Abstract
Distributed abnormalities of gray matter (GM) and white matter (WM) volume characterize individuals experiencing their first episode of schizophrenia. Regions of abnormality are present already, albeit less extensively, during the prodromal phase of illness. This study aimed to determine whether putatively at-risk children, aged 9–12 years, who present multiple antecedents of schizophrenia (ASz), display GM and WM volume abnormalities relative to typically developing (TD) children presenting no antecedents. Structural magnetic resonance images were acquired for 20 ASz children and 20 TD children matched on age, sex, and IQ. Whole-brain differences in GM and WM volume were determined using voxel-based morphometry. Relative to the TD group, ASz children showed significantly decreased GM volume in the right middle temporal gyrus (MTG) and increased GM volume in the left superior-middle temporal gyri (P < 0.05, cluster correction). WM volume was significantly increased in ASz children relative to TD children in a cluster encompassing the left inferior parietal lobe, occipital lobe, and superior temporal gyrus. Post-hoc analyses indicated that these abnormalities were not limited to ASz children who self-reported auditory hallucinations on questionnaire. Our findings suggest that children aged 9–12 years who present multiple ASz are characterized by abnormalities of GM and WM volume in the temporal lobes, comprising a subset of the regions affected in first-episode schizophrenia and in the prodromal phase of illness. These preliminary findings indicate that structural brain abnormalities associated with schizophrenia may be detected in putatively at-risk, preprodromal children. Prospective studies following the brain development of at-risk children are needed.
Key words: psychosis, high risk, MRI, VBM, biomarkers, brain structure
Introduction
Schizophrenia is characterized by widespread abnor malities of gray matter (GM) and white matter (WM) volume,1 a subset of which are already present by the first episode of illness. Studies employing voxel-based morphometry (VBM),2 a semi-automated, whole-brain approach to analyzing brain structure, indicate distributed GM volume abnormalities in first-episode patients in the frontal, temporal, cingulate, parietal, and subcortical regions and in the cerebellum.1 These abnormalities predominately reflect GM volume decreases in patients relative to healthy individuals, although relative increases have been observed. Widespread WM reductions in the frontal, temporal, cingulate, parietal, occipital, and subcortical regions have also been reported in first-episode patients.3,4
A subset of these neuroanatomical disturbances precede psychosis onset. Studies of at-risk youth with a family history of schizophrenia indicate that these individuals are characterized by progressive GM loss in the prefrontal and temporal lobes.5 However, because two-thirds of individuals with schizophrenia have no affected relatives,6 findings from studies examining these genetic high-risk youth may not generalize to the majority of patients with schizophrenia. Investigations of (prodromal) youth considered at ultra high risk (UHR) of psychosis due to their clinical presentation (attenuated psychotic symptoms; brief, limited intermittent psychotic symptoms; or genetic risk plus functional decline)7 indicate relative GM reductions in the hippocampus, insula, superior temporal gyrus (STG), and prefrontal cortex,8 and WM reductions in the superior temporal lobe9 relative to healthy youth. Among UHR youth who transition to psychosis, progressive GM decreases occur in the frontal, temporal, parietal, and cingulate cortices and in the cerebellum,10,11 with progressive WM decreases apparent in the parietal and occipital lobes.12
Recent efforts to delineate an earlier at-risk (preprodromal) phase of schizophrenia13–15 offer an exciting prospect for preventive intervention during childhood or early adolescence that might avert the functional decline, disability, and distress that characterizes the schizophrenia prodrome and limit the progression of structural brain abnormalities. The challenge, presently, is to accurately identify these children who may subsequently develop schizophrenia.13,15,16 A study that examined brain structure in 11 putatively at-risk children aged 11–13 years who presented subclinical psychotic symptoms on clinical interview showed increased GM volume in the left STG and in the left middle temporal, left angular, and right orbitofrontal gyri relative to healthy children (n = 14) and GM decreases in the left inferior temporal gyrus.17 WM abnormalities in the parietal and temporal lobes, including the inferior longitudinal fasciculus, were detected by diffusion tensor imaging. We have employed an alternative early identification strategy that might more specifically and sensitively identify putatively at-risk children by requiring the presence of multiple antecedents of schizophrenia (ASz) rather than a single risk factor.15 Our strategy identifies children aged 9–12 years who present a triad of replicated ASz,16,18 defined as (a) speech and/or motor developmental delays or abnormalities, (b) social, emotional, and/or behavioral problems, and (c) psychotic-like (or subclinical psychotic) experiences. While only longitudinal follow-up will ultimately determine the specificity and sensitivity of the triad in predicting later schizophrenia, preliminary evidence has demonstrated that ASz children present several neurobiological features that characterize adults with schizophrenia, including functional-brain abnormality following commission of behavioral errors,19 poorer intellectual and cognitive functioning,20 and increased involuntary dyskinetic movement abnormalities21 relative to typically developing (TD) children. Delineating biomarkers associated with a preprodromal at-risk phase may provide a means to refine identification strategies, track changes in disease phase, and monitor the impact of early therapeutic interventions. This preliminary investigation thus aimed to identify brain-structure abnormalities present in ASz children who are putatively at-risk of developing schizophrenia. This being the first study of brain structure in ASz children, we used VBM, rather than a region-of-interest approach, to examine whole-brain differences in GM and WM volume. Based on previous VBM studies of at-risk individuals, we hypothesized that ASz children would show volume abnormalities in temporal and prefrontal regions. Given that brain structure in healthy children is associated with sex and age,22 and also IQ,23 the ASz and TD groups were matched on these factors.
Methods
Participants
All children, aged 9–12 years, were identified via a community-screening procedure conducted in primary schools in London, UK.15,16 Children completed antecedent screening questionnaires independently at school, and corresponding caregiver questionnaires were completed at home and returned via reply-paid mail.15,16 Questionnaires included items to assess a triad of ASz, namely (a) a caregiver-reported delay or abnormality in speech and/or motor development, assessed via quantitative and qualitative questions; (b) an “abnormal” rating (ie, top 10th percentile on UK population norms) on at least 1 of the 4 Strengths and Difficulties Questionnaire24 psychopathology scales, including Emotional Symptoms (child reported) or Conduct Problems, Hyperactivity-Inattention or Peer Relationship Problems (caregiver reported); and (c) a child-reported “certain experience” of at least 1 psychotic-like experience (PLE) among 9 items.25 The PLE questionnaire included 5 items adapted from the Diagnostic Interview Schedule for Children26—auditory hallucinations, thoughts read, ideas of reference, paranoid ideas, and ideas of somatic changes—and 4 additional items assessing visual hallucinations, passivity phenomena, telepathic experiences, and grandiosity. Two large studies, 1 examining our 9-item PLE screening questionnaire25 and another investigating a similar 7-item PLE questionnaire,27 indicated that the item assessing auditory hallucinations demonstrated the best psychometric properties among PLE items. This item loaded strongly on a latent psychotic-like construct25 and had high specificity, sensitivity, and positive and negative predictive values for clinician-rated psychotic symptoms from interview.27 Screening of more than 1500 child-caregiver dyads indicates that 23% of children present none of the antecedents, 42% present a single antecedent, 26% present 2 antecedents, while 9% present the triad of antecedents. This is consistent with the finding that, in the inner-city south-east London neighborhoods where the majority of community screening was conducted, the incidence of schizophrenia among adults is elevated.28
Procedure
Ethical permission for the study was granted by the Joint South London and Maudsley and the Institute of Psychiatry National Health Service Research Ethics Committee. Children meeting ASz criteria (those presenting abnormalities in all 3 antecedent domains) and those eligible for the TD group (children who presented none of the 3 antecedents) were invited to participate in research assessments at the Institute of Psychiatry. Caregivers and children provided written informed consent and assent, respectively, for participation. Children completed a large battery of assessments, including the Wechsler Abbreviated Scale of Intelligence,29 the Pubertal Development Scale,30 and a structural magnetic resonance imaging (MRI) scan. Children were excluded from either group if they or their primary caregiver had insufficient English language ability to complete assessments, or if they had a neurological or physical condition that affected developmental milestone attainment and/or current functioning (eg, epilepsy and cerebral palsy). Family history of psychotic and mood disorders were assessed in first- and second-degree relatives using the Family Interview for Genetic Studies.31 Three ASz children had a second-degree relative with schizophrenia; 1 ASz and 2 TD children had a second-degree relative with bipolar disorder (without psychotic symptoms), and an additional ASz child had a first-degree relative with bipolar disorder with psychotic symptoms. Major depression was present in the relatives of 12 ASz children (8 first degree; 4 second degree) and 11 TD children (4 first degree; 7 second degree). None of the participants had ever received psychotropic medication or experienced a previous psychotic episode, as confirmed by clinical interview with a child psychiatrist or psychologist.
Structural MRI scans were obtained using a 3T GE Medical Systems MRI scanner. Due to excessive movement, images for 6 ASz children and 4 TD children were of insufficient quality to permit accurate segmentation and were thus excluded. Images from the remaining 20 ASz children were compared with those from 20 TD children who were matched to an ASz child on age (within 6 months) and IQ score (within 7 IQ points). All but 5 pairs were also matched for sex. By the time of scanning, 1 ASz child was aged 13 years, 4 months.
Imaging Parameters
Participants were scanned using a 5-min, three-dimensional Spoiled Gradient Recalled Echo sequence yielding 196 slices of 1.1mm thickness (time-to-repetition = 6.0ms, time-to-echo = 2.8ms, flip angle = 20°, field-of-view = 28 × 21cm, acquisition matrix = 256 × 256).
Image preprocessing and statistical analyses were conducted using Statistical Parametric Mapping 5 software (SPM5, www.fil.ion.ucl.ac.uk/spm/software/spm5). SPM5 utilizes a unified segmentation approach, which integrates segmentation, spatial normalization, and bias inhomogeneity within an iterative algorithm, thus increasing segmentation accuracy. We additionally employed the VBM5 toolbox implemented within SPM5 (http://dbm.neuro.uni-jena.de/vbm/vbm5-for-spm5), which includes the option to combine unified segmentation with a Hidden Markov Random Field (HMRF). By using information from neighboring voxels, which are likely to be of the same tissue class, the HMRF improves the signal-to-noise ratio and enables cluster-based statistical inference (http://dbm.neuro.uni-jena.de/vbm/vbm5-for-spm5). The VBM5 toolbox retains the option to use customized templates for segmentation. We used the matched pairs approach within the Template-O-Matic toolbox (https://irc.cchmc.org/software/tom.php) to create a study-specific paediatric template, derived from reference data obtained from 404 healthy children, selected according to the age and sex characteristics of the total sample (n = 40).32 Within SPM5, GM and WM images can be modulated with the Jacobian determinants of the deformation parameters to retain the original tissue volumes. We performed modulation by nonlinear effects only, which negates the need to correct for differences in brain size. Finally, modulated images were smoothed using a 4-mm full width at half-maximum (FWHM) filter. Smoothing kernels of 4–8mm are sensitive to structural brain abnormalities in schizophrenia populations, particularly in smaller regions.33 As modulation introduces additional smoothing of the brain images, a low FWHM is needed (http://dbm.neuro.uni-jena.de/vbm/vbm5-for-spm5).
Statistical Analyses
Group differences in demographic variables were examined using the Statistical Package for the Social Sciences (SPSS) version 15. Independent samples t-tests were used to compare continuous variables (age, IQ, Pubertal Development Scale score, and time lapsing between antecedent screening and MRI scan), and chi-square or Fisher’s Exact tests were used to compare categorical variables (sex, handedness, and ethnicity).
Two-sample t-tests were conducted within SPM5 to examine group differences (ASz vs TD) in GM and WM modulated images. An advantage of the VBM5 toolbox is the nonstationary isotropic correction that can be applied to account for the nonuniform smoothness of structural MRI data (http://dbm.neuro.uni-jena.de/vbm/vbm5-for-spm5). This allows cluster-level statistics to be conducted, which are more sensitive to the spatially extended signals than voxel-level statistics and address the mass-univariate, multiple comparison problem.34 An initial statistical threshold of P < .01 uncorrected was implemented at the voxel level, with a corrected family-wise error (FWE; P < .05) subsequently applied to identify significant clusters.34 Cohen’s D effect sizes were used to determine the magnitude of differences in these regions ((2 × T)/√df). We followed a similar procedure to that used by Meisenzahl and colleagues;34,35 effect sizes were computed from the maximum T statistic obtained for any voxel within the significant cluster and from the mean T statistic obtained across all voxels within the significant cluster.
Although ASz and TD children were matched on age, sex, and IQ, we conducted further analyses to account for variability in these factors within the entire sample. Mean GM and WM volumes for each participant were extracted from the clusters that distinguished the ASz and TD groups using the MarsBaR Statistical Parametric Mapping (SPM) toolbox (http://marsbar.sourceforge.net). Linear regression analyses were performed on these mean volumes in SPSS to determine whether group status predicted cluster volumes after accounting for any variance in brain volumes that was related to age, sex, and/or IQ (all variables entered simultaneously).
To localize clusters of significant volume differences between groups, the SPM (ie, Montreal Neurological Institute [MNI]) coordinates were translated into standard Talairach coordinates (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach) and localized according to the Talairach and Tournoux atlas.36 All spatial coordinates are reported in the original SPM (MNI) space.
Results
Sample Demographics
The ASz and TD groups did not differ with respect to age, sex, IQ, pubertal status, ethnicity, or time lapsing between completion of antecedent screening questionnaires and MRI scanning (table 1). The prevalences of specific components within the antecedent triad among 20 ASz children were speech (n = 16) and motor (n = 7) delays/abnormalities; emotional symptoms (n = 5), peer relationship problems (n = 12), conduct problems (n = 10), and hyperactivity inattention (n = 8); and multiple (>1) “certainly true” PLEs (n = 14).
Table 1.
Demographic Characteristics of Children Presenting ASz and TD Children
| Demographic Variable | ASz (n = 20) | TD (n = 20) | Statistical Test |
|---|---|---|---|
| Age at scan; mean (SD) | 11.1 (1.0) | 11.3 (0.8) | t =.5, P = .6 |
| Pubertal development scale score; mean (SD) | 2.0 (0.6) | 1.7 (0.6) | t = -1.4, P = .2 |
| Sex (male); n (%) | 14 (70) | 9 (45) | χ2 = 1.6, P = .2 |
| Handedness (right); n (%) | 16 (84) | 18 (90) | Fisher’s exact test, P = .7 |
| Full-scale IQ estimate; mean (SD) | 107 (13) | 113 (10) | t = 1.8, P = .08 |
| Ethnicity: WB/(BA/AC)/O; n (%) | 6/2/12 (30/10/60) | 11/3/6 (55/15/30) | Fisher’s exact test, P = .2 |
| Time lapsing between antecedent screening and MRI scan (months); mean (SD) | 9 (6) | 9 (6) | t = −.04, P = 1.0 |
Note: ASz, antecedents of schizophrenia; TD, typically developing; WB, white British; BA/AC, black African/African Caribbean; O, other ethnicity. Pubertal development scale total score is created as the average of scores obtained on 5 indices of pubertal status (range 1–5); higher scores indicate more advanced pubertal development (a score of 2 indicates an “Early Pubertal” stage). Missing data on pubertal developmental scale (ASz = 1).
GM Volume
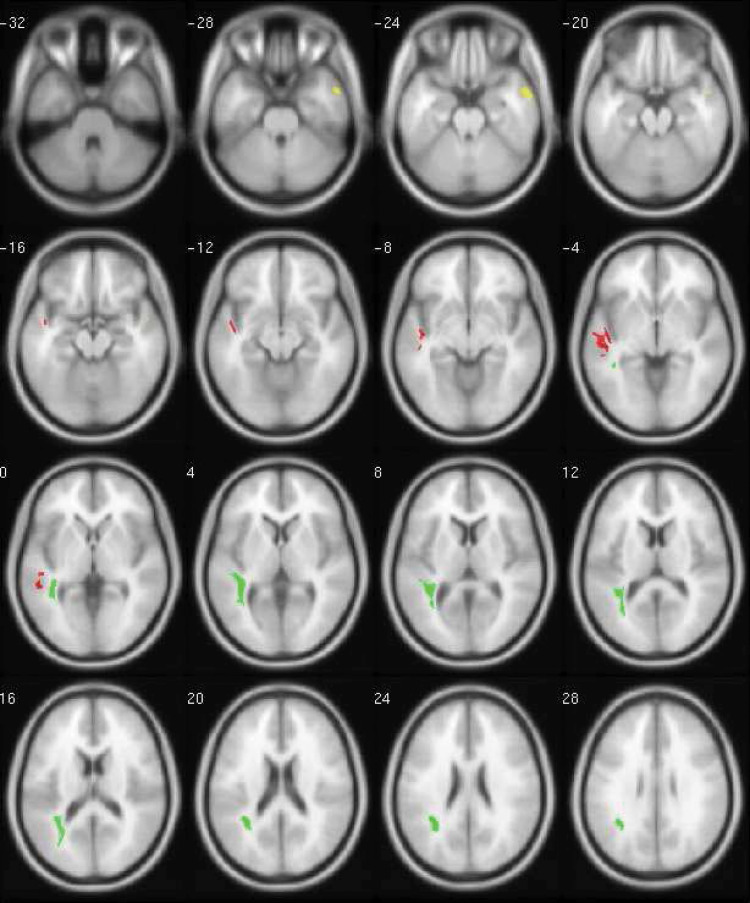
The ASz children were characterized by reduced GM volume relative to the TD group in a single, small cluster located in the right MTG, corresponding to Brodmann Area (BA) 21 (table 2). The effect size for this cluster was large (maximum = 1.8; mean = 1.4). In the left temporal lobe, a single, larger cluster of increased GM volume was observed in the ASz group relative to the TD group, which encompassed the left STG (BA 22) and extended into the MTG (BA 21) (table 2). A large effect size was also observed for this cluster (maximum = 1.4; mean = 1.2). Figure 1 illustrates the regions of increased (red) and decreased (yellow) GM volume in the ASz group relative to TD group (P < .05 corrected). Significant group differences in GM volume in the right MTG cluster (P < .001) and left STG-MTG cluster (P < .001) remained after adjustment for age, sex, and IQ.
Table 2.
Significant Clusters of Relatively Increased and Decreased Gray Matter Volume in Children With ASz Compared With TD Children
| Contrast | Hemisphere | MNI Coordinates Within the Cluster (x, y, z) | Size of Cluster (Voxels) | Cluster P (FWE corrected) | T Statistica, Maximum; Mean (SD) | Cohen’s D b, Maximum; Mean (SD) | ||
|---|---|---|---|---|---|---|---|---|
| Gray matter | ||||||||
| TD > ASz | R. middle temporal gyrus (BA 21) | 57 | 2 | −26 | 915 | .04 | 5.5; 4.2 (1.9) | 1.8; 1.4 (0.6) |
| R. middle temporal gyrus (BA21) | 51 | 3 | −19 | |||||
| ASz > TD | L. superior temporal gyrus (BA 22) | −44 | −13 | −8 | 2077 | <.001 | 4.3; 3.6 (0.5) | 1.4; 1.2 (0.2) |
| L. middle temporal gyrus (BA 22) | −51 | −38 | 0 | |||||
| L. middle temporal gyrus (BA 21) | −47 | −2 | −14 | |||||
| L. superior temporal gyrus (BA 21) | −60 | −13 | −4 | |||||
| White matter | ||||||||
| ASz > TD | L. inferior parietal lobule | −35 | −48 | 26 | 2737 | <.001 | 3.8; 3.2 (0.4) | 1.2; 1.0 (0.1) |
| L. superior temporal gyrus | −48 | −25 | 3 | |||||
| L. temporal lobe | −40 | −46 | 0 | |||||
| L. middle temporal gyrus | −32 | −70 | 16 | |||||
Note: BA, Brodmann Area; FWE, family-wise error; SD, standard deviation.
aMaximal and mean T statistic obtained within each cluster.
bCohen’s D effect size computed from the maximal and mean T statistic obtained within each cluster.
Fig. 1.
Significant clusters of relatively decreased gray matter volume (yellow: slices -28 through -20), increased gray matter volume (red: slices -16 through 0) and increased white matter volume (green: slices 0 through 28), in children presenting antecedents of schizophrenia compared with TD children (P < .05, FWE corrected for multiple comparisons). Data are presented in SPM Montreal Neurological Institute (MNI) space and displayed on an average template brain according to neurological convention (left hemisphere shown on the left). Transaxial slices are illustrated in 4-mm intervals from −32mm below to 28mm above, the AC-PC plane. Note: Color version available online only.
WM Volume
No areas of significantly decreased WM volume in the ASz group relative to the TD group were detected (table 2). A single cluster of increased WM volume in the ASz group relative to the TD group was identified, encompassing voxels in the left inferior parietal lobe, occipital lobe, and STG/MTG. Coordinates within this cluster corresponded to the superior longitudinal fasciculus, optic radiation, and inferior longitudinal fasciculus. As indicated in table 2, the effect size for this cluster was large (maximum = 1.2; mean = 1.0). Figure 1 illustrates the areas of increased WM volume (green) in the ASz group relative to the TD group (P < .05 corrected). After controlling for age, sex, and IQ, significant group differences in WM volume in this cluster remained (P = .001).
Post-hoc Examination of Associations With Auditory Hallucinations
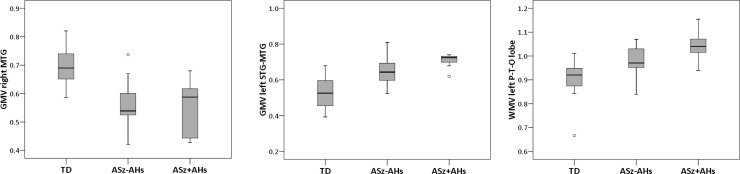
The STG and MTG have been associated with auditory hallucinations in both structural,37 and functional38 MRI studies of patients with schizophrenia. Thus, post-hoc analyses were conducted to determine whether the structural brain abnormalities observed in ASz children characterized only those children who self-reported auditory hallucinations on the screening questionnaire (answered “certainly true” to the item “Have you ever heard voices that other people can’t hear?”). Using the mean GM and WM values extracted from the significant clusters for each child, an ANOVA was used to investigate differences between the 20 TD children, the 7 ASz children who reported auditory hallucinations at screening, and the 13 ASz children who did not (figure 2). A significant main effect of group was observed within the right MTG GM cluster (F 2, 37 = 16.9; P < .01), in the left STG/MTG GM cluster (F 2, 37 = 16.8; P < .01) and in the left parietal, temporal, and occipital lobes WM cluster (F 2, 37 = 10.9; P < .01). Post-hoc pairwise comparisons (Bonferroni-corrected) indicated that the TD group mean in each cluster differed significantly from the group means of both ASz children who reported auditory hallucinations (P < .001) and the ASz children who did not (P < .05). No GM or WM volume differences were observed between the 2 ASz subgroups (P > .05). Due to the small subgroup sizes, these analyses were repeated using nonparametric tests (Mann-Whitney U), and the results were similar (for all 3 clusters: TD vs ASz without auditory hallucinations, P < .01; TD vs ASz with auditory hallucinations, P < .001; ASz without auditory hallucinations vs ASz with auditory hallucinations, P > .05).
Fig. 2.
Gray matter and white matter volumes (group median indicated by line) in TD children, children presenting antecedents of schizophrenia who additionally reported auditory hallucinations, and children presenting antecedents who did not report auditory hallucinations. TD, typically developing; ASz-AHs, antecedents of schizophrenia without auditory hallucinations; ASz + AHs, antecedents of schizophrenia with auditory hallucinations; GMV, gray matter volume; WMV, white matter volume; MTG, middle temporal gyrus; STG, superior temporal gyrus; P-T-O lobe, parietal, temporal, occipital lobe.
Discussion
We examined whole-brain differences in GM and WM volume in children presenting ASz and TD children matched on age, sex, and IQ, none of whom had ever received psychotropic medication or experienced a psychotic episode. As hypothesized, GM abnormalities were observed in the temporal regions, with ASz children showing relative GM decreases in the right MTG and relative GM increases in the left STG-MTG. ASz children also showed increased WM volume in a large cluster spanning the left parietal, temporal, and occipital lobes. Large effect sizes were observed for all 3 regions. Contrary to hypotheses, we did not observe abnormalities within the prefrontal cortex.
Relative to the TD group, ASz children showed increased GM in the left STG, extending into the MTG. Left STG volume reduction is one of the most consistently reported neuroanatomical findings among adults with schizophrenia.33 STG volume abnormalities may relate to illness stage, with a recent meta-analysis indicating GM volume reductions in the right STG of patients with chronic schizophrenia but GM volume increases in the left STG of first-episode patients.39 Longitudinal studies also indicate progressive decreases in STG volume after the first psychotic episode.40 Among UHR youth, compared with healthy youth, reduced GM in both the left and right STG has been observed,41 with progressive bilateral reductions among those who transition to psychosis.42 Our findings are consistent with those from a cross-sectional study of UHR youth who later transitioned to psychosis that reported reduced GM volume in the right STG relative to healthy controls but increased GM volume in the posterior portions of the left STG and MTG.43 GM volume increases were also reported in the left STG and MTG in children aged 11–13 years presenting subclinical psychotic symptoms,17 the only previous VBM study of children in a putative early at-risk phase.
We also observed reduced GM volume in the right MTG in ASz children relative to TD children. GM volume reductions in the right MTG have been observed in individuals with first-episode schizophrenia44 and UHR youth who later developed psychosis.43 In contrast, volume abnormalities in the right MTG were not observed in the study of children presenting subclinical psychotic symptoms.17
ASz children were additionally characterized by increased WM volume in a cluster encompassing the left parietal, temporal, and occipital lobes, corresponding to parts of the superior longitudinal fasciculus, inferior longitudinal fasciculus, and optic radiation. Reduced WM volume in the inferior longitudinal fasciculus has been reported in first-episode patients,4 while increased WM within this tract was observed in a study of patients with chronic schizophrenia.45 Furthermore, decreased volume in the left optic radiation was found to characterize UHR individuals who developed psychosis relative to those who did not.12 Diffusion tensor imaging studies have also detected abnormalities of WM integrity in the superior and inferior longitudinal fasciculus and optic radiation in adults with schizophrenia,46 and in the inferior longitudinal fasciculus in children presenting subclinical psychotic symptoms.17 Thus, while the superior and inferior longitudinal fasciculi are characterized by abnormality across illness phases, further investigation is needed to resolve inconsistencies in the direction of abnormality (relative decreases or increases) that may change with developmental stage and/or stage of illness.
The STG and MTG support auditory processing and both functional38 and structural37 MRI studies of schizophrenia patients indicate that these structures are involved in the production of auditory hallucinations. In this study, the GM and WM abnormalities we observed, which encompassed these regions, were not limited to the subgroup of ASz children who reported auditory hallucinations at screening. Rather, these abnormalities characterized both subgroups relative to TD children. No significant differences in GM or WM volume were detected between ASz children with and without auditory hallucinations. These post-hoc analyses imply that the GM and WM abnormalities indentified in ASz children do not merely index the presence of auditory hallucinations though investigations in larger samples are needed.
The left and right hemispheres in ASz children were characterized by contrasting patterns of relatively increased and decreased GM and WM volume abnormalities, which mimic the pattern observed in a study of UHR youth who later transitioned to psychosis.43 The reduced asymmetry hypothesis of schizophrenia,47 which proposes that the typical pattern of asymmetry across the left and right hemispheres is attenuated or reversed in individuals with schizophrenia, particularly in the auditory processing regions, may be relevant. In healthy adults, the volume and area of the temporal lobe structures show a left-dominant pattern, while individuals with schizophrenia show reduced asymmetry of these structures.47 Among healthy children, the right temporal lobe is larger than the left.48 The pattern of abnormality among ASz children in this study may imply an attenuation of the typical right-dominant pattern of childhood asymmetry in this region; investigations using region-of-interest approaches are needed to test this hypothesis.
Schizophrenia is thought to result from abnormal neural development, which may commence as early as conception.49,50 Despite being equivalent in age and pubertal stage to the TD group, the ASz children showed differences in brain structure, predominately in the temporal regions. GM volume development follows a regionally specific pattern. Total volume peaks in late childhood/early adolescence, with the temporal lobe, particularly the STG, being one of the last regions to achieve adult status.51 In contrast, WM volume increases linearly across the brain into adulthood.51 Thus, the abnormalities observed among the ASz children do not appear to reflect a pattern of delayed maturation.
UHR youth are characterized by reduced GM in the left hippocampus and insula and in the right STG and prefrontal cortex;8 however, abnormalities in these regions were not observed in ASz children. The absence of hippocampal volume reduction is particularly notable given the consistency of this finding in individuals with schizophrenia.52 It may be that hippocampal volume reductions associated with specific psychiatric disorders and psychopathological states do not emerge until later in development. For example, several studies of children who developed posttraumatic stress disorder after experiencing maltreatment indicate a lack of hippocampal volume reductions among these children, whereas studies of adults who were maltreated as children consistently report hippocampal volume reductions.53 Thus, while schizophrenia has been robustly associated with reduced hippocampal volume in adult populations, this feature may not yet be present in children who are at risk for the disorder. Our failure to identify GM volume differences in other regions that have been shown to be abnormal in individuals at UHR may also reflect illness stage and participant age. Based on early neurodevelopmental theories of schizophrenia,54,55 and updated theories that draw evidence from studies of childhood-onset schizophrenia,50,56 we anticipate that, as ASz children enter adolescence, neuroanatomical abnormalities may become more pronounced and extend into the prefrontal cortex. Indeed, more widespread GM abnormalities have been observed in an older sample of children presenting subclinical psychotic symptoms17 although this difference in findings might also reflect the alternative strategies used to define putatively at-risk children.
The differences in GM and WM volume that characterized ASz children were localized, but large in magnitude (mean effect size range: 1.0–1.4). Potentially, variability among ASz children in GM and/or WM volume may have precluded the detection of more subtle volume differences in other brain regions. The few previous VBM studies to report effect sizes, conducted with UHR youth who, by definition, are more proximal to illness onset than ASz children, indicate more distributed volume abnormalities, of medium-to-large magnitude.34,35 Prospective longitudinal studies are needed to determine the course of brain development in ASz children.
The high prevalence of PLEs in the general population57 suggests that not all individuals who are vulnerable for schizophrenia will develop illness. Markers of vulnerability may differ from markers of incipient illness. For example, cross-sectional analyses identified smaller hippocampal volumes among UHR youth relative to healthy youth, but relatively increased hippocampal volume was observed subsequently in those who developed psychosis.58 Until ASz children pass through the age of risk for schizophrenia, nothing is known about the proportion that will develop illness. Thus, only follow-up of the ASz children can distinguish between GM and WM abnormalities that represent biomarkers of oncoming illness and those that represent disease vulnerability.
There are several limitations to this study that should be addressed in future research. First, this study is limited by the relatively small sample, particularly with respect to the post-hoc comparison of ASz children with and without auditory hallucinations. Nevertheless, this constitutes the largest VBM study of putatively at-risk, never-medicated children. Further, future research might utilize alternative neuroimaging techniques to examine other features of brain structure in at-risk children, such as those measuring cortical thickness or complete diffusion tensor imaging to more accurately examine WM abnormalities. Second, the extent and/or magnitude of brain volume differences between ASz and TD children may have been underestimated by matching on, and adjusting for, IQ. This strategy was employed given the known association between IQ and brain structure,23 despite evidence that ASz children are characterized by significantly lower IQ scores than their TD peers,20 as are children who later develop schizophrenia.59 A third limitation, not unique to this study, concerns potentially inaccurate localization of SPM spatial coordinates. Due to differences in brain structure between adults and children, standard neuroimaging analysis techniques may be less appropriate for paediatric samples. At the preprocessing stage, a study-specific paediatric template was created for segmentation; however, the lack of standardized child atlases to localize SPM spatial coordinates necessitated the use of the international standard Talairach and Tournoux (1998) atlas derived from an adult brain.
We detected relatively localized structural brain abnormalities associated with schizophrenia vulnerability in putatively at-risk children aged 9–12 years. These findings are preliminary and require independent replication, as well as longitudinal follow-up, to determine the extent to which the ASz criteria afford a specific and sensitive prediction of later schizophrenia. Extending the ASz criteria to include the temporal lobe abnormalities identified here may provide a useful marker to track the effectiveness of early intervention to reduce schizophrenia risk and the associated neuroanatomical abnormalities.
Funding
National Institute for Health Research Career Development Fellowship (CDF/08/01/015 to KRL); Bial Foundation Research Grant (36/06); NARSAD Young Investigator Award (2005); British Medical Association Margaret Temple Award for schizophrenia research (2006).
Acknowledgments
The authors thank the children and caregivers who participated in the study and the researchers and students who contributed to data collection. All authors are affiliated with the National Institute for Health Research Specialist Biomedical Research Centre (BRC) for Mental Health at the South London and Maudsley National Health Service Foundation Trust and Institute of Psychiatry, King’s College London, United Kingdom. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Footnotes
The original version was incorrect. The figure is supposed to be in color. The publisher regrets the error.
The original figure 2 was incorrect. The author regrets the error.
References
- 1. Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36:1342–1356 [DOI] [PubMed] [Google Scholar]
- 2. Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821 [DOI] [PubMed] [Google Scholar]
- 3. Chua SE, Cheung C, Cheung V, et al. Cerebral grey, white matter and csf in never-medicated, first-episode schizophrenia. Schizophr Res. 2007;89:12–21 [DOI] [PubMed] [Google Scholar]
- 4. Whitford TJ, Grieve SM, Farrow TF, et al. Volumetric white matter abnormalities in first-episode schizophrenia: a longitudinal, tensor-based morphometry study. Am J Psychiatry. 2007;164:1082–1089 [DOI] [PubMed] [Google Scholar]
- 5. McIntosh AM, Owens DC, Moorhead WJ, et al. Longitudinal volume reductions in people at high genetic risk of schizophrenia as they develop psychosis. Biol Psychiatry. 2011;69:953–958 [DOI] [PubMed] [Google Scholar]
- 6. Gottesman II, Shields J. Schizophrenia, the Epigenetic Puzzle. Cambridge; New York: Cambridge University Press; 1982. [Google Scholar]
- 7. Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22:353–370 [DOI] [PubMed] [Google Scholar]
- 8. Fusar-Poli P, Borgwardt S, Crescini A, et al. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35:1175–1185 [DOI] [PubMed] [Google Scholar]
- 9. Witthaus H, Brüne M, Kaufmann C, et al. White matter abnormalities in subjects at ultra high-risk for schizophrenia and first-episode schizophrenic patients. Schizophr Res. 2008;102:141–149 [DOI] [PubMed] [Google Scholar]
- 10. Borgwardt SJ, McGuire PK, Aston J, et al. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106:108–114 [DOI] [PubMed] [Google Scholar]
- 11. Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288 [DOI] [PubMed] [Google Scholar]
- 12. Walterfang M, McGuire PK, Yung AR, et al. White matter volume changes in people who develop psychosis. Br J Psychiatry. 2008;193:210–215 [DOI] [PubMed] [Google Scholar]
- 13. Kelleher I, Cannon M. Psychotic-like experiences in the general population: characterizing a high-risk group for psychosis. Psychol Med. 2011;41:1–6 [DOI] [PubMed] [Google Scholar]
- 14. Keshavan MS, DeLisi LE, Seidman LJ. Early and broadly defined psychosis risk mental states. Schizophr Res. 2011; 126:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laurens KR, Hodgins S, Taylor E, Murray RM. Is earlier intervention for schizophrenia possible?: Identifying antecedents of schizophrenia in children aged 9–12 years. In: David AS, McGuffin P, Kapur S, eds. Schizophrenia: The Final Frontier. London: Psychology Press; 2011. 19–32 [Google Scholar]
- 16. Laurens KR, Hodgins S, Maughan B, Murray RM, Rutter ML, Taylor EA. Community screening for psychotic-like experiences and other putative antecedents of schizophrenia in children aged 9-12 years. Schizophr Res. 2007;90:130–146 [DOI] [PubMed] [Google Scholar]
- 17. Jacobson S, Kelleher I, Harley M, et al. Structural and functional brain correlates of subclinical psychotic symptoms in 11-13 year old schoolchildren. Neuroimage. 2010;49:1875–1885 [DOI] [PubMed] [Google Scholar]
- 18. Laurens KR, West SA, Murray RM, Hodgins S. Psychotic-like experiences and other antecedents of schizophrenia in children aged 9-12 years: a comparison of ethnic and migrant groups in the United Kingdom. Psychol Med. 2008;38:1103–1111 [DOI] [PubMed] [Google Scholar]
- 19. Laurens KR, Hodgins S, Mould GL, et al. Error-related processing dysfunction in children aged 9 to 12 years presenting putative antecedents of schizophrenia. Biol Psychiatry. 2010;67:238–245 [DOI] [PubMed] [Google Scholar]
- 20. Cullen AE, Dickson H, West SA, et al. Neurocognitive performance in children aged 9-12 years who present putative antecedents of schizophrenia. Schizophr Res. 2010;121:15–23 [DOI] [PubMed] [Google Scholar]
- 21. Macmanus D, Laurens KR, Walker EF, Brasfield JL, Riaz M, Hodgins S. Movement abnormalities and psychotic-like experiences in childhood: markers of developing schizophrenia? Psychol Med 2012;42:99–109. [DOI] [PubMed] [Google Scholar]
- 22. Giedd JN, Clasen LS, Lenroot R, et al. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;254-255:154–162 [DOI] [PubMed] [Google Scholar]
- 23. Wilke M, Sohn JH, Byars AW, Holland SK. Bright spots: correlations of gray matter volume with IQ in a normal pediatric population. Neuroimage. 2003;20:202–215 [DOI] [PubMed] [Google Scholar]
- 24. Goodman R. Psychometric properties of the strengths and difficulties questionnaire. J Am Acad Child Adolesc Psychiatry. 2001;40:1337–1345 [DOI] [PubMed] [Google Scholar]
- 25. Laurens KR, Hobbs MJ, Sutherland M, Green MJ, Mould GL. Psychotic-like experiences in a community sample of 8,000 children aged 9–11 years: an item response theory analysis. Psychol Med 2012;42:1495–1506 [DOI] [PubMed] [Google Scholar]
- 26. Costello A, Edelbrock C, Kalas R, Kessler M, Klaric S. NIMH Diagnostic Interview Schedule for Children: Child Version. Rockville, MD: National Institute of Mental Health; 1982. [Google Scholar]
- 27. Kelleher I, Harley M, Murtagh A, Cannon M. Are screening instruments valid for psychotic-like experiences? A validation study of screening questions for psychotic-like experiences using in-depth clinical interview. Schizophr Bull. 2011;37:362–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kirkbride JB, Fearon P, Morgan C, et al. Heterogeneity in incidence rates of schizophrenia and other psychotic syndromes: findings from the 3-center AeSOP study. Arch Gen Psychiatry. 2006;63:250–258 [DOI] [PubMed] [Google Scholar]
- 29. Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) Manual. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 30. Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14:190–195 [DOI] [PubMed] [Google Scholar]
- 31. Maxwell ME. Family Interview for Genetic Studies. St. Louis, MO: National Institute of Mental Health; 1992. [Google Scholar]
- 32. Wilke M, Holland SK, Altaye M, Gaser C. Template-O-Matic: a toolbox for creating customized pediatric templates. Neuroimage. 2008;41:903–913 [DOI] [PubMed] [Google Scholar]
- 33. Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245 [DOI] [PubMed] [Google Scholar]
- 34. Meisenzahl EM, Koutsouleris N, Gaser C, et al. Structural brain alterations in subjects at high-risk of psychosis: a voxel-based morphometric study. Schizophr Res. 2008;102:150–162 [DOI] [PubMed] [Google Scholar]
- 35. Koutsouleris N, Schmitt GJ, Gaser C, et al. Neuroanatomical correlates of different vulnerability states for psychosis and their clinical outcomes. Br J Psychiatry. 2009;195:218–226 [DOI] [PubMed] [Google Scholar]
- 36. Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Stuttgart: Georg Thieme; 1988. [Google Scholar]
- 37. Modinos G, Costafreda SG, van Tol MJ, McGuire PK, Aleman A, Allen P. Neuroanatomy of auditory verbal hallucinations in schizophrenia: A quantitative meta-analysis of voxel-based morphometry studies. Cortex. . In press. [DOI] [PubMed] [Google Scholar]
- 38. Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168:73–81 [DOI] [PubMed] [Google Scholar]
- 39. Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mané A, Falcon C, Mateos JJ, et al. Progressive gray matter changes in first episode schizophrenia: a 4-year longitudinal magnetic resonance study using VBM. Schizophr Res. 2009;114:136–143 [DOI] [PubMed] [Google Scholar]
- 41. Takahashi T, Wood SJ, Yung AR, et al. Superior temporal gyrus volume in antipsychotic-naive people at risk of psychosis. Br J Psychiatry. 2010;196:206–211 [DOI] [PubMed] [Google Scholar]
- 42. Takahashi T, Wood SJ, Yung AR, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366–376 [DOI] [PubMed] [Google Scholar]
- 43. Borgwardt SJ, McGuire PK, Aston J, et al. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. Br J Psychiatry Suppl. 2007;51:s69–s75 [DOI] [PubMed] [Google Scholar]
- 44. Lui S, Deng W, Huang X, et al. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry. 2009;166:196–205 [DOI] [PubMed] [Google Scholar]
- 45. Seok JH, Park HJ, Chun JW, et al. White matter abnormalities associated with auditory hallucinations in schizophrenia: a combined study of voxel-based analyses of diffusion tensor imaging and structural magnetic resonance imaging. Psychiatry Res. 2007;156:93–104 [DOI] [PubMed] [Google Scholar]
- 46. Mitelman SA, Torosjan Y, Newmark RE, et al. Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophr Res. 2007;92:211–224 [DOI] [PubMed] [Google Scholar]
- 47. Crow TJ. Temporal lobe asymmetries as the key to the etiology of schizophrenia. Schizophr Bull. 1990;16:433–443 [DOI] [PubMed] [Google Scholar]
- 48. Giedd JN, Vaituzis AC, Hamburger SD, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol. 1996;366:223–230 [DOI] [PubMed] [Google Scholar]
- 49. Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Molecular Psychiatry. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729 [DOI] [PubMed] [Google Scholar]
- 52. Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25 [DOI] [PubMed] [Google Scholar]
- 53. McCrory E, De Brito SA, Viding E. Research review: the neurobiology and genetics of maltreatment and adversity. J Child Psychol Psychiatry. 2010;51:1079–1095 [DOI] [PubMed] [Google Scholar]
- 54. Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J. 1987;295:681–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669 [DOI] [PubMed] [Google Scholar]
- 56. Vyas NS, Kumra S, Puri BK. What insights can we gain from studying early-onset schizophrenia? The neurodevelopmental pathway and beyond. Expert Rev Neurother. 2010;10:1243–1247 [DOI] [PubMed] [Google Scholar]
- 57. Nuevo R, Chatterji S, Verdes E, Naidoo N, Arango C, Ayuso-Mateos JL. The continuum of psychotic symptoms in the general population: a cross-national study. Schizophr Bull. 2012;38:475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Phillips LJ, Velakoulis D, Pantelis C, et al. Non-reduction in hippocampal volume is associated with higher risk of psychosis. Schizophr Res. 2002;58:145–158 [DOI] [PubMed] [Google Scholar]
- 59. Dickson H, Laurens KR, Cullen AE, Hodgins S. Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychol Med. 2012;42:743–755 [DOI] [PubMed] [Google Scholar]