Abstract
Psilocybin is a classic psychedelic and a candidate drug model of psychosis. This study measured the effects of psilocybin on resting-state network and thalamocortical functional connectivity (FC) using functional magnetic resonance imaging (fMRI). Fifteen healthy volunteers received intravenous infusions of psilocybin and placebo in 2 task-free resting-state scans. Primary analyses focused on changes in FC between the default-mode- (DMN) and task-positive network (TPN). Spontaneous activity in the DMN is orthogonal to spontaneous activity in the TPN, and it is well known that these networks support very different functions (ie, the DMN supports introspection, whereas the TPN supports externally focused attention). Here, independent components and seed-based FC analyses revealed increased DMN-TPN FC and so decreased DMN-TPN orthogonality after psilocybin. Increased DMN-TPN FC has been found in psychosis and meditatory states, which share some phenomenological similarities with the psychedelic state. Increased DMN-TPN FC has also been observed in sedation, as has decreased thalamocortical FC, but here we found preserved thalamocortical FC after psilocybin. Thus, we propose that thalamocortical FC may be related to arousal, whereas DMN-TPN FC is related to the separateness of internally and externally focused states. We suggest that this orthogonality is compromised in early psychosis, explaining similarities between its phenomenology and that of the psychedelic state and supporting the utility of psilocybin as a model of early psychosis.
Key words: serotonin, 5-HT, resting-state networks, default-mode network, psychedelics, consciousness, psychosis, at-risk mental state
Introduction
Background
Psilocybin is a tryptamine psychedelic and the prodrug of the major psychoactive component of magic mushrooms, psilocin. Psilocybin and psilocin were first isolated and synthesized by Albert Hofmann1 after which they were used in psychotherapy before this was curtailed by political pressure.2 Classic psychedelics like psilocybin produce a range of subjective effects from superficial perceptual changes to more profound existential-type experiences.3 Much has been written about the phenomenology of the psychedelic state, but we have only a limited understanding of how it is produced in the brain.
Functional MRI Measures of Spontaneous Brain Activity
There has been an increased interest in measures of spontaneous brain activity in recent years.4 In humans, fMRI measures of task-free- or “resting-state” functional connectivity (FC) have become popular. Measures of resting-state FC using independent components analysis (ICA) have identified a number of spatiotemporally coherent networks5 that closely resemble stimulus-evoked activation maps.6 Of particular interest is the default-mode network (DMN), a network of regions (including the posterior cingulate cortex; medial prefrontal cortex, mPFC; and lateral inferior parietal cortex) that show greater activity during internally oriented cognition than externally focused attention.7 The DMN receives more blood flow8 and consumes more energy7 than other brain regions, has undergone significant evolutionary expansion,9 and serves as an important convergence zone or “connector hub” in the cortex.10 The DMN is activated during high-level cognitions such as predicting the future11; making personal, social, and moral judgments12,13; and contemplating the past.14 These properties have led to speculations that the DMN is the biological system upon which our psychological notions of self15 or “ego”16 are based.
Between-Network FC and Cognitive Function
The DMN is known to deactivate during cognitive tasks, while a generic task-positive network (TPN) is activated; and the TPN deactivates during introspection, while the DMN becomes more active. Importantly, this competitive relationship between DMN and TPN activity may be preserved under task-free conditions,17 potentially implying that DMN-TPN competition, or at least orthogonality, is a fundamental property of global brain function.18
Spontaneous fluctuations in resting-state network (RSN) activity influence stimulus-evoked activity and predict behavioral variability.19 Task performance is more consistent or less variable if inverse coupling between the DMN and TPN is greater.20 Moreover, increased spontaneous DMN activity has been associated with increased mind wandering.21 Thus, a picture emerges of a fundamental orthogonality between the DMN and TPN, with the DMN serving explorative inner thought, and the TPN serving focused attention. Crucially, during normal waking consciousness, explorative inner thought and focused attention do not occur simultaneously, presumably because the systems that support these states are kept apart. If, however, activity in the DMN and TPN was to become less orthogonal, then this might cause a confusion of states and a disturbance of cognition such as is seen in early psychosis.
Pharmacological fMRI studies have discovered relationships between changes in DMN-TPN FC and changes in subjective experience. For example, abstinent smokers with improved cognition following nicotine replacement therapy showed increased inverse coupling between the DMN and TPN.22 Changes in DMN-TPN and thalamocortical coupling have also been measured after propofol infusion. Reduced conscious awareness correlated with reduced inverse coupling between the DMN and TPN and decreased thalamocortical FC.23
The present study sought to test the effect of psilocybin on DMN-TPN and thalamocortical FC. We hypothesized that thalamocortical FC would be preserved after psilocybin but DMN-TPN FC would be increased—consistent with reduced orthogonality between these networks in the psychedelic state.
Materials and Methods
Design
This was a within-subjects placebo-controlled study. The study was approved by a local NHS Research Ethics Committee and Research and Development department, and conducted in accordance with Good Clinical Practice guidelines. A Home Office Licence was obtained for storage and handling of a Schedule 1 drug. The University of Bristol sponsored the research.
Participants
This is a new analysis on a previously published data set.24 Fifteen healthy subjects took part: 13 males and 2 females (mean age = 32, SD = 8.9). Recruitment was via word of mouth. All subjects were required to give informed consent and undergo health screens prior to enrolment. Entry criteria were the following: at least 21 years of age, no personal or immediate family history of a major psychiatric disorder, substance dependence, cardiovascular disease, and no history of a significant adverse response to a hallucinogenic drug. All of the subjects had used psilocybin at least once before (mean number of uses per subject = 16.4, SD = 27.2) but not within 6 weeks of the study.
Anatomical Scans
Imaging was performed on a 3T GE HDx system. Anatomical scans were performed before each functional scan. These were 3D fast spoiled gradient echo scans in an axial orientation, with field of view = 256 × 256 × 192 and matrix = 256 × 256 × 192 to yield 1-mm isotropic voxel resolution (repetition time/echo time [TR/TE] = 7.9/3.0ms; inversion time = 450ms; flip angle = 20°).
Drug and Scanning Parameters
All subjects underwent two 12-min eyes-closed resting-state blood oxygen–level dependent (BOLD) fMRI scans on 2 separate occasions at least 7 days apart: placebo (10ml saline, 60-s intravenous injection) was given on 1 occasion and psilocybin (2mg dissolved in 10ml saline) on the other. Seven of the subjects received psilocybin in scan 1, and 8 received it in scan 2. Injections were given manually by a study doctor situated within the scanning suite. The 60-s infusions began exactly 6min after the start of the 12-min scans. Subjective ratings were given postscan using visual analog scales. The subjective effects of psilocybin were felt almost immediately after injection and were sustained for the duration of the scan.25
fMRI Data Acquisition
BOLD-weighted fMRI data were acquired using a gradient echo planar imaging sequence, TR/TE 3000/35ms, field-of-view = 192mm, 64 × 64 acquisition matrix, parallel acceleration factor = 2, 90° flip angle. Fifty-three oblique axial slices were acquired in an interleaved fashion, each 3mm thick with zero slice gap (3 × 3 × 3mm voxels).
Independent Components Analysis
All analyses were performed using the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL, www.fmrib.ox.ac.uk/fsl). FSLs MELODIC was used to derive 20 spatiotemporally coherent components from 30 concatenated data sets. Twenty preinjection “baseline” components were derived so that the effect of psilocybin on FC between these components could then be examined. Thus, the data sets from which the components were derived included the first 6min of each subjects’ placebo and psilocybin resting-state scans (ie, the 100 volumes that were acquired prior to the injection of saline or psilocybin). These data were motion corrected using FSLs MCFLIRT function and a high-pass filter of 100 s was applied. The 20 components were registered to the subjects’ T1-weighted high-resolution (1 × 1 × 1mm) anatomical scans that were themselves registered to the Montreal Neurological Institute standard brain (1 × 1 × 1mm).
Between-Network FC Using ICA
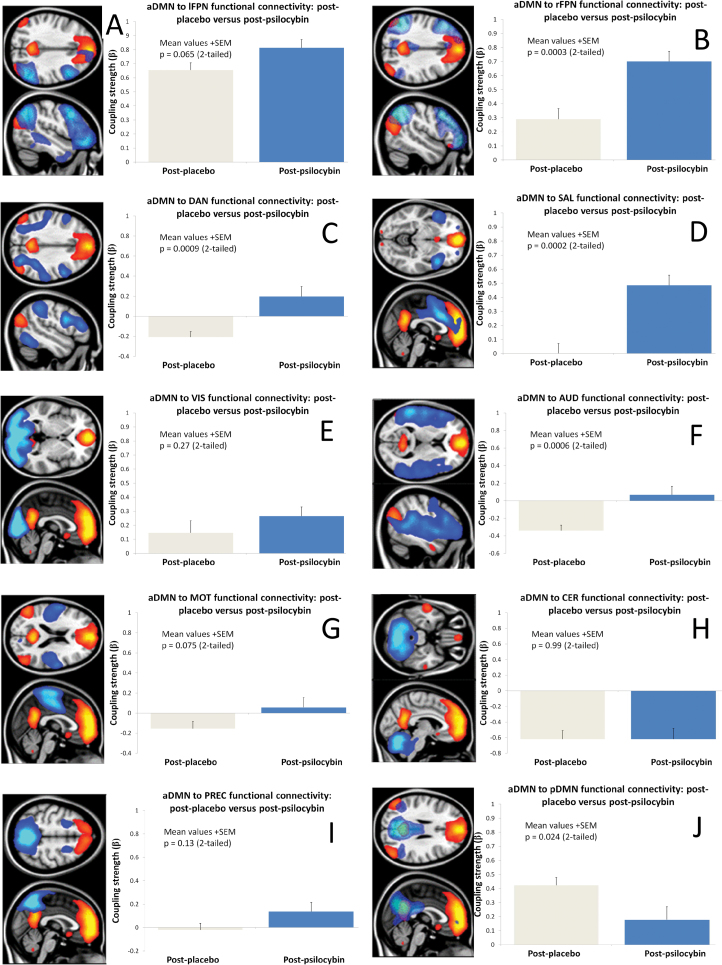
Of the 20 components derived from the group ICA, 11 were identified as “functionally meaningful” RSNs; explicitly, we excluded 9 components where the majority of the voxels were in white matter, ventricular space, or outside of the brain. Henceforth, we will refer to these as “noise” components because their signal was most likely nonneuronal. The remaining 11 RSNs included an anteriorly loaded DMN (aDMN), a posteriorly loaded DMN (pDMN), right- and left-lateralized frontoparietal networks (rFPN & lFPN), an auditory network (AUD), salience network (SAL), visual network, precuneus network, dorsal attention network (DAN), cerebellar network, and sensorimotor network. These 11 networks are shown in figure 1 with the aDMN in every image.
Fig. 1.
Default-mode network-resting-state network (DMN-RSN) connectivity after psilocybin vs after placebo. (A) anteriorly loaded DMN (aDMN)-left frontoparietal network connectivity, (B) aDMN-right frontoparietal connectivity, (C) aDMN-dorsal attention network connectivity, (D) aDMN-salience network connectivity, (E) aDMN-visual network connectivity, (F) aDMN-auditory network connectivity, (G) aDMN-motor network connectivity, (H) aDMN-cerebellar network connectivity, (I) aDMN-precuneus network connectivity, (J) aDMN-posterior DMN connectivity. Images show the aDMN in orange and the relevant RSN in blue with the adjacent chart displaying the regression coefficient or “functional connectivity (FC) strength” after placebo (gray) and psilocybin (blue). The betas on the y-axis refer to regression coefficients. Contrasts B, C, D and F were all statistically significant when corrected for multiple comparisons (corrected α = 0.005).
Time series for the last 100 volumes (postinjection) for all 20 components were extracted using multiple regression. Our analyses focused on the relationship between the DMN and other RSN in the psychedelic state. Thus, the aDMN was chosen as the dependent variable in regression analyses run in SPSS. One RSN at a time plus the 9 noise components were entered as independent variables. The noise components were included to remove nonneuronal variance. This process was repeated for each of the RSNs, with the aDMN as the dependent variable in every case. The unstandardized regression coefficient for the RSN of interest were plotted and compared across the 2 conditions (psilocybin vs placebo) in paired t tests. All t tests were 2 tailed. Pearson’s correlational analyses were used to test for relationships between aDMN-RSN FC and subjective ratings. Based on their relevance to the hypothesis that decreased orthogonality between the DMN and TPNs would predict experiences of disturbed ego boundaries and cognition, we chose the following 5 questionnaire items for correlational analyses: “I felt a sense of merging with my environment,” “I experienced a loss of separation from my surroundings,” “my thinking was muddled,” “I lost all sense of ego,” and the item that required subjects to rate the “intensity” of the drug effects. Results were corrected for multiple comparisons (Bonferonni).
Between-Network FC Using Seed-Based FC
In addition to the ICA approach, we assessed DMN-TPN FC using seed-based FC. The results of a ventromedial PFC (vmPFC) seed-based resting-state FC analysis were used to define the DMN (vmPFC-positive network) and TPN (vmPFC-negative network).24 Activity in these networks is sometimes referred to as being “anticorrelated,” but this can be misleading because “anticorrelations” can be introduced by regressing out the global grey matter signal.18 For this reason, we chose not to include global grey matter regression in any of the analyses presented in this article. The DMN and TPN (cluster threshold Z > 2.3, P < .05 whole brain corrected) were converted into spatial masks from which time series were extracted for each functional scan. We also extracted times series from white matter and cerebrospinal fluid (CSF) masks to serve as nonneuronal “noise regressors.”
Linear regression was performed to calculate the FC strengths between the DMN and TPN for all of the scanning sessions. Times series were separated into the first 100 volumes (preinjection) and the last 100 volumes (postinjection), with the 40 volumes surrounding the injection period excluded from the analysis. The DMN time series served as the dependent variable and the TPN, white matter, and CSF time series, plus motion parameters served as independent variables. The unstandardized regression coefficients for the DMN vs TPN were compared before vs after psilocybin injection, plus after placebo vs after psilocybin injection, using t tests.
Thalamic FC
To test for an effect of psilocybin on FC between the thalamus and high-level cortical networks, a bilateral thalamic mask was generated based on an anatomical template in FSL. This mask was thresholded and transformed into single-subject functional space, and time series were extracted for each subject. Linear regression was used to measure changes in thalamocortical connectivity after psilocybin. The thalamic time series served as the dependent variable, and the DMN and TPN time series served as independent variables. Thalamus-DMN and thalamus-TPN FC were calculated in 2 separate regression analyses. As before, white matter and CSF time series and motion variance were entered as regressors of no interest. Again, global grey matter signal regression was not included in this analysis. Regression coefficients for thalamus-DMN and thalamus-TPN FC were compared before and after psilocybin and placebo, and after psilocybin vs after placebo.
Results
Subjective Effects
The subjective effects of psilocybin have been documented elsewhere.24,25 Briefly, the subjective effects of 2mg psilocybin given as an intravenous injection over 60 s begin at the end of the injection period, reach a sustained peak after 4min, and subside completely after 45–60min. Primary subjective effects include altered visual perception (eg, hallucinated motion and geometric patterns), an altered sense of space and time, and vivified imagination.
ICA and Between-Network FC
Eleven RSNs were identified from the preinjection time series. These RSNs are listed in the Materials and Methods section and shown in figure 1. Of these 11 networks, there were 2 DMNs, an anteriorly loaded DMN (aDMN, figure 1, orange in all images) with all of the major DMN nodes present, and a posteriorly loaded DMN (pDMN, blue in figure 1J). FC between the aDMN and each of the 10 remaining networks was compared in turn. Charts in figure 1 show the strength of the FC between aDMN and the other RSNs after placebo and after psilocybin. Significant increases in FC between the aDMN and the SAL were evident after psilocybin (P = .0002), as well as the right frontoparietal network (P = .0003), the AUD (P = .0006), and the DAN (P = .0009). There was also a suggestion of decreased FC between the anterior and posterior DMNs (P = .02), but this did not survive correction for multiple comparisons (α = 0.005, Bonferonni corrected). An example of increased aDMN-SAL FC after psilocybin is shown in Figuer 2C.
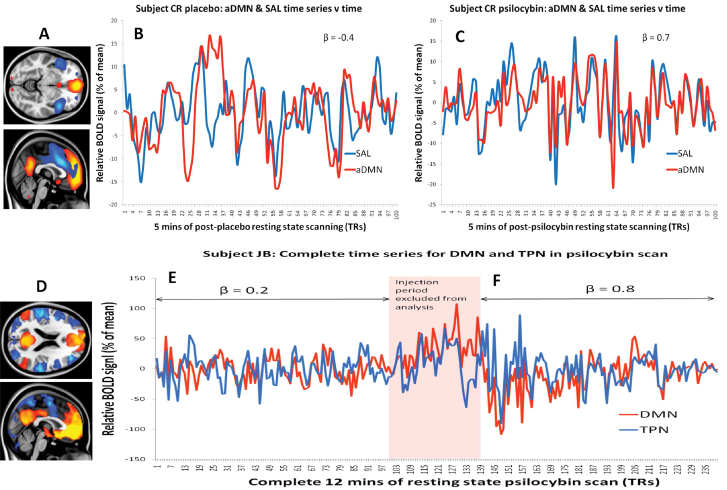
Fig. 2.
The effect of psilocybin on DMN connectivity seen at the single-subject level. (A) The aDMN (orange) and salience network (blue) derived from Independent Components Analysis. (B) An illustrative single-subject time series for the DMN (red) and salience network (blue) after placebo injection. (C) The same subject’s time series for the DMN and salience network after psilocybin injection. Note the increase in FC between the DMN and salience network after psilocybin vs after placebo. (D) Positive (orange) and “negative” (blue) FC with the ventromedial prefrontal cortex (vmPFC) based on data from fifteen placebo condition 12-min resting-state scans. This vmPFC-positive network is the DMN, and the vmPFC-negative network is the task-positive network. (E) The complete time series for the DMN and task-positive network (TPN) for a single subject’s psilocybin scan. Note the increase in DMN-TPN FC after psilocybin. Subject CR rated the intensity of the effects at 9/10 and JB 10/10.
Seed-Based FC and Between-Network FC
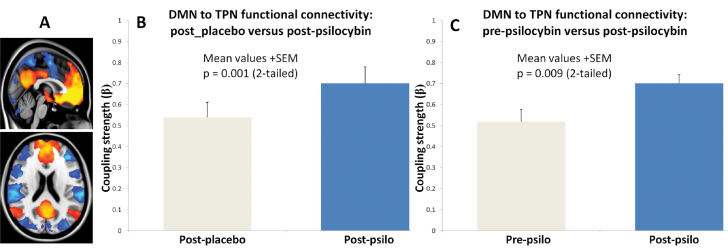
A vmPFC seed-based FC analysis24 was used to derive DMN and TPN spatial masks (figure 3A, orange and blue, respectively). Linear regression revealed significantly increased FC between the DMN and TPN after psilocybin vs placebo (figure 3B, P = .001) and post- vs prepsilocybin injection (figure 3C, P = 009).
Fig. 3.
Increased functional FC between the default-mode- and task-positive network under psilocybin. (A) vmPFC-positive- (DMN, orange) and vmPFC-negative regions (TPN, blue). (B) Increased FC between the DMN and TPN after psilocybin vs placebo (P = .001). (C) Increased FC between the DMN and TPN post vs prepsilocybin (P = .009).
Correlations Between DMN-RSN FC and Psychedelic Effects
Pearson’s correlational analyses were performed to test for relationships between altered DMN-RSN FC and psilocybin’s subjective effects. There were suggestions of positive correlations between DMN-TPN FC and ratings of psychedelic effects. The item “my thinking was muddled” showed suggestions of a relationship with increased DMN-rFPN FC, but this did not survive correction for multiple comparison (P = .02, revised α = 0.002); ratings of drug effects intensity showed suggestions of a relationship with increased DMN-TPN FC, but this was also not significant (P = .16).
DMN and TPN Connectivity With the Thalamus
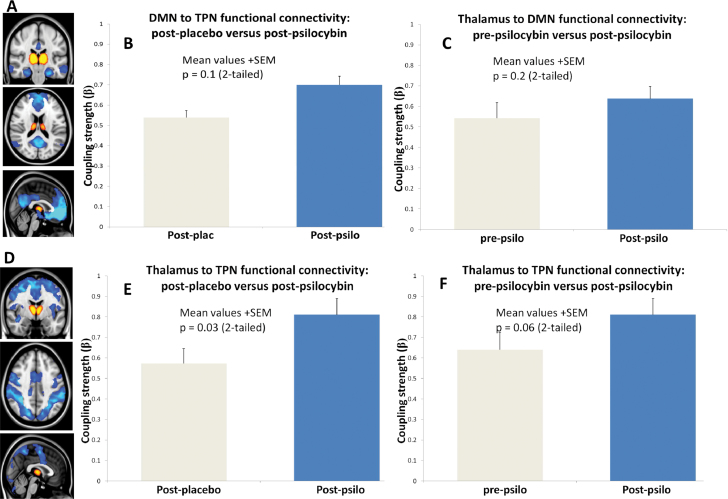
Previous work found a positive correlation between increases in DMN-TPN FC and propofol-induced reductions in consciousness23; however, none of our subjects reported reduced consciousness after psilocybin. The same study also reported reduced thalamocortical connectivity that correlated with reduced consciousness; thus, we tested to see if psilocybin caused similar reductions in thalamocortical connectivity. Thalamic-DMN FC showed a nonsignificant increase after psilocybin (figure 4D,E), and there was a significant increase in thalamic-TPN FC (figure 4G). The increases in thalamocortical FC after psilocybin did not correlate with ratings of the intensity of the subjective effects of psilocybin.
Fig. 4.
Thalamic connectivity with the DMN and TPN. (A) The thalamic (orange) and DMN (blue) masks from which time series were extracted. (B) Thalamic-DMN connectivity postplacebo vs postpsilocybin (P = .1). (C) Thalamic-DMN connectivity postpsilocybin vs prepsilocybin (P = .2). (D) The thalamic (orange) and TPN (blue) networks from which time series were extracted. (E) Thalamic-TPN connectivity postplacebo vs postpsilocybin (P = .03). (F) Thalamic-TPN connectivity post- vs prepsilocybin (P = .06).
Validity Tests
Between-Condition Differences in Movement. Movement regressors were included in all of our FC analyses, but it remains possible that the increases in FC between the aDMN and other RSNs may have been caused by different levels of movement in the 2 conditions. Thus, we calculated the mean movement per volume for each subject’s scan and compared the psilocybin and placebo scans in a paired t test. Significantly, greater movement was seen under psilocybin (mean movement per volume = 0.06mm [SD = 0.015] for placebo vs 0.1mm [SD = 0.05] for psilocybin, P < .01). However, there was considerable variability in movement under psilocybin, with some participants showing less movement under drug. Therefore, to test whether the between-condition differences in movement could explain the regression results shown in figure 1, we ran correlational analyses on all of the positive results from this analysis (ie, aDMN-DAN, aDMN-rFPN, aDMN-SAL, and aDMN-AUD). No relationships were found between the changes in between-network coupling under psilocybin and the differences in movement, so the argument that the observed changes in between-network coupling were caused by between-condition differences in movement is not supported by the data.
Discussion
ICA revealed 11 RSNs, including a canonical DMN. Increased FC was evident between this DMN and 4 RSNs. These 4 RSNs include well-characterized TPNs, ie, the dorsal attention, salience and right frontoparietal network, and the auditory network. Importantly, DMN and TPN activity is normally orthogonal, or even competitive,17 so increased DMN-TPN FC implies that these networks’ functionality became less distinct under psilocybin. Confirmatory results were found when we repeated the analysis with DMN and TPN masks derived from a seed-based FC analysis; increased DMN-TPN FC was evident after psilocybin. These results imply that increased DMN-TPN FC, and so decreased DMN-TPN orthogonality, is an important characteristic of the psychedelic state.
The question now arises, is increased DMN-TPN coupling specific to the psychedelic state? Boveroux and colleagues found a graded decrease in DMN-TPN inverse coupling (or increase in DMN-TPN FC) with increasing levels of propofol-induced sedation. However, none of our subjects reported sedation after psilocybin; in fact, psychedelics are often described as “mind expanding.” This inconsistency in phenomenology but consistency in physiology is intriguing. Because decreased thalamocortical excitation is closely linked to reduced arousal26 and large decreases in thalamocortical connectivity were evident in the propofol study, we tested to see if the same thalamocortical decoupling occurred under psilocybin. We hypothesized that if thalamocortical connectivity is preserved in the psychedelic state, then this may explain the psychological differences between the psychedelic and sedated state. As shown above, thalamic FC with the DMN was preserved under psilocybin. Moreover, while thalamic FC with a right frontoparietal network was decreased under propofol, thalamic-TPN connectivity was actually increased under psilocybin. In summary, the results of the present study strongly imply that increased DMN-TPN FC, especially in the presence of preserved thalamocortical FC, is not an index of reduced consciousness but rather a change in the specific mode or style of consciousness.
Increased DMN-TPN coupling (or decreased inverse coupling) has been observed in patients with schizophrenia27–30; however, it is not known how this relates to symptomatology. Increased DMN-TPN coupling has been found in people at high risk of psychosis31 and an inability to distinguish between one’s internal world and the external environment, sometimes referred to as “disturbed ego boundaries,” is a hallmark of early psychoses32 and the psychedelic state.33,34 For example, one of our volunteers reported the following after psilocybin: “It was quite difficult at times to know where I ended and where I melted into everything around me.” And the following account is from a patient experiencing early psychotic symptoms: “My personality is in danger … my ‘self’ is beginning to disappear.”35
It is intriguing to consider whether increased DMN-TPN FC can explain such phenomena. Disturbed ego boundaries is a key component of spiritual-type experiences.36 It is curious therefore that increased DMN-TPN coupling has been found in experienced mediators,37 especially those practicing a form of meditation known as “nondual awareness,” which specifically promotes a unitary state of awareness in which there is no distinction between the subject and object.38 Supplementing the mapping between DMN activity and the sense-of-self, decreased DMN activity has also been found in meditation37,39,40 and the psychedelic state.24 There is increasing evidence that DMN functioning is related to the sense-of-self15 or “the ego,”16 and “ego dissolution” is commonly described in meditation and the psychedelic state. For example, one of our volunteers reported after psilocybin: “That was real ego death stuff, a total dissolving of the ego-boundaries ... I only existed as a concept ... as an idea.”
There is a fundamental motivation towards organization in biological systems. However, it is also know that biological systems retain a degree of stochasticity in their processes, so to enable flexibility. An imbalance in the relative influence of these 2 factors may occur in the psychedelic and early psychotic states. Characterizing the psychedelic and early psychotic states as states of relative disorganization yet heightened plasticity may enable us to explain a range of phenomena. For example, focusing on the psychedelic state, it is known that psychedelics can promote suggestibility, spiritual and religious revelation, and delusional thoughts; and there is also evidence that they can be effective treatments for addiction and perhaps other mental illnesses.2 All of these phenomena presumably rely on heightened plasticity in the brain. Translating this to psychosis lends emphasis to the importance of early intervention because it suggests that there is a window of opportunity for changes in associative learning in the early phase of the disorder.
To conclude, this study found increased DMN-TPN FC using 2 complementary FC analyses. Secondary analyses of thalamocortical FC were carried out to explore differences between the psychedelic and sedated state. These analyses revealed preserved thalamocortical FC, which is opposite to the effects of sedation. We propose that increased DMN-TPN coupling in the presence of preserved thalamocortical connectivity is related to a state in which arousal is preserved but the distinction between inner thought and external focus becomes blurred.
This is the first time that between-network FC has been assessed after a psychedelic. The findings make an important contribution to our understanding of the brain effects of these drugs. The phenomenological and neurobiological association between the psychedelic state, early psychosis and spiritual-type experiences suggest that psychedelics may serve as models of the prodrome to psychosis, as well as tools to deconstruct abstract concepts such as “the ego” and scientifically study mystical-type experiences.
Funding
Beckley Foundation ; Neuropsychoanalysis Foundation; Multidisplinary Association for Psychedelic Studies; and Heffter Research Institute.
Acknowledgments
We are grateful to the reviewers for improving this manuscript. We are also grateful to Alison Diaper, Ann Rich, Sue Wilson for help with this research.
References
- 1. Hofmann A, Frey A, Ott H, Petr Zilka T, Troxler F. [Elucidation of the structure and the synthesis of psilocybin]. Experientia. 1958;14:397–399 [DOI] [PubMed] [Google Scholar]
- 2. Grinspoon L, Bakalar JB. Psychedelic drugs reconsidered. New York: Basic Books; 1979. [Google Scholar]
- 3. Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology. 2006;187:268–283 [DOI] [PubMed] [Google Scholar]
- 4. Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871 [DOI] [PubMed] [Google Scholar]
- 5. Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond, B, Biol Sci. 2005;360:1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37:1083–1090 [DOI] [PubMed] [Google Scholar]
- 8. Zou Q, Wu CW, Stein EA, Zang Y, Yang Y. Static and dynamic characteristics of cerebral blood flow during the resting state. Neuroimage. 2009;48:515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Essen DC, Dierker DL. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron. 2007;56:209–225 [DOI] [PubMed] [Google Scholar]
- 10. Hagmann P, Cammoun L, Gigandet X, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci (Regul Ed). 2007;11:49–57 [DOI] [PubMed] [Google Scholar]
- 12. Harrison BJ, Pujol J, López-Solà M, et al. Consistency and functional specialization in the default mode brain network. Proc Natl Acad Sci USA. 2008;105:9781–9786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network’s role in spontaneous cognition. J Neurophysiol. 2010;104:322–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510 [DOI] [PubMed] [Google Scholar]
- 15. Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57:1221–1233 [DOI] [PubMed] [Google Scholar]
- 16. Carhart-Harris RL, Friston KJ. The default-mode, ego-functions and free-energy: a neurobiological account of Freudian ideas. Brain. 2010;133:1265–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9:23–25 [DOI] [PubMed] [Google Scholar]
- 20. Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537 [DOI] [PubMed] [Google Scholar]
- 21. Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage. 2010;52:590–599 [DOI] [PubMed] [Google Scholar]
- 23. Boveroux P, Vanhaudenhuyse A, Bruno MA, et al. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology. 2010;113:1038–1053 [DOI] [PubMed] [Google Scholar]
- 24. Carhart-Harris RL, Erritzoe D, Williams T, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci USA. 2012;109:2138–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carhart-Harris RL, Williams TM, Sessa B, et al. The administration of psilocybin to healthy, hallucinogen-experienced volunteers in a mock-functional magnetic resonance imaging environment: a preliminary investigation of tolerability. J Psychopharmacol. 2011;25:1562–1567 [DOI] [PubMed] [Google Scholar]
- 26. Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386 [DOI] [PubMed] [Google Scholar]
- 27. Salvador R, Sarró S, Gomar JJ, et al. Overall brain connectivity maps show cortico-subcortical abnormalities in schizophrenia. Hum Brain Mapp. 2010;31:2003–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130:86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chai XJ, Whitfield-Gabrieli S, Shinn AK, et al. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36:2009–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shim G, Oh JS, Jung WH, et al. Altered resting-state connectivity in subjects at ultra-high risk for psychosis: an fMRI study. Behav Brain Funct. 2010;6:58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bowers MB, Jr, Freedman DX. “Psychedelic” experiences in acute psychoses. Arch Gen Psychiatry. 1966;15:240–248 [DOI] [PubMed] [Google Scholar]
- 33. SAVAGE C. Variations in ego feeling induced by D-lysergic acid diethylamide (LSD-25). Psychoanal Rev. 1955;42:1–16 [PubMed] [Google Scholar]
- 34. Klee GD. Lysergic acid diethylamide (LSD-25) and ego functions. Arch Gen Psychiatry. 1963;8:461–474 [DOI] [PubMed] [Google Scholar]
- 35. Chapman J. The early symptoms of schizophrenia. Br J Psychiatry. 1966;112:225–251 [DOI] [PubMed] [Google Scholar]
- 36. Stace WT. Mysticism and philosophy. 1st ed. Philadelphia: Lippincott; 1960. [Google Scholar]
- 37. Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci USA. 2011;108:20254–20259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Josipovic Z, Dinstein I, Weber J, Heeger DJ. Influence of meditation on anti-correlated networks in the brain. Front Hum Neurosci. 2011;5:183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Farb NA, Segal ZV, Mayberg H, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci. 2007;2:313–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hasenkamp W, Barsalou LW. Effects of meditation experience on functional connectivity of distributed brain networks. Front Hum Neurosci. 2012;6:38 [DOI] [PMC free article] [PubMed] [Google Scholar]