Abstract
Background: Impaired insight is a common feature in psychosis and an important predictor of variables such as functional outcome, prognosis, and treatment adherence. A cognitive process that may underlie insight in psychosis is self-reflection, or the conscious evaluation of one’s traits and characteristics. The current study aims to investigate the neural correlates of self-reflective processing and its relationship with insight in schizophrenia. Methods: Forty-seven schizophrenia patients and 21 healthy controls performed a self-reflection task in a functional magnetic resonance imaging (fMRI) scanner. The tasks comprised a self-reflection, close other-reflection, and a semantic (baseline) condition. Insight scores were obtained with the Schedule of Assessment of Insight Expanded. In addition, cognitive insight scores were obtained (Beck Cognitive Insight Scale [BCIS]). Results: Schizophrenia patients demonstrated less activation in the posterior cingulate cortex in the self- and other-reflection conditions and less activation in the precuneus in the other-reflection condition compared with healthy controls. Better insight was associated with greater response in the inferior frontal gyrus, anterior insula, and inferior parietal lobule during self-reflection. In addition, better cognitive insight was associated with higher activation in ventromedial prefrontal cortex during self-reflection. Conclusion: In the current study, evidence for a relationship between self-reflection and insight in patients with schizophrenia was found in brain areas related to self-reflection, self/other distinction and source attribution. The findings support the rationale for a treatment that is currently under evaluation, which attempts to increase insight by enhancing self-reflection.
Key words: self-reflection, schizophrenia, psychosis, insight, cognitive insight, fMRI
Introduction
A growing body of research has described impaired insight as an important feature of schizophrenia occurring in 50%–80% of schizophrenia patients.1 Insight can be distinguished into clinical insight and cognitive insight.
Clinical insight is defined as a multidimensional construct2 encompassing three dimensions: (1) awareness of illness, (2) relabeling symptoms, and (3) recognizing need for treatment. Impaired insight has been linked to poor treatment compliance, poorer treatment outcome, overall symptom severity, higher relapse, lower self-esteem, and impaired psychosocial functioning (see ref.1 for an overview). Insight can be studied as a set of descriptive beliefs regarding mental illness, the presence of symptoms or as a personal narrative.3 It is not merely the acceptance of a fact, patients create a narrative that contains a life story, which in varying degrees includes the mental illness and differs to the extent in which it is coherent and adaptive.4 Earlier findings reported insight being one of the best discriminators among psychotic disorders.5 Insight thus is an important factor for a good prognosis, indicating that increasing awareness is of potential clinical relevance. Despite the efforts to understand the underlying mechanisms, the determinants of poor insight remain unclear. Previous research demonstrated a relationship between insight and symptomatology6; however, insight change is not simply a consequence of changes in psychopathology.7 This indicates that other factors must play a role in the etiology of impaired insight. Some evidence is found for both the psychological defense model and the neurocognitive model as an explanation for poor insight.8 In the psychological defense model, impaired insight is viewed as a form of denial in order to cope with the diagnosis.8 In the neurocognitive model, impaired insight is seen as the result of neurocognitive deficit—either general or specific.8 Several studies show a relationship between a variety of cognitive functions and impaired insight, such as intelligence, memory and executive functioning; more specifically, mental flexibility and error monitoring are repeatedly associated with lack of insight (see ref.9 for a meta-analysis).
Cognitive insight is a construct referring to the ability to evaluate abnormal experiences and recognize incorrect interpretations.10 Patients with impaired cognitive insight may not distance themselves from abnormal experiences and use feedback to correct their conclusions. Two dimensions of cognitive insight can be distinguished: (1) self-reflectiveness: “the patients’ capacity and willingness to observe their mental productions and to consider alternative explanations,” and (2) self-certainty: “overconfidence in the validity of their beliefs.”10 Although self-certainty seems to be state independent, self-reflectiveness improves when psychotic symptoms diminish.11 Interestingly, although insight as defined by David2 and cognitive insight showed a significant relationship in the initial validation,10 recent results are mixed suggesting that they may measure different constructs.12
Insight and Self-Reflection
It has been suggested that impaired insight may result from an inability to self-reflect.13–15 Self-reflection is defined as the process by which a person decides whether or not a certain attribution or environmental cue is applicable to the self, eventually resulting in a representation of one’s traits, abilities, and attitudes.14 Discriminating between self and others, emotional awareness and a sense of control over one’s thoughts and actions form the basis of self-reflection.16 Impaired self-reflection demonstrates the difficulties in generating personal narratives that link the present and past, resulting in an inadequate self-image not incorporating a psychiatric disorder or psychiatric symptoms.13,14 Recent meta-analyses investigating the neural correlates of self-reflection revealed a network of brain regions associated with self-reflective processing.14,17 Most importantly, this network encompasses the dorsomedial and ventromedial prefrontal cortex (dMPFC and vMPFC), the anterior cingulate cortex (ACC), posterior cingulate cortex (PCC) (together termed the cortical midline structures [CMS]), anterior insula, inferior frontal gyrus (IFG), and temporo-parietal junction/angular gyrus/inferior parietal lobule (IPL). In a cognitive neuropsychiatric model for self-reflection, we described two reflection routes: (1) other-reflective processing (ACC, PCC, insula/IFG and dMPFC) and (2) self-reflective processing involving the same structures, but with an additional role for the vMPFC in the emotional tagging of stimuli for self-relevance.14 Murray and colleagues17 recently extended this model by demonstrating that close other-reflection activation closely resembled self-reflection activation in the vMPFC, whereas distant other-reflection activation was more dorsally located. Their results suggest that the closer the other the more ventral the MPFC activation. This cognitive neuropsychiatric model will serve as a theoretical framework for the current study. A body of research demonstrated a relationship between impaired insight and smaller brain volumes in lateral, medial and orbitofrontal cortex and temporal and parietal areas (see for a recent overview Gerretsen et al.).18 Furthermore, white-matter abnormalities in fronto-temporal networks have been related to impaired insight.19 Despite these findings, there is a paucity of functional neuroimaging research looking into the relationship between brain functioning and impaired insight in psychiatric patients. Only two functional magnetic resonance imaging (fMRI) studies assessed the relationship between social processes and insight in schizophrenia patients (unpublished data).12,20 Lee and colleagues20 showed increased left vMPFC activation in patients in a remitted compared with an acute state while evaluating empathic and forgivability judgements. This increase of activation was related to improvement of insight and social functioning. Bedford and colleagues21 showed that higher levels of insight were related to higher activation during self-reflection in inferior parietal, superior frontal, and superior temporal areas, whereas lower levels of insight were related to higher activation in the right middle frontal cortex and precuneus. However, this study included only 11 schizophrenia patients, which makes replication important. In patients with mild cognitive impairment, a self-appraisal task induced less activation in the MPFC and PCC, which was related to impaired awareness of their loss of functioning.22
In the current study, we tested a self-reflection task in a large sample of schizophrenia patients. We used the cognitive neuropsychiatric model for self-reflection14 as a theoretical framework and hypothesized that abnormal self-reflective processing or diminished introspection is a core problem in impaired insight. We expected that this would be reflected in a relationship between impaired insight and abnormal activation in this self-reflection network. In addition, we explored the relationship between cognitive insight and self-reflection in the brain.
Methods
Participants
A total of 47 patients with a diagnosis of schizophrenia participated in the study (35 male, 12 female). We aimed to include patients with varying degrees of good insight on the one hand and of poor insight on the other. Therefore 42 patients, inpatients as well as outpatients, were recruited from several mental health institutions in the North of the Netherlands. We asked treating clinicians to screen their caseload and select patients based on three questions of the multidimensional construct of insight2 to estimate insight level and to ensure that both patients with good and with poor insight would be selected. Five patients came to us through patient and family organizations or the website of our study. Apart from one patient, their psychopathology was stable and they were aware of the availability of treatment facilities. Their demographic and clinical variables did not differ from the other patients. The diagnosis was confirmed with the Mini International Neuropsychiatric Interview-Plus 5.0.0 (MINI-Plus,23 which was also used to exclude psychiatric disorders in the controls). Current severity and frequency of symptoms was assessed by trained raters with the positive and negative syndrome scale (PANSS).24 Twenty-one healthy control subjects (12 male, 9 female) were recruited by means of flyers and posters and matched for age, gender, and education.
Patients had to be free from other psychiatric disorders and from somatic or neurological disorders that may influence the central nervous system. Patients had to be stable on current medication; medication changes the week prior to scanning was considered an exclusion criterion. Healthy controls had to be free from current and past psychiatric disorders. Other exclusion criteria were MRI-contraindications such as metal implants, red ink tattoos, pregnancy, and claustrophobia. All participants gave written informed consent, and the study had full approval of the Medical Ethics Committee. Participants received a monetary compensation (45 euros) for their participation. See table 1 for clinical and demographical characteristics.
Table 1.
Demographic and Clinical Characteristics for the Patient Group and the Healthy Control Group
| Variable | Schizophrenia Patients Mean (SD) | N | Healthy Controls Mean (SD) | N |
|---|---|---|---|---|
| Age (years)a | 34.3 (10.7) | 47 | 30.0 (11.0) | 21 |
| Level of educationb | 5.3 (1.1) | 47 | 5.8 (0.8) | 21 |
| Gender (percentage male)c | 75% | 47 | 57% | 21 |
| PANSS (score)d | ||||
| Total | 58.0 (13.4) | 46 | ||
| Positive | 14.8 (4.9) | 46 | ||
| Negative | 13.7 (4.6) | 46 | ||
| General psychopathology | 29.6 (7.0) | 46 | ||
| Insight d | ||||
| SAI-E | ||||
| Total | 13.0 (5.7) | 46 | ||
| Awareness | 8.2 (3.5) | 46 | ||
| Relabeling | 3.3 (2.0) | 46 | ||
| Treatment | 1.5 (1.5) | 46 | ||
| BCIS | ||||
| Composite score | 6.6 (5.0) | 46 | ||
| Self-reflectiveness | 17.4 (6.2) | 46 | ||
| Self-certainty | 10.8 (4.7) | 46 | ||
| Antipsychotic medication (n)e | ||||
| Olanzapine | 14 | |||
| Aripiprazole | 14 | |||
| Clozapine | 10 | |||
| Quetiapine | 7 | |||
| Risperidone | 2 | |||
| Haloperidol | 1 | |||
| Perfenazine | 1 | |||
| Pemozide | 1 | |||
| None | 2 | |||
aBetween group differences in age were nonsignificant (F(1,66) = 2.368; P = 0.127).
bEducation level according to Verhage (1964). Median did not differ between groups (P = 0.082).
cBetween group differences in gender distribution were nonsignificant (χ2 = 2 041; P = 0.153).
dFor one patient, neither insight nor PANSS scores were available. This subject was not included in the regression analysis.
eFor four subjects, information on current medication use was missing.
Tests and Measures
Insight (SAI-E). Insight was measured with the Schedule of Assessment of Insight-Expanded version (SAI-E),25 a 12-item semistructured interview measuring insight along the three dimensions of David2: relabeling symptoms, awareness of illness, and need for treatment. For subscale, a separate score can be calculated. Because not all participants were in treatment or gave permission to contact the treating clinician, the last three questions directed at the treating clinician could not be completed for all participants. To avoid exclusion of this patient group, we chose to use the subtotal score (items 1–9) of the SAI-E for all analyses. SAI-E subtotal scores and PANSS-G12 (a single PANSS item measuring insight) scores correlated significantly, indicating that both instruments measured the same construct (r = −0.67; P < 0.0001). Because the SAI-E has a more detailed distribution, these scores were used for further analyses with self-reflection results.
Cognitive Insight (BCIS). The Beck Cognitive Insight Scale (BCIS)10 is a self-report questionnaire containing two subcomponents: self-reflectiveness (eg, “Some of my experiences that have seemed very real may have been due to my imagination.”) and self-certainty (eg, “When people disagree with me, they are generally wrong.”). A separate self-certainty and self-reflectiveness score is obtained and a composite score is computed by subtracting the self-certainty score from the self-reflectiveness score.
Insight and Cognitive Insight. Pearson correlations revealed a significant relationship between SAI-E subtotal and BCIS-self certainty only (r = −0.334; P = 0.024). No relationship was found for SAI-E subtotal (P > 0.05) or the PANSS item G12 (P > 0.05) with either BCIS-self reflectiveness or BCIS composite. This suggests that the BCIS measures a somewhat different construct than the SAI-E and the PANSS G12.
Self-Reflection. Prior to the experiment, subjects re ceived task instructions and explanation of the experimental procedure. Participants had the opportunity to ask questions and provided a name of a person close to them to present in the other-condition. We chose a close-other, so the two conditions (self-other) would not differ too much in terms of amount of stored (and potentially activated) person-knowledge.
In the scanner, task instructions were once more presented on the screen.
The self-reflection task contained 180 sentences, subdivided into three main conditions (self, other, and semantic) of 60 sentences each, presented in E-prime (Psychology Software Tools Inc., Pittsburgh, PA). We employed a version of the task that had previously been validated and used by Modinos and colleagues26 to investigate neural correlates of self-reflection. In the self-condition, sentences referred to the participant, using pronouns as “I” or “me”; in the other-condition, sentences referred to a relative or close friend of the participant, who remained the same throughout the task. The subject to whom the sentence referred to (I/me/name close-other) was always presented on the screen. Subjects were asked to indicate to what extent the sentences were applicable to themselves or the other person. In the semantic baseline condition, containing sentences of general knowledge, participants were asked to indicate to what extent they agreed with the statement. Stimuli in self- and other-conditions were balanced for valence (positive [eg “I am a good friend”] and negative [eg “I get mad easily”]) and quality (mental properties [eg “I am honest”] and physical properties [eg “I smell bad”]). Sentences in the semantic condition contained an equal number of true (eg “dogs run faster than snails”) and false (eg “snow is black”) statements. Responses were given on a four-button response box. Response options were displayed on the screen as a reminder, ranging from 1 (fully disagree) to 4 (fully agree). Stimulus presentation lasted 4000ms followed by a 500ms fixation cross. Total task duration was approximately 15min. The three main conditions (self/other/semantic) were organized in a block design, each block containing five trials; subconditions (valence/quality) were organized event related. To keep the task as similar as possible between subjects, presentation of the three main conditions was semirandomized so that no two blocks per condition would be presented consecutively. Subconditions were presented fully randomized.
Image Acquisition
Functional and anatomical images were acquired using a 3.0 Tesla whole body scanner (Philips Intera, Best, NL). The head was kept in position by an elastic band and foam cushions on each side of the head. Stimuli were projected on a screen visible through a mirror attached to the sense 8 head coil. Functional images were acquired by T2*-weighted echo planar images sequences. Each functional image consisted of 37 interleaved axial slices of 3.5mm thick (slice gap = 0mm; TR = 2.00 s; TE = 30ms; FOV = 224.0, 129.5, 224.0mm; 64×64 matrix of 3.5×3.5×3.5 voxels). To prevent artefacts due to nasal cavities, images were tilted approximately 10° to the AC–PC transverse plane. A T1-weighted 3D fast field echo (FFE) anatomical image was acquired parallel to the bicommissural plane, covering the whole brain (170 slices; TR = 9ms; TE = 3.5ms; FOV = 232, 170, 256; voxel size: (1×1 × 1mm).
Statistical Analyses
Behavioral Analysis. PASW Statistics 18 (SPSS Inc., Chicago, IL) was used for analyzing behavioral data. Gender differences were assessed with chi-square tests. Self-reflection data (reaction times [RT]) were tested with a repeated measures ANOVA with “condition” (self, other, and semantic) as a within-subjects factor and “group” (healthy controls and schizophrenia patients) as a between-subjects factor. Significance levels for all behavioral analyses was P < .05 two-tailed.
fMRI Analysis. After converting from Philips PAR to Analyze in MRI-cro, fMRI data were preprocessed and analyzed using Statistical Parametric Mapping (SPM 8) (www.fil.ion.ucl.ac.uk) run in Matlab7 (The MathWorks Inc., Natick, MA). Orientation of functional images was manually adjusted to the anatomical image for each participant separately. Then, the functional images were slicetime corrected, realigned, and coregistered. After manually checking the coregistrations, images were spatially normalized on an MNI T1 template and smoothed using a 3D isotropic 10mm full-width/half-maximum Gaussian kernel. In first-level analysis, three regressors were modeled: self, other, and semantic. A high-pass filter of 1.1 times the longest period between two subsequent trials of the same condition was calculated to remove systematic low-frequency noise. For each subject, two contrasts were defined: (1) self > semantic and (2) other > semantic.
For second-level analyses, contrast images were entered into a 2 × 2 full factorial model, with condition (self and other) and group (patients and healthy controls) as factors. To verify whether the task-elicited activation in self-reflection areas, the overall task effect was examined by calculating the main effect for (self > semantic), (other > semantic), [(self > semantic) – (other > semantic)], and [(other > semantic) – (self > semantic)] for all subjects combined. Results were corrected for multiple comparisons (few, P < 0.05), minimum cluster size was set to 10 voxels.
Subsequently, group differences were examined for the same contrasts in region of interest analyses limiting the area of interest to self-reflection regions. Regions of interest (ROI) were drawn based upon a recent self-reflection meta-analysis.17 For each contrast, a separate ROI template was made by drawing a sphere of 20 mm (radius) around the cluster center coordinates reported by Murray et al.17 A total of four ROI templates were made: (1) self > semantic, (2) other > semantic, (3) self > other, and (4) other > self. For ROI templates 2, 3, and 4, we chose to use the meta-analytic results for close-other, because this is most similar to our task conditions. Threshold for group comparisons was set to P < 0.001 (uncorrected) and a minimum clustersize of 10 voxels.
Finally, second-level multiple-regression analyses were conducted to assess the relationship between insight and self-reflection. The contrast images of the patient group (for one patient, no insight scores were available. Therefore, this subject was not included in the regression analysis) were entered in the model and scores on overall insight, relabeling symptoms, awareness of illness, and need for treatment (SAI-E) were separately added as covariates. The analyses were restricted to the same ROIs as the group comparisons. Thresholds were set to P < 0.001 (uncorrected) and a minimum cluster size of 10 voxels. The same regression analyses were conducted for cognitive insight (composite scores, self-reflectiveness scores, and self-certainty scores [BCIS] were separately added as covariates).
Results
Self-Reflection: Behavioral Results
The repeated measures ANOVA for RT revealed a main effect for condition (F(2,63) = 5.36; P = 0.007) and group (F(1,64) = 9.35; P = 0.007). No significant group × condition interaction was demonstrated (P > 0.05). Thus, patients as well as healthy controls responded faster in the self-condition compared with the other- and semantic conditions, but patients responded slower overall than healthy controls (see table 2).
Table 2.
Reaction Times for Schizophrenia Patients and Healthy Controls
| Schizophrenia Patients (N = 45)a | Healthy Controls (N = 21) | |||
|---|---|---|---|---|
| Condition | % Items Agreed | Mean RT Seconds (SD) | % Items Agreed | Mean RT Seconds (SD) |
| Self | — | 2.38 (0.3) | — | 2.15 (0.4) |
| Positive | 59 | 2.31 (0.3) | 81 | 2.08 (0.4) |
| Negative | 22 | 2.46 (0.4) | 14 | 2.19 (0.3) |
| Other | — | 2.44 (0.4) | — | 2.25 (0.4) |
| Positive | 68 | 2.48 (0.4) | 79 | 2.22 (0.4) |
| Negative | 16 | 2.41 (0.4) | 17 | 2.32 (0.4) |
| Semantic | 53 | 2.52 (0.3) | 57 | 2.25 (0.4) |
aDue to technical problems, behavioral data were unavailable for two patients.
No significant differences were demonstrated when analyzing the valence of the participants’ attribution of positive and negative qualities to self or to other (P > 0.05).
Neuroimaging Results
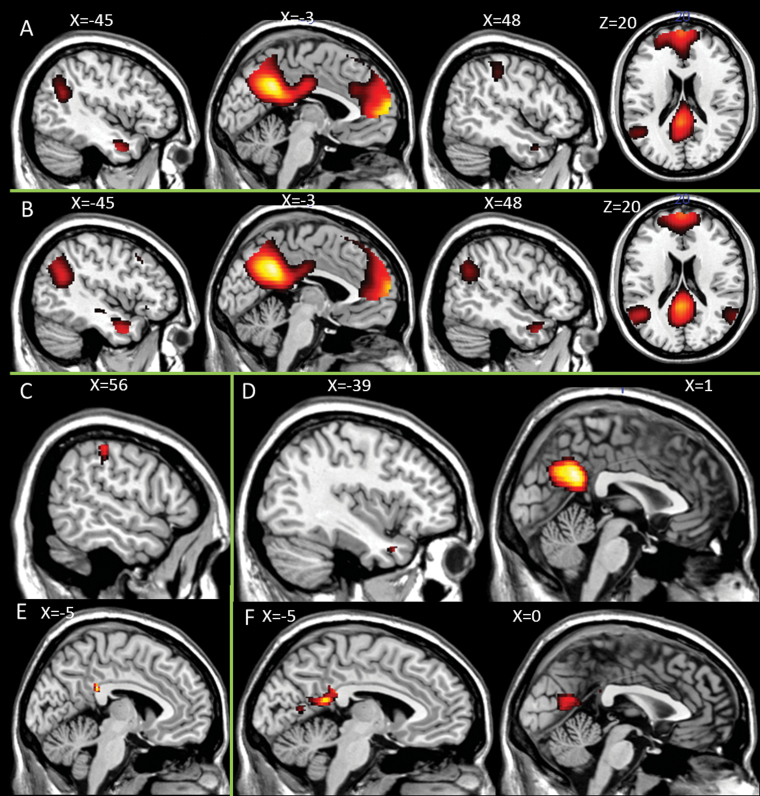
Overall Task Effect. The activation elicited by the self > semantic contrast for all subjects revealed significant activation in self-reflection areas: PCC, precuneus, vMPFC and dMPFC, superior temporal gyrus (STG), insula and IFG (figure 1A). The other > semantic contrast revealed activation patterns that were very similar to the self > semantic contrast: PCC, precuneus, vMPFC and dMPFC, middle temporal gyrus (MTG) and STG (figure 1B). The contrast self > other only revealed one cluster of activation including the supramarginal gyrus (SMG) and IPL (figure 1C). The inverse contrast other > self showed significant clusters of activation in the PCC, middle temporal gyrus (MTG), and STG (figure 1D; see table 3 for all peak activations).
Fig. 1.
Brain activation self-reflection task. Main effects for (A) self > semantic (B) other > semantic (C) self > other (D) other > self. Group differences healthy controls > patients. (E) self > semantic; (F) other > semantic.
Table 3.
Peak Activations Self-Reflection Task
| Region | BA | Clustersize (voxels) | T-value | MNI Coordinates | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Main task effect all subjects | |||||||
| Self > semantic | PCC/precuneus | 7/23/31 | 6578 | 16.10 | −6 | −52 | 34 |
| 9.09 | 0 | −20 | 32 | ||||
| vMPFC/dMPFC | 8/9/10/24/32 | 5135 | 13.8 | −2 | 64 | 12 | |
| 12.47 | −4 | 54 | 6 | ||||
| 9.55 | −12 | 58 | 32 | ||||
| MTG | 20/21/38 | 209 | 8 | −46 | −2 | −30 | |
| SMG/STG | 39/40 | 740 | 7.28 | −48 | −56 | 24 | |
| 7.12 | −52 | −62 | 36 | ||||
| IPL/SMG | 40/2/3 | 748 | 6.76 | 58 | −26 | 50 | |
| 6.01 | 62 | −20 | 40 | ||||
| 5.82 | 44 | −32 | 42 | ||||
| MTG | 21 | 35 | 5.72 | 50 | 8 | −30 | |
| IFG/insula | 47 | 70 | 5.61 | −28 | 18 | −14 | |
| STG | 39 | 15 | 4.99 | 58 | −60 | 26 | |
| Other > semantic | PCC/precuneus | 7/23/31 | 6825 | 21.77 | −6 | −52 | 34 |
| 8.56 | 0 | −22 | 30 | ||||
| vMPFC/dMPFC | 8/9/10/24/32 | 5250 | 16.31 | −2 | 64 | 14 | |
| 12.41 | −6 | 60 | 32 | ||||
| 10.68 | −8 | 50 | 44 | ||||
| STG/SMG | 22/39/40 | 1368 | 10.6 | −48 | −58 | 24 | |
| 10.33 | −52 | −64 | 34 | ||||
| STG/MTG/temporal pole | 13/21/38 | 982 | 10.41 | −50 | 0 | −28 | |
| 10.4 | −42 | 10 | −32 | ||||
| 8.94 | −56 | −8 | −16 | ||||
| STG/MTG/temporal pole | 21/38 | 335 | 8.82 | 50 | 8 | −30 | |
| 8.03 | 60 | −4 | −20 | ||||
| 4.98 | 36 | 16 | −20 | ||||
| SMG/STG | 39/40 | 778 | 8.38 | 58 | −62 | 26 | |
| 7.96 | 50 | −60 | 30 | ||||
| Self > other | SMG/IPL | 2 | 105 | 5.2 | 58 | −28 | 50 |
| 4.82 | 60 | −26 | 40 | ||||
| Other > self | PCC/Precuneus | 7/23/31 | 1460 | 7.14 | −2 | −52 | 30 |
| MTG | 21 | 92 | 5.61 | −54 | −4 | −18 | |
| 5.4 | −60 | −10 | −12 | ||||
| STG | 38 | 25 | 5.2 | −40 | 12 | −30 | |
| STG | 15 | 4.87 | 46 | −58 | 28 | ||
| Group differences healthy controls > schizophrenia patients | |||||||
| self > semantic | PCC | 23 | 21 | 3.42 | −6 | −38 | 28 |
| other > semantic | PCC/precuneus | 31 | 585 | 4.46 | −8 | −46 | 18 |
| 3.94 | −6 | −38 | 24 | ||||
| 3.76 | 4 | −68 | 16 | ||||
| Associations with insight for schizophrenia patients only | |||||||
| Self > semantic | |||||||
| SAI-E subtotal | Insula/IFG | 48 | 47 | 4.57 | −38 | 18 | 12 |
| IPL/angular gyrus | 39 | 110 | 4.40 | −36 | −64 | 42 | |
| SAI−E awareness of illness | Insula/IFG | 48 | 20 | 3.94 | −38 | 18 | 12 |
| IPL/angular gyrus | 39/40 | 126 | 4.58 | −36 | −66 | 44 | |
| 3.96 | −32 | −36 | 34 | ||||
| SAI-E relabeling symptoms | Insula/IFG | 48 | 66 | 4.18 | −38 | 16 | 12 |
| IPL/angular gyrus | 39/40 | 45 | 3.47 | −38 | −62 | 42 | |
| 18 | 3.21 | −44 | −50 | 30 | |||
| Associations with cognitive insight for schizophrenia patients only | |||||||
| Self > semantic | |||||||
| Self-reflectiveness | Left vMPFC | 32 | 71 | 4.06 | −14 | 44 | 10 |
| Right vMPFC | 10 | 62 | 3.81 | 10 | 54 | 12 | |
Group Differences. Healthy control subjects revealed increased activation in the PCC for the self > semantic contrast (figure 1E). The other > semantic contrast revealed increased activation for the healthy controls in the PCC and precuneus (figure 1F). No group differences were observed for the contrasts self > other or other > self. Patients did not reveal increased activation compared with the healthy controls in any of the contrasts (see table 3 for all peak activations).
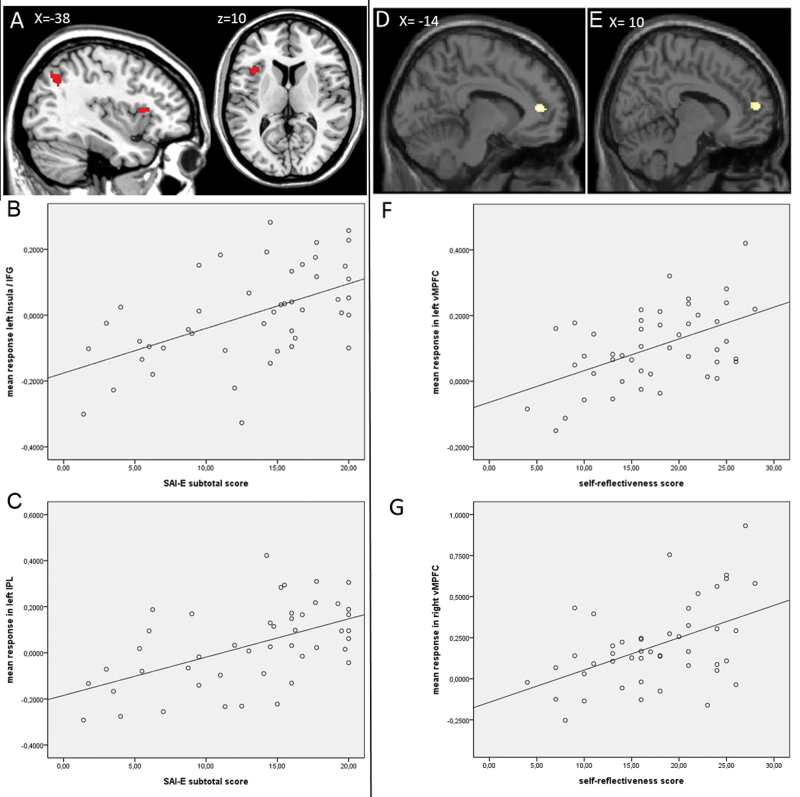
Self-Reflection and Insight. For the contrast self > semantic, a positive relationship was demonstrated for subtotal insight score in the left insula, left IFG, and left IPL/angular gyrus (figure 2A–C; table 3 for all peak activations). The subscales awareness of illness and relabeling of symptoms did not reveal any additional areas of activation; we did not find areas of activation related to need for treatment. No negative relationship with overall insight or any of the subscales was found. For the contrasts other > semantic, self > other, and other > self, no relationships with insight could be demonstrated.
Fig. 2.
Associations with insight and brain activation for self > semantic. (A) Activation positively related to insight subtotal score. Correlation mean response of activation with subtotal score of insight in (B) insula/IFG (r = 0.53) and (C) IPL/angular gyrus (r = 0.54). Activation positively related to self-reflectiveness in (D) left vMPFC and (E) right vMPFC. Correlation mean response of activation with self-reflectiveness in (F) left vMPFC (r = 0.51) and (G) right vMPFC (r = 0.48).
Self-Reflection and Cognitive Insight. FMRI analyses revealed a relationship between BCIS self-reflectiveness and activation in the bilateral vMPFC for the contrast self > semantic (see figure 2D–G; table 3 for all peak activations). No relationship was found in any of the other contrasts, for BCIS self-certainty, or the BCIS composite score related to activation in any of the contrasts.
Discussion
The current study aimed to investigate the relationship between the neural underpinnings of self-reflection and insight in a relatively large and heterogenuous sample of schizophrenia patients. Most importantly, we demonstrated that impaired insight is related to brain activation during self-reflection in the left insula, left IFG and left IPL/angular gyrus. Furthermore, we demonstrated a relationship between self-reflection activation and cognitive insight (BCIS) in the bilateral vMPFC. Schizophrenia patients in general showed hypoactivation for self-reflection, as well as other-reflection, in the PCC and precuneus compared with healthy control subjects. Task effects in the whole group analysis revealed activation that is consistent with the literature on self-reflection, which implies that our paradigm was valid.
Self-Reflection, Other-Reflection, and Schizophrenia
Both self- and other-reflection induced more brain activation in the PCC and precuneus in healthy controls compared with schizophrenia patients. PCC and precuneus have previously been related to self- and other-reflective processings,14,17 experiencing a sense of self,27 the evaluation of other people’s qualities,14,17 and the retrieval of autobiographical memories.28 Decreased anatomical and functional connectivity for schizophrenia patients in the PCC has been demonstrated, which points toward functional abnormalities in this region for this patient group.29 According to the cognitive neuropsychiatric self-reflection model, the PCC is involved in coupling past experiences to current self-relevant stimuli and may be required for decision-making processing regarding the self, as well as a close other.14 D’Argembeau and colleagues30 showed more activation in the PPC during reflection upon current self compared with past self. Decreased functioning of the PCC may hamper this coupling of experiences, limiting the ability to compare current and past self-relevant information and subsequently the ability to update the self-image and the image of the close-other. We found larger between group activation in other-reflection vs self-reflection. Modinos and colleagues26 also found this in the PCC, suggesting that retrieving information about another and mentalizing about another takes more effort than about self.
Other studies investigating similar constructs in patients with schizophrenia reported varying results (see also David et al.).12 Some studies demonstrated hypoactivation for patients with schizophrenia in the self- reflection network. Bedford and colleagues21 showed decreased activation for schizophrenia patients in medial and lateral frontal areas for self- and other-reflection vs baseline. Murphy and colleagues31 found similar results as the current study: hypoactivation in patients with schizophrenia for self vs baseline in the precuneus and lingual gyrus and for other vs baseline in IPL and lingual gyrus. They concluded that schizophrenia patients in general have an impaired awareness of self and others. Other studies showed hyperactivation in the PCC in schizophrenia patients for self vs baseline32 and self vs other33 contrasts. Such differences may be caused by slightly differing experimental paradigms and baseline control conditions. Holt and colleagues32 used positive vs negative valence evaluation and Shad and colleagues33 did not use a baseline condition. In our study, we used nonvalenced statements of general knowledge. This dimishes chances of associations with either self or other making it more reliable as a baseline condition. Moreover, for all four studies sample sizes were below 20 per group, so the results may not be robust.
Self-Reflection and Insight
Most importantly, we demonstrated a relationship between level of insight and brain activation in the left IFG, anterior insula, and left IPL/angular gyrus activation; better insight was related to higher activation in these areas. The region of activation in the left IFG and left anterior insula, also together termed ventrolateral prefrontal cortex (vLPFC), has been related to many cognitive processes, including the cognitive control of affective and nonaffective stimuli,34 theory of mind processing,35 and the integration of bodily and cognitive information.36 Direct relationships between subjective bodily and emotional feelings have been associated with activation in the anterior insular cortex.37 Modinos et al.38 demonstrated higher insula activation in subjects prone to psychosis during self-reflection, which was interpreted as a stronger emotional and interoceptive responses. The insula may be activated as an emotional response evoked by self-reflection.39 As such activation in the anterior insula may reflect an emotional reaction to self-reflection, rather than self-reflection itself. In patients with impaired insight, such an emotional response may fail to occur because the self-reflective process is hampered to begin with. Decreased activation in the vLPFC in patients with schizophrenia has been related to misattributions of self-generated speech and40 misattributions of mental states.41 Habel and colleagues42 showed that activation in the vLPFC in schizophrenia patients increased after following a training program for the improvement of affect recognition. Thus, the vLPFC seems important in the cognitive control of emotional and self-related processes. Reduced insight may result from an impairment in the integration of information coming from the outside world and internal information (eg beliefs about the self from autobiographical memory), resulting in a personal narrative that lacks complexity and does not include being ill or in need of treatment. It is not the mere acceptance of a fact, but the construction of a sufficiently rich narrative by integrating information into a coherent and adaptive account. A lack of such integration may be caused by malfunctioning of the vLPFC.
Besides the vLPFC, we found a relationship between insight and the IPL/angular gyrus. Literature suggests that the IPL plays an important role in movement evaluation and the inhibition of automatic actions43 and distinguishing the attribution of an action between the self and the another person.44 Ruby and Decety45 demonstrated that specifically the left IPL is involved in first-person perspective taking, whereas the right IPL is important in taking the perspective of others. Together, this suggests that this process of agency and distinguising between self and other may be hampered in patients with impaired insight.
Mapping these results on the cognitive neuropsychiatric model of self-reflection suggests that at least one node within the self-reflection network seems to be of importance in explaining impaired insight in schizophrenia: the IFG/insula or vLPFC, important in the integration of external and internal information. Furthermore, our results suggest that distinguishing between self and other, mediated by the IPL, is an important additional process for gaining insight in schizophrenia.
Self-Reflection and Cognitive Insight
Results demonstrated that self-reflectiveness is related to activation in the bilateral vMPFC during self-reflection. Hence, it appears that in our sample BCIS self-reflectiveness (SR) is, perhaps not surprisingly, much closer to the construct measured by our experimental self-reflectiveness task than the broader concept of insight and has activated the expected neural substrate. This could be further explored in healthy subjects in whom self-reflectiveness may be treated as a cognitive style or trait with the prediction that higher scores on the SR subscale would correlate with greater activation of cortical midline systems.
Another way of looking at this pattern comes from our previous work suggesting that the vMPFC may be specifically related to emotionally tagging stimuli for self-relevance.14 Murray and colleagues17 complemented our work and demonstrated that activation in the vMPFC is implicated in close-other reflection, as well as self-reflection, suggesting that the area may be salience related. Our results thus suggest that the ability to observe one’s own mental production may depend upon the ability to emotionally tag stimuli and recognize that a stimulus refers to the self. If the patient does not recognize this, then observing one’s own thoughts and evaluating alternative explanations is problematic. Insight is likely a multifactorial construct. In addition to self-reflection processes, other factors such as coping mechanisms and attributional style may contribute to insight. Further research could look at the role of these mechanisms in relation to the present findings.
Limitations
Some limitations need to be addressed. Antipsychotic medication may have influenced brain activation. However, in the most important analyses in the current study regarding insight, only patients were included. Because most patients did use some type of antipsychotics, possible medication effects will be averaged out. Possibly, the overall slower RTs in patients may have been caused by medication; however, this was not our main area of interest. Furthermore, for practical reasons, patients had to be stable on their current medication resulting in a patient sample with rather low levels of psychopathology. However, patients did show a wide variability of insight. In fact, it may be even more interesting to test the neural basis of insight in a stable group of patients. Although the relationship between symptomatology and insight is small, minimizing the moderative effect of symptoms should increase our chances on finding other underlying mechanisms of poor insight. Finally, measuring brain activation in the ventromedial prefrontal areas is prone to scanner artifacts. Although we tilted the scans 10 degrees, this may not have been enough to prevent the artifact. The study should be replicated with scanner parameters that limit chances on this artifact even more.
Conclusion
In sum, we propose that self-reflective processing is related to level of insight. More specifically, activation in areas important for the integrating internal and external stimuli (vLPFC) and distinguishing between self and other (IPL) show a positive relationship with level of insight. In addition, cognitive insight may be positively related to activation in the vMPFC. These results suggest that the process of self-reflection may contribute to gaining insight in one’s symptoms and one’s psychiatric condition. Further research should investigate whether teaching patients to closely evaluate their own personality, qualities, and characteristics may help them achieve insight in their disorder. Pijnenborg and colleagues46 examine a new treatment that attempts to enhance insight based on a model where self-reflection is increased. This is currently under evaluation.
Funding
European Science Foundation EURYI (NWO no. 044035001) awarded to A.A.
Acknowledgment
All authors deny any conflicts of interest or commercial associations connected to the submitted manuscript.We thank Drs R. Knegtering and R. Bruggeman and Mrs L. Bais for help with inclusion of patients. We also thank Dr R. Renken for advice and Mrs A. Sibeijn for scanning the participants.
References
- 1. Amador XF, David AS. Insight and Psychosis: Awareness of Illness in Schizophrenia and Related Disorders, Vol 2. Oxford: Oxford University Press; 2004. [Google Scholar]
- 2. David AS. Insight and psychosis. Br J Psychiatry. 1990;156:798–808 [DOI] [PubMed] [Google Scholar]
- 3. Roe D, Kravetz S. Different ways of being aware of a psychiatric disability: a multifunctional narrative approach to insight into mental disorder. J Nerv Ment Dis. 2003;191:417–424 [DOI] [PubMed] [Google Scholar]
- 4. Lysaker PH, Clements CA, Plascak-Hallberg CD, Knipscheer SJ, Wright DE. Insight and personal narratives of illness in schizophrenia. Psychiatry. 2002;65:197–206 [DOI] [PubMed] [Google Scholar]
- 5. Carpenter WT, Jr, Strauss JS, Bartko JJ. Flexible system for the diagnosis of schizophrenia: report from the WHO International Pilot Study of Schizophrenia. Science. 1973;182:1275–1278 [DOI] [PubMed] [Google Scholar]
- 6. Mintz AR, Dobson KS, Romney DM. Insight in schizophrenia: a meta-analysis. Schizophr Res. 2003;61:75–88 [DOI] [PubMed] [Google Scholar]
- 7. Wiffen BD, Rabinowitz J, Lex A, David AS. Correlates, change and ‘state or trait’ properties of insight in schizophrenia. Schizophr Res. 2010;122:94–103 [DOI] [PubMed] [Google Scholar]
- 8. Cooke MA, Peters ER, Kuipers E, Kumari V. Disease, deficit or denial? Models of poor insight in psychosis. Acta Psychiatr Scand. 2005;112:4–17 [DOI] [PubMed] [Google Scholar]
- 9. Aleman A, Agrawal N, Morgan KD, David AS. Insight in psychosis and neuropsychological function: meta-analysis. Br J Psychiatry. 2006;189:204–212 [DOI] [PubMed] [Google Scholar]
- 10. Beck AT, Baruch E, Balter JM, Steer RA, Warman DM. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68:319–329 [DOI] [PubMed] [Google Scholar]
- 11. Bora E, Erkan A, Kayahan B, Veznedaroglu B. Cognitive insight and acute psychosis in schizophrenia. Psychiatry Clin Neurosci. 2007;61:634–639 [DOI] [PubMed] [Google Scholar]
- 12. David AS, Bedford N, Wiffen B, Gilleen J. Failures of metacognition and lack of insight in neuropsychiatric disorders. Philos Trans R Soc Lond, B, Biol Sci. 2012;367:1379–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. David AS. “To see oursels as others see us.” Aubrey Lewis’s insight. Br J Psychiatry. 1999;175:210–216 [DOI] [PubMed] [Google Scholar]
- 14. van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev. 2010;34:935–946 [DOI] [PubMed] [Google Scholar]
- 15. Lysaker PH, Dimaggio G, Buck KD, et al. Poor insight in schizophrenia: links between different forms of metacognition with awareness of symptoms, treatment need, and consequences of illness. Compr Psychiatry. 2011;52:253–260 [DOI] [PubMed] [Google Scholar]
- 16. Dimaggio G, Vanheule S, Lysaker PH, Carcione A, Nicolò G. Impaired self-reflection in psychiatric disorders among adults: a proposal for the existence of a network of semi independent functions. Conscious Cogn. 2009;18:653–664 [DOI] [PubMed] [Google Scholar]
- 17. Murray RJ, Schaer M, Debbané M. Degrees of separation: a quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neurosci Biobehav Rev. 2012;36:1043–1059 [DOI] [PubMed] [Google Scholar]
- 18. Gerretsen P, Chakravarty MM, Mamo D, et al. Frontotemporoparietal asymmetry and lack of illness awareness in schizophrenia. Hum Brain Mapp. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Antonius D, Prudent V, Rebani Y, et al. White matter integrity and lack of insight in schizophrenia and schizoaffective disorder. Schizophr Res. 2011;128:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee KH, Brown WH, Egleston PN, et al. A functional magnetic resonance imaging study of social cognition in schizophrenia during an acute episode and after recovery. Am J Psychiatry. 2006;163:1926–1933 [DOI] [PubMed] [Google Scholar]
- 21. Bedford N, Surguladze S, Giampietro V, Brammer MJ, David AS . Self-evaluation in schizophrenia: an fMRI study with implications for the understanding of insight. BMC Psychiatry 2012;12:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ries ML, Jabbar BM, Schmitz TW, et al. Anosognosia in mild cognitive impairment: Relationship to activation of cortical midline structures involved in self-appraisal. J Int Neuropsychol Soc. 2007;13:450–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33;quiz 34 [PubMed] [Google Scholar]
- 24. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
- 25. Kemp R, Davis AS. Insight and compliance. In: Treatment Compliance and the Therapeutic Alliance. Amsterdam: Harwood academic publishers; 1997. 61–84 [Google Scholar]
- 26. Modinos G, Ormel J, Aleman A. Activation of anterior insula during self-reflection. PLoS ONE. 2009;4:e4618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814 [DOI] [PubMed] [Google Scholar]
- 28. Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–676 [DOI] [PubMed] [Google Scholar]
- 29. Skudlarski P, Jagannathan K, Anderson K, et al. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. D’Argembeau A, Feyers D, Majerus S, et al. Self-reflection across time: cortical midline structures differentiate between present and past selves. Soc Cogn Affect Neurosci. 2008;3:244–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murphy ER, Brent BK, Benton M, et al. Differential processing of metacognitive evaluation and the neural circuitry of the self and others in schizophrenia: a pilot study. Schizophr Res. 2010;116:252–258 [DOI] [PubMed] [Google Scholar]
- 32. Holt DJ, Cassidy BS, Andrews-Hanna JR, et al. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biol Psychiatry. 2011;69:415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shad MU, Keshavan MS, Steinberg JL, et al. Neurobiology of self-awareness in schizophrenia: an fMRI study. Schizophr Res. 2012;138:113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci (Regul Ed). 2005;9:242–249 [DOI] [PubMed] [Google Scholar]
- 35. Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum Brain Mapp. 2009;30:2313–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Damasio A. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York, NY: Harcourt Brace; 1999. [Google Scholar]
- 37. Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 2011;1225:72–82 [DOI] [PubMed] [Google Scholar]
- 38. Modinos G, Renken R, Ormel J, Aleman A. Self-reflection and the psychosis-prone brain: an fMRI study. Neuropsychology. 2011;25:295–305 [DOI] [PubMed] [Google Scholar]
- 39. Fossati P, Hevenor SJ, Graham SJ, et al. In search of the emotional self: an fMRI study using positive and negative emotional words. Am J Psychiatry. 2003;160:1938–1945 [DOI] [PubMed] [Google Scholar]
- 40. Allen PP, Amaro E, Fu CH, et al. Neural correlates of the misattribution of self-generated speech. Hum Brain Mapp. 2005;26:44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Russell TA, Rubia K, Bullmore ET, et al. Exploring the social brain in schizophrenia: left prefrontal underactivation during mental state attribution. Am J Psychiatry. 2000;157:2040–2042 [DOI] [PubMed] [Google Scholar]
- 42. Habel U, Koch K, Kellermann T, et al. Training of affect recognition in schizophrenia: Neurobiological correlates. Soc Neurosci. 2010;;5:92–104 [DOI] [PubMed] [Google Scholar]
- 43. Grezes J. Top down effect of strategy on the perception of human biological motion: a pet investigation. Cogn Neuropsychol. 1998;15:553–582 [DOI] [PubMed] [Google Scholar]
- 44. Brunet-Gouet E, Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Res. 2006;148:75–92 [DOI] [PubMed] [Google Scholar]
- 45. Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nat Neurosci. 2001;4:546–550 [DOI] [PubMed] [Google Scholar]
- 46. Pijnenborg GH, Van der Gaag M, Bockting CL, Van der Meer L, Aleman A. REFLEX, a social-cognitive group treatment to improve insight in schizophrenia: study protocol of a multi-center RCT. BMC Psychiatry. 2011;11:161 [DOI] [PMC free article] [PubMed] [Google Scholar]