Abstract
Persons with multiple sclerosis (MS) suffer memory impairment, but research on the nature of MS-related memory problems is mixed. Some have argued for a core deficit in retrieval, while others have identified deficient initial learning as the core deficit. We used a selective reminding paradigm to determine whether deficient initial learning or delayed retrieval represents the primary memory deficit in 44 persons with MS. Brain atrophy was measured from high-resolution MRIs. Regression analyses examined the impact of brain atrophy on (a) initial learning and delayed retrieval separately, and then (b) delayed retrieval controlling for initial learning. Brain atrophy was negatively associated with both initial learning and delayed retrieval (ps < .01), but brain atrophy was unrelated to retrieval when controlling for initial learning (p > .05). In addition, brain atrophy was associated with inefficient learning across initial acquisition trials, and brain atrophy was unrelated to delayed recall among MS subjects who successfully acquired the word list (although such learning frequently required many exposures). Taken together, memory deficits in MS are a result of deficits in initial learning; moreover, initial learning mediates the relationship between brain atrophy and subsequent retrieval, thereby supporting the core learning-deficit hypothesis of memory impairment in MS.
Keywords: Multiple Sclerosis, Learning, Memory, Neuropsychology, Cognitive Disorders, Atrophy
INTRODUCTION
Learning and memory impairments are common among persons with Multiple Sclerosis (MS) (Chiaravalloti & DeLuca, 2008; Thornton & Raz, 1997) with negative consequences for employment and overall quality of life (Kessler, Cohen, Lauer, & Kausch, 1992; S. M. Rao et al., 1991). There has been disagreement, however, over the nature of MS-related memory impairment. Some have argued for deficient retrieval processes as the core memory deficit (Bobholz et al., 2006; S. M. Rao et al., 1993; S. M. Rao, Leo, & St Aubin-Faubert, 1989), while others have identified inefficient initial learning as the core deficit (DeLuca, Barbieri-Berger, & Johnson, 1994; DeLuca, Gaudino, Diamond, Christodoulou, & Engel, 1998; Demaree, Gaudino, DeLuca, & Ricker, 2000; Thornton, Raz, & Tucke, 2002), which secondarily results in poor performance on delayed recall tasks. Determination of the core memory deficit is critical for development of effective treatments to address memory impairment.
The predominance of evidence for memory impairment in MS comes from behavioral studies that examined neuropsychological test performance (Bobholz, et al., 2006; DeLuca, et al., 1994; DeLuca, et al., 1998; Demaree, et al., 2000; S. M. Rao, et al., 1993; Thornton, et al., 2002). Recent MRI research estimated that brain atrophy accounts for about 10 – 15% of the variance in performance on memory tasks of initial learning and delayed retrieval in persons with MS (Benedict et al., 2006; Benedict et al., 2004; Christodoulou et al., 2003;Sanfilipo, Benedict, Weinstock-Guttman, & Bakshi, 2006). Although this may appear to suggest that MS disease-related atrophy (henceforth referred to simply as ‘MS disease’) impacts learning and retrieval equally, the relationship between MS disease and retrieval may actually be mediated through initial learning. That is, MS disease may impair initial learning, which secondarily leads to poor delayed recall. As such, MS disease may not directly impact retrieval. The current research (a) examines the relationship between MS disease (brain atrophy) and initial learning and delayed retrieval separately, and then (b) investigates whether initial learning mediates the relationship between MS disease and retrieval. That is, we examined whether learning or retrieval represents the core memory deficit in MS.
METHODS
Subjects
Subjects were 44 persons (39 women, 5 men) with MS(McDonald et al., 2001) without an exacerbation in the last four weeks, no current corticosteroid use, and no history of serious psychiatric illness, substance abuse, learning disability, or other neurologic condition. Demographics were as follows: age: 44.9 ± 6.9 years; education: 16.1 ± 2.3 years; disease duration: 10.5 ± 7.0 years; disease course: 34 relapsing-remitting, 7 secondary-progressive, 3 primary progressive. Physical disability assessed with Ambulation Index (Hauser et al., 1983) was relatively mild (N=43, 2.3 ± 2.2). Institutional review boards at UMDNJ and the Kessler Foundation Research Center approved this study. Informed consent was obtained from all subjects prior to participation.
Learning and Retrieval
Initial learning and delayed retrieval were assessed with the open-trial Selective Reminding Test (SRT) (Chiaravalloti, Balzano, Moore, & DeLuca, 2009). Subjects were presented with a list of 10 words to learn over 15 trials, or until a learning criterion of complete recall (all 10 words) on two consecutive trials was achieved. Initial learning was defined as the total number of words recalled across the 15 trials (SRT Total Learning). If the learning criterion was achieved, full credit was awarded for all subsequent trials up to trial 15. Delayed retrieval was defined as free recall of the word list after a 30-minute delay (SRT 30-Minute Recall). For the purposes of characterizing this sample’s memory function, we compared their Total Learning and Delayed Recall scores to a normative sample of 40 healthy controls (age: 44.8 ± 10.2; 28 women). Relative to healthy persons, our MS sample performed below average on both Total Learning (mean z = −0.95) and Delayed Recall (mean z = −0.77).
Brain Atrophy
Consistent with previous research (Benedict, et al., 2006; Benedict, et al., 2004), brain atrophy was estimated with third ventricle width (TVW) defined as the distance in mm between the left and right boundaries of the third ventricle as imaged in the axial plane of high-resolution 3D images of the brain acquired from magnetization prepared rapid gradient echo (MP-RAGE) scans performed in a 3.0T Siemens Allegra scanner. Detailed procedures are provided elsewhere (Sumowski, Chiaravalloti, Wylie, & Deluca, 2009) with high reported interrater and intrarater reliabilities (r’s > .96). Mean TVW was 4.9 ± 2.0 mm.
Statistical Analyses
Separate hierarchical regression analyses were performed to predict initial learning (SRT Total Learning) and delayed retrieval (SRT 30-Minute Recall), controlling for age and education in step one, and brain atrophy (TVW) in step two. We were also interested in the impact of brain atrophy on learning efficiency (i.e., learning curve) across the 15 SRT learning trials. After dividing the sample into brain atrophy subgroups via a median split (lower, higher), we performed a 2 (brain atrophy: lower, higher) × 15 (SRT Learning Trial) repeated measures ANCOVA, controlling for age and education. The brain atrophy × SRT Learning Trial interaction was analyzed to examine differences in learning efficiency across levels of brain atrophy.
The aforementioned analyses evaluate the effect of MS disease on initial learning and delayed retrieval in isolation, but we also wanted to test whether brain atrophy is uniquely related to retrieval independent of initial learning. A second hierarchical regression was performed predicting delayed retrieval (SRT 30-Minute Recall), with age and education controlled for in step one, and initial learning (SRT Total Learning) and brain atrophy (TVW) entered in a stepwise fashion in step two. If brain atrophy does not uniquely predict retrieval, then the relationship between brain atrophy and retrieval is likely mediated through initial learning. In a follow-up analysis, we examined correlations between initial learning and delayed retrieval with and without controlling for brain atrophy. If MS disease does not weaken the relationship between initial learning and retrieval, then there is little support for a direct effect of MS disease on retrieval processes.
As described above, subjects practiced the SRT word list until either (a) the learning criterion was met, or (b) 15 learning trials were administered. This procedure afforded multiple opportunities to learn the word list. After identifying a subgroup of subjects (n=38) who met the learning criterion, we reanalyzed the hierarchical regression predicting delayed retrieval (SRT 30-Minute Recall), with age and education controlled for in step one, and brain atrophy entered in step two. If learning difficulty represents the core MS-related memory deficit, then brain atrophy should be unrelated to delayed retrieval in subjects who met the learning criterion, regardless of how many trials they needed.
RESULTS
Impact of MS disease on learning and retrieval
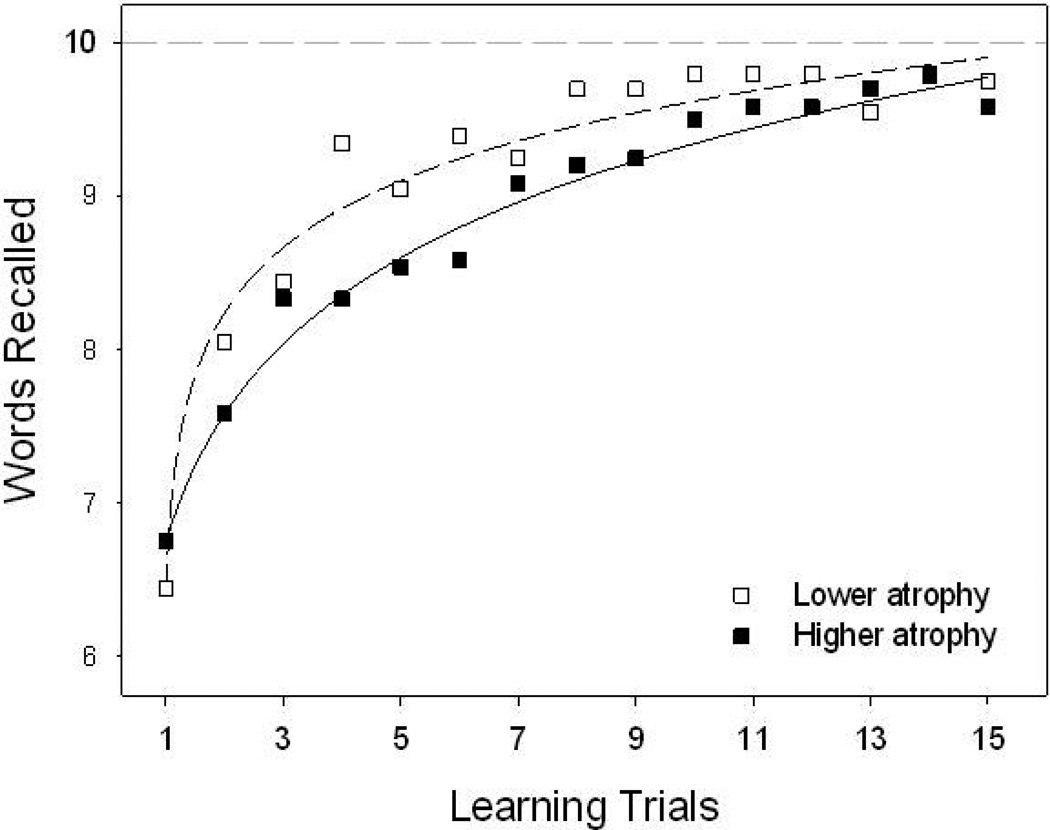
The full regression equation predicting SRT Total Learning accounted for 24% of the variance in initial learning (F [3, 40] = 4.28, p = .01). Controlling for age and education in step one (F [2, 41] = 1.99, p > .10, R2 = .09), brain atrophy was negatively associated with learning in step two (F [1, 40] = 8.17, p < .01, R2Δ = .16), indicating that MS disease is associated with worse initial learning. The full regression equation predicting SRT 30-Minute Recall accounted for 30% of the variance in delayed retrieval (F [3, 40] = 5.79, p < .01). Controlling for age and education in step one (F [2, 41] = 2.62, p = .09, R2 = .11), brain atrophy was negatively associated with retrieval in step two (F [1, 40] = 10.87, p < .01, R2Δ = .19), indicating that MS disease is associated with worse delayed retrieval. Regarding the impact of MS disease on learning efficiency (i.e., learning curve) across trials, the 2 (atrophy group: lower: TVW = 3.3 ± 0.6 mm; higher: TVW = 6.3 ± 1.6 mm) × 15 (SRT Learning Trials) repeated measures ANCOVA revealed a significant interaction (F [14, 588] = 2.86, p < .001). That is, subjects with greater disease required more trials to meet the learning criterion than subjects with lesser disease (see Figure 1), indicating that MS disease is associated with inefficient initial learning. Post hoc analyses showed that the points where the atrophy groups differed were found on trials 2,4,5,6,8,9.
Figure 1.
Negative impact of brain atrophy on initial learning efficiency across SRT trials.
Initial learning as a mediator between MS disease and delayed retrieval
Although brain atrophy is related to both learning and retrieval when analyzed separately, we tested the hypothesis that the relationship between brain atrophy and retrieval is mediated through initial learning. After controlling for age and education in step one (F [2, 41] = 2.62, p = .09, R2 = .11), only SRT Total Learning was a significant predictor of SRT 30-Minute Recall in step two (F [1, 40] = 33.83, p < .001, R2Δ = .41). Brain atrophy did not uniquely predict delayed retrieval over and above initial learning (F = 3.17, p = .08). To examine the R2 change value for brain atrophy after accounting for initial learning, entering brain atrophy in the model as a third block showed an R2 change of only 0.036, p = .083 for this factor. These data suggest that the relationship between MS disease and retrieval processes is mediated primarily through initial learning. To confirm the primary involvement of SRT Total Learning as a mediator, we performed a similar hierarchical regression with age and education in step one, and brain atrophy and a number of other neuropsychological tasks (symbol digit modalities test, paced auditory serial addition task, controlled oral word association task, judgment of line orientation)(Benedict et al., 2002) entered in a stepwise fashion in step two. Eight subjects with missing data on the aforementioned tasks were excluded from this follow-up analysis. As expected, brain atrophy remained the only significant predictor of SRT 30-Minute Recall (F [1, 32] = 8.27, p < .01, R2Δ = .18). When SRT Total Learning was added to the list of neuropsychological tasks in step two, brain atrophy was no longer significant (t = −0.29, p > .50) and SRT Total Learning remained the only predictor (F [1, 32] = 36.53, p < .001, R2Δ = .48).
If MS disease impacts retrieval processes over and above initial learning, then the relationship between initial learning and delayed retrieval should be weakened when controlling for brain atrophy. There was a strong positive relationship between SRT Total Learning and SRT 30-Minute Recall (r = .706, p < .001), which remained even when controlling for brain atrophy (rp = .671, p < .001). MS disease does not disrupt the strong relationship between initial learning and delayed retrieval, indicating that MS disease has little direct impact on retrieval processes.
Relationship between MS disease and retrieval after successful learning
Given several opportunities to learn the word list, most subjects eventually met the learning criterion (N = 38), indicating successful acquistion. If weak initial learning is the core memory deficit associated with MS, then MS disease should have minimal or no effect on delayed retrieval among subjects who successfully acquire the word list during the learning phase. Consistent with this hypothesis, brain atrophy was unrelated to delayed retrieval within this subsample (r = −.167, p > .10). We also repeated the initial regression predicting SRT 30-Minute Recall in this subsample. After controlling for age and education in step one (F [2, 35] = 0.37, p > .50, R2 = .02), brain atrophy did not uniquely predict delayed retrieval in step two (F [1, 34] = 2.34, p > .10, R2Δ = .06). That is, MS disease was unrelated to retrieval processes in persons who adequately learned the word list.
DISCUSSION
Memory impairment is prevalent among persons with MS, but there has been disagreement over whether this deficit is due to weak initial learning (DeLuca, et al., 1994; DeLuca, et al., 1998; Demaree, et al., 2000; Thornton, et al., 2002) or deficient retrieval (Bobholz, et al., 2006; S. M. Rao, et al., 1993; S. M. Rao, et al., 1989). Our findings indicate that MS disease is associated with weak initial learning, which then leads to poor retrieval. Brain atrophy was associated with a pattern of inefficient learning across acquisition trials, as well as lower total learning. Although brain atrophy was also associated with lower retrieval, this virtually relationship disappeared when controlling for initial learning. That is, the relationship between MS disease and retrieval was mediated primarily through initial learning. Finally, MS disease was unrelated to delayed retrieval in persons who successfully learned the word list (although several opportunities were required to accomplish this).
These findings support the learning-deficit hypothesis of memory impairment in MS, and they are consistent with previous research showing that delayed recall performance in persons with MS is comparable to healthy controls when controlling for initial learning (DeLuca, et al., 1994; DeLuca, et al., 1998; Demaree, et al., 2000). More specifically, DeLuca and colleagues found that persons with MS required more opportunities to learn information initially; however, once the information was adequately learned, they had no difficulty retrieving that information during delayed recall tasks. The current results extend previous research by incorporating a more direct measure of MS disease, namely, brain atrophy.
Memory rehabilitation research provides converging evidence for the learning-deficit hypothesis of memory impairment in MS (Chiaravalloti & Deluca, 2002; Chiaravalloti, DeLuca, Moore, & Ricker, 2005; O'Brien, Chiaravalloti, Goverover, & DeLuca, 2008; Sumowski, Chiaravalloti, & Deluca, 2010). Specifically, memory strategies designed to improve initial learning have led to notable gains in delayed retrieval (e.g., generation effect (Chiaravalloti & Deluca, 2002), visual imagery (Chiaravalloti, et al., 2005), testing effect (Sumowski, et al., 2010)). In fact, when using the testing effect to improve initial learning, memory-impaired MS subjects achieved delayed retrieval comparable to the baseline retrieval performance of healthy controls (Sumowski, et al., 2010). These interventions targeting initial learning would be ineffective if retrieval difficulty was the core memory deficit in MS; rather, these strategies are effective because they treat weak initial learning, which appears to be the core deficit.
Others have argued that deficient retrieval represents the primary memory deficit in MS (Bobholz, et al., 2006; S. M. Rao, et al., 1993; S. M. Rao, et al., 1989), based principally on a neuropsychological profile of poor delayed retrieval despite intact recognition. This argument is contingent on the use of recognition performance as a proxy of initial learning; however, there are notable problems with this methodology. First, this strategy assumes that learning is dichotomous (learned versus unlearned) and can therefore be adequately measured quantitatively. This is inconsistent with a great deal of research demonstrating notable variability in the quality of initial learning, with better learning quality leading to better retrieval (e.g., depth of processing framework (Craik & Lockhart, 1972), encoding specificity principle (Tulving & Thomson, 1973), transfer appropriate processing (Morris, Bransford, & Franks, 1977). Indeed, qualitative differences in initial learning also impact retrieval among persons with MS (Thornton, et al., 2002). Given that recognition is cognitively less challenging than retrieval (Craik & McDowd, 1987), it is clearly possible for weak initial learning to be adequate enough to support recognition, but at the same time, too weak to support retrieval (Westerberg et al., 2006). It is not surprising, therefore, that researchers have found tremendous recognition capacity in both experimental (Shepard, 1967) and clinical settings (e.g., symptom validity testing(Bianchini, Mathias, & Greve, 2001)). Given that even weak initial learning can support subsequent recognition, intact recognition performance provides little information about the quality of initial learning. Conversely, persons who fail to recognize previously presented stimuli likely do have notable learning impairments. Contrary to the frequently cited claim that recognition accuracy among persons with MS is comparable to healthy persons, the evidence has actually been mixed, with intact performance in some studies (S. M. Rao, et al., 1993; S. M. Rao, et al., 1989) and deficits in others (Beatty, MacInnes, Porphyris, Troster, & Cermak, 1988; S. M. Rao, Hammeke, McQuillen, Khatri, & Lloyd, 1984). Furthermore, consistent with the learning-deficit hypothesis, persons with MS have consistently produced lower total learning scores than healthy controls across many studies (Beatty, Goodkin, Monson, Beatty, & Hertsgaard, 1988; DeLuca, et al., 1994; DeLuca, et al., 1998; S. M. Rao, et al., 1993; S. M. Rao, et al., 1984; S. M. Rao, et al., 1989).
The current results stand in contrast to findings from a recent fMRI study by Bobholz and colleagues (Bobholz, et al., 2006) investigating the relationship between MS disease (T2 lesion load) and cerebral activity during learning and delayed recognition (used as a proxy of retrieval) (Bobholz, et al., 2006). Stronger correlations were found between MS disease and cerebral activity during recognition than during learning, from which the authors inferred that MS disease is more related to retrieval than initial learning. Unfortunately, this conclusion is marred by conceptual and methodological difficulties. First, given that the quality of initial learning was not assessed, we cannot know whether the relative absence of disease-related cerebral activation during the learning phase was due to (a) ease of initial learning regardless of disease, or (b) failure of the MS subjects with advanced disease to recruit the necessary resources to support good quality learning. Although these two scenarios are equally plausible in the absence of data on learning quality, the weak initial learning of MS subjects in our study and previous research (Beatty, Goodkin, et al., 1988; Benedict, et al., 2006; Benedict, et al., 2004; Christodoulou, et al., 2003; DeLuca, et al., 1994; DeLuca, et al., 1998; S. M. Rao, et al., 1993; S. M. Rao, et al., 1984; S. M. Rao, et al., 1989; Sanfilipo, et al., 2006) suggests that learning was weaker in the MS subjects with more advanced disease. Regarding recognition, disease-related cerebral activation may indicate greater challenge among MS subjects with more advanced disease; however, this increased challenge is likely the product of weak initial learning. Indeed, weak initial learning leads to greater cerebral recruitment during recognition in healthy persons (Benedict, et al., 2002). Our findings indicate that the relationship between MS disease and retrieval is primarily mediated through initial learning, and may explain at least in part the findings of Bobholz et al (2006).
The present study has several limitations. First, the relationship between strength of acquisition and retrieval is a complex one and our analysis offers a non-sophisticated approach to this complex phenomenon. The present results do not provide a definitive analysis concerning this complex interaction, and future work is needed to continue to examine this issue. Second, in the present study high and low atrophy was determined using a median split of the MS data. It would have been desirable to derive brain volume measures from healthy controls, but this data was not available in the current study. Third, while third ventricular width has been shown to be highly correlated with cognition, more sophisticated techniques designed for greater specificity (e.g., widespread grey and white matter damage) may be required to further test acquisition and retrieval interactions. Lastly, the small sample size, heavy distribution of females, and preponderance of relapsing remitting subjects limit the generalizability of the current findings to the broader MS population.
In conclusion, MS disease is associated with weak initial learning, which explains, at least in part, poor delayed retrieval. These findings support the learning-deficit hypothesis of memory impairment in MS, and suggest that effective interventions for memory problems in persons with MS should focus on improving the quality of initial learning.
ACKNOWLEDGEMENTS
This project was supported by grants from the National Multiple Sclerosis Society (RG3330A1/3 to N.C., MB0003 to J.D.) and the National Institutes of Health (HD045798 to N.C.).
Footnotes
The authors have no conflicts of interest to report.
REFERENCES
- Beatty WW, Goodkin DE, Monson N, Beatty PA, Hertsgaard D. Anterograde and retrograde amnesia in patients with chronic progressive multiple sclerosis. Arch Neurol. 1988;45(6):611–619. doi: 10.1001/archneur.1988.00520300029013. [DOI] [PubMed] [Google Scholar]
- Beatty WW, MacInnes WD, Porphyris HS, Troster AI, Cermak LS. Preserved topographical memory following right temporal lobectomy. Brain Cogn. 1988;8(1):67–76. doi: 10.1016/0278-2626(88)90039-5. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Bruce JM, Dwyer MG, Abdelrahman N, Hussein S, Weinstock-Guttman B, et al. Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch Neurol. 2006;63(9):1301–1306. doi: 10.1001/archneur.63.9.1301. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Fischer JS, Archibald CJ, Arnett PA, Beatty WW, Bobholz J, et al. Minimal neuropsychological assessment of MS patients: a consensus approach. Clin Neuropsychol. 2002;16(3):381–397. doi: 10.1076/clin.16.3.381.13859. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Weinstock-Guttman B, Fishman I, Sharma J, Tjoa CW, Bakshi R. Prediction of neuropsychological impairment in multiple sclerosis: comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol. 2004;61(2):226–230. doi: 10.1001/archneur.61.2.226. [DOI] [PubMed] [Google Scholar]
- Bianchini KJ, Mathias CW, Greve KW. Symptom validity testing: a critical review. Clin Neuropsychol. 2001;15(1):19–45. doi: 10.1076/clin.15.1.19.1907. [DOI] [PubMed] [Google Scholar]
- Bobholz JA, Rao SM, Lobeck L, Elsinger C, Gleason A, Kanz J, et al. fMRI study of episodic memory in relapsing-remitting MS: correlation with T2 lesion volume. Neurology. 2006;67(9):1640–1645. doi: 10.1212/01.wnl.0000242885.71725.76. 10.1212/01.wnl.0000242885.71725.76. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti ND, Balzano J, Moore NB, DeLuca J. The Open-Trial Selective Reminding Test (OT-SRT) as a tool for the assessment of learning and memory. Clin Neuropsychol. 2009;23(2):231–254. doi: 10.1080/13854040802121158. 10.1080/13854040802121158. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti ND, Deluca J. Self-generation as a means of maximizing learning in multiple sclerosis: an application of the generation effect. Arch Phys Med Rehabil. 2002;83(8):1070–1079. doi: 10.1053/apmr.2002.33729. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7(12):1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti ND, DeLuca J, Moore NB, Ricker JH. Treating learning impairments improves memory performance in multiple sclerosis: a randomized clinical trial. Mult Scler. 2005;11(1):58–68. doi: 10.1191/1352458505ms1118oa. [DOI] [PubMed] [Google Scholar]
- Christodoulou C, Krupp LB, Liang Z, Huang W, Melville P, Roque C, et al. Cognitive performance and MR markers of cerebral injury in cognitively impaired MS patients. Neurology. 2003;60(11):1793–1798. doi: 10.1212/01.wnl.0000072264.75989.b8. [DOI] [PubMed] [Google Scholar]
- Craik FI, Lockhart RS. Levels of processing: A framework for memory research. J Verb Learn Verb Be. 1972;11:671–684. [Google Scholar]
- Craik FI, McDowd JM. Age differences in recall and recognition. J exp Psychol Learn. 1987;13:474–479. [Google Scholar]
- DeLuca J, Barbieri-Berger S, Johnson SK. The nature of memory impairments in multiple sclerosis: acquisition versus retrieval. J Clin Exp Neuropsychol. 1994;16(2):183–189. doi: 10.1080/01688639408402629. [DOI] [PubMed] [Google Scholar]
- DeLuca J, Gaudino EA, Diamond BJ, Christodoulou C, Engel RA. Acquisition and storage deficits in multiple sclerosis. J Clin Exp Neuropsychol. 1998;20(3):376–390. doi: 10.1076/jcen.20.3.376.819. [DOI] [PubMed] [Google Scholar]
- Demaree HA, Gaudino EA, DeLuca J, Ricker JH. Learning impairment is associated with recall ability in multiple sclerosis. J Clin Exp Neuropsychol. 2000;22(6):865–873. doi: 10.1076/jcen.22.6.865.961. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Dawson DM, Lehrich JR, Beal MF, Kevy SV, Propper RD, Mills JA, Weiner HL. Intensive immunosuppression in progressive multiple sclerosis. A randomized, three arm study of high-dose intravenous cyclophosphamide, plasma exchange, and ACTH. N Engl J Med. 1983;308:173–180. doi: 10.1056/NEJM198301273080401. [DOI] [PubMed] [Google Scholar]
- Kessler HR, Cohen RA, Lauer K, Kausch DF. The relationship between disability and memory dysfunction in multiple sclerosis. Int J Neurosci. 1992;62(1–2):17–34. doi: 10.3109/00207459108999754. [DOI] [PubMed] [Google Scholar]
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- Morris C, Bransford JD, Franks J. Levels of processing versus transfer appropriate processing. J Verb Learn Verb Be. 1977;16:519–533. [Google Scholar]
- O'Brien AR, Chiaravalloti N, Goverover Y, DeLuca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: a review of the literature. Arch Phys Med Rehabil. 2008;89(4):761–769. doi: 10.1016/j.apmr.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Rao SM, Grafman J, DiGiulio D, Mittenberg W, Bernardin L, Leo GJ, et al. Memory dysfunction in multiple sclerosis: Its relation to working memory, semantic encoding, and implicit learning. Neuropsychology. 1993;Vol 7(3):364–374. [Google Scholar]
- Rao SM, Hammeke TA, McQuillen MP, Khatri BO, Lloyd D. Memory disturbance in chronic progressive multiple sclerosis. Arch Neurol. 1984;41(6):625–631. doi: 10.1001/archneur.1984.04210080033010. [DOI] [PubMed] [Google Scholar]
- Rao SM, Leo GJ, Ellington L, Nauertz T, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology. 1991;41(5):692–696. doi: 10.1212/wnl.41.5.692. [DOI] [PubMed] [Google Scholar]
- Rao SM, Leo GJ, St Aubin-Faubert P. On the nature of memory disturbance in multiple sclerosis. J Clin Exp Neuropsychol. 1989;11(5):699–712. doi: 10.1080/01688638908400926. [DOI] [PubMed] [Google Scholar]
- Sanfilipo MP, Benedict RH, Weinstock-Guttman B, Bakshi R. Gray and white matter brain atrophy and neuropsychological impairment in multiple sclerosis. Neurology. 2006;66(5):685–692. doi: 10.1212/01.wnl.0000201238.93586.d9. [DOI] [PubMed] [Google Scholar]
- Shepard RN. Recognition memory for words, sentences, and pictures. J Verb Learn Verb Be. 1967;6:156–163. [Google Scholar]
- Sumowski JF, Chiaravalloti N, Deluca J. Retrieval practice improves memory in multiple sclerosis: clinical application of the testing effect. Neuropsychology. 2010;24(2):267–272. doi: 10.1037/a0017533. 10.1037/a0017533. [DOI] [PubMed] [Google Scholar]
- Sumowski JF, Chiaravalloti N, Wylie G, Deluca J. Cognitive reserve moderates the negative effect of brain atrophy on cognitive efficiency in multiple sclerosis. J Int Neuropsychol Soc. 2009;15(4):606–612. doi: 10.1017/S1355617709090912. [DOI] [PubMed] [Google Scholar]
- Thornton AE, Raz N. Memory impairment in multiple sclerosis: a quantitative review. Neuropsychology. 1997;11(3):357–366. doi: 10.1037//0894-4105.11.3.357. [DOI] [PubMed] [Google Scholar]
- Thornton AE, Raz N, Tucke KA. Memory in multiple sclerosis: contextual encoding deficits. J Int Neuropsychol Soc. 2002;8(3):395–409. doi: 10.1017/s1355617702813200. [DOI] [PubMed] [Google Scholar]
- Tulving E, Thomson DM. Encoding specificity and retrieval processes in episodic memory. Psychol Rev. 1973;80:352–373. [Google Scholar]
- Westerberg CE, Paller KA, Weintraub S, Mesulam MM, Holdstock JS, Mayes AR, et al. When memory does not fail: familiarity-based recognition in mild cognitive impairment and Alzheimer's disease. Neuropsychology. 2006;20(2):193–205. doi: 10.1037/0894-4105.20.2.193. 10.1037/0894-4105.20.2.193. [DOI] [PubMed] [Google Scholar]