activation, consistent with results in LCMV infected IL-21R−/− mice (Elsaesser et al., 2009, Frohlich et al., 2009, Yi et al., 2009). However, IL-21R independent CNS chemokine expression and mobilization of activated T cells to the CNS contrasted the reduced chemokine production and T cell recruitment into the lung of pneumovirus infected IL-21R−/− mice (Spolski et al., 2012). The modest effect of IL-21 on T cell effector molecules specifically in the CNS was indicated by decreased granzyme B, as well as perforin and IFN-γ transcript expression in purified CNS-derived IL-21R−/− CD8 T cells analyzed directly ex vivo. The more severely diminished IL-10 transcripts further supported fewer highly cytotoxic IL-10+ CD8 T cells within the CNS (Trandem et al., 2011). However, this is difficult to confirm in ex vivo T cell assays, as the infected target cells in vivo express distinct levels of inhibitory molecules (Phares et al., 2009, Phares et al., 2012a). Notably, the decreases in granzyme B and IL-10 transcripts were similar in magnitude to CNS-derived CD8 T cells from CD4 T cell depleted versus WT JHMV-infected mice (Phares et al., 2012b). Whether IL-21 directly promotes granzyme B and IL-10 production by T cells, or indirectly via effects on local antigen presenting cells, remains unclear. Nevertheless, transcript levels of IL-2, CCL5 and tumor necrosis factor (data not shown) were similar in CNS-derived CD8 T cells from WT and IL-21R−/− mice, suggesting IL-21 selective enhances specific functions. In this context it is of interest to note that IL-21 can support CD8 T cell activity via the transcription factor T-box expressed in T cells (T-bet) (Sutherland et al., 2013), which binds to the promoters of IFN-γ, granzyme B, and perforin (Szabo et al., 2002, Pearce et al., 2003, Sullivan et al., 2003). However, we found no differences in T-bet mRNA levels in purified CNS-derived WT and IL-21R−/− CD8 T cells (data not shown), suggesting IL-21 mediated effects on CD8 T cells are not associated with altered T-bet activity. Similar to JHMV infection, no differences in peripheral CD8 T cell expansion or expression of T cell-derived IFN-γ were detected between T. gondii infected IL-21−/− and WT mice; however, during chronic infection loss of IL-21 reduced CNS-derived T cell-mediated IFN-γ and IL-10 production (Stumhofer et al., 2013) arguing IL-21 to be essential for maintenance of local T cell responses in the CNS. Lastly, although Annexin-V+ CD8 T cells increased ~ 2.5 fold in the CNS of JHMV-infected WT mice following CD4 T cell depletion (Phares et al., 2012b) and IL-21 is known to promote CD8 T cell survival (Novy et al., 2011), IL-21R deficiency did not alter frequencies of CNS-derived Annexin-V+ CD8 T cells. These results indicated CD4 T cell-mediated survival of CNS-derived CD8 T cells during JHMV infection is IL-21 independent contrasting chronic T. gondii infection (Stumhofer et al., 2013).
Regulation of CD4 T cell function by IL-21 depends on the model and site of inflammation. During JHMV infection higher expression of IL-21R transcripts in CNS-derived CD8 relative to CD4 T cells with time suggested that CD8 T cells may be more dependent on IL-21 signaling at the effector site. This is supported by greater reductions of IFN-γ and IL-10 transcripts in CNS-derived IL-21R−/− CD8 than CD4 T cells relative to their respective WT counterparts. By contrast, during peripheral LCMV infection IL-21R deficiency increases the numbers of IFN-γ+ and IL-2+ CD4 T cells (Elsaesser et al., 2009), suggesting that IL-21 suppresses distinct facets of CD4 T cell function. During EAE IL-21R deficiency is associated with a defect in CD25+CD4 T regulatory cell generation resulting in severe neurological impairment (Liu et al., 2008, Piao et al., 2008). CD25+Foxp3+ CD4 T regulatory cells are a prominent source of IL-10 in the CNS of JHMV infected mice (Puntambekar et al., 2011). However, distinct from EAE (Liu et al., 2008, Piao et al., 2008), IL-21R deficiency did not alter frequencies of CD25+Foxp3+ CD4 T cells compared to WT mice. Similar accumulation of CD25+Foxp3+ CD4 T cells, yet reduced IL-10 mRNA in IL-21R−/− CD4 T cells, thus supports diminished IL-10 production on a cellular basis, similar to CD8 T cells. While diminished IL-10 is associated with enhanced CNS viral clearance and tissue damage (Trandem et al., 2011), reduced IL-10 in JHMV infected IL-21R−/− mice coincided with impaired control of infectious virus without affecting demyelination or clinical disease over the first 3 weeks of infection. These data support additional functions of IL-21 on CNS resident cells as suggested by detection of IL-21R on some neurons (Tzartos et al., 2011).
Although IL-21 promotes germinal center reactions and generation of ASC following protein immunization (Ozaki et al., 2002, Linterman et al., 2010, Zotos et al., 2010, Rankin et al., 2011), Toll-like receptor signaling can overcome deficits in IL-21 dependent humoral responses (Bessa et al., 2010), suggesting IL-21 dependency of B cells for protective humoral responses may depend on the type of infection. Similar to impaired serum Ab responses following LCMV infection (Elsaesser et al., 2009, Yi et al., 2009, Rasheed et al., 2013), IL-21R deficiency diminished peripheral ASC activation and antiviral serum Ab following JHMV infection. However, this was not associated with impaired generation of TFH cells, which corroborates similar results during LCMV (Rasheed et al., 2013), T. gondii (Stumhofer et al., 2013) and Heligmosomoides polygyrus (King et al., 2010) infection as well as following live recombinant rabies-based vaccination (Dorfmeier et al., 2013). The significant increases in TFH cells compared to WT mice were also noted during H. polygyrus infection and live recombinant rabies-based vaccination in IL-21R−/− mice (King et al., 2010, Dorfmeier et al., 2013), suggesting that IL-21 signaling may play an inhibitory role in TFH cell development. Whether the severely impaired Ab production in the CNS is a direct consequence of suboptimal peripheral humoral responses or whether IL-21 further acts directly on B cells within the CNS warrants further investigation. Similarly, the characteristics of the IL-21 producing CD4 T cells in the CNS remain to be identified, as preliminary analysis shows no evidence for TFH cell phenotype.
In summary these results demonstrate that IL-21 plays a vital role in optimizing antiviral T and B cell responses within the CNS, thus promoting control of infectious virus during acute and persistent encephalomyelitis. Similar recent observations during T. gondii infection (Stumhofer et al., 2013) confirm the notion that targeted enhancement of T and B cell responses by IL-21 may provide an early intervention strategy against infections prone to establishing persistence.
Acknowledgments
This work was supported by U.S. National Institutes of Health grants NS064932 and AI 47249 (C.C.B) and AI49360 and AI082966 (A.J.Z). We sincerely thank Wenqiang Wei, Eric Barron and Ernesto Barron for exceptional technical assistance with immunohistochemistry and Jennifer Powers for FACS purification.
References
- Barker B.R., Gladstone M.N., Gillard G.O., Panas M.W., Letvin N.L. Critical role for IL-21 in both primary and memory anti-viral CD8(+) T-cell responses. Eur. J. Immunol. 2010;40:3085–3096. doi: 10.1002/eji.200939939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann C.C., Altman J.D., Hinton D., Stohlman S.A. Inverted immunodominance and impaired cytolytic function of CD8 + T cells during viral persistence in the central nervous system. J. Immunol. 1999;163:3379–3387. [PubMed] [Google Scholar]
- Bergmann C.C., Parra B., Hinton D.R., Ramakrishna C., Dowdell K.C., Stohlman S.A. Perforin and gamma interferon-mediated control of coronavirus central nervous system infection by CD8 T cells in the absence of CD4 T cells. J. Virol. 2004;78:1739–1750. doi: 10.1128/JVI.78.4.1739-1750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann C.C., Lane T.E., Stohlman S.A. Coronavirus infection of the central nervous system: host-virus stand-off. Nat. Rev. Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa J., Kopf M., Bachmann M.F. Cutting edge: IL-21 and TLR signaling regulate germinal center responses in a B cell-intrinsic manner. J. Immunol. 2010;184:4615–4619. doi: 10.4049/jimmunol.0903949. [DOI] [PubMed] [Google Scholar]
- Brandt K., Bulfone-Paus S., Foster D.C., Ruckert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. 2003;102:4090–4098. doi: 10.1182/blood-2003-03-0669. [DOI] [PubMed] [Google Scholar]
- Brandt K., Bulfone-Paus S., Jenckel A., Foster D.C., Paus R., Ruckert R. Interleukin-21 inhibits dendritic cell-mediated T cell activation and induction of contact hypersensitivity in vivo. J. Invest. Dermatol. 2003;121:1379–1382. doi: 10.1046/j.1523-1747.2003.12603.x. [DOI] [PubMed] [Google Scholar]
- Casey K.A., Mescher M.F. IL-21 promotes differentiation of naive CD8 T cells to a unique effector phenotype. J. Immunol. 2007;178:7640–7648. doi: 10.4049/jimmunol.178.12.7640. [DOI] [PubMed] [Google Scholar]
- Dorfmeier C.L., Tzvetkov E.P., Gatt A., Mcgettigan J.P. Investigating the role for IL-21 in rabies virus vaccine-induced immunity. PLoS Negl. Trop. Dis. 2013;7:e2129. doi: 10.1371/journal.pntd.0002129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaesser H., Sauer K., Brooks D.G. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J.O., Trousdale M.D., El-Zaatari F.A., Stohlman S.A., Weiner L.P. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J. Virol. 1986;58:869–875. doi: 10.1128/jvi.58.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich A., Kisielow J., Schmitz I., Freigang S., Shamshiev A.T., Weber J., Marsland B.J., Oxenius A., Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- Gonzalez J.M., Bergmann C.C., Ramakrishna C., Hinton D.R., Atkinson R., Hoskin J., Macklin W.B., Stohlman S.A. Inhibition of interferon-gamma signaling in oligodendroglia delays coronavirus clearance without altering demyelination. Am. J. Pathol. 2006;168:796–804. doi: 10.2353/ajpath.2006.050496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamo L., Stohlman S.A., Otto-Duessel M., Bergmann C.C. Distinct regulation of MHC molecule expression on astrocytes and microglia during viral encephalomyelitis. Glia. 2007;55:1169–1177. doi: 10.1002/glia.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland D.D., Stohlman S.A., Hinton D.R., Kapil P., Silverman R.H., Atkinson R.A., Bergmann C.C. RNase L mediated protection from virus induced demyelination. PLoS Pathog. 2009;5:e1000602. doi: 10.1371/journal.ppat.1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapil P., Atkinson R., Ramakrishna C., Cua D.J., Bergmann C.C., Stohlman S.A. Interleukin-12 (IL-12), but not IL-23, deficiency ameliorates viral encephalitis without affecting viral control. J. Virol. 2009;83:5978–5986. doi: 10.1128/JVI.00315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King I.L., Mohrs K., Mohrs M. A nonredundant role for IL-21 receptor signaling in plasma cell differentiation and protective type 2 immunity against gastrointestinal helminth infection. J. Immunol. 2010;185:6138–6145. doi: 10.4049/jimmunol.1001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M.T., Stohlman S.A., Hinton D.R. Mouse hepatitis virus is cleared from the central nervous systems of mice lacking perforin-mediated cytolysis. J. Virol. 1997;71:383–391. doi: 10.1128/jvi.71.1.383-391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M.T., Hinton D.R., Marten N.W., Bergmann C.C., Stohlman S.A. Antibody prevents virus reactivation within the central nervous system. J. Immunol. 1999;162:7358–7368. [PubMed] [Google Scholar]
- Linterman M.A., Beaton L., Yu D., Ramiscal R.R., Srivastava M., Hogan J.J., Verma N.K., Smyth M.J., Rigby R.J., Vinuesa C.G. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Bai Y., Vollmer T.L., Bai X.F., Jee Y., Tang Y.Y., Campagnolo D.I., Collins M., Young D.A., La Cava A., Shi F.D. IL-21 receptor expression determines the temporal phases of experimental autoimmune encephalomyelitis. Exp. Neurol. 2008;211:14–24. doi: 10.1016/j.expneurol.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Malone K.E., Stohlman S.A., Ramakrishna C., Macklin W., Bergmann C.C. Induction of class I antigen processing components in oligodendroglia and microglia during viral encephalomyelitis. Glia. 2008;56:426–435. doi: 10.1002/glia.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques C.P., Kapil P., Hinton D.R., Hindinger C., Nutt S.L., Ransohoff R.M., Phares T.W., Stohlman S.A., Bergmann C.C. CXCR3-dependent plasma blast migration to the central nervous system during viral encephalomyelitis. J. Virol. 2011;85:6136–6147. doi: 10.1128/JVI.00202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marten N.W., Stohlman S.A., Zhou J., Bergmann C.C. Kinetics of virus-specific CD8 +-T-cell expansion and trafficking following central nervous system infection. J. Virol. 2003;77:2775–2778. doi: 10.1128/JVI.77.4.2775-2778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief C.J., Schoenberger S.P. Enhancement of proliferation and downregulation of TRAIL expression on CD8 + T cells by IL-21. Eur. J. Immunol. 2010;40:2990–2992. doi: 10.1002/eji.201041076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohra R., Beyeen A.D., Guo J.P., Khademi M., Sundqvist E., Hedreul M.T., Sellebjerg F., Smestad C., Oturai A.B., Harbo H.F., Wallstrom E., Hillert J., Alfredsson L., Kockum I., Jagodic M., Lorentzen J., Olsson T. RGMA and IL21R show association with experimental inflammation and multiple sclerosis. Genes Immun. 2010;11:279–293. doi: 10.1038/gene.2009.111. [DOI] [PubMed] [Google Scholar]
- Novy P., Huang X., Leonard W.J., Yang Y. Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection. J. Immunol. 2011;186:2729–2738. doi: 10.4049/jimmunol.1003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R., Yang X.O., Martinez G., Zhang Y., Panopoulos A.D., Ma L., Schluns K., Tian Q., Watowich S.S., Jetten A.M., Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Ostiguy V., Allard E.L., Marquis M., Leignadier J., Labrecque N. IL-21 promotes T lymphocyte survival by activating the phosphatidylinositol-3 kinase signaling cascade. J. Leukoc. Biol. 2007;82:645–656. doi: 10.1189/jlb.0806494. [DOI] [PubMed] [Google Scholar]
- Ozaki K., Spolski R., Feng C.G., Qi C.F., Cheng J., Sher A., Morse H.C., III, Liu C., Schwartzberg P.L., Leonard W.J. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- Pallikkuth S., Parmigiani A., Pahwa S. The role of interleukin-21 in HIV infection. Cytokine Growth Factor Rev. 2012;23:173–180. doi: 10.1016/j.cytogfr.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra B., Hinton D.R., Marten N.W., Bergmann C.C., Lin M.T., Yang C.S., Stohlman S.A. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J. Immunol. 1999;162:1641–1647. [PubMed] [Google Scholar]
- Pearce E.L., Mullen A.C., Martins G.A., Krawczyk C.M., Hutchins A.S., Zediak V.P., Banica M., Dicioccio C.B., Gross D.A., Mao C.A., Shen H., Cereb N., Yang S.Y., Lindsten T., Rossant J., Hunter C.A., Reiner S.L. Control of effector CD8 + T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- Phares T.W., Ramakrishna C., Parra G.I., Epstein A., Chen L., Atkinson R., Stohlman S.A., Bergmann C.C. Target-dependent B7-H1 regulation contributes to clearance of central nervous system infection and dampens morbidity. J. Immunol. 2009;182:5430–5438. doi: 10.4049/jimmunol.0803557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares T.W., Stohlman S.A., Hinton D.R., Atkinson R., Bergmann C.C. Enhanced antiviral T cell function in the absence of B7-H1 is insufficient to prevent persistence but exacerbates axonal bystander damage during viral encephalomyelitis. J. Immunol. 2010;185:5607–5618. doi: 10.4049/jimmunol.1001984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares T.W., Marques C.P., Stohlman S.A., Hinton D.R., Bergmann C.C. Factors supporting intrathecal humoral responses following viral encephalomyelitis. J. Virol. 2011;85:2589–2598. doi: 10.1128/JVI.02260-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares T.W., Stohlman S.A., Hinton D.R., Bergmann C.C. Enhanced CD8 T-cell anti-viral function and clinical disease in B7-H1-deficient mice requires CD4 T cells during encephalomyelitis. J. Neuroinflammation. 2012;9:269. doi: 10.1186/1742-2094-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares T.W., Stohlman S.A., Hwang M., Min B., Hinton D.R., Bergmann C.C. CD4 T cells promote CD8 T cell immunity at the priming and effector site during viral encephalitis. J. Virol. 2012;86:2416–2427. doi: 10.1128/JVI.06797-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao W.H., Jee Y.H., Liu R.L., Coons S.W., Kala M., Collins M., Young D.A., Campagnolo D.I., Vollmer T.L., Bai X.F., La Cava A., Shi F.D. IL-21 modulates CD4 + CD25 + regulatory T-cell homeostasis in experimental autoimmune encephalomyelitis. Scand. J. Immunol. 2008;67:37–46. doi: 10.1111/j.1365-3083.2007.02035.x. [DOI] [PubMed] [Google Scholar]
- Puntambekar S.S., Bergmann C.C., Savarin C., Karp C.L., Phares T.W., Parra G.I., Hinton D.R., Stohlman S.A. Shifting hierarchies of interleukin-10-producing T cell populations in the central nervous system during acute and persistent viral encephalomyelitis. J. Virol. 2011;85:6702–6713. doi: 10.1128/JVI.00200-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishna C., Stohlman S.A., Atkinson R.D., Shlomchik M.J., Bergmann C.C. Mechanisms of central nervous system viral persistence: the critical role of antibody and B cells. J. Immunol. 2002;168:1204–1211. doi: 10.4049/jimmunol.168.3.1204. [DOI] [PubMed] [Google Scholar]
- Ramakrishna C., Bergmann C.C., Atkinson R., Stohlman S.A. Control of central nervous system viral persistence by neutralizing antibody. J. Virol. 2003;77:4670–4678. doi: 10.1128/JVI.77.8.4670-4678.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin A.L., Macleod H., Keegan S., Andreyeva T., Lowe L., Bloom L., Collins M., Nickerson-Nutter C., Young D., Guay H. IL-21 receptor is critical for the development of memory B cell responses. J. Immunol. 2011;186:667–674. doi: 10.4049/jimmunol.0903207. [DOI] [PubMed] [Google Scholar]
- Rasheed M.A., Latner D.R., Aubert R.D., Gourley T., Spolski R., Davis C.W., Langley W.A., Ha S.J., Ye L., Sarkar S., Kalia V., Konieczny B.T., Leonard W.J., Ahmed R. IL-21 is a critical cytokine for the generation of virus-specific long-lived plasma cells. J. Virol. 2013;87:7737–7746. doi: 10.1128/JVI.00063-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman Y., Spolski R., Leonard W.J. New insights into the regulation of T cells by gamma(c) family cytokines. Nat. Rev. Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarin C., Stohlman S.A., Atkinson R., Ransohoff R.M., Bergmann C.C. Monocytes regulate T cell migration through the glia limitans during acute viral encephalitis. J. Virol. 2010;84:4878–4888. doi: 10.1128/JVI.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolski R., Wang L., Wan C.K., Bonville C.A., Domachowske J.B., Kim H.P., Yu Z., Leonard W.J. IL-21 promotes the pathologic immune response to pneumovirus infection. J. Immunol. 2012;188:1924–1932. doi: 10.4049/jimmunol.1100767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strengell M., Lehtonen A., Matikainen S., Julkunen I. IL-21 enhances SOCS gene expression and inhibits LPS-induced cytokine production in human monocyte-derived dendritic cells. J. Leukoc. Biol. 2006;79:1279–1285. doi: 10.1189/jlb.0905503. [DOI] [PubMed] [Google Scholar]
- Stumhofer J.S., Silver J.S., Hunter C.A. IL-21 Is Required for Optimal Antibody Production and T Cell Responses during Chronic Toxoplasma gondii Infection. PLoS One. 2013;8:e62889. doi: 10.1371/journal.pone.0062889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan B.M., Juedes A., Szabo S.J., Von Herrath M., Glimcher L.H. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland A.P., Joller N., Michaud M., Liu S.M., Kuchroo V.K., Grusby M.J. IL-21 promotes CD8 + CTL activity via the transcription factor T-bet. J. Immunol. 2013;190:3977–3984. doi: 10.4049/jimmunol.1201730. [DOI] [PubMed] [Google Scholar]
- Szabo S.J., Sullivan B.M., Stemmann C., Satoskar A.R., Sleckman B.P., Glimcher L.H. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- Trandem K., Zhao J., Fleming E., Perlman S. Highly activated cytotoxic CD8 T cells express protective IL-10 at the peak of coronavirus-induced encephalitis. J. Immunol. 2011;186:3642–3652. doi: 10.4049/jimmunol.1003292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschen S.I., Bergmann C.C., Ramakrishna C., Morales S., Atkinson R., Stohlman S.A. Recruitment kinetics and composition of antibody-secreting cells within the central nervous system following viral encephalomyelitis. J. Immunol. 2002;168:2922–2929. doi: 10.4049/jimmunol.168.6.2922. [DOI] [PubMed] [Google Scholar]
- Tschen S.I., Stohlman S.A., Ramakrishna C., Hinton D.R., Atkinson R.D., Bergmann C.C. CNS viral infection diverts homing of antibody-secreting cells from lymphoid organs to the CNS. Eur. J. Immunol. 2006;36:603–612. doi: 10.1002/eji.200535123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos J.S., Craner M.J., Friese M.A., Jakobsen K.B., Newcombe J., Esiri M.M., Fugger L. IL-21 and IL-21 receptor expression in lymphocytes and neurons in multiple sclerosis brain. Am. J. Pathol. 2011;178:794–802. doi: 10.1016/j.ajpath.2010.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer T.L., Liu R., Price M., Rhodes S., La Cava A., Shi F.D. Differential effects of IL-21 during initiation and progression of autoimmunity against neuroantigen. J. Immunol. 2005;174:2696–2701. doi: 10.4049/jimmunol.174.5.2696. [DOI] [PubMed] [Google Scholar]
- Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis. Adv. Virus Res. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A., Zhong X., Wang H., Xu W., Cheng C., Dai Y., Bao J., Qiu W., Lu Z., Hu X. Cerebrospinal fluid IL-21 levels in neuromyelitis optica and multiple sclerosis. Can. J. Neurol. Sci. 2012;39:813–820. doi: 10.1017/s0317167100015663. [DOI] [PubMed] [Google Scholar]
- Yi J.S., Du M., Zajac A.J. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J.S., Cox M.A., Zajac A.J. Interleukin-21: a multifunctional regulator of immunity to infections. Microbes Infect. 2010;12:1111–1119. doi: 10.1016/j.micinf.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J.S., Ingram J.T., Zajac A.J. IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection. J. Immunol. 2010;185:4835–4845. doi: 10.4049/jimmunol.1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng R., Spolski R., Finkelstein S.E., Oh S., Kovanen P.E., Hinrichs C.S., Pise-Masison C.A., Radonovich M.F., Brady J.N., Restifo N.P., Berzofsky J.A., Leonard W.J. Synergy of IL-21 and IL-15 in regulating CD8 + T cell expansion and function. J. Exp. Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Hinton D.R., Stohlman S.A., Liu C.P., Zhong L., Marten N.W. Maintenance of CD8 + T cells during acute viral infection of the central nervous system requires CD4 + T cells but not interleukin-2. Viral Immunol. 2005;18:162–169. doi: 10.1089/vim.2005.18.162. [DOI] [PubMed] [Google Scholar]
- Zotos D., Coquet J.M., Zhang Y., Light A., D'costa K., Kallies A., Corcoran L.M., Godfrey D.I., Toellner K.M., Smyth M.J., Nutt S.L., Tarlinton D.M. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]