Abstract
Background: Amongst schizophrenia patients, a large subgroup of up to 25% also suffers from comorbid obsessive-compulsive symptoms (OCSs). The association between comorbid OCSs in these patients and neuropsychological impairment remains unclear and somewhat contradictory. Longitudinal approaches investigating the stability of OCS-associated cognitive deficits are missing. Methods: Thirty-seven patients with schizophrenia and comorbid OCSs and 43 schizophrenia patients without OCS were assessed with a comprehensive cognitive test battery and compared at baseline and, again, 12 months later. Results: Schizophrenia patients with comorbid OCSs showed significant pronounced deficits, with increasing effect sizes over the 12-month assessment period in specific cognitive areas such as visuospatial perception and visual memory (WAIS-R block design, Rey–Osterrieth Complex Figure Test), executive functioning (perseveration in the Wisconsin Card Sorting test), and cognitive flexibility (Trail Making test B). These cognitive domains are correlated with OCS severity and are known to be candidate cognitive domains in obsessive-compulsive disorder (OCD). Conclusions: OCSs in schizophrenia is associated with specific and longitudinally stable cognitive deficits, strongly arguing for at least partially overlapping neurobiological mechanisms with OCD. Prospective studies involving patients with at-risk mental states for psychosis are necessary to decipher the interaction of cognitive impairment and the clinical manifestations of schizophrenia and OCSs. This might facilitate the definition of patients at high risk for OCSs, an early detection of subclinical levels, therapeutic interventions, and clinical monitoring.
Key words: schizophrenia, obsessive-compulsive symptoms, cognitive deficits, comorbidity, neuropsychology, psychosis
Introduction
Patients with schizophrenia have a high life-time risk to suffer from comorbid obsessive-compulsive symptoms (OCSs). Epidemiological studies estimate that 12% of schizophrenia patients also fulfill the criteria for obsessive-compulsive disorder (OCD) and that every fourth patient reports obsessive, distressing, intrusive thoughts and related compulsions.1,2
During the past decade, an increasing body of evidence showed that the existence of comorbid OCSs in schizophrenia patients is associated with high subjective burden of disease, poorer social and vocational function,3 greater service utilization,4 and heightened levels of anxiety and depression5 and that these additional impairments may contribute to a poor overall prognosis. A recent meta-analysis by Cunill et al reported more severe global, positive, and negative symptoms in the presence of OCSs.6
However, a large variability and heterogeneity within the investigated comorbid samples should be mentioned. Within these limitations, some studies on the early course of schizophrenia suggested a protective effect of specific OCS on other clinical variables or overall functionality,7–9 whereas others reported lower subject well-being and quality of life even in the early course of the illness.10
Apart from investigating clinical impairment and social functioning among comorbid patients, the question arose whether OCSs in schizophrenia might also be linked to a specific pattern of cognitive deficits.11 Subsequent efforts to differentiate schizophrenia patients with vs without co-occurring OCSs on levels of neuropsychological variables produced contradicting results. Some studies did not find any significant differences,1,12–17 whereas others suggested that OCSs may be associated with better performance on some cognitive functions in schizophrenia.18,19 Most findings, however, reported larger deficits in specific cognitive domains for schizophrenia patients with comorbid OCSs. These include executive functioning,11,20,21 cognitive flexibility,22,23 and visual memory.24 Furthermore, these studies found correlations between higher levels of OCSs and poorer delayed visual memory, as well as poorer executive functioning and decreased cognitive flexibility as measured with the Wisconsin Card Sorting test (WCST) and the Trail Making test B (TMT-B).
Reasons for inconsistent results may be heterogeneity within the clinical samples and the applied neurocognitive tests, a lack of power due to small sample sizes, or the fact that often only 1 or 2 specific cognitive domains have been evaluated within the same sample. In addition, it remains unknown whether the reported cognitive deficits in the comorbid sample are stable over time, because longitudinal assessments are almost completely missing. Only Lysaker et al. prospectively analyzed executive functioning in comorbid patients and reported that the presence of deficits in the inhibition domain of executive function was linked with greater concurrent and prospective self-report of OCSs among patients with schizophrenia.20
The aim of this longitudinal study was to investigate how schizophrenia patients with or without comorbid OCSs differ in a comprehensive cognitive test battery and whether these differences persist over time. We, therefore, evaluated cognitive domains normally impaired in schizophrenia patients without OCSs25 and, in addition, included cognitive areas described to be impaired in primary OCD patients such as visual–spatial abilities, visual memory, inhibitory control, and cognitive shifting abilities.26,27
Methods
Hypothesis and Primary Endpoint
We hypothesized that patients with schizophrenia and comorbid OCSs (OCS+) would show larger deficits in specific cognitive domains known to be impaired in primary OCD patients, when compared to schizophrenia patients without OCSs (OCS–) and that these pronounced deficits would be stable over time. As part of a comprehensive longitudinal project, wherein we recruited schizophrenia patients in antipsychotic monotherapy, we investigated cross-sectional between-group differences in a cognitive test battery and performed longitudinal analyses over 12 months. In addition, we examined whether the presented cognitive deficits were correlated with OCS severity.
Participants
Recruitment details and the inclusion and exclusion criteria have been reported elsewhere (Schirmbeck F, Rausch F, Englisch S, et al., in preparation),28 where a subsample of 70 patients was stratified according to pharmacological aspects. In short, the criteria were diagnosis of schizophrenia or schizoaffective disorder according to DSM IVR (Diagnostic and Statistical Manual, version IV, Research), stable antipsychotic monotherapy with second-generation antipsychotic agents (SGA; namely, clozapine, olanzapine, amisulpride, or aripiprazole), absence of treatment with serotonergic antidepressant, and substance abuse or addiction (with the exception of nicotine). The investigation was approved by the ethical committee of the University of Heidelberg (2008-235N-MA) and performed in complete agreement with the guidelines of good clinical practice. After providing the participants with a complete description of the study, written informed consent was obtained.
Clinical Assessments
Sociodemographic data on age, gender, level of education, age of onset, duration of illness, and pharmacological treatment were collected. Patients were assessed at baseline and 12 months later by a trained and certified rater (F.S.) using a set of clinical rating scales: OCSs were measured with the Yale Brown Obsessive-Compulsive Scale (YBOCS). Compulsions and obsessions are rated separately on five 5-point (0–4) scale items (time, handicap, frequency, controllability, and discomfort), yielding subtotal scores for compulsions and for obsessions (each ranging from 0 to 20).29 In addition, a self-rating questionnaire with 72 items assessing 6 different subtypes of obsessions and compulsions, including checking, washing, ordering, and counting behavior, as well as nonaggressive and aggressive obsessions, was administered (Hamburger–Zwangsinventar, HZI).30 Current severity of the psychotic disorder was measured with the Positive and Negative Syndrome Scale (PANSS). We further applied the Calgary Depression Scale for Schizophrenia (CDSS), the Clinical Global Impression Scale (CGI), and the Personal and Social Performance Scale (PSP).
Neuropsychological Assessment
At both assessments, patients completed a neuropsy chological test battery investigating cognitive domains that are often reported to be impaired in patients with schizophrenia, such as processing speed, working memory, executive functioning, attention, and verbal learning and memory. At the same time, we included those cognitive functions that have been reported impaired in patients with OCD, such as tests of cognitive shifting abilities, inhibitory control, visual–spatial skills, and delayed visual memory. The following instruments were included in the battery and predefined outcome measures selected:
The Trail Making Test (TMT) Part A measures psychomotor speed and attention, whereas Part B requires additional cognitive shifting abilities. The key measures were the times needed to complete each task.
The WCST evaluates executive functions and requires strategic planning, the ability to use feedback, to shift cognitive sets, and the ability to modulate impulsive responding. Number of categories completed and percent of perseverative errors constituted outcome measures.
The Stroop Color and Word test was used as a measure of semantic interference. The interference score evaluates the inhibitory capacity to name the color of ink a word is printed in by ignoring the word itself, which spells out a different color. The time spent on this task was used as our outcome variable.
To assess further aspects of attention, 4 computer-based subtests of the Test Battery for the Assessment of Attentional Dysfunction (TAP) were applied: a Go/NoGo task, which measured response inhibition; a set-shift task, assessing the ability to shift attention between 2 modalities; an N-back task, measuring working memory based on the “2-back” principle, and a Continuous Performance Task (CPT) measuring sustained attention. Accuracy scores as the number of mistakes were selected for each subtest.
In addition, the d2 test was administered to evaluate selective attention by detecting the target letter d with 2 dashes as quickly and as accurately as possible. We selected the concentration index as our outcome measure, which takes accuracy and processing speed into account.
The Rey–Osterrieth Complex Figure Test (ROCFT) was administered to evaluate visuospatial memory. The test involves the copying and the immediate and 30-min delayed drawing of a complex line figure. The immediate reproduction and memory score were selected as the outcome measure. In addition, the Block Design task of the Wechsler Adult Intelligence Scale (Revised) was also used to measure visual–spatial skills.
The German version of the Rey Auditory-Verbal Learning Test (AVLT) was included to measure verbal learning and memory. The test consists of the immediate recall of a word list, as well as the recall after the presentation of a distraction list, and a delayed recall after a 30-min period. Of the several memory parameters, we selected the immediate recall score, the interference score (number of words lost after distraction), and the delayed recall score (number of words lost after a 30-min delay).
Finally, a multiple-choice vocabulary intelligence spot-the-word test (Mehrfachwahl-Wortschatz-Intelligenz test; MWT-B) assessed premorbid verbal intelligence. Available parallel test versions were used for the reassessment at follow-up.
Statistical Analysis
Statistical analyses were performed using the Statistical package for Social Sciences (IBM SPSS version 20.0). Nonnormal distributed parameters were compared between groups with Mann-Whitney U tests. Sociodemographic and psychopathological characteristics were compared between treatment groups using Student t tests and χ2-tests. Differences in neuropsychological characteristics were assessed using analysis of covariance (ANCOVA). We further estimated the standardized difference (effect size) between groups using Cohen’s d (d = mean OCS+ minus mean OCS–/pooled SD). Pearson’s correlation analyses were performed between assessed OCS severity (YBOCS, HZI) and clinical variables as well as performance in neurocognitive tasks.
Outliers were defined as extreme scores that were greater than 3 box lengths away from the upper or lower edge of the box values (indicating the middle 50% of scores).
To investigate the course of OCSs over the 12-month period, between-group comparisons were investigated with a repeated-measure analysis of variances (rmANOVA) and post hoc paired t test. Regarding these longitudinal comparisons, we calculated analyses for the sample of patients who completed the neuropsychological tasks at both time points of assessment (per protocol, PP; n = 50) and also analyzed data for the initial sample size (n = 80) with the baseline date carried forward (last observations carried forward or LOCF samples).
Results
Sociodemographic Characteristics and Clinical Assessments
We included 80 patients with schizophrenia (n = 75) or schizoaffective disorder (n = 5), as diagnosed by DSM IVR, in our study. Patients were grouped according to OCS severity: OCS+ (YBOCS total score ≥8) vs OCS– (YBOCS total score <8). Based on interpretation guidelines, we defined presence of OCSs as an YBOCS total score of at least 8, representing mild symptom severity.31 Table 1 summarizes the sociodemographic characteristics and antipsychotic treatment. The cohorts did not differ regarding age, gender, age of onset of the psychotic disorder, years of education, or estimated premorbid verbal intelligence. However, patients with comorbid OCSs reported longer illness duration. All patients received second-generation antipsychotics (SGA) in monotherapy, but the representation of the individual substances varied between groups.
Table 1.
Sociodemographic Characteristics and Antipsychotic Medication
| OCS+ (n = 37) | OCS– (n = 43) | Between-Group Differences | |
|---|---|---|---|
| Sociodemographics | |||
| Age | 39.7±10.8 | 35.8±10.0 | T = 1.674; P > .05 |
| Male/female ratio | 27:10 | 29:14 | χ2 = 0.290; P > .05 |
| Age of onset (years of age) | 26.4±6.3 | 29.0±8.1 | T = –1.557; P > .05 |
| Duration of illness (years) | 13.2±9.2 | 7.0±7.1 | T = 3.405; P = .001 |
| Education (years) | 10.7±1.7 | 11.1±1.9 | T = –1.078; P > .05 |
| Premorbid intelligence | 103.3±14.4 | 106.6±14.9 | T = –1.003; P > .05 |
| Antipsychotic medication | |||
| Clozapine | 22 | 7 | χ2 = 33.518; P < .001 |
| Olanzapine | 12 | 5 | |
| Amisulpride | 1 | 15 | |
| Aripiprazole | 2 | 16 | |
Note: The table summarizes group-specific means and standard deviations, as well as evidence for group-dependent differences regarding sample size, age, gender, age at first psychotic manifestation, duration of illness, education, premorbid intelligence, and current intake of antipsychotic medication. OCS, obsessive-compulsive symptom.
As a consequence of group definition, OCS severity scores significantly differed regarding the YBOCS subscores obsessions (Z = –7.740, P < .001) and compulsions (Z = –6.988, P < .001), as well as the total score (Z = –7.964, P < .001) and the 2 HZI subscales “checking” (Z = –4.512, P < .001) and “counting” (Z = –3.315, P = .001). The mean YBOCS score of 16.5±5.7 in OCS+ patients reflects a mean severity of illness commonly considered an indication for treatment. The large majority of these patients (82%) reported onset of OCSs after the onset of psychosis, whereas the remaining experienced OCS development before or at the same time as the first psychotic manifestation. Patients with comorbid OCSs showed significantly higher scores in the PANSS negative and general psychopathology (GPP) subscales but not in the section assessing positive symptoms or in comorbid depressive symptoms, as depicted by the CDSS. They also scored higher in clinical global impression and lower on measures of psychosocial functioning. Impairment in these variables significantly correlated with OCS severity, as measured with the YBOCS total score (table 2).
Table 2.
Symptom Severity and General Functioning
| OCS+ (n = 37) | OCS– (N = 43) | Between-Group Differences | Correlations With YBOCS Total Score | |
| PANSS | ||||
| Positive Scale | 13.9±2.7 | 13.3±3.1 | T = 0.981; P > .05 | r = .10; P > .05 |
| Negative Scale | 18.0±4.1 | 14.7±4.8 | T = 3.249; P = .002 | r = .29; P = .009 |
| General Psychopathology | 34.9±3.8 | 32.6±5.2 | T = 2.170; P = .033 | r = .22; P = .049 |
| CDSS | 1.7±1.9 | 1.3±2.2 | T = 0.822; P > .05 | r = .09; P > .05 |
| General Functioning | ||||
| CGI | 3.8±0.6 | 3.2±0.7 | χ 2 = 14.249; P = .003 | r = .35; P = .001 |
| PSP | 65.9±5.8 | 69.6±7.9 | T = –2.510; P = .014 | r = –.26; P = .021 |
Note: Significant values are noted in bold. The table summarizes group-specific means and standard deviations and evidence for group-dependent differences regarding psychotic and affective symptoms as measured with the PANSS and CDSS, as well as the general psychosocial functioning. CDSS, Calgary Depression Scale for schizophrenia; CGI, Clinical global impression; OCS, obsessive-compulsive symptom; PANSS, Positive and Negative Syndrome Scale; PSP, Personal and Social Performance Scale; YBOCS, Yale Brown Obsessive Compulsive Scale.
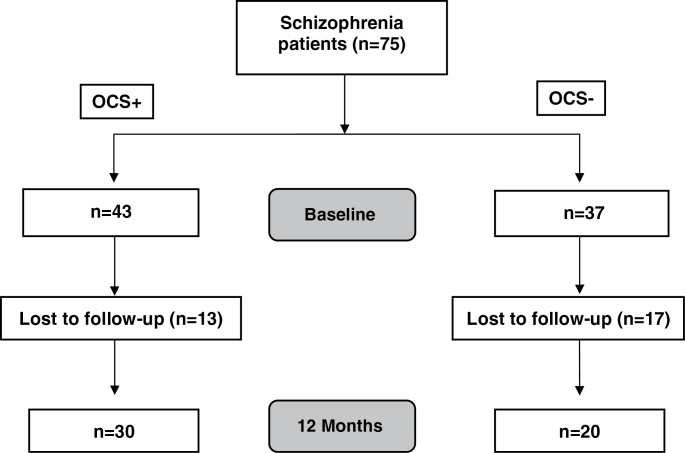
During the course of the study, several patients were lost to follow-up (see figure 1). Within groups, dropouts did not significantly differ from completers regarding sociodemographic variables such as age, education in years, MWT-B, age of onset, or duration of illness. However, patients who dropped out of OCS– showed higher scores in the 3 PANSS subscales (positive: T = –3.460, P = .001; negative: T = –3.115, P = .003; and GPP: T = –2.136, P = .039) and higher illness severity as measured with the CGI (χ2 = 9.161, P = .010). We did not observe any significant differences between dropouts and completers within the OCS+ group.
Fig. 1.
Consort diagram of sample flow. OCS, obsessive-compulsive symptom.
Pharmacological details for OCS+ and OCS– at follow-up did not change significantly regarding antipsychotic treatment, but 3 patients in OCS+ were treated with serotonergic antidepressants at follow-up. With the mean YBOCS total score increasing to 18.2±6.0 in the OCS+ group, the difference in OCS severity remained highly significant between groups (YBOCS: obsessions: Z = –5.699, P < .001; compulsions: Z = –5.984, P < .001; total: Z = –5.961, P < .001; HZI: checking: Z = –3.679, P < .001; and counting: Z = –2.285, P = .022). Due to the loss of more severely impaired patients within OCS–, an additional between-group difference in the PANSS positive subscale (T = 2.579, P = .013) was observed at the 12-month follow-up and subsequently inserted as a covariate for the second assessment period.
Longitudinal Assessment of Comorbid OCSs and Associated Cognitive Deficits
All between-group comparisons for the 16 cognitive domains are presented for baseline (table 3) and follow-up after 12 months (table 4). To account for significant between-group differences in duration of illness and the PANSS subscales, analyses were performed by means of ANCOVA integrating these factors as covariates. We also calculated Cohen’s d effect sizes to present standardized magnitudes of between-group differences. Cross-sectional analysis revealed comparable levels of performance in several neuropsychological domains commonly attributed to the cognitive deficits of psychosis. These were verbal memory as measured with the delayed recall and interference scores in the AVLT, attention and continuous performance according to the d2 and CPT tests, as well as working memory assessed with the N-back task.
Table 3.
Group-Dependent Performance in Neuropsychological Tests at Baseline
| Baseline | Group I (OCS; N = 37) | Group II (Non-OCS; N = 43) | Between-Group Differences ANCOVA Illness Duration and PANSS Negative /GPP | Effect Size Cohen’s d | Pearson Correlation With YBOCS Total Scores | Pearson Correlation With HZI “Checking” | Pearson Correlation With HZI “Counting” |
| Processing speed | |||||||
| TMT-A | 38.3±16.3 | 33.7±14.8 | F = 5.700; P < .001 | d = .30 | r = .15; P > .05 | r = .13; P > .05 | r = –.07; P > .05 |
| Executive function & working memory | |||||||
| WCST | |||||||
| Categories completed | 5.8±1.7 | 6.4±1.2 | F = 1.418; P > .05 | d = .41 | r = –.06; P > .05 | r = .01; P > .05 | r = .01; P > .05 |
| Perseveration errors (%) | 32.6±16.7 | 19.1±16.6 | F = 4.381; P = .003 | d = .81 | r = .36; P = .002 | r = .31; P = .007 | r = .09; P > .05 |
| Stroop | |||||||
| Interference | 100.8±21.8 | 89.4±29.5 | F = 3.838; P = .008 | d = .44 | r = .14; P > .05 | r = .22; P >.05 | r =.17; P >.05 |
| Go/NoGo | 2.0±2.3 | 1.0±1.7 | F = 4.464; P = .003 | d = .49 | r = .37; P = .001 | r = .30; P =.009 | r =.14; P >.05 |
| Set shift | 4.2±5.7 | 1.5±2.4 | F = 3.443; P = .013 | d = .62 | r = .44; P < .001 | r = .29; P =.015 | r =.2;1 P >.05 |
| TMT-B | 97.6±39.6 | 76.9±48.0 | F = 5.086; P = .001 | d = .47 | r = .28; P = .017 | r = .20; P >.05 | r =.21; P >.05 |
| N-back | 3.8±4.2 | 3.4±3.7 | F = 0.577; P > .05 | d = .10 | r = .08; P > .05 | r = .25; P =.039 | r = .07; P >.05 |
| Verbal learning and memory | |||||||
| AVLT | |||||||
| Immediate recall | 45.9±11.4 | 50.0±10.2 | F = 3.003; P = .025 | d = .38 | r = –.17; P > .05 | r = –.18; P > .05 | r = –.07; P > .05 |
| Interference | 1.7±3.1 | 1.9±1.9 | F = 0.292; P > .05 | d = .08 | r = .05; P > .05 | r = .08; P > .05 | r = .21; P > .05 |
| Delayed recall | 1.9±2.5 | 2.4±2.5 | F = 0.849; P > .05 | d = .20 | r = –.16; P > .05 | r = –.08; P > .05 | r = .03; P > .05 |
| Visual memory and perception | |||||||
| Rey figure test | |||||||
| Reproduction | 34.0±14.9 | 44.4±15.8 | F = 4.469; P = .003 | d = .68 | r = –.34; P = .003 | r = –.39; P = .001 | r = .17; P > .05 |
| Memory | 98.8±40.7 | 127.7±41.1 | F = 5.232l; P = .001 | d = .71 | r = –.37; P = .001 | r = –.42; P <.001 | r = –.23; P = .05 |
| WAIS block design | 29.3±12.8 | 34.8±10.0 | F = 4.695; P = .002 | d = .48 | r = –.20; P = .05 | r = –.32; P = .008 | r = –.07; P > .05 |
| Attention | |||||||
| d2 | 132.1±48.6 | 137.5±40.9 | F = 0.573; P > .05 | d = .12 | r = .03; P > .05 | r = .01; P > .05 | r = .18; P > .05 |
| CPT | 12.7±9.1 | 12.1±8.7 | F = 2.085; P > .05 | d = .07 | r = –.11; P > .05 | r = –.15; P > .05 | r = –.16; P > .05 |
Table 4.
Group-Dependent Performance in Neuropsychological Tests at 12-Month Follow-up
| Follow-Up (PP) | OCS+ (N = 20) | OCS– (N = 30) | Between-Group Differences ANCOVA Illness Duration And PANSS Positive/Negative/GPP | Effect Size Cohen’s d | Pearson Correlation With YBOCS Total Score | Pearson Correlation With HZI “Checking” | Pearson Correlation With HZI “Counting” |
|---|---|---|---|---|---|---|---|
| Processing speed | |||||||
| TMT-A | 39.8±18.8 | 31.1±10.7 | F = 3.026; P = .020 | d = .57 | r = .35; P = .015 | r = .15; P > .05 | r = –.05; P > .05 |
| Executive function & working memory | |||||||
| WCST | |||||||
| Categories completed | 6.0±1.7 | 6.6±1.0 | F = 3.005; P = .021 | d = .43 | r = .05; P > .05 | r = –.14; P > .05 | r = .09; P > .05 |
| Perseveration errors (%) | 34.4±16.0 | 15.6±13.6 | F = 2.762; P = .030 | d = 1.27 | r = .46; P = .001 | r = .25; P > .05 | r = .08; P > .05 |
| Stroop | |||||||
| Interference | 98.4±21.0 | 86.1±22.7 | F = 1.281; P > .05 | d = .56 | r = .22; P > .05 | r = .16; P > .05 | r = –.04; P > .05 |
| Go/NoGo | 1.4±1.3 | 0.7±0.8 | F = 1.230; P > .05 | d = .65 | r = .26; P = .066 | r = .13; P > .05 | r = .09; P > .05 |
| Set shift | 1.9±1.7 | 1.3±1.6 | F = 0.622; P > .05 | d = .36 | r = .12; P > .05 | r = .12; P > .05 | r = –.03; P > .05 |
| TMT–B | 99.4±47.6 | 63.0±22.0 | F = 8.646; P < .001 | d = .98 | r = .46; P = .001 | r = .42; P = .003 | r = .19; P > .05 |
| N-back | 2.7±2.7 | 2.6±2.5 | F = 0.331; P > .05 | d = .04 | r = .03; P > .05 | r = –.01; P > .05 | r = –.01; P > .05 |
| Verbal learning and memory | |||||||
| AVLT | |||||||
| Immediate recall | 42.7±11.8 | 51.9±10.3 | F = 2.976; P = .022 | d = .83 | r = –.37; P = .011 | r = –.18; P > .05 | r = –.20; P > .05 |
| Interference | 2.3±1.6 | 1.7±1.5 | F = 1.108; P > .05 | d = .39 | r = .02;; P > .05 | r = –.02; P > .05 | r = –.13; P > .05 |
| Delayed recall | 3.0±1.5 | 2.6±2.0 | F = 0.554; P > .05 | d = .23 | r = .08; P > .05 | r = –.08; P > .05 | r = –.20; P > .05 |
| Visual memory and perception | |||||||
| Rey figure test | |||||||
| Reproduction | 35.8±13.3 | 47.7±13.4 | F = 2.510; P = .44 | d = .89 | r = –.44; P = .001 | r = .35; P = .013 | r = –.12; P > .05 |
| Memory | 106.2±34.1 | 136.8±33.6 | F = 2.000; P > .05 | d = .90 | r = –.41; P = .003 | r = .34; P = .016 | r = –.10; P > .05 |
| WAIS – Block design | 28.0±12.6 | 37.6±8.7 | F = 4.365; P = .003 | d = .89 | r = –.39; P = .007 | r = –.26; P > .05 | r = –.13; P > .05 |
| Attention | |||||||
| d2 | 150.5±51.4 | 153.3±38.3 | F = 1.378; P > .05 | d = .06 | r = .04; P > .05 | r = –.05; P > .05 | r = .24; P > .05 |
| CPT | 15.1±11.1 | 10.0±9.8 | F = 1.518; P > .05 | d = .49 | r = .15; P > .05 | r = .02; P > .05 | r = –.07; P > .05 |
Note: Tables 3 and 4 summarize means and standard deviations of the 2 groups in 16 neurocognitive variables. Comparisons between groups were calculated using ANCOVAs, and Pearson’s correlation analyses were performed with YBOCS total scores and the HZI subscales checking and counting. AVLT, Rey Auditory-Verbal Learning Test; CPT, Continuous Performance test; GPP, general psychopathology; HZI, Hamburger–Zwangsinventar; OCS, obsessive-compulsive symptom; PP, per protocol; TMT-A and -B, Trail Making tests A and B; WAIS, Wechsler Adult Intelligence Scale, subtest block design; WCST, Wisconsin Card Sorting test; YBOCS, Yale Brown Obsessive Compulsive Scale.
However, the OCS+ group showed significantly higher deficits in visuospatial perception and visual memory (block design, Rey figure), more frequent perseveration errors (WCST), lower inhibitory control (Stroop, Go/NoGo), poorer cognitive flexibility (TMT-B, Set-shift task), and also lower levels in the immediate recall of verbal stimuli (AVLT; see table 3). One year later, the repeated cross-sectional neuropsychological assessment showed similar results within the sample of completers (OCS+: n = 20, OCS–: n = 30). Except for differences in the Go/NoGo task, set-shift task, Stroop and Rey visuospatial memory score, all between-group comparisons remained significant (see table 4). Calculated Cohen’s d showed large effect sizes at follow-up for perseveration errors (WCST), performance in the TMT-B, immediate verbal recall (AVLT), and the 3 scores assessing visuospatial abilities and visual memory (block design, Rey figure).
Because our OCS+ group showed greater incidence with clozapine treatment, we calculated additional analyses within the OCS+ group, comparing individuals under clozapine treatment (n = 22) to those receiving another SGA (n = 15). We did not find any significant differences between these 2 subgroups with comorbid OCSs in any of the 16 cognitive domains, except for a better performance of the no-clozapine group in the TMT-A at baseline (F = 4.603, P = .009) and at follow-up (F = 3.601, P = .025).
We were interested in the longitudinal course of neurocognitive performance in the study samples and calculated repeated-measure ANOVAs (rmANOVA), both for patients with complete data sets (PP) and in a conservative intend-to-observe analysis, where the last observations were carried forward (LOCF). In line with the cross-sectional analyses, PP rmANOVAs showed significant Group and Time effects, whereas significant interaction effects of Group × Time were only found for the set-shift task (F = 4.810, P = .033) due to a trend of improvement in the OCS+ group (paired t test: T = 6.257, P = .064). LOCF analyses of the 80 patients who were initially included in the study revealed equivalent Group and Time effects, but the interaction effects of Group × Time in the set-shift task (F = 3.747, P = .057) did not reach significance.
In order to analyze the association between pronounced cognitive impairments and comorbid OCS severity, correlation analyses within the complete study sample were performed. Results revealed significant associations with the above-reported cognitive domains of executive functioning (WCST perseveration scores), visual perception and memory (Rey reproduction and Rey memory), inhibitory control (Go/NoGo), and cognitive flexibility (set-shift and TMT-B; see table 3). Repeated correlation analyses 1 year later again showed similar results, with the exceptions of the Go/NoGo task, which just missed statistical significance, and the set-shift task. At this assessment time, additional significant correlations between OCS severity and immediate verbal recall (VLMT) as well as performance in the block design task were found (table 4). We also calculated correlation analyses between the HZI subscales “checking” and “counting,” representing the most frequent compulsions in our sample, and performance in the cognitive tasks (tables 3 and 4). The results for the subscale “checking” at baseline remarkably reflected the correlations with the YBOCS total score, except for nonsignificant results for performance in the TMT-B and additional significant correlations with the block design task. However at follow-up, only correlations with the TMT-B and Rey figure remained significant. None of the correlations with the subscale “counting” reached significance at either assessment (tables 3 and 4). Furthermore, we did not observe any significant correlations between changes in the YBOCS total score (difference between scores at 12 months and baseline) and changes in any of the 16 cognitive domains.
Discussion
Previous studies comparing schizophrenia patients with vs without comorbid OCSs described inconsistent differences in cognitive impairment, most likely due to a high degree of heterogeneity within the OCS+ samples. Reasons for this heterogeneity are diverse and range from different severity scores defining groups for comparison and presented OCS subtypes to the usage of different clinical rating scales and neurocognitive tests. The aim of this study was to investigate the neurocognitive profile of OCSs in schizophrenia in a well-defined sample of clinically stable patients using a comprehensive set of psychometric and neuropsychological instruments in a longitudinal perspective over a period of 12 months. In line with our hypothesis, the 2 groups did not significantly differ in their performance in some cognitive domains commonly associated with schizophrenia.25 However, at both assessment times, OCS+ patients showed more deficits in tasks measuring visuospatial perception and visual memory, cognitive shifting abilities, and immediate verbal learning as well as higher perserveration tendencies with increasing effect sizes over time. Subsequent correlation analyses revealed persistent associations between the severity of comorbid OCSs and impaired performance in these cognitive domains.
In contrast to our findings, a number of previous studies did not find any significant differences in cognition between OCS+ and OCS– patients with schizophrenia.12,14–17 In order to understand the possible reasons for these contradictory findings, we evaluated differences in study designs and outcomes in more detail. Whitney et al compared schizophrenia patients with and without comorbid OCSs to 11 primary OCS patients. Although they did not find any significant differences between the 2 schizophrenia groups, the OCS+ group showed a clear trend to higher impairments than the other 2 groups in most neuropsychological domains. The lack of significance could be explained by smaller sample sizes and consequently lower power.12 Tumkaya et al compared OCS+ and OCS– patients with schizophrenia to OCD patients with and without insight and did not find significant differences on any neuropsychological tasks between OCS+ and OCS– in their post hoc analyses. However, with only 16 patients, the OCS+ group was rather underrepresented and a second-order error cannot be excluded.15 The work group around Hermesh et al investigated between-group differences in orbitofrontal cortex functioning using an alternation learning task. They did not find significant differences between their OCS+ and OCS– groups, due to a curvilinear relationship between the severity of OCSs and task performance. Although low OCS severity correlated negatively with deficits in alternation learning, they did find a positive correlation with severer OCSs.16 Therefore, their results are in line with our findings of more impaired difficulties in tasks measuring orbitofrontal functioning in schizophrenia patients with clinically relevant OCSs. Meijer et al mainly focused their comparisons on schizophrenia-associated cognitive domains and did not find any differences.17 Accordingly, we did not observe a general poorer performance within our OCS+ group, but found very specific pronounced deficits in certain other cognitive domains. These observations confirm other studies that also pointed to a particular pattern of neuropsychological deficits. Especially higher impairment in executive functioning, such as a higher tendency for perseveration errors in the WCST, more difficulties in cognitive flexibility tasks, and lower inhibitory control have been reported before.11,20,22,24,33,33 In summary, the majority of reports show more pronounced cognitive deficits in the OCS+ group and results suggest that these patients could be characterized by their performance in a specific set of cognitive domains rather than by poorer overall cognitive functioning. Until now, investigations assessing the longitudinal course and stability of these deficits have however been limited to the above-mentioned study by Lysaker et al.20 In line with their findings on executive function, reported deficits in our OCS+ group appeared highly stable during the 12-month observational period.
The link between comorbid OCSs in schizophrenia and cognitive impairment raises the question of causality. It has been suggested that higher deficits in these cognitive domains reflect the additive presence of both the structural and functional abnormalities known to be associated with schizophrenia and OCD per se.34 Accordingly, greater deficits of our comorbid patients appeared in domains commonly associated with the functional connectivity between prefrontal or orbitofrontal cortex, the striatum and thalamus, in line with current pathomechanistic theories of OCD and converging results of neuroimaging studies.35 This assumption may lead to the hypothesis that these impairments reflect an underlying endophenotype and risk factor to develop OCSs. It has been proposed previously by Poyurovski et al that candidate endophenotypic markers, including cognitive functioning, could be used to characterize the so-called schizo-obsessive subtype of schizophrenia.2 Following this hypothesis, we would assume cognitive impairments to precede the actual clinical onset of comorbid OCSs. So far, prospective studies are missing and because none of our patients developed de novo OCSs during the course of the study and most of them suffered from schizophrenia prior to their secondary OCSs, our data do not contribute to this assumption. In order to answer this question of causal interactions, further prospective examination of at-risk mental state and first-episode patients are needed.
Another hypothesis might see additional cognitive impairment as a consequence of comorbid OCSs. Observed covariations between changes in OCS severity and subsequent increase or decrease of cognitive deficits would support this explanation. We did not find significant interaction effects in our analyses nor significant correlations between changes in OCS severity and changes in cognitive performance. This is most likely due to a lack of variance, because our OCS+ group reported persisting OCSs and only slight changes in their YBOCS scores. Other studies, however, showed high percentages of fluctuation in reported comorbid OCSs,3 especially in the early course of schizophrenia. It would be interesting to see whether cognitive deficits covary accordingly over time.
One of the above-mentioned reasons for the heterogeneity of previous findings has been linked to the presence of different subtypes of OCSs within the studied samples. Even studies in primary OCD patients showed that different OCS subtypes were associated with different neuropsychological impairment.36 Omori et al reported poorer performance on tests of response inhibition and cognitive flexibility in patients with OCD characterized by prominent checking symptoms when compared with patients with primarily washing symptoms.37 Similarly, we found significant correlations between pronounced cognitive deficits in perseveration, cognitive flexibility, and visuospatial memory and reported checking behavior, whereas these associations did not reach significance when looking at counting compulsions. However, larger studies are needed to investigate possible OCS subtype-specific associations with cognitive processes in schizophrenia. Because our patients mainly reported checking and counting symptoms, a generalization of our findings to schizophrenia patients with other subtypes of comorbid OCSs, such as hoarding or cleaning, is not substantiated.
Regarding further limitations, the 2 cohorts differed in relevant clinical variables at baseline as reflected in higher PANSS scores and longer illness duration within the OCS+ group. However, by including these factors as covariates, we account for group differences. It is, therefore, very unlikely that the observed specific pattern of cognitive deficits was a mere consequence of severer psychotic symptoms. We also carefully controlled for intellectual abilities in order to exclude another potential confounding factor. Our study selected for patients in stable SGA monotherapy with 4 broadly used substances; therefore, a generalization to patients in polypharmacy or under treatment with first-generation antipsychotics is limited and needs further exploration. Furthermore, our groups differed with regard to the type of atypical antipsychotic treatment. Because treatment with antiserotonergic SGAs (especially clozapine) is perceived as an important causative factor for second-onset OCSs in schizophrenia (Schirmbeck F, Zink M. Obsessive-compulsive syndromes in schizophrenia: A case for polypharmacy? In: Ritsner M, ed. Polypharmacy in Psychiatric Practice, in preparation),38 we cannot rule out that medication effects influenced our results. However, the link between OCSs and cognitive impairment seems to be largely independent, because we did not observe significant differences between OCS comorbid patients being treated with clozapine vs those receiving another SGA, except for a slower reaction time of the first group in the TMT-A. In addition, in a previously published article, we found similar correlations between OCSs and cognitive deficits within the pharmacologically more homogeneous subgroup of patients treated with clozapine and olanzapine.28 Our study samples consisted mostly of schizophrenia patients in later disease stages who reported persistent clinically relevant OCS severity. Therefore, our results are limited to this cohort and associations between OCSs and cognitive domains in earlier disease stages need further replication.
In conclusion, schizophrenia patients with comorbid OCSs suffer from stable and somewhat specific neurocognitive deficits. An improved knowledge about OCSs in schizophrenia and their association with pronounced psychopathological and cognitive impairment is of clinical importance, as it is a possible predictor for a poorer prognosis. Further studies should try to unravel the involved genetic risk factors, pharmacological triggers, and neural correlates using functional magnetic imaging. In perspective, understanding the neuropsychological profile of OCSs in schizophrenia might facilitate risk definition, early detection of subclinical levels, therapeutic interventions and clinical monitoring. The multimodal treatment of these comorbid OCSs in schizophrenia should be an important focus of interventional studies using pharmacological methods as well as cognitive behavioral therapy.
Funding
This work was funded by an unrestricted scientific grant to F. Schirmbeck by the Evangelisches Studienwerk. No further third-party-funding.
Acknowledgments
S. Eifler, F. Rausch., and Dr Esslinger were not supported by third parties. Dr Englisch received travel expenses and consultant fees from AstraZeneca, Bristol-Myers Squibb GmbH & CoKGaA, Eli-Lilly, Janssen Cilag, Lundbeck, Otsuka Pharma, Pfizer Pharma, and Servier. Prof. Meyer-Lindenberg received consultant fees and travel expenses from AstraZeneca, Hoffmann-La Roche, and Lundbeck Foundation, in addition to speaker′s fees from Pfizer Pharma, Lilly Deutschland, Glaxo SmithKline, Janssen Cilag, Bristol-Myers Squibb, Lundbeck, and AstraZeneca. Prof. Zink received unrestricted scientific grants of the European Research Advisory Board (ERAB), German Research Foundation (DFG), Pfizer Pharma GmbH, Servier and Bristol Myers Squibb Pharmaceuticals; further speaker and travel grants were provided from Astra Zeneca, Lilly, Pfizer Pharma GmbH, Bristol Myers Squibb Pharmaceuticals, and Janssen Cilag. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Achim AM, Maziade M, Raymond E, Olivier D, Mérette C, Roy MA. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull. 2011;37:811–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Poyurovsky M, Zohar J, Glick I, et al. Obsessive-compulsive symptoms in schizophrenia: implications for future psychiatric classifications. Compr Psychiatry. 2012;53:480–483 [DOI] [PubMed] [Google Scholar]
- 3. de Haan L, Sterk B, Wouters L, Linszen DH. The 5-year course of obsessive-compulsive symptoms and obsessive-compulsive disorder in first-episode schizophrenia and related disorders [published online ahead of print July 28, 2011]. Schizophr Bull10.1093/schbul/sbr077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berman I, Kalinowski A, Berman SM, Lengua J, Green AI. Obsessive and compulsive symptoms in chronic schizophrenia. Compr Psychiatry. 1995;36:6–10 [DOI] [PubMed] [Google Scholar]
- 5. Lysaker PH, Whitney KA. Obsessive-compulsive symptoms in schizophrenia: prevalence, correlates and treatment. Expert Rev Neurother. 2009;9:99–107 [DOI] [PubMed] [Google Scholar]
- 6. Cunill R, Castells X, Simeon D. Relationships between obsessive-compulsive symptomatology and severity of psychosis in schizophrenia: a systematic review and meta-analysis. J Clin Psychiatry. 2009;70:70–82 [DOI] [PubMed] [Google Scholar]
- 7. Poyurovsky M, Fuchs C, Weizman A. Obsessive-compulsive disorder in patients with first-episode schizophrenia. Am J Psychiatry. 1999;156:1998–2000 [DOI] [PubMed] [Google Scholar]
- 8. Üçok A, Ceylan ME, Tihan AK, Lapçin S, Ger C, Tükel R. Obsessive compulsive disorder and symptoms may have different effects on schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:429–433 [DOI] [PubMed] [Google Scholar]
- 9. de Haan L, Hoogenboom B, Beuk N, van Amelsvoort T, Linszen D. Obsessive-compulsive symptoms and positive, negative, and depressive symptoms in patients with recent-onset schizophrenic disorders. Can J Psychiatry. 2005;50:519–524 [DOI] [PubMed] [Google Scholar]
- 10. de Haan L, Sterk B, van der Valk R. Presence of obsessive compulsive symptoms in first-episode schizophrenia or related disorders is associated with subjective well-being and quality of life. Early Interv Psychiatry. 2012; 10.1111/j.1751-7893.2012.00377 [DOI] [PubMed] [Google Scholar]
- 11. Lysaker PH, Bryson GJ, Marks KA, Greig TC, Bell MD. Association of obsessions and compulsions in schizophrenia with neurocognition and negative symptoms. J Neuropsychiatry Clin Neurosci. 2002;14:449–453 [DOI] [PubMed] [Google Scholar]
- 12. Whitney KA, Fastenau PS, Evans JD, Lysaker PH. Comparative neuropsychological function in obsessive-compulsive disorder and schizophrenia with and without obsessive-compulsive symptoms. Schizophr Res. 2004;69:75–83 [DOI] [PubMed] [Google Scholar]
- 13. Ongür D, Goff DC. Obsessive-compulsive symptoms in schizophrenia: associated clinical features, cognitive function and medication status. Schizophr Res. 2005;75:349–362 [DOI] [PubMed] [Google Scholar]
- 14. Tiryaki A, Ozkorumak E. Do the obsessive-compulsive symptoms have an effect in schizophrenia? Compr Psychiatry. 2010;51:357–362 [DOI] [PubMed] [Google Scholar]
- 15. Tumkaya S, Karadag F, Oguzhanoglu NK, et al. Schizophrenia with obsessive-compulsive disorder and obsessive-compulsive disorder with poor insight: a neuropsychological comparison. Psychiatry Res. 2009;165:38–46 [DOI] [PubMed] [Google Scholar]
- 16. Hermesh H, Weizman A, Gur S, et al. Alternation learning in OCD/schizophrenia patients. Eur Neuropsychopharmacol. 2003;13:87–91 [DOI] [PubMed] [Google Scholar]
- 17. Meijer JH, Swets M, Keeman S, Nieman DH, Meijer CJ. Genetic Risk and Outcome of Psychosis (GROUP) Investigators Does a schizo-obsessive subtype exist from a cognitive perspective? Results from a large cross-sectional study in patients with psychosis and their unaffected relatives. J Nerv Ment Dis In press [DOI] [PubMed] [Google Scholar]
- 18. Lee MJ, Shin YB, Sunwoo YK, et al. Comparative Analysis of Cognitive Function in Schizophrenia with and without Obsessive Compulsive Disorder. Psychiatry Investig. 2009;6:286–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borkowska A, Pilaczyñska E, Rybakowski JK. The frontal lobe neuropsychological tests in patients with schizophrenia and/or obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 2003;15:359–362 [DOI] [PubMed] [Google Scholar]
- 20. Lysaker PH, Whitney KA, Davis LW. Associations of executive function with concurrent and prospective reports of obsessive-compulsive symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci. 2009;21:38–42 [DOI] [PubMed] [Google Scholar]
- 21. Hwang MY, Morgan JE, Losconzcy MF. Clinical and neuropsychological profiles of obsessive-compulsive schizophrenia: a pilot study. J Neuropsychiatry Clin Neurosci. 2000;12:91–94 [DOI] [PubMed] [Google Scholar]
- 22. Patel DD, Laws KR, Padhi A, et al. The neuropsychology of the schizo-obsessive subtype of schizophrenia: a new analysis. Psychol Med. 2010;40:921–933 [DOI] [PubMed] [Google Scholar]
- 23. Kumbhani SR, Roth RM, Kruck CL, Flashman LA, McAllister TW. Nonclinical obsessive-compulsive symptoms and executive functions in schizophrenia. J Neuropsychiatry Clin Neurosci. 2010;22:304–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berman I, Merson A, Viegner B, Losonczy MF, Pappas D, Green AI. Obsessions and compulsions as a distinct cluster of symptoms in schizophrenia: a neuropsychological study. J Nerv Ment Dis. 1998;186:150–156 [DOI] [PubMed] [Google Scholar]
- 25. Kern RS, Gold JM, Dickinson D, et al. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126:124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuelz AK, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: a critical review. Biol Psychol. 2004;65:185–236 [DOI] [PubMed] [Google Scholar]
- 27. Rajender G, Bhatia MS, Kanwal K, Malhotra S, Singh TB, Chaudhary D. Study of neurocognitive endophenotypes in drug-naïve obsessive-compulsive disorder patients, their first-degree relatives and healthy controls. Acta Psychiatr Scand. 2011;124:152–161 [DOI] [PubMed] [Google Scholar]
- 28. Schirmbeck F, Esslinger C, Rausch F, Englisch S, Meyer-Lindenberg A, Zink M. Antiserotonergic antipsychotics are associated with obsessive-compulsive symptoms in schizophrenia. Psychol Med. 2011;41:2361–2373 [DOI] [PubMed] [Google Scholar]
- 29. Woody SR, Steketee G, Chambless DL. Reliability and validity of the Yale-Brown Obsessive-Compulsive Scale. Behav Res Ther. 1995;33:597–605 [DOI] [PubMed] [Google Scholar]
- 30. Zaworka W, Hand I, Jauernig G, Lünenschloss K. Hamburger Zwangsinventar. Manual. Weinheim, Federal Republic of Germany: Beltz, 1983. [Google Scholar]
- 31. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–1011 [DOI] [PubMed] [Google Scholar]
- 32. Hwang MY, Kim SW, Yum SY, Opler LA. Management of schizophrenia with obsessive-compulsive features. Psychiatr Clin North Am. 2009;32:835–851 [DOI] [PubMed] [Google Scholar]
- 33. Lysaker PH, Lancaster RS, Nees MA, Davis LW. Patterns of obsessive-compulsive symptoms and social function in schizophrenia. Psychiatry Res. 2004;125:139–146 [DOI] [PubMed] [Google Scholar]
- 34. Gross-Isseroff R, Hermesh H, Zohar J, Weizman A. Neuroimaging communality between schizophrenia and obsessive compulsive disorder: a putative basis for schizo-obsessive disorder? World J Biol Psychiatry. 2003;4:129–134 [DOI] [PubMed] [Google Scholar]
- 35. Del Casale A, Kotzalidis GD, Rapinesi C, et al. Functional neuroimaging in obsessive-compulsive disorder. Neuropsychobi ology. 2011;64:61–85 [DOI] [PubMed] [Google Scholar]
- 36. Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564–576 [DOI] [PubMed] [Google Scholar]
- 37. Omori IM, Murata Y, Yamanishi T, et al. The differential impact of executive attention dysfunction on episodic memory in obsessive-compulsive disorder patients with checking symptoms vs. those with washing symptoms. J Psychiatr Res. 2007;41:776–784 [DOI] [PubMed] [Google Scholar]
- 38. Schirmbeck F, Zink M. Clozapine-induced obsessive-compulsive symptoms in schizophrenia: a critical review. Curr Neuropharmacol. 2012;10:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]