Abstract
Introduction
Whereas deficits in muscle function, particularly power production, develop in old age and are risk factors for mobility impairment, a complete understanding of muscle fatigue during dynamic contractions is lacking. We tested hypotheses related to torque-producing capacity, fatigue resistance, and variability of torque production during repeated maximal contractions in healthy older, mobility-impaired older, and young women.
Methods
Knee extensor fatigue (decline in torque) was measured during 4 min of dynamic contractions. Torque variability was characterized using a novel 4-component logistic regression model.
Results
Young women produced more torque at baseline and during the protocol than older women (P < 0.001). Although fatigue did not differ between groups (P = 0.53), torque variability differed by group (P = 0.022) and was greater in older impaired compared with young women (P = 0.010).
Conclusions
These results suggest that increased torque variability may combine with baseline muscle weakness to limit function, particularly in older adults with mobility impairments.
Keywords: aging, contraction, physical function, power, sarcopenia
It is well documented that neuromuscular function declines with advancing age. Although isometric strength often is used to evaluate muscular function, its static nature fails to capture the age-related reductions in performance and power that occur with increasing contraction velocities.1–7 Likewise, because many activities of daily living involve active muscle shortening, it is logical to include dynamic contractions in studies of age-related losses in muscular performance. Recent work suggests that power may predict mobility impairments, such as reduced gait speed, difficulty ascending or descending stairs, and difficulty getting into and out of a chair, better than isometric strength.8–10 More recently, a focus on the potential role of muscle fatigue, the acute loss of power as a result of contractile activity, in mobility function is emerging as an important topic of study in aging research.
A recent systematic review of the literature11 revealed that, although healthy older adults fatigue relatively less than young adults during isometric and low-velocity contraction protocols, less is known about aging and fatigue during protocols in which power is the outcome variable. Indeed, there is new evidence that age-related fatigue resistance does not extend to high-velocity dynamic contractions.12,13 Coupled with low baseline power output, a propensity for greater fatigue during high-velocity contractions likely predisposes older adults to problems with physical function. This may be particularly true for older women, whose muscle weakness and risk for mobility impairment generally exceed that of men.14,15 Critically, maximal knee extensor power in older adults with mild-to-moderate mobility impairment is perilously close to the minimum power necessary to perform some activities of daily living.16,17
In addition to loss of power, the ability to produce torque consistently during a task can decline with age.18,19 Further, poor torque production consistency is associated with poor performance on functional tasks.20,21 Thus, age-related increases in voluntary torque production variability may limit the capacity of mobility-impaired older adults to perform the high-velocity contractions necessary to recover from a trip or stumble.22 Performance variability in the context of muscle fatigue has not been examined closely in older adults, and a consistent approach to quantifying variability during fatigue is lacking. Indeed, little information about the interactions between muscle weakness, fatigue, and the reliability of muscle performance in older adults, especially those with mobility impairments, is currently available.
Therefore, the purpose of this study was to investigate muscle performance during a fatiguing bout of maximal knee extensions in groups of young, healthy older, and older mobility-impaired women. Quantification of knee extensor performance was determined as: (1) torque-generating capacity, measured both as baseline, absolute peak torque, and the sum of the peak torques from all contractions during the bout; (2) fatigue, quantified as the loss in torque at the end of the bout relative to baseline; and (3) variability of torque production, evaluated using a novel, non-linear regression model of peak torque produced during each contraction. We hypothesized that: (1) healthy and mobility-impaired older women would have progressively lower baseline and summed torques compared with young women; (2) fatigue would be similar in young and healthy older women, but greater in mobility-impaired older women; and (3) older women would exhibit greater torque variability throughout the fatigue protocol than young women, and older mobility-impaired women would show the greatest variability.
METHODS
Study Participants
Twenty-five community-dwelling women (21–35 or 65–85 years) were studied. Eight young and 9 older, healthy women comprised 2 of the study groups, and 8 older women with mild-to-moderate mobility impairments constituted the third group. Mobility impairment was identified using the Short Physical Performance Battery,23 which evaluates balance, walking speed, and repeated chair rise time. Participants in the older impaired group scored between 8 and 10 on the 12-point scale, indicating the presence of mild-to-moderate mobility impairment.23
All participants were relatively sedentary (participating in less than 2 structured exercise sessions per week), otherwise healthy by self-report, and not taking any medications known to affect neuromuscular function. Orthopedic problems were self-identified: 1 older impaired woman reported a hip replacement, and 1 reported bilateral knee osteoarthritis. In both cases, the participants’ muscle fatigue and variability data placed them within 1 standard deviation of their group means. There were no knee replacements in the cohort, and no participants reported knee pain in the leg studied on the study days. All older participants provided written consent from their personal physician prior to beginning the study. All participants provided signed informed consent approved by the institutional review board at the University of Massachusetts, Amherst, and testing procedures were in accordance with the Declaration of Helsinki for the protection of human subjects. Portions of these data have been published elsewhere.24
Physical Function
To characterize the study groups, physical function was assessed using timed performances of repeated chair rises, stair ascent, and descent. The time to complete 10 chair rises was performed using a lightly padded, straight-backed chair without armrests (seat height = 46 cm). Participants were instructed to rise fully and sit as quickly as possible without the use of their arms. The Short Physical Performance Battery23 includes a 5-times chair rise task, but we used the 10-times chair rise test to better distinguish the healthy older group from the young group. This decision was based on the work of Csuka and McCarty, who used 10-times repeated chair stands to assess physical function in adults ranging from 20 to 85 years of age.25 Next, each participant was asked to perform separate timed stair ascent and descent tasks. These tasks were completed using a flight of 8 steps, and the fastest time of 2 attempts in each direction was recorded. All participants climbed 1 step at a time and kept at least 1 foot in contact with the surface at all times. To ensure safety, participants were monitored closely by study personnel during the physical function measures.
Muscle Torque Measures
Prior to baseline measurements of muscle torque, participants warmed up by pedaling lightly on a recumbent cycle ergometer (Schwinn, Nautilus, Inc., Vancouver, Washington) for 5 min, followed by light stretching of the knee flexor muscles. Next, knee extension torque measures were collected using a dynamometer (Biodex System 3; Biodex Medical, Shirley, New York). The left leg was tested in all participants except the 3 older women who reported left knee pain. The right leg of these individuals was tested instead. Participants were seated in 90° of hip flexion and 105° of knee flexion (straight leg = 180°), with their shoulders, hips, and ankle strapped firmly to the dynamometer. Analog recordings were obtained using Lab-View software (National Instruments, Austin, Texas), developed in-house for this purpose. A sampling rate of 2500 Hz was used for baseline measures of torque, and a rate of 1000 Hz was used during the fatigue protocol.
To evaluate baseline isometric strength, each participant performed 3–5 maximal voluntary isometric contractions, with the knee fixed at 105° of flexion. Participants were instructed to contract “as fast and hard as possible.” Each contraction lasted 3–4 s, and at least 1 min of rest was provided between trials. Baseline isometric torque was defined as the peak torque (Nm) obtained from these contractions. To elicit a maximal effort from all participants, strong verbal encouragement and visual torque feedback were provided.
After the isometric measures were obtained, participants completed a series of 3 maximal voluntary dynamic contractions through a 60° range of motion to establish baseline torque-generating capacity. Extension velocity was limited to 120°/s. This velocity was selected because, while rapid, it is attainable by both younger and older participants.1,12 Because the dynamometer's isovelocity mode was used, torque thus approximates power in this study. Contractions were cued verbally every 3 s, and peak torque (Nm) was defined as the highest torque obtained from the 3 contractions. Sets of 3 contractions were recorded to account for the increase in torque that is often observed with repeated dynamic contractions.26 To evaluate the potential impact of body size on baseline torque-producing capacity, peak torque also was normalized to body mass (Nm/kg).
One of our goals was to quantify the coupled effect of baseline weakness and the development of fatigue on the absolute torque output of our study groups. The rationale for this goal is the working hypothesis that, when muscle fatigue develops in individuals with baseline weakness, the potential impact on their mobility function would be far greater than fatigue in stronger individuals. The summed torque variable (ΣNm), which reflects both strength and fatigue, was used as an overall measure of torque-producing capacity during the 4-min fatigue protocol (described below). Summed torque (ΣNm) was calculated for each participant by summing the peak torques for each contraction during the fatigue task. Finally, to allow comparison of torque production at fatigue with torque values reported for activities of daily living,16,17 peak torque during the final contraction of the fatigue protocol was determined and expressed in absolute terms (Nm) as well as relative to body mass (Nm/kg).
Fatigue Measures
After baseline testing, participants began a 4-min fatigue protocol consisting of 1 maximal dynamic contraction every 2 s. Knee extension velocity was limited to 120°/s, and knee flexion was passive. Fatigue was quantified as: 100 (average of final 5 contractions) / (average of peak dynamic torque at baseline and peak torque achieved during the fatigue protocol), as described previously.24 This approach accounted for the tendency of individuals to achieve the highest torque 2–3 contractions into the protocol. After the 4-min protocol, the recovery of torque was evaluated with a series of 3 maximal dynamic contractions obtained at 2, 5, and 10 min of recovery.
Torque Variability Measures
The pattern of torque production during the fatigue protocol was generally sigmoid (Fig. 1, top). Thus, a 4-parameter, non-linear regression model was fit to each participant's relative torque data to evaluate the variability of performance during the fatigue protocol. For each individual, the model was fit to all contractions from 5 to 240 s; the first 2 contractions were excluded to eliminate the tendency for peak torque to increase during the first few contractions of the protocol in this type of protocol. The model used a 4-parameter logistic of the form:
| (1) |
where Y = predicted torque (% baseline); Asinitial and Asend are the asymptotes of torque at the beginning and end of the fatigue task, respectively; tmid is the time at which predicted fatigue is midway between Asinitial and Asend; and slope is the slope of the curve at t = tmid.
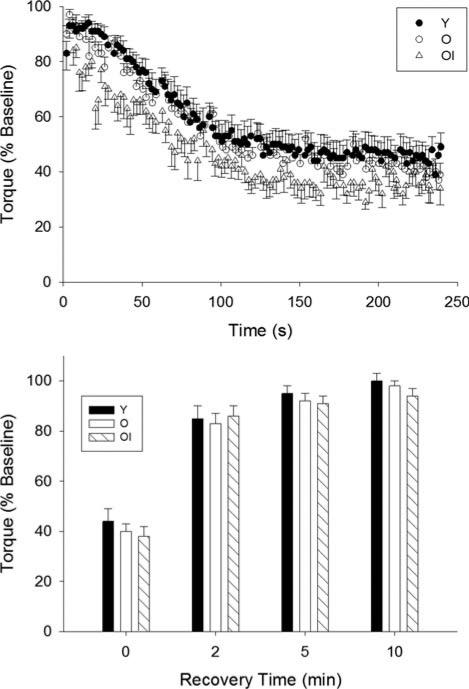
FIGURE 1.
Mean ± SE torque (% of baseline) for each group during the fatigue protocol (top) and recovery period (bottom). As expected, no differences in fatigue were observed between young and healthy older women. Contrary to our hypothesis, however, older impaired women did not differ in fatigue compared with the young and healthy older groups. There were no differences between groups in the recovery of torque. Y, young group; O, healthy older group; OI, older impaired group.
Performance variability was assessed using squared residuals from the model, where the residual at each time-point is the difference between peak relative torque and the value predicted by the model at the same time-point. This normalized performance variability was expressed as percent MVC squared and quantified for each individual as the average of the squared residuals within each 30-s epoch (or 25 s in the case of the first epoch), as well as for overall variability during the fatigue protocol (5–240s).
Statistical Analyses
A 1-way analysis of variance was used to evaluate descriptive characteristics (age, height, body mass, physical function), baseline torque, and fatigue among the 3 study groups. A 1-way (group) repeated-measures analysis of variance was used to evaluate torque recovery following the fatigue task. The Tukey post hoc analysis was conducted where differences were detected. This same method was used to compare the coefficients resulting from the 4-parameter logistic fits, described above, among the 3 groups.
Two-factor (group, time) repeated measures analysis of variance (ANOVA) was used to compare performance variability in 30-s bins throughout the protocol. A weighted, 1-factor ANOVA (group) with post hoc comparisons using Bonferroni correction was applied to evaluate pairwise differences in overall variability across groups. The weighting accounted for the fact that, across groups, there were some differences in within-group variance for the overall variability measure. Linear regression analysis was used to explore associations between the measure of fatigue (% baseline) and end-exercise torque determined from the 4-parameter fit (Asend), as well as between baseline power and both fatigue and torque variability. Data are presented as mean and standard error (SE), and significance was established when P ≤ 0.05.
RESULTS
Group characteristics are summarized in Table 1. All groups were different from one another in age. There were no differences in body mass across groups, although the older impaired group was shorter than both the young and healthy older groups. Measures of physical function are summarized in Table 2. The older impaired women were slower than both the young and healthy older groups in all measures of physical function. The healthy older group was slower than the young group only for stair descent time.
Table 1.
Group characteristics.
| Variable | Young (n = 8) | Healthy older (n = 9) | Older impaired (n = 8) | P-value |
|---|---|---|---|---|
| Age (years)*†‡ | 25.9 ± 1.3 | 69.8 ± 1.1 | 74.8 ± 1.2 | <0.01 |
| Height (m)†‡ | 1.67 ± 0.03 | 1.64 ± 0.01 | 1.56 ± 0.02 | <0.01 |
| Body mass (kg) | 69.5 ± 5.9 | 65.1 ± 2.1 | 68.0 ± 5.2 | 0.79 |
Data expressed as mean ± SE, with group size in parentheses. P-values are for main effect of group. Statistical significance P ≤ 0.05 for all comparisons.
Difference between young and healthy older (post hoc analysis).
Difference between young and older impaired (post hoc analysis).
Difference between healthy older and older impaired (post hoc analysis).
Table 2.
Physical function.
| Variable | Young (n = 8) | Healthy older (n = 9) | Older impaired (n = 8) | P-value |
|---|---|---|---|---|
| Chair rise (s)†‡ | 13.7 ± 1.1 | 15.9 ± 0.9 | 23.5 ± 2.1 | <0.01 |
| Stair ascent (s)†‡ | 2.9 ± 0.1 | 3.4 ± 0.1 | 4.9 ± 0.3 | <0.01 |
| Stair descent (s)*†‡ | 2.6 ± 0.1 | 3.3 ± 0.1 | 4.5 ± 0.3 | <0.01 |
Data expressed as mean ± SE, with group size in parentheses. P-values are for main effect of group. Statistical significance P ≤ 0.05 for all comparisons.
Difference between young and healthy older (post hoc analysis).
Difference between young and older impaired (post hoc analysis).
Difference between healthy older and older impaired (post hoc analysis).
Muscle Torque and Fatigue
At baseline, the young group had greater isometric torque than both older groups (144.4 ± 9.2, 112.3 ± 6.6, and 95.6 ± 8.0 Nm, respectively; P < 0.01). Likewise, dynamic torque was higher in young than both older groups, with no difference between the healthy and older impaired groups (Table 3). The differences in dynamic torque at baseline persisted after normalizing to body mass (Table 3).
Table 3.
Muscle function during dynamic knee extensions.
| Variable | Young (n = 8) | Healthy older (n = 9) | Older impaired (n = 8) | P-value |
|---|---|---|---|---|
| Baseline | ||||
| Torque (Nm)*† | 119.7 ± 9.1 | 72.0 ± 3.6 | 59.2 ± 4.1 | <0.001 |
| Normalized torque (Nm/kg)*† | 1.79 ± 0.16 | 1.11 ± 0.06 | 0.89 ± 0.07 | <0.001 |
| Fatigue protocol | ||||
| Fatigue (end torque/baseline torque) | 0.44 ± 0.05 | 0.40 ± 0.03 | 0.38 ± 0.04 | 0.53 |
| Summed torque (ΣNm)*†‡ | 7804 ± 590 | 4585 ± 274 | 3117 ± 341 | <0.001 |
| Torque at fatigue (Nm)*† | 51.4 ± 4.5 | 28.6 ± 2.5 | 22.3 ± 2.8 | <0.001 |
| Normalized torque at fatigue (Nm/kg)*† | 0.76 ± 0.08 | 0.44 ± 0.04 | 0.34 ± 0.05 | <0.001 |
Data expressed as mean ± SE, with group size in parentheses. P-values are for main effect of group. Statistical significance P ≤ 0.05 for all comparisons.
Difference between young and healthy older (post hoc analysis)
Difference between young and older impaired (post hoc analysis)
Difference between healthy older and older impaired (post hoc analysis).
Fatigue was not different between groups (Table 3 and Fig. 1, top), and all groups recovered from fatigue similarly in the 10 min after conclusion of the contraction protocol (Fig. 1, bottom). In contrast, there were marked differences in absolute torque-generating capacity across the groups in response to the fatiguing contractions. Summed torque (ΣNm) was higher in the young group than either older group, and it was higher in healthy older compared with older impaired women (Fig. 2 and Table 3). Peak torques produced at the conclusion of the fatiguing bout (both Nm and Nm/kg) were lower in both older groups compared with the young group (Table 3). The lack of association between baseline power and fatigue (r = 0.024, P = 0.91, n = 24) supports the concept that weakness and fatigue are physiologically independent variables.
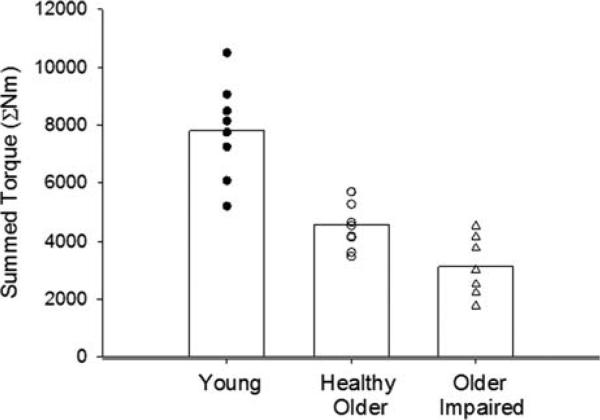
FIGURE 2.
Summed torque. The sum of peak torques (ΣNm) for all contractions during the fatigue protocol showed a progressive reduction for both older groups, such that young-> healthy older > older impaired (P < 0.001). Individual values are given for each study group; bars indicate group means.
Torque Variability
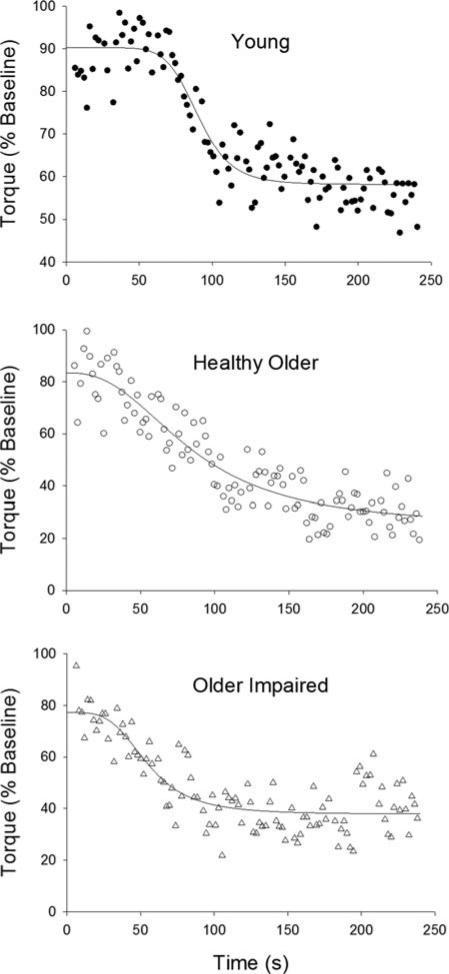
During the contraction protocol, torque fell in a sigmoidal manner for all groups (Fig. 1, top). Nonlinear regression fits of data from representative young, healthy older, and older impaired women are shown in Figure 3. The mean coefficient data are listed in Table 4. The fits reveal that torque produced at the onset of the contraction protocol (Asinitial) was lower in the older impaired group than in the young and healthy older groups (Table 4). As with the traditional measure of fatigue (Table 3), Asend was not different between groups (Table 4), and regression analysis revealed a significant association between Asend and fatigue (r = 0.96, P < 0.01).
FIGURE 3.
Non-linear regression analyses. A 4-parameter logistic regression was applied to the peak torque values for each participant, from 5 to 240 s of the fatigue protocol. Data and fits from representative young (top), healthy older (middle), and older impaired (bottom) participants are shown. Asymptotes at the onset and end of the fatigue protocol, as well as coefficients for the curvature and slope of the line, were obtained from each fit. See Methods for details and Table 4 for results.
Table 4.
Coefficients from 4-parameter logistic regression.
| Variable | Young (n = 8) | Healthy older (n = 9) | Olderimpaired (n = 8) | P-value |
|---|---|---|---|---|
| Asinitial (% baseline)* | 0.93 ± 0.02 | 0.92 ± 0.02 | 0.80 ± 0.04 | <0.01 |
| Asend (% baseline) | 0.44 ± 0.05 | 0.38 ± 0.03 | 0.33 ± 0.04 | 0.23 |
| Variability* | 0.0026 ± 0.0004 | 0.0048 ± 0.0011 | 0.0081 ± 0.0019 | 0.022 |
Data expressed as mean ± SE, with group size in parentheses. P-values are for main effect of group. Statistical significance P ≤ 0.05 for all comparisons. Coefficients are averaged from fits to individual torque data. As, asymptote (see text for details).
Difference between young and older impaired (post hoc analysis).
†Difference between healthy older and older impaired (post hoc analysis).
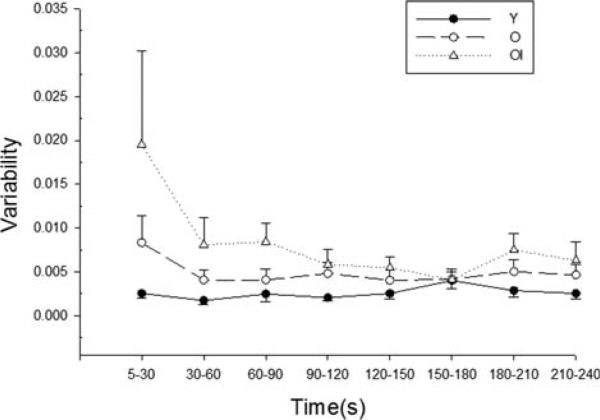
There was a main effect of group for torque variability assessed for the duration of the fatigue protocol (Table 4), and post hoc analysis revealed that variability during the 4-min protocol was higher in the older impaired group than in the young group. Repeated-measures analysis, using the data from all epochs, suggested trends for a main effect of time (P = 0.079) and a group-by-time interaction (P = 0.075) for variability during the protocol (Fig. 4). The large SE associated with the older impaired group at the first time point (Fig. 4) was due to a very high value (~9-fold higher than the group mean) in 1 individual. Repeated-measures ANOVA with data from this individual excluded substantially reduced the main effect of time (P = 0.42) and had a more limited impact on significance for the group-by-time interaction (P = 0.12). Likewise, if the first time-point for all groups was eliminated from the repeated measures analysis, the group effect remains significant (P = 0.026), but time (P = 0.481) and the group-by-time interaction (P = 0.159) do not. There is no particular explanation for the convergence of all groups at 150–180 s. Baseline muscle power was associated with torque variability (r = –0.57, n = 24, P = 0.005). The fit coefficients tmid and slope did not differ between groups (data not shown).
FIGURE 4.
Torque variability. Group mean variability during the 4-min fatigue protocol. Variability was calculated for each individual based on the distance of each peak torque value (in “y” direction) from the nonlinear fit of all contractions between 5 and 240 s, and expressed as percent MVC squared (see text for details). There was a main effect of group (Table 4), and the older impaired group had higher variability than the young group (P = 0.010). There were trends for an effect of time (P = 0.079) and a time-by-group interaction (P = 0.075; see Discussion). Data are in 30-s bins, with the exception of the first time-point, which is a 25-s bin. Error bars are SE.
DISCUSSION
The aim of this study was to quantify knee extensor muscle torque production, fatigue, and variability during repeated maximal contractions in young, healthy older, and older mobility-impaired women. In agreement with our first hypothesis, healthy and impaired older women were weaker than young women both in absolute terms and when peak torque was normalized to body mass. Further, this baseline weakness was evident in the markedly lower summed torque (ΣNm) of the older women compared with the young women during the fatigue protocol—a result that was even more apparent in the impaired group. In contrast to our second hypothesis, there were no differences in fatigue between the groups, indicating that fatigue during dynamic contractions at a moderate velocity was not excessive in these older women. Finally, our third hypothesis was supported in that there were differences in torque variability between groups during the fatigue task, and post hoc analysis indicated greater variability in the older impaired group compared with the young group. This combination of deficits in voluntary torque production may have significant implications for functional performance in this population.
Muscle Torque Production and Fatigue
Consistent with our first hypothesis, the maximal capacity to produce dynamic torque, corresponding to power production under our conditions, was significantly lower in the older groups (Table 3). This was the case both for baseline torque production and the summed torque calculated for the contraction protocol (Fig. 2). Further, these differences by age remained even after the data were adjusted for body mass.
A number of investigators have suggested a minimum threshold for torque production (~1.0–1.5 Nm/kg per leg) necessary to accomplish tasks such as walking up stairs or rising from a chair.16,17,27 At baseline, torque production in our older groups was near or below this threshold. For our measures of physical function, the healthy older women were slower than young women only in stair descent time (Table 2), consistent with reports that older adults are closer to their maximal strength during stair descent compared with stair ascent or chair rise tasks.17
The mobility-impaired older women, on the other hand, were slower in all measures of physical function. These results illustrate the importance of understanding the relationship between knee extensor weakness and physical function in aging. Reeves et al.28 demonstrated that older adults employ alternative strategies relative to young in order to operate within their maximal capacity while ascending stairs. In their study, older adults used ~90% of their maximal joint moments to climb stairs. Kinematic analysis of this task showed that the older adults changed their movement pattern to allow greater translocation of energy from the knee to the ankle, thus distributing the demand of stair ascent across 2 joints.28 However, despite this and other compensatory biomechanical strategies,29 the functional reserve of older adults is limited17,30 and may have dramatic effects on the development of physical dysfunction. Our results suggest that the additional stress placed on this functional reserve by fatiguing activities may further burden the neuromuscular system and elevate the risks for falling or other mobility problems in this population.
Until relatively recently, the literature regarding muscle fatigue and aging largely has focused on the use of isometric contractions in healthy older men and women.11 Our study extends previous work to include groups of healthy and mobility-impaired older women and to evaluate dynamic torque (i.e., power) during and after a task of repeated, maximal dynamic contractions. In contrast to our second hypothesis, there were no differences between groups in fatigue during the dynamic knee extension task (Fig. 1 and Table 3). Likewise, recovery of torque after fatigue was not different between groups. Although the angular velocity used in this study (120°/s) was somewhat higher than that identified from individual torque–velocity curves as “intermediate” (104°/s in young adults and 78°/s in older adults), we found a result similar to that reported in an earlier study.12 Specifically, fatigue did not differ between young and older women, even those with mobility impairments, during repeated knee extensions. These results are consistent with other studies of fatigue in aging,11,31 and reinforce the concept that age-related impairments in the ability to resist fatigue are not evident at low and moderate contraction intensities.
A key finding was the importance of potential interactions between weakness and fatigue in older adults, which are physiologically distinct characteristics of muscle function. Coupled with their baseline weakness, the fatigue induced by the contraction protocol resulted in mean knee extensor torques per body mass of ≤0.44 Nm/kg per leg in the older women (Table 3). In a population where peak torque and contractile velocity are low,12,13,32,33 working at or near maximal could leave these individuals at risk for additional functional impairment or even task failure. Support for this possibility is given by the summed torque measure (ΣNm), which illustrates the impact of repeated contractions on the capacity to produce torque in the older women (Fig. 2 and Table 3). The summed torque measure thus captures the functional impact of both weakness and fatigue development. These results provide additional evidence and illustrate the profound consequences of low knee extensor power in older women, including healthy older women without signs of mobility impairment and minimal changes in physical function (Table 2). Overall, the data suggest that there is little room for fatigue if adequate physical function is to be achieved by older women throughout the day.
Variability of Torque Production
This study was designed to investigate not only the torque production and fatigue of young and older women during a dynamic knee extension task, but to examine the fidelity with which the task was completed. The use of a novel, 4-parameter logistic regression analysis revealed a main effect of group on overall torque variability (Table 4). Specifically, torque variability was higher in the older impaired group than in the young group, thus providing some support for our third hypothesis. The healthy older women fell between the young and impaired older groups in terms of variability.
The planned analyses revealed trends for an effect of time and a group-by-time interaction for variability, which could suggest that variability was influenced by fatigue, particularly in the older impaired group. However, this possibility seems tenuous, given that force continued to fall after the first time period, whereas, for the most part, variability leveled out. Low enthusiasm for this interpretation is also supported by the abolishment of the trends for effects of time and group-by-time interactions when the first time period was eliminated from the analyses. Rather, the high variability in the older impaired group during the initial part of the protocol, which was largely attributable to the extremely high variability of 1 individual, seems likely to have been due to an adjustment to the task early in the protocol. It may be that the typical adjustments made at the onset of such a task26 are in some way more difficult for older adults with impairments. This possibility remains to be tested.
The existence and significance of potential interactions between the development of fatigue and changes in variability during maximal voluntary contractions such as those used here is not clear. When assessed over the duration of the fatigue protocol, variability of peak torque production was higher in the impaired older group (Table 4), revealing a notable age-related deficit in neuromuscular function. Further, our data indicate that performance variability during maximal contractions is most evident in those already experiencing mobility impairment. This interpretation is consistent with the observation of a negative association between baseline power and torque variability, which suggests that weaker individuals have greater performance variability. Thus, it appears that muscle weakness may place older adults at risk not only in tasks that require significant power production, but also for tasks that require consistent performance at relatively high intensities.
The effect of age on the variability of torque production, or rather the extent to which torque varies about a submaximal target value, has been studied extensively.20,34–36 These studies suggest that older adults have greater variability while attempting to produce consistent, submaximal isometric torques18,34,36 or maintain consistent torque trajectories during dynamic contraction tasks.20,35 Other investigators have identified age-related limitations in attaining “target” torque during repeated voluntary contractions.34 In these studies, torque or force variability is typically calculated as the coefficient of variation or standard deviation about the mean, submaximal force. To our knowledge, the variability of peak torque production has not been examined during a maximal dynamic contraction task to fatigue. For this purpose, we developed a new method for calculating torque variability that can be used under conditions in which torque is changing. The results from this analysis suggest that poor consistency of performance in older adults may be a problem, particularly given the potential impact of performance variability on mobility function in the context of increased relative effort during activities of daily living.17,37 A useful feature of our 4-parameter fit was the calculation of torque variability for each individual that was based on each contraction's distance on the y-axis from the line-fit of the total data set, with “Y” expressed as torque relative to MVC. This approach allows a focus on variability in the face of changes in maximal torque due to fatigue. This characteristic is particularly useful for fatigue protocols that use maximal contractions, as the loss of torque generally becomes evident early in these protocols. Thus, we can begin to disentangle fatigue from performance variability.
In addition to increased variability of torque production, the older impaired group had a difficult time achieving peak baseline torque from the beginning of the task (Asinitial; Table 4). It is possible that this group elected to “pace” their effort at the outset of the task in order to assure its completion. However, full voluntary activation of the muscle was encouraged in this study with verbal and visual feedback during the protocol. Further, any pacing that might have occurred could be expected to decrease the variability of torque production, but this group instead exhibited increased torque variability at this point in the task (Table 4). It is notable that, despite not achieving peak baseline torque at the onset of the protocol, fatigue in the impaired group was similar in magnitude to that of the other groups (Table 3). Likewise, the lack of difference between groups in Asend (Table 4), modeled using all of each subject's peak torques, is consistent with the lack of difference in fatigue observed between groups. Given these results, it is tempting to speculate that fatigue might have been greater in the older impaired group had they managed to hit target torques from the beginning of the protocol. Overall, the difference in performance by the older impaired group in terms of attaining and maintaining target torque at the onset of dynamic contractions provides insight as to their level of dysfunction even prior to the onset of fatigue. Finally, the strong association between observed fatigue and that predicted by the 4-parameter fit (r2 = 0.93) lends support for the use of this model for these data.
An intent of this study was to advance our understanding of the link between laboratory-based studies of fatigue, which quantify the relative fall in torque, and “real-world” situations that require some minimum level of absolute torque output. As a first approximation, we have: (1) evaluated fatigue and physical function in 3 groups with differing torque-generating capacity; and (2) compared our torque data at fatigue with torque or power values for stair climbing and chair rising drawn from the literature. Certainly, more work needs to be done in this area, but these results provide additional rationale for this need, as well as evidence to suggest that both the capacity to produce power and power variability should be considered in future studies.
In conclusion, although fatigue during maximal dynamic contractions did not differ by age, older women exhibited lower torque-producing capacity (i.e., power) and greater torque production variability than young women. These results provide support for the concept that, although excess muscular fatigue may not occur in older adults under conditions such as these, the addition of fatigue to baseline muscle weakness may lead to power output that is lower than the minimum necessary to achieve some mobility tasks, thus imposing or exacerbating functional limitations in the real-world setting for this population. Finally, the combination of increased torque production variability and lower capacity for absolute torque output during repeated contractions likely has profound implications for fall risk and mobility impairment in older adults.
Acknowledgments
This work was supported by the NIH/NIA (K02 AG023582).
Abbreviations
- ANOVA
analysis of variance
- Asinitial
asymptote in torque at the beginning of the fatigue task, from logistic regression analysis
- Asend
asymptote in torque at the end of the fatigue task, from logistic regression analysis
- O
older group
- OI
older-impaired group
- SPPB
Short Physical Performance Battery
- tmid
time at which fatigue predicted by logistic regression analysis is midway between Asinitial and Asend
- Y
young group
- ΣNm
sum of peak torques from all contractions during the fatigue protocol
REFERENCES
- 1.Lanza IR, Towse TF, Caldwell GE, Wigmore DM, Kent-Braun JA. Effects of age on human muscle torque, velocity, and power in two muscle groups. J Appl Physiol. 2003;95:2361–2369. doi: 10.1152/japplphysiol.00724.2002. [DOI] [PubMed] [Google Scholar]
- 2.Petrella J, Kim J, Tuggle SC, Hall S, Bamman M. Age differences in knee extension power, contractile velocity, and fatigability. J Appl Physiol. 2005;98:211–220. doi: 10.1152/japplphysiol.00294.2004. [DOI] [PubMed] [Google Scholar]
- 3.van Roie E, Verschueren S, Boonen S, Bogaerts A, Kennis E, Coudyzer W, et al. Force–velocity characteristics of the knee extensors: an indication of the risk for physical frailty in elderly women. Arch Phys Med Rehabil. 2011;92:1827–1832. doi: 10.1016/j.apmr.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol Series A Biol Sci Med Sci. 1997;52:B267–B276. doi: 10.1093/gerona/52a.5.b267. [DOI] [PubMed] [Google Scholar]
- 5.Hortob agyi T, Zheng D, Weidner M, Lambert NJ, Westbrook S, Houmard JA. The influence of aging on muscle strength and muscle fiber characteristics with special reference to eccentric strength. J Gerontol Series A Biol Sci Med Sci. 1995;50:B399–B406. doi: 10.1093/gerona/50a.6.b399. [DOI] [PubMed] [Google Scholar]
- 6.Pousson M, Lepers R, van Hoecke J. Changes in isokinetic torque and muscular activity of elbow flexors muscles with age. Exp Gerontol. 2001;36:1687–1698. doi: 10.1016/s0531-5565(01)00143-7. [DOI] [PubMed] [Google Scholar]
- 7.Thom J, Morse C, Birch K, Narici M. Influence of muscle architecture on the torque and power–velocity characteristics of young and elderly men. Eur J Appl Physiol. 2007;100:613–619. doi: 10.1007/s00421-007-0481-0. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki T, Bean JF, Fielding RA. Muscle power of the ankle flexors predicts functional performance in community-dwelling older women. J Am Geriatr Soc. 2001;49:1161–1167. doi: 10.1046/j.1532-5415.2001.49232.x. [DOI] [PubMed] [Google Scholar]
- 9.Bean J, Leveille S, Kiely D, Bandinelli S, Guralnik J, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol Series A Biol Sci Med Sci. 2003;58:728–733. doi: 10.1093/gerona/58.8.m728. [DOI] [PubMed] [Google Scholar]
- 10.Cuoco A, Callahan D, Sayers S, Frontera W, Bean J, Fielding R. Impact of muscle power and force on gait speed in disabled older men and women. J Gerontol. 2004;59:1200–1206. doi: 10.1093/gerona/59.11.1200. [DOI] [PubMed] [Google Scholar]
- 11.Christie A, Snook E, Kent Braun J. Systematic review and meta-analysis of skeletal muscle fatigue in old age. Med Sci Sports Exerc. 2011;43:568–577. doi: 10.1249/MSS.0b013e3181f9b1c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callahan D, Kent Braun J. Effect of old age on human skeletal muscle force-velocity and fatigue properties. J Appl Physiol. 2011;111:1345–1352. doi: 10.1152/japplphysiol.00367.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalton B, Power G, Vandervoort A, Rice C. The age-related slowing of voluntary shortening velocity exacerbates power loss during repeated fast knee extensions. Exp Gerontol. 2012;47:85–92. doi: 10.1016/j.exger.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Janssen I, Heymsfield S, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 15.Leveille SG, Resnick HE, Balfour J. Gender differences in disability: evidence and underlying reasons. Aging. 2000;12:106–112. doi: 10.1007/BF03339897. [DOI] [PubMed] [Google Scholar]
- 16.Ploutz Snyder L, Manini T, Ploutz Snyder R, Wolf D. Functionally relevant thresholds of quadriceps femoris strength. J Gerontol. 2002;57:B144–B152. doi: 10.1093/gerona/57.4.b144. [DOI] [PubMed] [Google Scholar]
- 17.Hortob agyi T, Mizelle C, Beam S, DeVita P. Old adults perform activities of daily living near their maximal capabilities. J Gerontol. 2003;58:M453–M460. doi: 10.1093/gerona/58.5.m453. [DOI] [PubMed] [Google Scholar]
- 18.Tracy B, Enoka R. Older adults are less steady during submaximal isometric contractions with the knee extensor muscles. J Appl Physiol. 2002;92:1004–1012. doi: 10.1152/japplphysiol.00954.2001. [DOI] [PubMed] [Google Scholar]
- 19.Marmon A, Pascoe M, Schwartz R, Enoka R. Associations among strength, steadiness, and hand function across the adult life span. Med Sci Sports Exerc. 2011;43:560–567. doi: 10.1249/MSS.0b013e3181f3f3ab. [DOI] [PubMed] [Google Scholar]
- 20.Kornatz K, Christou E, Enoka R. Practice reduces motor unit discharge variability in a hand muscle and improves manual dexterity in old adults. J Appl Physiol. 2005;98:2072–2080. doi: 10.1152/japplphysiol.01149.2004. [DOI] [PubMed] [Google Scholar]
- 21.Marmon A, Gould J, Enoka R. Practicing a functional task improves steadiness with hand muscles in older adults. Med Sci Sports Exerc. 2011;43:1531–1537. doi: 10.1249/MSS.0b013e3182100439. [DOI] [PubMed] [Google Scholar]
- 22.Madigan M. Age-related differences in muscle power during single-step balance recovery. J Appl Biomech. 2006;22:186–193. doi: 10.1123/jab.22.3.186. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 24.Callahan D, Foulis S, Kent-Braun J. Age-related fatigue resistance in the knee extensor muscles is specific to contraction mode. Muscle Nerve. 2009;39:692–702. doi: 10.1002/mus.21278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Csuka M, McCarty DJ. Simple method for measurement of lower extremity muscle strength. Am J Med. 1985;78:77–81. doi: 10.1016/0002-9343(85)90465-6. [DOI] [PubMed] [Google Scholar]
- 26.Baudry S, Klass M, Pasquet B, Duchateau J. Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. Eur J Appl Physiol. 2007;100:515–525. doi: 10.1007/s00421-006-0206-9. [DOI] [PubMed] [Google Scholar]
- 27.Kotake T, Dohi N, Kajiwara T, Sumi N, Koyama Y, Miura T. An analysis of sit-to-stand movements. Arch Phys Med Rehabil. 1993;74:1095–1099. doi: 10.1016/0003-9993(93)90068-l. [DOI] [PubMed] [Google Scholar]
- 28.Reeves ND, Spanjaard M, Mohagheghi AA, Baltzopoulos V, Maganaris CN. Older adults employ alternative strategies to operate within their maximum capabilities when ascending stairs. J Electromyogr Kinesiol. 2009;19:e57–e68. doi: 10.1016/j.jelekin.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 29.DeVita P, Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. J Appl Physiol. 2000;88:1804–1811. doi: 10.1152/jappl.2000.88.5.1804. [DOI] [PubMed] [Google Scholar]
- 30.Buchner DM, Larson EB, Wagner EH, Koepsell TD, de Lateur BJ. Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing. 1996;25:386–391. doi: 10.1093/ageing/25.5.386. [DOI] [PubMed] [Google Scholar]
- 31.Lindström B, Lexell J, Gerdle B, Downham D. Skeletal muscle fatigue and endurance in young and old men and women. J Gerontol. 1997;52:B59–B66. doi: 10.1093/gerona/52a.1.b59. [DOI] [PubMed] [Google Scholar]
- 32.Ng AV, Kent Braun JA. Slowed muscle contractile properties are not associated with a decreased EMG/force relationship in older humans. J Gerontol A Biol Sci Med Sci. 1999;54:B452–B458. doi: 10.1093/gerona/54.10.b452. [DOI] [PubMed] [Google Scholar]
- 33.Larsson L, Li X, Frontera WR. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol. 1997;272:C638–649. doi: 10.1152/ajpcell.1997.272.2.C638. [DOI] [PubMed] [Google Scholar]
- 34.Vaillancourt D, Larsson L, Newell K. Effects of aging on force variability, single motor unit discharge patterns, and the structure of 10, 20, and 40 Hz EMG activity. Neurobiol Aging. 2003;24:25–35. doi: 10.1016/s0197-4580(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 35.Christou E, Shinohara M, Enoka R. Fluctuations in acceleration during voluntary contractions lead to greater impairment of movement accuracy in old adults. J Appl Physiol. 2003;95:373–384. doi: 10.1152/japplphysiol.00060.2003. [DOI] [PubMed] [Google Scholar]
- 36.Galganski ME, Fuglevand AJ, Enoka RM. Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. J Neurophysiol. 1993;69:2108–2115. doi: 10.1152/jn.1993.69.6.2108. [DOI] [PubMed] [Google Scholar]
- 37.Bieryla K, Anderson D, Madigan M. Estimations of relative effort during sit-to-stand increase when accounting for variations in maximum voluntary torque with joint angle and angular velocity. J Electromyogr Kinesiol. 2009;19:139–144. doi: 10.1016/j.jelekin.2007.07.002. [DOI] [PubMed] [Google Scholar]