Summary
Neisseria gonorrhoeae regulates the expression of epithelial cell genes, activates cytoprotective pathways in the infected cell and protects it from apoptosis. Many of these responses are enhanced by the Type IV pilus (Tfp). We tested the hypothesis that N. gonorrhoeae modulates the innate immune response by inducing expression of ATF3, a transcription factor that negatively regulates the expression of many cytokine genes. We further determined whether Tfp are involved in these events. We found that N. gonorrhoeae induces ATF3 expression in mucosal epithelial cells through activation of mitogen activated protein kinases. Maximal ATF3 expression requires Tfp retraction. Knocking down endogenous levels of ATF3 results in higher levels of IL-6 transcript. Our findings strongly suggest that ATF3 is involved in suppressing cytokine expression during gonococcal infection. We propose a model for the role of ATF3 in the context of N. gonorrhoeae infection.
Introduction
Neisseria gonorrhoeae is a human-specific pathogen that infects over 106 million people annually (WHO, 2012). While gonorrhea is treatable with antibiotics, a large number of infections are asymptomatic, and therefore go untreated. Asymptomatic infections are of particular concern for women; over 60% of women infected with N. gonorrhoeae do not show obvious symptoms of disease (Gerbase et al., 2006). Left untreated, gonorrhea can lead to pelvic inflammatory disease, ectopic pregnancy and infertility (Trigg et al., 2008). The high incidence of asymptomatic infections indicates that N. gonorrhoeae has developed strategies to minimize the host response to infection.
The primary site of gonococcal infection is the mucosal epithelium (Janda et al., 2003). Although N. gonorrhoeae is most frequently isolated from the urogenital tract, it will also readily infect other sites, including rectal and pharyngeal epithelia (Hook, 1999). Attachment to the epithelial cell is mediated by fimbriate structures on the bacterial surface called Type IV pili (Tfp) (McGee et al., 1981, Merz et al., 1996, Mosleh et al., 1997, Swanson, 1973).
Tfp mediate additional interactions between gonococci and their environment, including DNA uptake; twitching motility (crawling of bacteria over the cell surface) and aggregation into microcolonies; and activation of signaling pathways and cortical plaque formation in the infected cell. These interactions require or are enhanced by Tfp retraction. This process, which requires the ATPase PilT, exerts mechanical forces in the nanonewton range on the attached surface (Biais et al., 2008, Maier et al., 2004, Merz et al., 2000, Wolfgang et al., 2000).
Tfp retraction-induced host cell signaling has a number of consequences for the infected cell. Activation of the presenilin/γ-secretase complex triggers proteolytic cleavage of CD46, a regulator of innate and adaptive immunity (Weyand et al., 2010). Pilus retraction also enhances signaling of the mitogen activated protein kinases (MAPKs) and phosphoinositide-3 kinase pathways (Howie et al., 2005, Lee et al., 2005). Activation of these signaling cascades may play an important role in protecting host cells from the stress of infection. Both Tfp retraction and artificial pulling on the plasma membrane can activate cytoprotective pathways that protect epithelial cells from apoptosis (Higashi et al., 2007, Howie et al., 2005); this protection is mediated in part by signaling through the MAPK ERK (Howie et al., 2008).
Tfp retraction also influences host gene expression. Microarray analysis has identified over 300 human epithelial cell genes whose expression is differentially regulated in response to N. gonorrhoeae infection (Howie et al., 2005). For a subset of these genes, the degree of up- or downregulation is further enhanced by Tfp retraction. Upregulation of some retraction-enhanced host genes can be blocked by inhibiting MAPK signaling (Howie et al., 2005), thus linking a retraction-enhanced signaling pathway to gene expression. Many retraction-enhanced host genes encode proteins involved in cell stress and immune responses. Intriguingly, one of these genes is activating transcription factor 3 (ATF3), which encodes a 23 kDa protein involved in transcriptional regulation of the immune response.
ATF3 belongs to the activating transcription factor/cyclic AMP response element-binding (ATF/CREB) family of transcriptional regulators. ATF/CREB family members respond to a variety of environmental stimuli, but share the common function of preserving cellular homeostasis via transcriptional regulation (Persengiev et al., 2003). Like other ATF/CREB proteins, ATF3 contains a basic-region leucine zipper domain that allows it to form dimers and bind DNA (Hai et al., 1991, Hai et al., 1989). While most ATF/CREB proteins are involved in activating transcription, ATF3 acts as a transcriptional repressor.
ATF3 is an important regulator of inflammation. In a mouse model of allergic inflammation, ATF3 expression is upregulated in response to T cell activation (Gilchrist et al., 2008). ATF3 then represses the transcription of several allergen-induced cytokines. ATF3 is also expressed by natural killer cells during murine cytomegalovirus (MCMV) infection (Rosenberger et al., 2008). In this model, ATF3 suppresses production of IFN-γ, an important cytokine for clearing viral infection. Thus, ATF3 expression actually promotes MCMV persistence by preventing the infection from being eliminated. ATF3 also negatively regulates innate immune responses. Binding of different microbe-associated molecular patterns (MAMPs) to their respective Toll-like receptors (TLRs) leads to elevated levels of ATF3 in bone marrow-derived macrophages (Gilchrist et al., 2006, Khuu et al., 2007, Whitmore et al., 2007). Furthermore, TLR activation induces elevated levels of CCL4, IL-6, IL-12, and TNFα in atf3-deficient macrophages, indicating that ATF3 is required to repress transcription of these pro-inflammatory cytokines. Collectively, these studies demonstrate that ATF3 is an important regulator of cytokine expression in immune cells.
N. gonorrhoeae infection upregulates the expression of several cytokines in mucosal epithelial tissues (Fichorova et al., 2001, Hedges et al., 1998, McGee et al., 1992, Naumann et al., 1997, Ramsey et al., 1995). Of these, IL-6 and TNFα are known to be regulated by ATF3 (Gilchrist et al., 2006, Whitmore et al., 2007). However, the role of ATF3 in regulating the immune response of N. gonorrhoeae-infected epithelial cells has not been explored. We hypothesize that ATF3 is upregulated in mucosal epithelial cells during infection by piliated N. gonorrhoeae and that ATF3 helps dampen the host response to infection by inhibiting the transcription of pro-inflammatory cytokines. In this paper, we demonstrate that N. gonorrhoeae infection upregulates ATF3 transcript and protein levels. We further show that ATF3 upregulation is enhanced by Tfp retraction, and requires signaling via MAPK pathways. Finally, we present evidence that ATF3 regulates expression of the pro-inflammatory cytokine IL-6 during N. gonorrhoeae infection.
Results
ATF3 is upregulated during N. gonorrhoeae infection
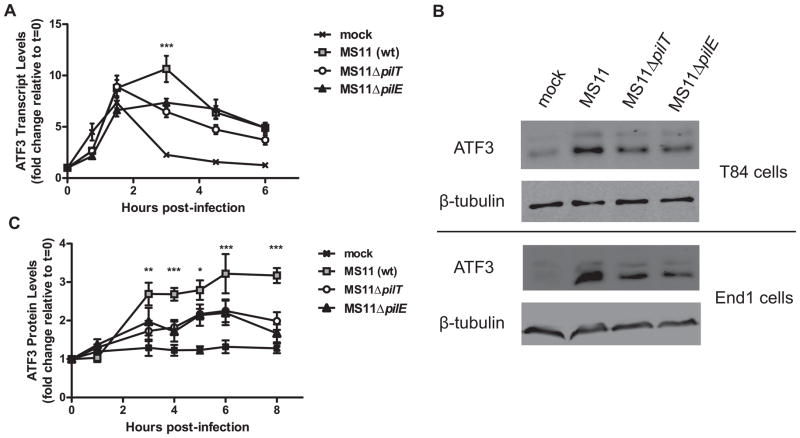
Microarray analysis by our lab suggests that the expression of a large number of epithelial cell genes is altered during N. gonorrhoeae infection of T84 human colorectal cells (Howie et al., 2005). Furthermore, this regulation is enhanced by Tfp retraction. One target identified by the previous study was the immunoregulatory factor ATF3. To verify these results, T84 cells were infected with the wild type (wt) N. gonorrhoeae strain MS11, and relative ATF3 transcript levels assessed by RT-PCR (Figure 1A). Mock-infection with medium alone induced a brief increase in ATF3 transcript levels that peaked at 1.5 hours post-infection and returned to baseline by 3 hours post-infection. Infection with MS11 significantly increased ATF3 expression compared to mock-infected cells. ATF3 levels peaked at 3 hours post-infection and remained elevated for the remainder of the time course (P<0.001 for 3, 4.5 and 6 hour time points).
Figure 1.
ATF3 is upregulated in response to N. gonorrhoeae infection and its expression is enhanced by Tfp retraction. T84 cells were infected with wt MS11, MS11ΔpilT, or MS11ΔpilE. (A) ATF3 transcript levels at 0.75, 1.5, 3, 4.5 and 6 hours post-infection were assessed by RT-PCR. Values are expressed as mean relative transcript levels, normalized to GAPDH and expressed relative to the 0 hour time point, which is set at 1 (±SEM, n=4). ***P<0.001 for MS11-infected samples vs. MS11ΔpilT and MS11ΔpilE. (B) Representative western blots of ATF3 protein levels in T84 cells (upper panel) or End1 cells (lower panel) infected with MS11, MS11ΔpilT, or MS11ΔpilE. Total β-tubulin was used as a loading control. (C) ATF3 protein levels over the course of infection. Samples were collected at the indicated time points, subjected to western blot, and relative ATF3 levels assessed by densitometry. Values represent mean ATF3 protein levels, normalized to β-tubulin and expressed relative to the 0 hour time point, which is set at 1 (±SEM, n=6). *P<0.05, **P<0.01, ***P<0.001 for MS11-infected samples vs. MS11ΔpilT and MS11ΔpilE.
To demonstrate that ATF3 protein levels were also increased during infection, western blots of T84 whole cell lysates were probed with an antibody specific for ATF3. A representative western blot is shown in Figure 1B; the two ATF3 isoforms migrated as a doublet (Miyazaki et al., 2009) and both were upregulated by infection. ATF3 protein levels were significantly higher in MS11-infected samples than mock-infected samples as early as 3 hours post-infection and remain elevated throughout the course of infection (Figure 1C, P<0.001 for 3 hours and greater post-infection). To confirm that this finding was not strain or cell line specific, we assayed multiple bacterial strains and epithelial cell lines for ATF3 protein levels. ATF3 expression was induced in T84 cells infected with N. gonorrhoeae strain FA1090 and Neisseria meningitidis strain 8013 (data not shown). ATF3 upregulation was also observed in MS11-infected End1 endocervical cells (Figure 1B), as well as nasopharyngeal (Detroit 562) and bronchial (16HBE14o-) epithelial cells (data not shown).
Tfp retraction enhances the expression of stress response genes that are upregulated by N. gonorrhoeae infection (Howie et al., 2005). To determine whether pilus retraction plays a role in ATF3 expression, we compared ATF3 levels in cells infected with wt MS11; MS11ΔpilT, a piliated but retraction-deficient mutant (Friedrich et al., 2007); and MS11ΔpilE, which does not produce Tfp (Merz et al., 1996) (Figure 1A). Beginning at 3 hours post-infection, ATF3 transcript levels were elevated in ΔpilT- and ΔpilE-infected cells compared to mock-infected cells (P<0.001). However, ATF3 transcript levels were significantly lower in mutant- than wt-infected cells. Similarly, ATF3 protein levels in mutant-infected cells were intermediate between uninfected and wt-infected cells (Figure 1B and 1C). Thus, while ATF3 upregulation can occur in the absence of Tfp, maximal upregulation requires Tfp expression and retraction. Collectively, these data demonstrate that ATF3 is upregulated in response to Neisseria infection in a variety of human epithelial cell types, and that ATF3 upregulation is enhanced by Tfp retraction.
ATF3 expression is induced by N. gonorrhoeae outer membrane protein(s)
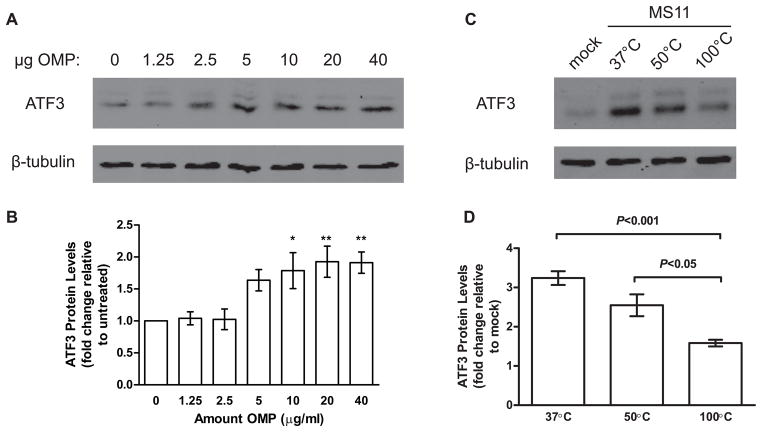
Purified Escherichia coli lipopolysaccharide (LPS) is sufficient to induce ATF3 expression in macrophages (Gilchrist et al., 2006, Khuu et al., 2007, Whitmore et al., 2007), suggesting that live bacteria are not essential for ATF3 upregulation. We therefore examined whether live bacteria are necessary to upregulate ATF3 in epithelial cells. T84 cells were treated with increasing amounts of a crude MS11 outer membrane preparation (OMP), which contains LPS as well as membrane proteins, and ATF3 protein levels assessed by western blotting. As little as 5 μg/ml of OMP was sufficient to induce ATF3 upregulation in epithelial cells (Figure 2A and 2B).
Figure 2.
N. gonorrhoeae outer membrane protein(s) induce ATF3 upregulation. T84 cells were treated with increasing amounts of crude OMP isolated from MS11 and assessed for ATF3 protein levels at 4 hours post-treatment. (A) Representative western blot of ATF3 protein levels in T84 cells treating with various amounts of OMP. Total β-tubulin was used as a loading control. (B) Densitometry values from (A) are expressed as mean ATF3 protein levels normalized to β-tubulin, with values from untreated cells set at 1 (±SEM, n=4). *P<0.05, **P<0.01 vs. untreated samples. (C) ATF3-inducing factor is heat sensitive. MS11 was incubated at 37°C, 50°C, or 100°C for 30 minutes prior to infection of T84 cells. Samples were collected at 3 hours post-infection and protein levels assessed by western blot. (D) Densitometry values from (C) represent mean ATF3 proteins levels normalized to β-tubulin and relative to mock-infected cells, which are set at 1 (±SEM, n=4).
In macrophages, ATF3 expression can be activated by multiple TLRs, including TLR4 in response to LPS treatment, and TLR2 in response to zymosan (Gilchrist et al., 2006, Whitmore et al., 2007). As our cell lines express little to no TLR4 (Abreu et al., 2001, Fichorova et al., 2002, Rydberg et al., 2009), LPS is unlikely to be important for ATF3 upregulation during gonococcal infection. However, at least two neisserial outer membrane proteins, PorB and Lip, have been shown to induce TLR2-mediated signaling (Fisette et al., 2003, Massari et al., 2002). To determine whether gonococcal proteins are important for upregulating ATF3 expression, we infected epithelial cells with wt MS11 that were heated at 37°C, 50°C (a temperature sufficient to kill bacteria, but maintain the structure of most proteins), or 100°C (which will denature proteins but not glycans such as LPS). ATF3 protein levels were still elevated in cells infected with N. gonorrhoeae heated at 50°C (Figure 2C and 2D); this is consistent with the earlier finding that live bacteria are not required to upregulate ATF3. In contrast, cells infected with 100°C-treated bacteria had significantly lower levels of ATF3 compared to cells infected with bacteria that had undergone other temperature treatments. As heating at 100°C is sufficient to denature proteins, this finding suggests that gonococcal protein may be necessary to induce ATF3 upregulation. This interpretation is supported by experiments with live and heat-killed (50°C) N. gonorrhoeae pre-treated with proteinase K (PK) to degrade surface proteins. T84 cells infected with live or heat-killed PK-treated bacteria had reduced levels of ATF3 when compared to their respective controls (Figure S1). Taken together, these results indicate that N. gonorrhoeae outer membrane proteins are responsible for inducing a significant fraction of the ATF3 that is expressed upon infection.
Tfp are a major component of gonococcal outer membranes. To test whether Tfp are required for upregulating ATF3, we incubated T84 cells with crude OMPs from the ΔpilE mutant, which are devoid of pili. ΔpilE OMPs were still able to increase ATF3 protein expression (Figure S2A). Similarly, ATF3 levels were higher in cells inoculated with heat-killed ΔpilE compared to mock-infected cells (Figure S2B and C). Thus, N. gonorrhoeae upregulates ATF3 through other outer membrane proteins in addition to Tfp.
MAPK signaling is required for N. gonorrhoeae-induced ATF3 expression
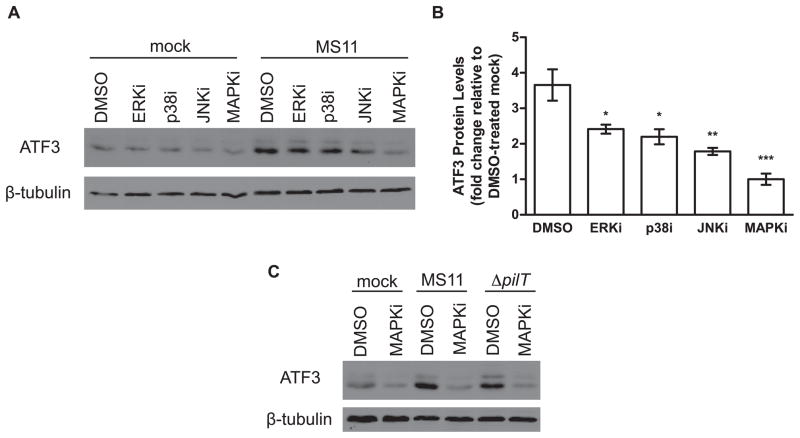
MAPKs are a family of serine/threonine kinases that are activated by environmental stimuli, including growth-promoting mitogens, environmental stresses such as heat shock and radiation, and pro-inflammatory stimuli such as cytokines and microbial products (Ashwell, 2006, Hazzalin et al., 2002). The three major classes of MAPK, ERK, p38, and JNK, help maintain cellular homeostasis by upregulating expression of immediate early stress response genes, including ATF3 (Hartman et al., 2004, Lu et al., 2007, Xie et al., 2005). Both N. gonorrhoeae and N. meningitidis activate MAPK signaling (Naumann et al., 1998, Sokolova et al., 2004); in the case of N. gonorrhoeae this activation is enhanced by Tfp retraction (Howie et al., 2005). Several neisserial outer membrane proteins have also been shown to activate MAPKs (Hauck et al., 1998, MacLeod et al., 2008, Sokolova et al., 2004). Based on these observations, we hypothesize that MAPKs act upstream of N. gonorrhoeae-induced ATF3 expression. We therefore determined whether inhibitors of ERK, p38, and JNK could block ATF3 expression during infection. T84 cells were pre-incubated with inhibitors U0126 (ERKi), 203580 (p38i) and SP600125 (JNKi) individually or together (MAPKi), then infected with MS11. We have previously shown that these inhibitors block N. gonorrhoeae-induced MAPK signaling in T84 cells (Howie et al., 2005). Each inhibitor blocked ATF3 protein expression to a significant but partial extent (Figure 3A and 3B). Together, the three inhibitors completely blocked ATF3 upregulation. MAPKi inhibition also blocked ATF3 upregulation in ΔpilT-infected cells (Figure 3C). Thus, MAPK signaling is required to induce both Tfp retraction-dependent and retraction-independent ATF3 expression.
Figure 3.
MAP kinase signaling is required for ATF3 induction. T84 cells were incubated with the chemical inhibitors U0126, 203580, and SP600125 (ERKi, p38i, and JNKi, respectively), individually or pooled (MAPKi) at a final concentration of 10 μM for each inhibitor for 1 hour prior to infection with MS11 or MS11ΔpilT for 4 hours. (A) Representative western blot of ATF3 protein levels in T84 cells infected with MS11 in the presence and absence of MAPK inhibitors. Total β-tubulin was used as a loading control. (B) Densitometry values from (A) are expressed as mean ATF3 protein levels in infected samples, normalized to β-tubulin and relative to mock-infected/DMSO-treated samples, which are set at 1 (±SEM, n=4). *P<0.05, **P<0.01, ***P<0.001 vs. DMSO-treated samples. (C) Representative western blot of ATF3 protein levels in T84 cells infected with MS11ΔpilT in the presence or absence of MAPKi, with β-tubulin serving as a loading control.
Protein kinase R (PKR), another stress response kinase, also regulates ATF3 expression (Garcia et al., 2006). Unfortunately, we were unable to determine the role of PKR in our system. Infection with MS11 induced PKR phosphorylation at Thr451 (Figure S3A), a step that is essential for PKR activity (Romano et al., 1998). Treatment with a chemical PKR inhibitor blocked infection-induced ATF3 expression. However, knocking down PKR levels with siRNA had no effect on ATF3 expression during infection (Figure S3B). These results suggest that the activity of the PKR inhibitor on ATF3 expression is likely due to an off-target effect.
ATF3 negatively regulates IL-6 expression during N. gonorrhoeae infection
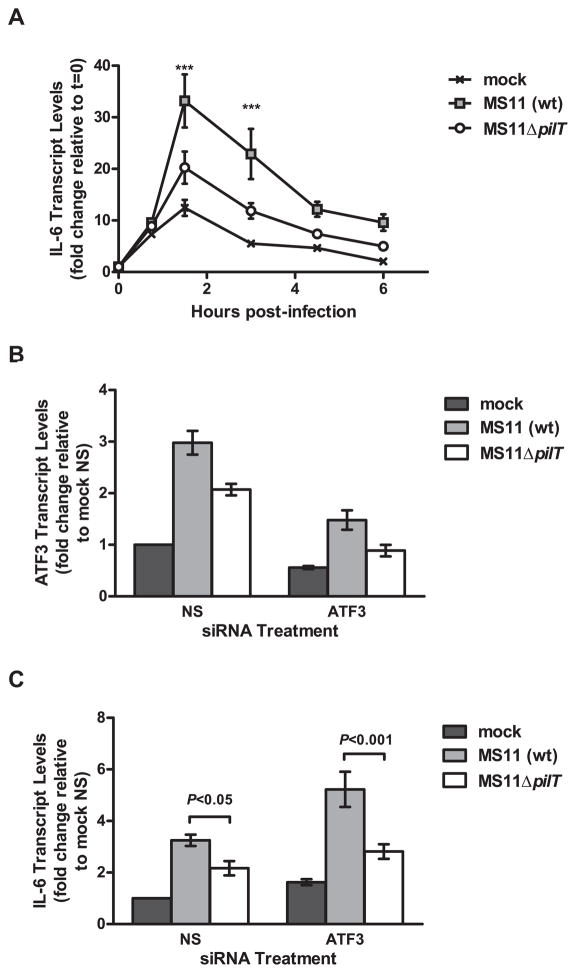
Given the role of ATF3 in negatively regulating cytokine expression by immune cells, we hypothesize that ATF3 may function similarly in mucosal epithelial cells. To test this hypothesis, we knocked down ATF3 expression in T84 cells using siRNA, then measured the transcript levels of TNFα and IL-6, two cytokines known to be induced by N. gonorrhoeae and regulated by ATF3. T84 cells were nucleofected with an ATF3-specific siRNA or a nonsense (NS) siRNA and infected with MS11 at 72 hours post-nucleofection. ATF3 expression was successfully knocked down by ATF3 siRNA, but not by NS siRNA (Figure 4A and 4B). Infection of ATF3 siRNA-treated cells resulted in higher levels of IL-6 transcript than NS siRNA-treated cells (Figure 4C). Knocking down ATF3 with siRNA did not alter the ability of N. gonorrhoeae to induce TNFα. These results strongly suggest that ATF3 is involved in suppressing infection-induced expression of IL-6.
Figure 4.
ATF3 knockdown results in higher levels of IL-6 transcript during infection. T84 cells were nucleofected with nonsense (NS) or ATF3-specific (ATF3) siRNA. At 72 hours post-infection cells were infected with MS11 for 4 hours. (A) ATF3 transcript levels assessed by RT-PCR. Results are expressed as the mean fold-change in transcript levels relative to mock-infected cells treated with nonsense siRNA (mock NS) which is set at 1 (±SEM, n=5). (B) Representative western blot of ATF3 protein levels in siRNA-treated cells. (C) IL-6 and TNFα transcript levels from infected, siRNA-treated cells were assessed by RT-PCR (±SEM, n=5). Results are expressed as described in (A).
Tfp retraction enhances IL-6 expression
The pilT mutant, which produces nonretractible Tfp, induces lower levels of ATF3 than its wt parent (Figure 1). This finding suggests that the pilT mutant is also less able to induce IL-6 expression. We therefore compared IL-6 transcript levels in T84 cells infected with MS11 and MS11ΔpilT. For these experiments we employed the same samples that were used to compare ATF3 transcript levels (see Figure 1). Mock-infection with medium alone induced a brief increase in IL-6 expression (Figure 5A). Compared to mock-infected cells, both wt and mutant strains induced significant IL-6 upregulation, with transcript levels peaking at 1.5 hours post-infection (P<0.01). However, wt-infected cells had significantly more IL-6 transcript than ΔpilT-infected at 1.5 and 3 hours post-infection. These results indicate that IL-6 expression is induced by N. gonorrhoeae and enhanced by Tfp retraction.
Figure 5.
Tfp retraction enhances IL-6 transcription independent of ATF3 expression. (A) T84 cells were infected with MS11 or MS11ΔpilT and assessed for IL-6 transcript levels at 0.75, 1.5, 3, 4.5 and 6 hours post-infection. Values are expressed as mean relative transcript levels, normalized to GAPDH and expressed relative to the 0 hour time point, which is set at 1 (±SEM, n=4). (B and C) ATF3 (B) and IL-6 (C) transcript levels in cells treated with nonsense siRNA (NS) or ATF3-specific siRNA (ATF3) prior to infection with MS11 or MS11ΔpilT for 4 hours. Results are expressed as the mean fold-change in transcript levels relative to mock-infected cells treated with nonsense siRNA (mock NS) which is set at 1 (±SEM, n=4).
Our results show that wt gonococci induce higher levels of ATF3 (Figure 1) and IL-6 (Figure 5A) than the pilT mutant. These findings are surprising, given the negative regulatory role of ATF3 in IL-6 transcription (Figure 4). We hypothesize that the reduced ability of the pilT mutant to induce IL-6 is independent of ATF3 expression. To test this hypothesis, we knocked down ATF3 expression using siRNA, infected cells with MS11 or MS11ΔpilT and compared IL-6 transcript levels. ATF3 knockdown was confirmed by RT-PCR (Figure 5B). Compared to wt, the pilT mutant induced lower levels of IL-6 transcript in cells treated with NS as well as ATF3-specific siRNA (Figure 5C). These results indicate that the effects of pilus retraction on IL-6 transcription are not influenced by ATF3.
Discussion
We have provided evidence that N. gonorrhoeae upregulates expression of ATF3, a member of the ATF/CREB family of transcriptional regulators, through the ERK, p38, and JNK MAPK cascades (Figures 1 through 3). Further, we identified a role for ATF3 in N. gonorrhoeae infection; specifically, it negatively regulates expression of the pro-inflammatory cytokine IL-6 (Figure 4). The role of ATF3 in regulating the innate response to infection is well characterized in professional immune cells (Thompson et al., 2009) but has not been studied in mucosal epithelial cells, which represent the first line of defense against microbes. To our knowledge, this study is the first to demonstrate a role for ATF3 in regulating epithelial cell responses to infection.
Tfp retraction is necessary to induce maximal expression of ATF3 during N. gonorrhoeae infection (Figure 1). ATF3 expression can also be induced by crude OMPs (Figure 2) and by infection with the non-piliated, relatively non-adherent MS11ΔpilE mutant (Figure 1). These results suggest that N. gonorrhoeae induces ATF3 through both Tfp-dependent and -independent pathways. In this model, outer membrane components other than Tfp initially induce ATF3 expression at low levels, but Tfp retraction is required for maximum ATF3 expression. Thus ATF3 belongs to the group of retraction-enhanced genes that promote a cytoprotective state in the epithelial cell.
One potential Tfp-independent mechanism for ATF3 induction is Toll receptor signaling. Purified bacterial components such as LPS can induce ATF3 expression by activating TLR pathways (Gilchrist et al., 2006, Khuu et al., 2007, Whitmore et al., 2007). Moreover, Neisseria outer membrane proteins are known to activate TLR2 signaling (Fisette et al., 2003, Massari et al., 2002), Thus, the Tfp-independent gonococcal outer membrane proteins observed to induce ATF3 (Figures 2 and S2) may exert their effect through TLR signaling. Future research will define the role of TLR signaling in ATF3 upregulation during gonococcal infection.
MAPK signaling is required for N. gonorrhoeae upregulation of ATF3 (Figure 3). Previous work has shown that Neisseria infection activates MAPK signaling (Howie et al., 2005, Naumann et al., 1998, Sokolova et al., 2004). Importantly, activation of all three MAPK pathways is enhanced by Tfp retraction, and by mechanical forces generated artificially on the epithelial cell membrane (Howie et al., 2005). We demonstrated that ATF3 levels are significantly higher in wt-infected cells compared to ΔpilT-infected cells (Figure 1), suggesting that Tfp retraction augments ATF3 upregulation. Consistent with this notion, mechanical forces can induce ATF3 expression during ventilation-induced lung injury (Akram et al., 2010). Enhanced ATF3 expression in N. gonorrhoeae-infected cells may therefore result from increased MAPK activation by mechanical forces exerted on the epithelial cell by Tfp retraction.
Complete inhibition of ATF3 expression requires blocking activation of all three MAPKs. However, small reductions in ATF3 expression were observed in cells treated separately with ERK, p38, or JNK inhibitors, suggesting that all three kinases play a role in ATF3 upregulation. Interestingly, activation of MAPKs, particularly p38 and JNK, is also important for upregulation of several pro-inflammatory cytokines, including IL-6 (Liu et al., 2007). Indeed, p38 activation is required for IL-6 expression during N. meningitidis infection (Sokolova et al., 2004). Given the temporal differences in the expression of ATF3 and IL-6 (compare Figure 1A and Figure 5A), it is tempting to speculate that these differences are the result of differential MAPK activation. This notion is supported by the observation that temporal activation of p38 and JNK during Legionella pneumophila infection induces expression of IL-1β (Shin et al., 2008), a target of ATF3 regulation (Zmuda et al., 2010).
Knocking down endogenous ATF3 levels resulted in higher IL-6 transcript levels in wt-infected cells (Figure 4), suggesting that ATF3 is involved in suppressing IL-6 expression during infection. Inhibition of IL-6 transcription during N. gonorrhoeae infection could occur via multiple mechanisms. In LPS-treated macrophages, ATF3 recruits histone deacetylase 1 to the IL-6 promoter and this recruitment is associated with increased deacetylase activity (Gilchrist et al., 2006). Histone deacetylation is known to alter chromatin structure, which reduces access of RNA polymerase and represses transcription (Medzhitov et al., 2009). Indeed, ovalbumin treatment induces higher levels of histone H4 acetylation at the IL-4, IL-5, and IL-13 promoters in atf3-deficient T cells compared to wild type T cells (Gilchrist et al., 2008). Thus, one means by which ATF3 represses IL-6 expression during N. gonorrhoeae infection may be to promote a less accessible chromatin structure via histone deacetylation. Alternatively, ATF3 may regulate IL-6 expression indirectly. For example, in mouse macrophages, ATF3 inhibits expression of the transcription factor CEBPδ, which is a positive regulator of IL-6 transcription (Litvak et al., 2009). These two mechanisms are not mutually exclusive; ATF3 inhibition of IL-6 during N. gonorrhoeae infection may result from a combination of direct and indirect mechanisms.
ATF3 binding sites are present in the promoters of over 1000 genes (Gilchrist et al., 2008, Tanaka et al., 2011). At least 30 of these sites have been verified as targets of ATF3 regulation (Hai et al., 2010, Thompson et al., 2009). It is therefore reasonable to hypothesize that ATF3 regulates the expression of additional genes during gonococcal infection. Among the genes known to be regulated by ATF3 in epithelial cells, a few stand out as possible candidates. In fibroblasts, ATF3 inhibits the expression of cyclin D1, which regulates progression of the cell cycle from G1 to S phase (Lu et al., 2006). N. gonorrhoeae causes G1 arrest in endocervical cells by downregulating cyclin D1. (Jones et al., 2007). Thus, it is possible that ATF3 plays a role in these events through its regulatory effects on cyclin D1. In breast cancer cells, ATF3 induces expression of FN-1, which encodes the extracellular matrix glycoprotein fibronectin (Yin et al., 2008). Fibronectin serves as a receptor for the gonococcal outer membrane protein OpaA, and is required for OpaA-mediated invasion of epithelial cells (van Putten et al., 1998). These findings suggest a scenario where N. gonorrhoeae upregulation of ATF3 leads to production of fibronectin, which in turn facilitates OpaA-mediated gonococcal binding and invasion of the epithelial cell. ATF3 also suppresses expression of Id1 (Kang et al., 2003), a transcription factor whose expression may be upregulated by gonococcal infection in a retraction-enhanced manner (Howie et al., 2005). Thus Id1 represents another potential target for ATF3 regulation in N. gonorrhoeae-infected cells. Finally, it should be noted that ATF3 regulates the expression of multiple cytokines in leukocytes (Hai et al., 2010, Thompson et al., 2009). Given that N. gonorrhoeae interacts with leukocytes during the course of infection, it is possible that the bacterium may regulate cytokine expression in this cell type via ATF3.
In contrast to IL-6, knocking down ATF3 levels did not alter N. gonorrhoeae-induced expression of TNFα. This finding may be specific to epithelial cells. While ATF3 has been shown to suppress expression of IL-6 in lung tissue (Akram et al., 2010), ATF3 regulation of TNFα expression has only been observed in white blood cells and pancreatic cells (Hai et al., 2010).
Wt bacteria induced significantly higher levels of IL-6 transcript than the pilT mutant at early time points (Figure 5). Previous reports have also shown that Tfp retraction exerts its effect on cytokine expression early in infection (Dietrich et al., 2011, Howie et al., 2005). Furthermore, Tfp retraction enhances activation of NFκB p65 (Dietrich et al., 2011), a transcription factor that upregulates the expression of many pro-inflammatory cytokine genes during gonococcal infection, including IL-6 (Naumann et al., 1997). These observations appear to be inconsistent with the role of Tfp in promoting cytoprotection. However, the induction of specific cytokines may actually be beneficial for N. gonorrhoeae. For example, IL-6 secretion inhibits apoptosis of cervical cancer cells by upregulating expression of the pro-survival protein MCL-1 (Wei et al., 2001). Given that pilT mutants induce greater levels of apoptosis (Higashi et al., 2007, Howie et al., 2005, Howie et al., 2008), it is tempting to speculate that the cytoprotective effects of Tfp retraction are mediated in part through a transient upregulation of IL-6 expression. IL-6 levels peaks at 1.5 hours post-infection and drop considerably by 3 hours post-infection (Figure 5). This drop in IL-6 transcript is consistent with the increase in ATF3 protein at this time point (Figure 1C). It will be interesting to test whether IL-6 expression helps to protect infected cells from apoptosis in future experiments. IL-6 production is also important for the recruitment and differentiation of immature polymorphonuclear leukocytes (PMNs). Interestingly, N. gonorrhoeae can survive and replicate within PMNs (Criss et al., 2009, Witt et al., 1976); indeed, a purulent discharge containing PMNs and gonococci is a hallmark of gonorrhea infection. Given these observations, it has been proposed that N. gonorrhoeae may preferentially recruit a cell type (PMNs) in which it has adapted to survive (Feinen et al., 2010, Liu et al., 2011).
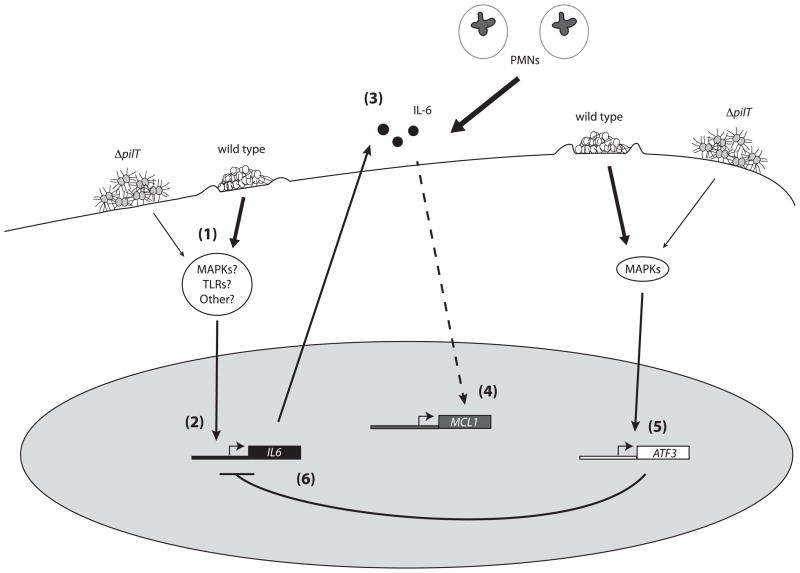
Based on data presented in this paper and previous findings, we propose a model to describe the role of ATF3 during N. gonorrhoeae infection of mucosal epithelial cells (Figure 6). N. gonorrhoeae infecton activates host cell signaling pathways that stimulate increased IL-6 expression. Secreted IL-6 can then recruit PMNs to the site of infection and possibly induce expression of the anti-apoptotic protein MCL-1. Infection also stimulates ATF3 expression in a MAPK-dependent manner and ATF3 expression is further enhanced by Tfp retraction. ATF3 protein can then suppress transcription of IL-6. Thus, early expression of IL-6 may help N. gonorrhoeae establish infection by preventing epithelial cell death and recruiting new cells (PMNs) to infect, while ATF3 expression at later time points prevents excessive inflammation that would be damaging to both host and microbe.
Figure 6.
Model for the role of ATF3 in N. gonorrhoeae infection of mucosal epithelial cells. (1) Infection with N. gonorrhoeae activates multiple host cell signaling cascades. Tfp retraction amplifies these signaling events. Activation of these pathways upregulates the expression of multiple host stress response genes. (2) IL-6 is upregulated early during infection and its expression is enhanced by Tfp retraction. (3) IL-6 is secreted and helps recruit PMNs to the site of infection. (4) IL-6 is proposed to induce expression of the pro-survival protein MCL-1, which would facilitate infection by preventing epithelial cell death. (5) Subsequently, ATF3 expression is upregulated by Tfp-dependent and independent activation of MAPK pathways. (6) Increased production of ATF3 protein leads to transcriptional repression of IL-6. Solid arrows represent events shown to occur in this paper or previous work. Dashed arrows represent hypothesized events. Thicker arrows represent enhanced signaling due to Tfp retraction.
Mucosal epithelial cells are the primary site of infection for many microbes. The ability of N. gonorrhoeae to induce a cytoprotective state in these cells is likely to be a key step in establishing asymptomatic infections. We have demonstrated that N. gonorrhoeae upregulates expression of the transcriptional regulator ATF3 during infection of epithelial cells; in turn, ATF3 inhibits production of the pro-inflammatory cytokine IL-6. These studies provide the basis for future work examining how repression of the host immune response can promote asymptomatic N. gonorrhoeae infections.
Materials and Methods
Reagents
The following antibodies were used in this study: rabbit anti-ATF3 polyclonal (C-19, Santa Cruz Biotechnology) mouse anti-β-tubulin monoclonal (E7, University of Iowa Developmental Studies Hybridoma Bank), and goat anti-rabbit and goat anti-mouse DyLight 800 fluorophore-conjugated secondary antibodies (Pierce). Assays on Demand TaqMan probes for GAPDH (Hs99999905_m1), ATF3 (Hs00231069_m1), IL-6 (Hs00174131_m1), and TNFα (Hs00174128_m1) were obtained from Applied Biosystems. ON-TARGETplus SMARTpool siRNAs for ATF3 (L-008663-00), PKR (L-003527-00), and nonsense control (D-001810-10) were purchased from Dharmacon RNAi Technologies. The MAP kinase inhibitors U0126 (ERK), 203580 (p38) and SP600125 (JNK) were purchased from Calbiochem and used as described previously (Howie et al., 2005) at a final concentration of 10 μM for each inhibitor. PKR inhibitor (52740) was obtained from Calbiochem and used at a final concentration of 5 μM.
Tissue culture
T84 colorectal epithelial cells (ATCC #CCL-248) were maintained in Dulbecco’s modified Eagle medium containing Ham’s F12 nutrient mixture and 5% heat-inactivated fetal bovine serum (FBS). End 1 endocervical cells (Fichorova et al., 1997) were grown in EpiLife supplemented with human keratinocyte growth supplement (Invitrogen) and 0.4 mM calcium chloride. Detroit 562 nasopharyngeal cells (ATCC #CCL-138) were grown in modified Eagle medium supplemented with non-essential amino acids and 10% FBS. The bronchial epithelial cell line 16HBE14o- (Cozens et al., 1994) was maintained in modified Eagle medium supplemented with 10% FBS and 2 mM L-glutamine, and grown in tissue culture dishes coated with a solution of LHC basal medium (Invitrogen) containing 0.1 mg/ml bovine serum albumin (BSA), 0.029 mg/ml bovine collagen (BD Biosciences), and 0.01 mg/ml human fibronectin (BD Biosciences). All cells were maintained at 37°C with 5% CO2 and passaged every three to five days. Cells were seeded into 6-well tissue culture plates and used at approximately 80–90% confluency, except for End1 cells, which were used at approximately 50% confluency.
Bacterial strains and infections
The N. gonorrhoeae strains MS11, MS11ΔpilT (Friedrich et al., 2007), MS11ΔpilE1::ErmΔpilE2 (Merz et al., 1996) and FA1090, as well as the N. meningitidis strain 8013 (Nassif et al., 1993) were used in this study. Strains were grown on gonococcal medium base (GCB) agar (Difco) with Kellogg’s supplements I and II at 37°C with 5% CO2. Piliation and Opa phenotypes were determined by observing colony morphology using light microscopy. Only Opa non-expressing bacteria were used. For infections, 18-hour-old bacteria were swabbed from GCB agar and resuspended in liquid GCB medium. Epithelial cells were infected with bacteria at a multiplicity of infection (MOI) of 25 (End1 cells) or 50 (all other cell types), or mock-infected with GCB liquid medium.
Crude outer membrane preparations
N. gonorrhoeae outer membrane preparations (OMPs) were prepared as previously described (Ayala et al., 2001). Briefly, 18-hour-old bacteria were swabbed from GCB agar and resuspended in Hank’s Balanced Salt Solution (HBSS). Samples were vigorously vortexed for two minutes followed by centrifugation at 16,100 × g for five minutes at 4°C to remove whole bacteria. Clarified supernatants were used immediately or stored at −80°C. Total protein was quantified by BCA assay (Pierce).
Heat-treated bacteria
Bacteria were resuspended in GCB liquid medium and incubated at 37°C, 50°C, or 100°C for 30 minutes. Samples were then briefly vortexed and epithelial cells infected with bacteria equivalent to an MOI of 50. Aliquots of heat-treated bacteria were plated onto supplemented GCB agar and incubated at 37°C with 5% CO2 for 48 hours to ensure complete killing. For some experiments, live or heat-killed bacteria were treated with three or six units of proteinase K (New England Biolabs) for one hour at 37°C and washed three times with pre-warmed GCB to remove residual enzyme.
MAP kinase inhibition
T84 cells were incubated in serum-free DMEM/F-12 for 18 hours prior to infection. On the day of infection, medium was removed by aspiration and replaced with fresh pre-warmed serum-free medium containing either the ERK, p38, or JNK chemical inhibitors individually, or with medium containing all three inhibitors. Control samples were treated with serum-free medium containing the vehicle (DMSO) alone. Cells were pre-incubated with inhibitor(s) for one hour prior to infection.
RNA isolation
T84 cells were lysed in buffer RLT (Qiagen) and scraped into Qiashredder columns to homogenize the samples. Total RNA was purified using the RNeasy mini-kit (Qiagen). Purified RNA samples were eluted in RNA Storage Solution (Ambion) and stored at −80°C.
Real-time PCR
For each sample, one microgram of total RNA was transcribed into cDNA using the iScript Select cDNA Synthesis kit (Biorad) according to manufacturer’s instructions. Semi-quantitative real-time PCR was performed on an ABI Prism 7300 using TaqMan Universal master mix (Applied Biosystems) and pre-designed TaqMan probes, per manufacturer’s protocols. Reactions were performed in a 20 μl volume, in triplicate. Transcript levels were normalized to the housekeeping gene GAPDH, and relative expression values determined using the comparative Ct method (Applied Biosystems).
Preparation of protein lysates
Cells were washed twice with ice-cold PBS and then lysed in ice-cold RIPA buffer (10 mM sodium phosphate, pH 7.2, 150 mM NaCl, 5 mM EDTA, 50 mM NaF, 0.1% SDS, 1% deoxycholate, 1% Triton X-100) supplemented with 1X Complete, EDTA-free protease inhibitor cocktail (Roche) and 1X PhosSTOP phosphatase inhibitor cocktail (Roche). Samples were incubated on ice for 20 minutes, then scraped into microfuge tubes. Nuclei and insoluble debris was removed by centrifugation at 16,100 × g for 20 minutes at 4°C. Cleared lysates were stored at −80°C until further processing.
Western blotting
Protein samples were resolved by SDS-PAGE and transferred onto a 0.45 μm nitrocellulose membrane (GE Osmonics). Membranes were blocked for one hour in Tris-buffered saline (TBS) containing 0.1% Tween and 5% nonfat dry powdered milk. Primary antibodies were diluted in either 5% milk/TBST (β-tubulin) or 5% BSA/TBST (ATF3) and incubated overnight at 4°C. Secondary antibodies were diluted in 5% milk/TBST and incubated for two hours at room temperature. Blots were imaged on the LICOR Odyssey Infrared Imaging System, and band intensities measured by densitometry using ImageJ v1.36b.
RNA interference
T84 cells were nucleofected with siRNA using the Amaxa Nucleofector II as described (Howie et al., 2008). For each nucleofection, approximately 1 × 106 cells were aliquotted into a microfuge tube and centrifuged at 180 × g for two minutes. Residual medium was removed by aspiration and the cell pellet resuspended in 100 μl of Nucleofector Solution R (Lonza). For each sample, 2.56 nmol of siRNA was added and mixed by gentle flicking. The suspension was transferred to an electroporation cuvette and nucleofected on setting T-016. Following nucleofection, 400 μl of pre-warmed RPMI/10% NCS was added; the mixture was then transferred to a clean microfuge tube and incubated in a heat block at 37°C for 5–10 minutes. Cells were seeded into one well of a 12-well plate containing an additional 1.5 ml of pre-warmed DMEM/F12/5% FBS. At 48 (PKR) or 72 (ATF3) hours post-nucleofection, the cells were infected with MS11 or MS11ΔpilT at an MOI of 50 for four hours. For PKR knockdown experiments, samples were collected in RIPA buffer and processed by western blot. For ATF3 knockdown experiments, RNA was isolated and relative transcript levels were assessed by RT-PCR. After binding nucleic acid to the column, the remaining flow through was saved. One part of flow through was combined with four parts ice-cold acetone, briefly vortexed and incubated overnight at −20°C. The precipitate was then centrifuged at 16,100 × g for 5 minutes at 4°C. The pellet was washed once with ice-cold 100% ethanol, dried briefly and resuspended in 100 μl 1X SDS-PAGE sample buffer supplemented with protease and phosphatase inhibitors. ATF3 protein levels were then assessed by western blotting.
Statistics
Statistical analyses were performed using Prism 5 (GraphPad Software). Significant differences were determined by one-way or two-way ANOVA, followed by Bonferroni’s post hoc comparison test. Outliers were eliminated using Grubbs’ test.
Supplementary Material
Figure S1. Gonococcal protein is important for ATF3 induction. MS11 was incubated at 37°C (Live) or 50°C (HK) for 30 minutes and then treated with 3 or 6 units of proteinase K (PK) for one hour. After washing out residual PK, T84 cells were infected for 4 hours. Representative western blot shows ATF3 levels after PK treatment. Total β-tubulin serves as a loading control.
Figure S2. Tfp are not required to induce ATF3 expression. (A) Representative western blot of ATF3 protein levels in T84 cells treated with increasing amounts of OMP isolated from MS11ΔpilE. β-tubulin was used as a loading control. (B) Representative western blot of ATF3 levels in cells infected with ΔpilE bacteria that were incubated at 37°C, 50°C, or 100°C for 30 minutes prior to infection. (C) Densitometry values from (B) represent mean ATF3 proteins levels, normalized to β-tubulin and set relative to mock-infected samples, which are set at 1 (±SEM, n=4).
Figure S3. PKR is phosphorylated by N. gonorrhoeae infection, but not required for ATF3 upregulation. (A) Representative western blot showing treatment with a chemical PKR inhibitor blocks gonococcal-induced ATF3 upregulation. T84 cells were treated with vehicle DMSO or 5 μM of PKR inhibitor 52740 (PKRi) for one hour and then infected with MS11 for four hours. Cell lysates were harvested at three hours post-infection and ATF3 levels determined by western blot. (B) Representative western blot of PKR knockdown by RNA interference. T84 cells were nucleofected with nonsense (NS) or PKR-specific (PKR) siRNA. At 72 hours post-nucleofection, the cells were infected with MS11 for 3 hours and protein levels determined by western blot.
Acknowledgments
We would like to thank D. Vercelli for use of the AMAXA nucleofector for our RNA interference experiments. We are grateful to D.R. Hernández for help with statistical analysis, and J.S. Wilbur and M.A. Rendón for their thoughtful suggestions about this manuscript. This work was supported in part by NIH grant RO1 AI068033 to M. So.
Footnotes
The authors declare no conflicts of interest.
References
- Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167:1609–1616. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- Akram A, Han B, Masoom H, Peng C, Lam E, Litvack ML, et al. Activating transcription factor 3 confers protection against ventilator-induced lung injury. Am Jl Respir Crit Care Med. 2010;182:489–500. doi: 10.1164/rccm.200906-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell JD. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat Rev Immunol. 2006;6:532–540. doi: 10.1038/nri1865. [DOI] [PubMed] [Google Scholar]
- Ayala BP, Vasquez B, Clary S, Tainer JA, Rodland K, So M. The pilus-induced Ca2+ flux triggers lysosome exocytosis and increases the amount of Lamp1 accessible to Neisseria IgA1 protease. Cell Microbiol. 2001;3:265–275. doi: 10.1046/j.1462-5822.2001.00112.x. [DOI] [PubMed] [Google Scholar]
- Biais N, Ladoux B, Higashi D, So M, Sheetz M. Cooperative retraction of bundled type IV pili enables nanonewton force generation. PLoS Biol. 2008;6:e87. doi: 10.1371/journal.pbio.0060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, et al. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- Criss AK, Katz BZ, Seifert HS. Resistance of Neisseria gonorrhoeae to non-oxidative killing by adherent human polymorphonuclear leucocytes. Cell Microbiol. 2009;11:1074–1087. doi: 10.1111/j.1462-5822.2009.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich M, Bartfeld S, Munke R, Lange C, Ogilvie LA, Friedrich A, Meyer TF. Activation of NF-kappaB by Neisseria gonorrhoeae is associated with microcolony formation and type IV pilus retraction. Cell Microbiol. 2011;13:1168–1182. doi: 10.1111/j.1462-5822.2011.01607.x. [DOI] [PubMed] [Google Scholar]
- Feinen B, Jerse AE, Gaffen SL, Russell MW. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol. 2010;3:312–321. doi: 10.1038/mi.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of toll-like receptor 4-mediated signaling. J Immunol. 2002;168:2424–2432. doi: 10.4049/jimmunol.168.5.2424. [DOI] [PubMed] [Google Scholar]
- Fichorova RN, Desai PJ, Gibson FC, 3rd, Genco CA. Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect Immun. 2001;69:5840–5848. doi: 10.1128/IAI.69.9.5840-5848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichorova RN, Rheinwald JG, Anderson DJ. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod. 1997;57:847–855. doi: 10.1095/biolreprod57.4.847. [DOI] [PubMed] [Google Scholar]
- Fisette PL, Ram S, Andersen JM, Guo W, Ingalls RR. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kappaB activation in epithelial cells in a Toll-like receptor 2-dependent manner. J Biol Chem. 2003;278:46252–46260. doi: 10.1074/jbc.M306587200. [DOI] [PubMed] [Google Scholar]
- Friedrich A, Arvidson CG, Shafer WM, Lee EH, So M. Two ABC transporter operons and the antimicrobial resistance gene mtrF are pilT responsive in Neisseria gonorrhoeae. J Bacteriol. 2007;189:5399–5402. doi: 10.1128/JB.00300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, Esteban M. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev. 2006;70:1032–1060. doi: 10.1128/MMBR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbase A, Stein C, Levison J, Htun Y. Global burden of sexually transmitted diseases (excluding HIV) in the year 2000. 2006 http://www.who.int.
- Gilchrist M, Henderson WR, Jr, Clark AE, Simmons RM, Ye X, Smith KD, Aderem A. Activating transcription factor 3 is a negative regulator of allergic pulmonary inflammation. J Exp Med. 2008;205:2349–2357. doi: 10.1084/jem.20072254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist M, Thorsson V, Li B, Rust AG, Korb M, Roach JC, et al. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature. 2006;441:173–178. doi: 10.1038/nature04768. [DOI] [PubMed] [Google Scholar]
- Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad of Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T, Wolford CC, Chang YS. ATF3, a hub of the cellular adaptive-response network, in the pathogenesis of diseases: is modulation of inflammation a unifying component? Gene Expr. 2010;15:1–11. doi: 10.3727/105221610x12819686555015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai TW, Liu F, Coukos WJ, Green MR. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989;3:2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Hartman MG, Lu D, Kim ML, Kociba GJ, Shukri T, Buteau J, et al. Role for activating transcription factor 3 in stress-induced beta-cell apoptosis. Mol Cell Biol. 2004;24:5721–5732. doi: 10.1128/MCB.24.13.5721-5732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck CR, Meyer TF, Lang F, Gulbins E. CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. The EMBO J. 1998;17:443–454. doi: 10.1093/emboj/17.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazzalin CA, Mahadevan LC. MAPK-regulated transcription: a continuously variable gene switch? Nat Rev Mol Cell Biol. 2002;3:30–40. doi: 10.1038/nrm715. [DOI] [PubMed] [Google Scholar]
- Hedges SR, Sibley DA, Mayo MS, Hook EW, 3rd, Russell MW. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J Infect Dis. 1998;178:742–751. doi: 10.1086/515372. [DOI] [PubMed] [Google Scholar]
- Higashi DL, Lee SW, Snyder A, Weyand NJ, Bakke A, So M. Dynamics of Neisseria gonorrhoeae attachment: microcolony development, cortical plaque formation, and cytoprotection. Infect Immun. 2007;75:4743–4753. doi: 10.1128/IAI.00687-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook EW. Gonococcal infections in the adult. In: Holmes KKea., editor. Sexually Transmitted Diseases. 3. New York, NY: McGraw-Hill; 1999. pp. 451–463. [Google Scholar]
- Howie HL, Glogauer M, So M. The N. gonorrhoeae type IV pilus stimulates mechanosensitive pathways and cytoprotection through a pilT-dependent mechanism. PLoS Biol. 2005;3:e100. doi: 10.1371/journal.pbio.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie HL, Shiflett SL, So M. Extracellular signal-regulated kinase activation by Neisseria gonorrhoeae downregulates epithelial cell proapoptotic proteins Bad and Bim. Infect and Immun. 2008;76:2715–2721. doi: 10.1128/IAI.00153-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda WM, Knapp JS. Neisseria and Moraxella catarrhalis. In: Murray PR, editor. Manual of Clinical Microbiology. 8. Washington DC: ASM Press; 2003. pp. 585–608. [Google Scholar]
- Jones A, Jonsson AB, Aro H. Neisseria gonorrhoeae infection causes a G1 arrest in human epithelial cells. FASEB J. 2007;21:345–355. doi: 10.1096/fj.06-6675com. [DOI] [PubMed] [Google Scholar]
- Kang Y, Chen CR, Massague J. A self-enabling TGFbeta response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol Cell. 2003;11:915–926. doi: 10.1016/s1097-2765(03)00109-6. [DOI] [PubMed] [Google Scholar]
- Khuu CH, Barrozo RM, Hai T, Weinstein SL. Activating transcription factor 3 (ATF3) represses the expression of CCL4 in murine macrophages. Mol Immunol. 2007;44:1598–1605. doi: 10.1016/j.molimm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Lee SW, Higashi DL, Snyder A, Merz AJ, Potter L, So M. PilT is required for PI(3,4,5)P3-mediated crosstalk between Neisseria gonorrhoeae and epithelial cells. Cell Microbiol. 2005;7:1271–1284. doi: 10.1111/j.1462-5822.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- Litvak V, Ramsey SA, Rust AG, Zak DE, Kennedy KA, Lampano AE, et al. Function of C/EBPdelta in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nat Immunol. 2009;10:437–443. doi: 10.1038/ni.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Feinen B, Russell MW. New concepts in immunity to Neisseria gonorrhoeae: innate responses and suppression of adaptive immunity favor the pathogen, not the host. Front Microbiol. 2011;2:52. doi: 10.3389/fmicb.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases--regulating the immune response. Nat Rev Immunol. 2007;7:202–212. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- Lu D, Chen J, Hai T. The regulation of ATF3 gene expression by mitogen-activated protein kinases. The Biochemical journal. 2007;401:559–567. doi: 10.1042/BJ20061081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Wolfgang CD, Hai T. Activating transcription factor 3, a stress-inducible gene, suppresses Ras-stimulated tumorigenesis. J Biol Chem. 2006;281:10473–10481. doi: 10.1074/jbc.M509278200. [DOI] [PubMed] [Google Scholar]
- MacLeod H, Bhasin N, Wetzler LM. Role of protein tyrosine kinase and Erk1/2 activities in the Toll-like receptor 2-induced cellular activation of murine B cells by neisserial porin. Clin Vaccine Immunol. 2008;15:630–637. doi: 10.1128/CVI.00435-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier B, Koomey M, Sheetz MP. A force-dependent switch reverses type IV pilus retraction. Proc Natl Acad of Sci USA. 2004;101:10961–10966. doi: 10.1073/pnas.0402305101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari P, Henneke P, Ho Y, Latz E, Golenbock DT, Wetzler LM. Cutting edge: Immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J Immunol. 2002;168:1533–1537. doi: 10.4049/jimmunol.168.4.1533. [DOI] [PubMed] [Google Scholar]
- McGee ZA, Clemens CM, Jensen RL, Klein JJ, Barley LR, Gorby GL. Local induction of tumor necrosis factor as a molecular mechanism of mucosal damage by gonococci. Microb Pathog. 1992;12:333–341. doi: 10.1016/0882-4010(92)90096-7. [DOI] [PubMed] [Google Scholar]
- McGee ZA, Johnson AP, Taylor-Robinson D. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J Infect Dis. 1981;143:413–422. doi: 10.1093/infdis/143.3.413. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- Merz AJ, Rifenbery DB, Arvidson CG, So M. Traversal of a polarized epithelium by pathogenic Neisseriae: facilitation by type IV pili and maintenance of epithelial barrier function. Mol Med. 1996;2:745–754. [PMC free article] [PubMed] [Google Scholar]
- Merz AJ, So M, Sheetz MP. Pilus retraction powers bacterial twitching motility. Nature. 2000;407:98–102. doi: 10.1038/35024105. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Inoue S, Yamada K, Watanabe M, Liu Q, Watanabe T, et al. Differential usage of alternate promoters of the human stress response gene ATF3 in stress response and cancer cells. Nucleic Acids Res. 2009;37:1438–1451. doi: 10.1093/nar/gkn1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosleh IM, Boxberger HJ, Sessler MJ, Meyer TF. Experimental infection of native human ureteral tissue with Neisseria gonorrhoeae: adhesion, invasion, intracellular fate, exocytosis, and passage through a stratified epithelium. Infect Immun. 1997;65:3391–3398. doi: 10.1128/iai.65.8.3391-3398.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif X, Lowy J, Stenberg P, O’Gaora P, Ganji A, So M. Antigenic variation of pilin regulates adhesion of Neisseria meningitidis to human epithelial cells. Mol Microbiol. 1993;8:719–725. doi: 10.1111/j.1365-2958.1993.tb01615.x. [DOI] [PubMed] [Google Scholar]
- Naumann M, Rudel T, Wieland B, Bartsch C, Meyer TF. Coordinate activation of activator protein 1 and inflammatory cytokines in response to Neisseria gonorrhoeae epithelial cell contact involves stress response kinases. J Exp Med. 1998;188:12771286. doi: 10.1084/jem.188.7.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann M, Wessler S, Bartsch C, Wieland B, Meyer TF. Neisseria gonorrhoeae epithelial cell interaction leads to the activation of the transcription factors nuclear factor kappaB and activator protein 1 and the induction of inflammatory cytokines. J Exp Med. 1997;186:247–258. doi: 10.1084/jem.186.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persengiev SP, Green MR. The role of ATF/CREB family members in cell growth, survival and apoptosis. Apoptosis. 2003;8:225–228. doi: 10.1023/a:1023633704132. [DOI] [PubMed] [Google Scholar]
- Ramsey KH, Schneider H, Cross AS, Boslego JW, Hoover DL, Staley TL, et al. Inflammatory cytokines produced in response to experimental human gonorrhea. J Infect Dis. 1995;172:186–191. doi: 10.1093/infdis/172.1.186. [DOI] [PubMed] [Google Scholar]
- Romano PR, Garcia-Barrio MT, Zhang X, Wang Q, Taylor DR, Zhang F, et al. Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2alpha kinases PKR and GCN2. Mol Cell Biol. 1998;18:2282–2297. doi: 10.1128/mcb.18.4.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger CM, Clark AE, Treuting PM, Johnson CD, Aderem A. ATF3 regulates MCMV infection in mice by modulating IFN-gamma expression in natural killer cells. Proc Natl Acad of Sci USA. 2008;105:2544–2549. doi: 10.1073/pnas.0712182105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydberg C, Mansson A, Uddman R, Riesbeck K, Cardell LO. Toll-like receptor agonists induce inflammation and cell death in a model of head and neck squamous cell carcinomas. Immunology. 2009;128:e600–611. doi: 10.1111/j.1365-2567.2008.03041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Case CL, Archer KA, Nogueira CV, Kobayashi KS, Flavell RA, et al. Type IV secretion-dependent activation of host MAP kinases induces an increased proinflammatory cytokine response to Legionella pneumophila. PLoS Pathog. 2008;4:e1000220. doi: 10.1371/journal.ppat.1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova O, Heppel N, Jagerhuber R, Kim KS, Frosch M, Eigenthaler M, Schubert-Unkmeir A. Interaction of Neisseria meningitidis with human brain microvascular endothelial cells: role of MAP- and tyrosine kinases in invasion and inflammatory cytokine release. Cell Microbiol. 2004;6:1153–1166. doi: 10.1111/j.1462-5822.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973;137:571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Nakamura A, Morioka MS, Inoue S, Tamamori-Adachi M, Yamada K, et al. Systems analysis of ATF3 in stress response and cancer reveals opposing effects on pro-apoptotic genes in p53 pathway. PLoS One. 2011;6:e26848. doi: 10.1371/journal.pone.0026848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MR, Xu D, Williams BR. ATF3 transcription factor and its emerging roles in immunity and cancer. J Mol Med. 2009;87:1053–1060. doi: 10.1007/s00109-009-0520-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigg BG, Kerndt PR, Aynalem G. Sexually transmitted infections and pelvic inflammatory disease in women. Med Clin North Am. 2008;92:1083–1113. x. doi: 10.1016/j.mcna.2008.04.011. [DOI] [PubMed] [Google Scholar]
- van Putten JP, Duensing TD, Cole RL. Entry of OpaA+ gonococci into HEp-2 cells requires concerted action of glycosaminoglycans, fibronectin and integrin receptors. Mol Microbiol. 1998;29:369–379. doi: 10.1046/j.1365-2958.1998.00951.x. [DOI] [PubMed] [Google Scholar]
- Wei LH, Kuo ML, Chen CA, Chou CH, Cheng WF, Chang MC, et al. The anti-apoptotic role of interleukin-6 in human cervical cancer is mediated by up-regulation of Mcl-1 through a PI 3-K/Akt pathway. Oncogene. 2001;20:5799–5809. doi: 10.1038/sj.onc.1204733. [DOI] [PubMed] [Google Scholar]
- Weyand NJ, Calton CM, Higashi DL, Kanack KJ, So M. Presenilin/gamma-secretase cleaves CD46 in response to Neisseria infection. J Immunol. 2010;184:694–701. doi: 10.4049/jimmunol.0900522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore MM, Iparraguirre A, Kubelka L, Weninger W, Hai T, Williams BR. Negative regulation of TLR-signaling pathways by activating transcription factor-3. J Immunol. 2007;179:3622–3630. doi: 10.4049/jimmunol.179.6.3622. [DOI] [PubMed] [Google Scholar]
- WHO. Global action plan: to control the spread and impact of antimicrobial resistance to Nesseria gonorrhoeae. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- Witt K, Veale DR, Smith H. Resistance of Neisseria gonorrhoeae to ingestion and digestion by phagocytes of human buffy coat. J Med Microbiol. 1976;9:112. doi: 10.1099/00222615-9-1-1. [DOI] [PubMed] [Google Scholar]
- Wolfgang M, van Putten JP, Hayes SF, Dorward D, Koomey M. Components and dynamics of fiber formation define a ubiquitous biogenesis pathway for bacterial pili. EMBO J. 2000;19:6408–6418. doi: 10.1093/emboj/19.23.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Bliss SP, Nett TM, Ebersole BJ, Sealfon SC, Roberson MS. Transcript profiling of immediate early genes reveals a unique role for activating transcription factor 3 in mediating activation of the glycoprotein hormone alpha-subunit promoter by gonadotropin-releasing hormone. Mol Endocrinol. 2005;19:2624–2638. doi: 10.1210/me.2005-0056. [DOI] [PubMed] [Google Scholar]
- Yin X, Dewille JW, Hai T. A potential dichotomous role of ATF3, an adaptive-response gene, in cancer development. Oncogene. 2008;27:2118–2127. doi: 10.1038/sj.onc.1210861. [DOI] [PubMed] [Google Scholar]
- Zmuda EJ, Viapiano M, Grey ST, Hadley G, Garcia-Ocana A, Hai T. Deficiency of Atf3, an adaptive-response gene, protects islets and ameliorates inflammation in a syngeneic mouse transplantation model. Diabetologia. 2010;53:1438–1450. doi: 10.1007/s00125-010-1696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Gonococcal protein is important for ATF3 induction. MS11 was incubated at 37°C (Live) or 50°C (HK) for 30 minutes and then treated with 3 or 6 units of proteinase K (PK) for one hour. After washing out residual PK, T84 cells were infected for 4 hours. Representative western blot shows ATF3 levels after PK treatment. Total β-tubulin serves as a loading control.
Figure S2. Tfp are not required to induce ATF3 expression. (A) Representative western blot of ATF3 protein levels in T84 cells treated with increasing amounts of OMP isolated from MS11ΔpilE. β-tubulin was used as a loading control. (B) Representative western blot of ATF3 levels in cells infected with ΔpilE bacteria that were incubated at 37°C, 50°C, or 100°C for 30 minutes prior to infection. (C) Densitometry values from (B) represent mean ATF3 proteins levels, normalized to β-tubulin and set relative to mock-infected samples, which are set at 1 (±SEM, n=4).
Figure S3. PKR is phosphorylated by N. gonorrhoeae infection, but not required for ATF3 upregulation. (A) Representative western blot showing treatment with a chemical PKR inhibitor blocks gonococcal-induced ATF3 upregulation. T84 cells were treated with vehicle DMSO or 5 μM of PKR inhibitor 52740 (PKRi) for one hour and then infected with MS11 for four hours. Cell lysates were harvested at three hours post-infection and ATF3 levels determined by western blot. (B) Representative western blot of PKR knockdown by RNA interference. T84 cells were nucleofected with nonsense (NS) or PKR-specific (PKR) siRNA. At 72 hours post-nucleofection, the cells were infected with MS11 for 3 hours and protein levels determined by western blot.