Abstract
Schizophrenia is associated with a high prevalence of smoking. Functional connectivity between the dorsal anterior cingulate (dACC) and limbic regions including the ventral striatum, extended amygdala and parahippocampal areas has been previously implicated in the genetics and clinical severity of smoking. In this study, we test the hypothesis that dACC functional circuits are key paths for the high risk of smoking comorbidity in schizophrenia. Resting state functional magnetic resonance imaging (fMRI) was performed using the dACC as a seed region in smoking and nonsmoking patients with schizophrenia (n = 54), matched controls (n = 65), and nonpsychotic first-degree relatives (n = 24). Multiple regions had decreased connectivity with the dACC in schizophrenia patients when compared with matched controls (n = 65). Several of these functional circuits were also associated with nicotine addiction severity; the largest cluster included limbic areas such as the parahippocampal, extended amygdala, ventral striatal, and posterior insula regions, indicating an overlap of schizophrenia and nicotine addiction on to this circuit. These same functional connectivity–defined circuits were also significantly impaired in schizophrenia nonsmokers compared with control nonsmokers and in nonpsychotic first-degree relatives. Functional connectivity between the dACC and limbic regions is inherently abnormal in schizophrenia, related to its genetic liability regardless of smoking, and overlaps with a nicotine addiction–related circuit. Our findings establish a biologically defined brain circuit mechanism that contributes to the high prevalence of smoking.
Key words: nicotine, anterior cingulate, functional connectivity, limbic
Introduction
Rates of tobacco smoking are elevated in individuals with a mental illness, and are highest in schizophrenia, where smoking prevalence is ~60%–88%.1,2 Schizophrenia is associated with excess mortality, with smoking-related illnesses being the number one cause of preventable mortality.3 The link between smoking and mental illness is well established epidemiologically, but its biological basis remains unclear.
Current conceptualization of schizophrenia–nicotine addiction comorbidity centers on smoking as self-medication to overcome neurocognitive deficits or temper antipsychotic medication side effects.4 While these factors likely contribute to the high smoking prevalence in schizophrenia, ~80% of individuals at risk for schizophrenia smoke prior to disease onset.5 Unaffected twins of patients with schizophrenia have higher rates of smoking and more difficulty quitting than controls.6 The close association between smoking and schizophrenia occurs across continents where environment and base rates of smoking differ.7 Therefore, increased risk of smoking in schizophrenia may not be entirely due to environmental or medication effects and may instead be due to shared neurobiology.
One approach to understanding comorbidity is to model its brain circuitry using resting state functional connectivity (rsFC). rsFC corresponds to functional networks utilized by the brain in action,8 which are constrained by fiber connectivity,9 and predicts working memory and other cognitive functions.10 In addition, specific alterations in rsFC have been found in a variety of neuropsychiatric diseases.11 In this study, we define rsFC “circuit” as a measure of synchronization of intrinsic low-frequency fluctuations between brain regions at rest9 that provides a way to assay in vivo network-level functions.
Recent rsFC analyses revealed that decreased rsFC between dorsal anterior cingulate (dACC)–ventral striatum (VS) is a key pathway associated with nicotine addiction12 and its genetics.13 Of note, the area noted as “VS” in prior work13 included adjacent limbic areas (substantia innominata, extended amygdala, and parahippocampal regions). Of relevance to this study, structural and functional differences in dACC and basal forebrain regions are commonly reported in schizophrenia. Abnormal ACC function in schizophrenia occurs during many cognitive tasks.14 Abnormal VS functions have been reported in prodromal, medicated, and unmedicated patients.15 Decreased volume of medial temporal lobe structures including parahippocampal gyrus and amygdala is a common finding in schizophrenia.16 Decreased gray matter in the amygdala, hippocampus, limbic lobe, and ACC has been found in first-episode schizophrenia,17 and decreased ACC and parahippocampal gray matter volume has been found in tobacco smokers.18 Concurrent ACC, striatal, and amygdala fMRI abnormalities have been observed within the same task in schizophrenia patients.19
Here, we hypothesized that the dACC-VS/extended amygdala/parahippocampal circuit is a key path responsible for the high prevalence of nicotine addiction in schizophrenia. Specifically, we hypothesized that dysfunction of this circuit is independently shared by nicotine addiction and schizophrenia. Testing this hypothesis relies on demonstrating that (1) dACC circuits are impaired in schizophrenia independent of smoking in schizophrenia nonsmokers compared with control nonsmokers, (2) dACC circuits are impaired in nicotine addiction, (3) schizophrenia-related dACC circuits overlap onto circuits linked to nicotine addiction, (4) circuits associated with nicotine addiction severity are fundamentally similar in schizophrenia smokers and control smokers, (5) disease-related effects in dACC circuits are fundamentally similar in schizophrenia smokers and schizophrenia nonsmokers, and (6) such circuit impairments are present in nonpsychotic first-degree relatives of schizophrenia patients and therefore are related to the genetic liability of schizophrenia and are not due to antipsychotic medication.
Methods
Subjects
Sixty-five controls and 54 schizophrenia patients participated in the study. We frequency-matched nonsmokers vs smokers in each group (28:37 in controls and 18:36 in patients; χ2 = 0.81, P = .37) (see online supplementary table 1). Local Institutional Review Board panels approved the study. All subjects gave written informed consent. Subjects were 18–58 years of age. Controls were recruited through media advertisements. Patients were recruited through Maryland Psychiatric Research Center outpatient and neighboring clinics. All patients were clinically stable on antipsychotic medications. We also recruited 24 nonpsychotic, unmedicated first-degree relatives of schizophrenia patients. Major medical and neurological conditions and current substance abuse or dependence in the past 12 months were exclusionary. All participants were evaluated with Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.20 Controls had no Axis I diagnosis except nicotine dependence. First-degree relatives had no history of psychosis and no Axis I diagnoses except for a past single episode of depression. Clinical symptoms in patients were measured by the 20-item Brief Psychiatric Rating Scale (BPRS), scored 1–7 on each item. Total and symptom scores on thought disorder, withdrawal, anxiety, activation, and psychosis symptoms were calculated. Smokers were current daily smokers for ≥1 year of any number of cigarettes. Nonsmokers did not currently smoke and have never been regular or daily smokers. Nicotine addiction severity was assessed by the Fagerstrom Test for Nicotine Dependence (FTND).21 Smokers were encouraged to smoke a cigarette prior to arriving at the study site to avoid withdrawal-related imaging changes. Every subject refrained from smoking for 30min before the scan to avoid the immediate peak of nicotine from the last cigarette. We measured end-expiration carbon monoxide (CO) prior to scanning as an objective measure of recent smoking. Lifetime exposure to smoking was calculated by pack-year (# packs smoked/day × # years as smoker). Subjects could not drink more than 4 oz of any caffeinated drink on the day of scanning. Alcohol breath test and urine toxicology were performed immediately prior to scanning. Positive results were exclusionary.
MRI and Processing
Subjects were instructed to rest with their eyes open and not to think of anything specific. Resting-state blood oxygenation level dependent (BOLD) data were acquired for 5min on a 3 Tesla Siemens Allegra scanner over 39, 4mm axial, interleaved slices using a gradient echo echoplanar imaging sequence (150vol/vol, echo time/repetition time [TR] = 27/2000ms; flip angle = 80°; field of view = 220 × 220mm2). T1-weighted magnetization prepared rapid acquisition gradient echo images (1 × 1 × 1mm3) were acquired. Volumes were slice-timing aligned, motion corrected, linear detrended, spatially normalized, and resampled to Talairach space at 3 × 3 × 3mm3, and spatially smoothed (full width at half maximum 6mm) in AFNI.22 Data were temporally low-pass filtered (f cutoff = 0.1 Hz). The dACC seed was manually identified on coronal sections for each subject starting one slice anterior to the disappearance of the corpus callosum to the anterior commissure posteriorly.12,13 Cross-correlation coefficients between the averaged time course in the dACC and each voxel’s time course in the brain were calculated, including 6 rigid head-motion parameter time courses and average time course in white matter as nuisance covariates to regress out fluctuations unlikely to be relevant to neuronal activity.23 Correlation coefficients were Fisher transformed to z scores as a measure of rsFC.23 Because head motion can lead to spurious differences in functional connectivity,24 we censored time points with significant motion.25 We calculated the Euclidean norm of the derivatives of the 6 motion parameters (comparing current to previous TR) to obtain a single measure of head motion for each TR and excluded time points (and 1 preceding) with a value ≥0.7.26 ANOVA was used to evaluate percent time points censored using diagnosis and smoking status as factors.
Statistical Analysis
Circuit Identification: Overlapping Circuits in Schizophrenia and Nicotine Addiction.
To test whether schizophrenia and nicotine addiction independently affect overlapping brain circuits, we performed the following 3 steps. (1) Comparing schizophrenia nonsmokers (n = 18) with control nonsmokers (n = 28) to identify dACC circuits associated with schizophrenia independent of smoking. (2) Using FTND as a measure of nicotine addiction severity,21 we performed regression analysis with FTND in smokers (n = 73: schizophrenia and control smokers combined) to identify circuits associated with nicotine addiction severity. Note that disease-related and smoking-related circuits were identified from 2 independent groups (nonsmokers and smokers, respectively). (3) To select the final candidate circuits, we created an intersection mask from the individually statistically thresholded (family-wise error corrected, P corrected < .05) disease-related maps from nonsmokers and nicotine addiction–related maps from smokers. Any circuit(s) identified should be independently affected by, but also shared between, schizophrenia and nicotine addiction (table 1).
Table 1.
Analysis Pipeline
| Hypotheses | ||||
| a. Dorsal anterior cingulate (dACC) circuits are impaired in schizophrenia independent of smoking in schizophrenia nonsmokers compared with control nonsmokers. | ||||
| b. dACC circuits are impaired in nicotine addiction. | ||||
| c. Schizophrenia-related dACC circuits overlap onto circuits linked to nicotine addiction. | ||||
| d. Circuits associated with nicotine addiction severity are fundamentally similar in schizophrenia smokers and control smokers. | ||||
| e. Disease-related effects in dACC circuits are fundamentally similar in schizophrenia smokers and schizophrenia nonsmokers. | ||||
| f. Such circuit impairments are present in nonpsychotic first-degree relatives of schizophrenia patients and therefore are related to the genetic liability of schizophrenia and are not due to antipsychotic medication. | ||||
| Step | Analysis | Subjects | Number of Subjects | Hypothesis |
| Hypothesis testing— circuit identification | ||||
| 1 | Identify disease-related circuits (t test: control vs schizophrenia) | Nonsmokers | Control: 28; schizophrenia: 18 | a |
| 2 | Identify smoking-related circuits (FTND regression) | Smokers | Control: 37; schizophrenia: 36 | b |
| 3 | Candidate circuits: intersection of disease- and smoking-related maps from steps 1 and 2 | c | ||
| Hypothesis testing— validity of candidate circuits | ||||
| 4 | rsFC-FTND correlations (control smokers vs schizophrenia smokers) | Smokers | Control smokers: 37; schizophrenia smokers: 36 | d |
| 5 | Identify disease-related differences in smokers (control vs schizophrenia, controlling for FTND) | Smokers | Control smokers: 37; schizophrenia smokers: 36 | e |
| 6 | Identify disease-related differences in first-degree relative smokers (control vs relative smokers, controlling for FTND) | First-degree relative smokers, control smokers | Control smokers: 37; first-degree relative smokers: 8 | f |
| 7 | Identify disease-related differences in first-degree relative nonsmokers (control vs relative nonsmokers) | First-degree relative nonsmokers, control nonsmokers | Control nonsmokers: 28; first-degree relative nonsmokers: 16 | f |
Note: FTND, Fagerstrom Test for Nicotine Dependence; rsFC, resting state functional connectivity.
Step 1 is based on voxel-wise 2-tailed independent sample t tests for group comparisons, correcting for whole brain comparisons at P corrected < .05, corresponding to a P threshold of .001 and a minimum cluster size of 324mm3 for the control vs schizophrenia nonsmoker analysis determined by Monte Carlo simulations. Step 2 is based on a voxel-wise linear regression using FTND as the independent variable, corrected at P corrected < .05 (P threshold of .001; minimum cluster size of 351mm3). In step 3, results are combined by an “AND” function to identify circuits fulfilling both analyses. Because small clusters could spuriously arise in the intersection mask (step 3) due to resampling and/or smoothing, only clusters ≥100mm3 were subjected to further analysis.
Schizophrenia Smokers vs Control Smokers—Nicotine Addiction Severity.
Because smoking-related circuits were identified from all smokers (patients and controls), to ensure that the relationship between nicotine addiction severity and rsFC in dACC circuits was fundamentally similar in schizophrenia smokers and control smokers, we extracted mean rsFC separately from the final candidate circuits in schizophrenia and control smokers. Pearson correlations were performed between rsFC and FTND for each group. We statistically performed group comparison of rsFC-FTND correlations using Fisher r-to-z transformation.
Schizophrenia Smokers vs Control Smokers—Disease-Related Effects.
Disease-related effects in candidate circuits were obtained from nonsmoker schizophrenia patients only. Therefore, disease-related rsFC could be unique to schizophrenia nonsmokers rather than representative of schizophrenia in general. To ensure this was not the case, we extracted mean rsFC for schizophrenia smokers and control smokers for the candidate circuits. We compared rsFC from the schizophrenia vs control smoker groups and included FTND as a covariate to control for smoking severity.
First-Degree Relatives.
In order to demonstrate that circuit abnormalities are related to the liability for schizophrenia and are not due to antipsychotic medication effects, we compared first-degree relatives of schizophrenia patients to controls. Mean rsFC values in relatives were extracted from candidate circuits described in step 3. Because the relative sample contained a higher percentage of nonsmokers than patient and control groups, we performed these analyses separately for smoking and nonsmoking relative groups because rsFC could potentially be confounded by the greater frequency of nonsmokers in the combined relative group.
Clinical Correlations.
Pearson’s correlations were applied to examine relationships between BPRS and rsFC, correcting for the number of correlations using Bonferroni correction. Correlations between BPRS and rsFC in candidate circuits would add further support that these circuits are related to schizophrenia pathophysiology.
Supportive Analyses.
Smoking-related analyses were based on nicotine addiction severity, which by definition involves smokers but not nonsmokers. For the primary analyses, we used correlation with nicotine addiction severity (FTND) for circuit identification because our sample included both light and heavy smokers, making a categorical (smoker vs nonsmoker) approach potentially less sensitive to detecting smoking-related circuits. We also wanted to use independent groups to generate the smoking-related and disease-related maps. We therefore performed a supportive analysis using 2-way ANOVA with rsFC as the dependent variable and diagnosis (control vs patient) and smoking status (heavy smoker vs nonsmoker) and their interaction as factors. We compared heavy smokers with nonsmokers by excluding light smokers and including only smokers with FTND ≥ 6, a common criterion for defining high levels of nicotine dependence.27
Results
Schizophrenia patients and controls did not significantly differ in age, gender, or smoking characteristics (see online supplementary table 1).
Circuit Identification: Overlapping Circuits in Schizophrenia and Nicotine Addiction
We identified dACC rsFC circuits associated with schizophrenia independent of smoking by comparing control and schizophrenia nonsmokers, yielding 8 large clusters with decreased rsFC in schizophrenia (figure 1A). We next identified 15 dACC rsFC circuits associated with nicotine addiction severity in smokers with voxel-wise regression analysis with FTND (P corrected < .05; figure 1C). In all 15 circuits, higher FTND was correlated with reduced circuit strength.
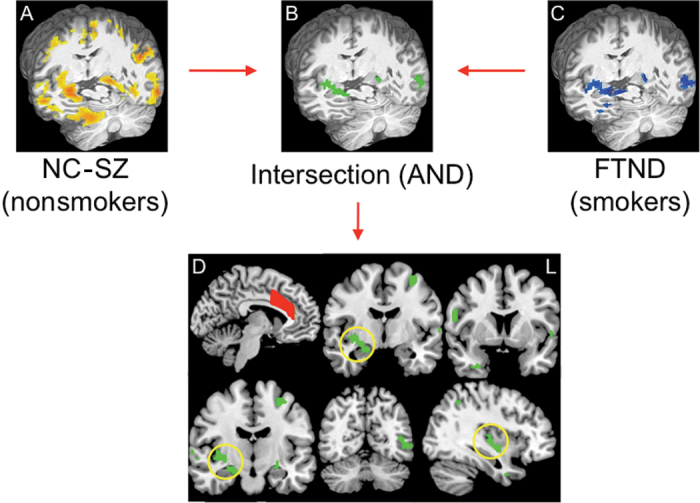
Fig. 1.
(A) Reductions in resting state functional connectivity (rsFC) were seen between dorsal anterior cingulate (dACC) and widespread areas of the brain (yellow) in schizophrenia (SZ) nonsmokers (n = 18) compared with normal control (NC) nonsmokers (n = 28) (P corrected < .05). (C) Reduced rsFC in smokers that negatively correlated with nicotine addiction severity (blue) as measured by the Fagerstrom Test for Nicotine Dependence (FTND; P corrected < .05). (B and D) Candidate circuits identified by overlapping disease-related circuits in (A) and smoking-related circuits in (C). Yellow circles highlight prominent dACC-right limbic rsFC. The manually drawn dACC seed region (D) is depicted in red.
To identify circuits shared between disease-related effects in nonsmokers and nicotine addiction severity effects in smokers, we found the intersection of disease-related and nicotine addiction–related maps and identified 12 candidate circuits significantly associated with both schizophrenia and smoking (figures 1B and 1D). The largest cluster encompassed key basal forebrain/limbic areas28: right parahippocampal gyrus, extended amygdala, VS, and posterior insula and will be referred to as the “dACC-right limbic” circuit. Other clusters included right inferior frontal gyrus, uncus, inferior parietal and left anterior and posterior portions of the inferior temporal gyrus, precuneus, precentral gyrus, parahippocampal, and fusiform gyri and bilateral superior temporal gyri (see online supplementary table 2).
Schizophrenia Smokers vs Control Smokers—Nicotine Addiction Severity
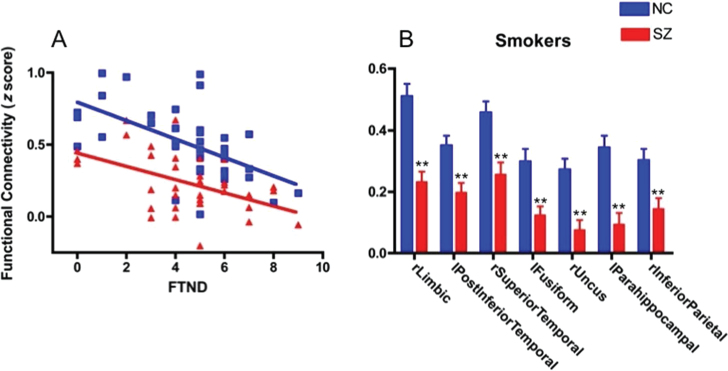
For all candidate circuits, all FTND-rsFC relationships were significant in control smokers after Bonferroni correction for 12 circuits (P = .05/12 = .004), verifying that these circuits were representative of nicotine addiction in controls and were not biased by inclusion of schizophrenia subjects. In schizophrenia smokers, 10 of 12 FTND-rsFC relationships were significant but only 1 remained significant after correction: the dACC-right limbic circuit (P < .001; online supplementary table 2). Importantly, there was no significant difference in the relationship between nicotine addiction severity and rsFC between the 2 smoker groups for the dACC-right limbic circuit (control: r = −.58; schizophrenia: r = −.50; P = .65; figure 2A) or any of the other circuits (all P > .05; online supplementary table 2) with lower circuit strength associated with greater nicotine addiction severity. Correlation analyses between CO, pack-year, and rsFC in all circuits were not significant (all r < .23, all P > .05), suggesting that our findings were specific to addiction severity rather than recent or chronic smoking.
Fig. 2.
(A) Decreased resting state functional connectivity (rsFC) associated with increased nicotine addiction severity (Fagerstrom Test for Nicotine Dependence [FTND]) in smokers for dorsal anterior cingulate (dACC)-right limbic cluster. Note the similar relationship between rsFC and FTND for schizophrenia (SZ) (n = 36) and normal control (NC) (n = 37) smokers but overall decreased rsFC at all levels of smoking severity in SZ. (B) Decreased rsFC in smokers with SZ compared with NC smokers (**P < .004, Bonferroni corrected for number of circuit comparisons). Cluster locations represent areas of decreased rsFC using dACC as seed listed on x-axis in order of size, with right limbic (rLimbic) being the largest.
Schizophrenia Smokers vs Control Smokers— Disease-Related Effects
rsFC in 7 of the 12 circuits were decreased in schizophrenia smokers compared with control smokers after Bonferroni correction (P = .05/12 = P < .004): dACC-right limbic, dACC-left posterior inferior temporal gyrus, dACC-right superior temporal gyrus, dACC-left fusiform gyrus, dACC-right uncus, dACC-left parahippocampal, and dACC-right inferior parietal lobule (figure 2B).
Further analysis will be restricted to these 7 circuits to ensure findings are representative of schizophrenia smokers and nonsmokers. To summarize results thus far, dACC rsFC circuits were identified from abnormalities in schizophrenia independent of smoking, and simultaneously were associated with nicotine addiction severity in both control smokers and smokers with schizophrenia. Thus, common circuits appear to exist between schizophrenia and nicotine addiction.
First-Degree Relatives
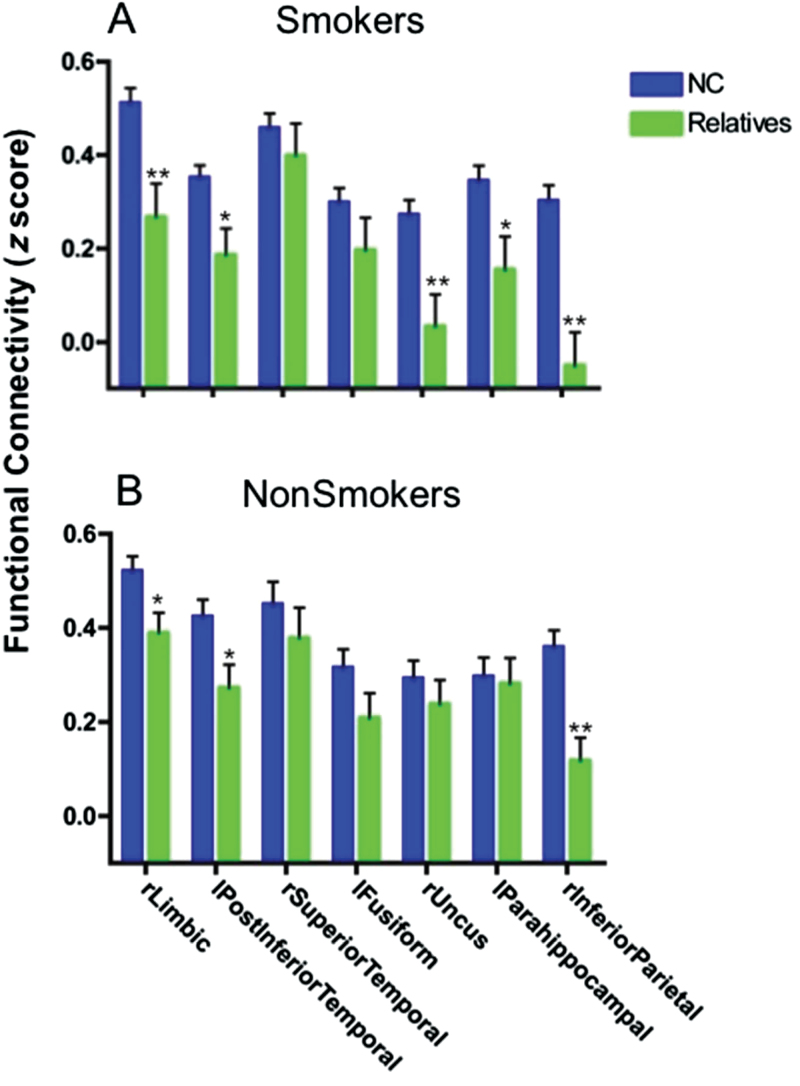
We further examined these 7 circuits in 24 unmedicated nonpsychotic first-degree relatives of schizophrenia patients, who have increased genetic risk for schizophrenia. Unlike patient and control groups, who were predominantly smokers, relatives were mostly nonsmokers (16/24). Thus, we compared relative smokers and relative nonsmokers to their respective control groups separately. We first extracted mean rsFC from each of the 7 remaining candidate circuits to compare relative smokers (n = 8) to control smokers (n = 37), controlling for nicotine addiction severity. Although we used candidate circuits derived from the intersection of disease and smoking-related maps, this test uses groups (relative smokers vs control smokers) independent from the subjects (schizophrenia nonsmokers vs control nonsmokers) used to generate and define disease-related effects in the candidate circuits. Five circuits showed significantly reduced rsFC in relative smokers, and 3 of these circuits survived Bonferroni correction for 7 circuits (P = .05/7 = P = .007) (figure 3A): dACC-right limbic, dACC-right uncus, and dACC-right inferior parietal lobule. Similar to control and schizophrenia smokers, correlations between FTND and rsFC were consistently negative in relative smokers (eg, dACC-right limbic circuit: r = −.74, P = .04).
Fig. 3.
Dorsal anterior cingulate (dACC) resting state functional connectivity (rsFC) in non-ill unmedicated first-degree relatives of schizophrenia patients. Relatives showed significantly (*P < .05; **P < .007, Bonferroni correction for 7 circuit comparisons) reduced rsFC in several dACC circuits (A. smokers [n = 8], B. nonsmokers [n = 16]), suggesting that these circuit abnormalities are related to familial liability for schizophrenia.
Nonsmoker relatives (n = 16) showed significantly reduced rsFC in 3 circuits compared with control nonsmokers (n = 28), again including dACC-right limbic (figure 3B). After Bonferroni correction, only the dACC-right inferior parietal lobule circuit remained significant. Here, the finding of decreased rsFC in relative nonsmokers may be influenced by inflated control nonsmoker rsFC values because control nonsmokers were used to isolate disease-related effects in the initial analysis that generated the regions of interest (ROIs).29 However, we performed Monte Carlo simulations to confirm that our findings are unlikely to be due to inflated control rsFC values (P = .02; online supplementary methods). Overall, our results suggest that shared schizophrenia/nicotine addiction rsFC reductions in dACC-right limbic and other circuits may contribute to the familial/genetic liability for schizophrenia.
Clinical Correlations
Exploring correlations of rsFC circuit measures with clinical symptoms (BPRS scores), we found modest correlations between the dACC-right limbic rsFC and psychosis (r = −.31, P = .02) and thought disorder (r = −.34, P = .01) scores, although significance did not survive Bonferroni correction for 42 comparisons (7 circuits × 6 BPRS measures).
Supportive Analyses
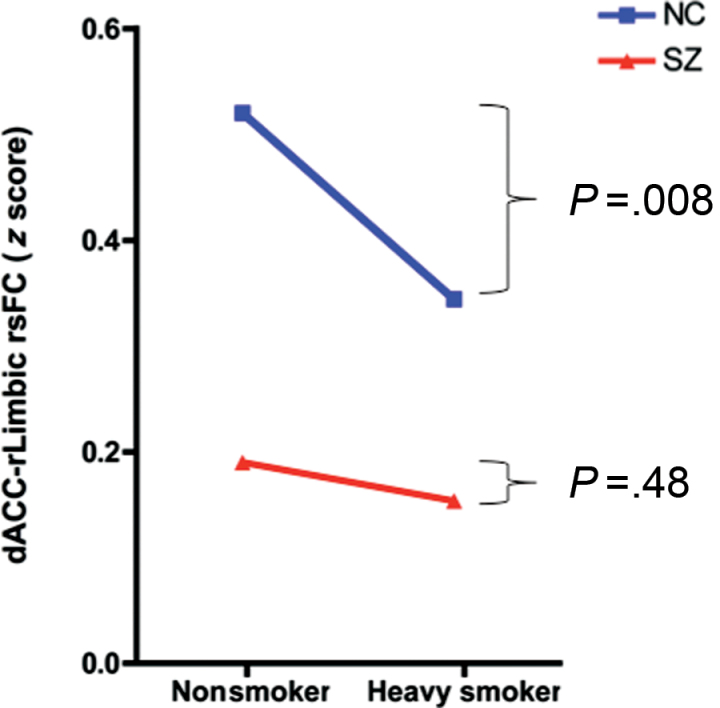
Using smoking status in a 2-way ANOVA of diagnosis (control vs patient) × smoking status (heavy smoker [FTND ≥ 6] vs nonsmoker) analysis for the dACC-right limbic circuit, we found significant main effects of diagnosis (F = 35.2, P < .001) and a significant smoking × diagnosis interaction (F = 4.0, P = .05; figure 4). The significant smoking × diagnosis interaction supports the hypothesis of a coincidental overlap of decreased rsFC in the dACC-right limbic circuit in schizophrenia and nicotine addiction. Subjects with schizophrenia have reduced dACC-right limbic rsFC regardless of whether or not they smoke.
Fig. 4.
Significant smoking × diagnosis interaction (F = 4.0, P = .05) in which subjects with schizophrenia have reduced functional connectivity of the dorsal anterior cingulate-right limbic (dACC-rLimbic) circuit regardless of smoking status (heavy smokers [n = 11] vs nonsmokers [n = 18], t = 0.7, P = .48). In contrast, normal control (NC) heavy smokers (n = 12) have significantly decreased dACC-rLimbic rsFC compared with NC nonsmokers (n = 28) (t = 2.8, P = .008). (Figure depicts heavy smokers vs nonsmokers with heavy smoking defined as Fagerstrom Test for Nicotine Dependence [FTND] ≥ 6).
Decreases in rsFC could potentially be caused by group differences in head motion.24,26 In a 2-way ANOVA, we found a significant effect of increased head motion (% of censored time points) in smokers vs nonsmokers (F = 4.9, P = .03) but no significant effect of diagnosis (P = .27) or smoking × diagnosis interaction (P = .91). Importantly, we found a near-zero correlation between FTND and head motion in control (r = .01, P = .94) and schizophrenia (r = .06, P = .74) smokers, suggesting that selecting circuits based on FTND as our primary analysis was relatively more immune to effects of head motion than the categorical approach of smoking status in our sample.
Discussion
This study showed that schizophrenia is associated with reduced rsFC in a dACC-right limbic circuit that included VS, parahippocampal, amygdalar, and posterior insular areas spatially similar to circuits previously identified in control smokers and by a smoking-related nicotinic acetylcholine receptor (nAChR) alpha5 receptor genotype.13 Furthermore, reduced circuit strength is present in nonsmoker schizophrenia patients and in first-degree relatives, suggesting that this circuit abnormality in schizophrenia is present regardless of smoking status or neuroleptic use, and instead is associated with the liability for and the presence of schizophrenia. In addition, we found a significant smoking × diagnosis interaction in which control heavy smokers had reduced rsFC in the dACC-right limbic circuit compared with healthy nonsmokers, whereas both nonsmokers and heavy smokers with schizophrenia had even further reductions in rsFC. Based on these supports to our hypothesis, we propose that individuals with schizophrenia are predisposed to smoking due to abnormalities inherent to schizophrenia in dACC-right limbic and perhaps several other dACC circuits associated with nicotine addiction. Abnormal findings identified in first-degree relatives in several of these circuits, including the dACC-right limbic, suggest that reduced strength in these circuits cannot be explained by medication or disease state–related confounds.
Smoking in schizophrenia patients is often attributed to variants of the self-medication theory, ie, due to individual needs for regulating mood, reducing stress, improving cognition, or suppressing medication side effects.30 While many of these effects of smoking are likely valid, these hypotheses may not explain other aspects of smoking in schizophrenia, such as increased smoking before psychosis onset5,31 or in family members.6 Our finding of reduced dACC-right limbic rsFC in patients and first-degree relatives supports a genetic predisposition mechanism. The high risk of smoking in schizophrenia can be construed as an unfortunate “biological coincidence” at a brain circuit level, such that a genetic predisposition to schizophrenia may affect the dACC-right limbic and perhaps other circuits in a manner similar to, but more severe, as to how this circuit predisposes nonpsychiatrically ill individuals to smoking. An alternative explanation may be that the finding of dACC-right limbic circuit impairments is complementary to self-medication hypotheses. For example, cognitive deficits in schizophrenia, also present in prodromal patients32 and in nonpsychotic family members,33 may be mediated by genetically determined dACC circuit impairments. Consistent with this view, nicotine administration in schizophrenia improves cognitive performance in association with increased rsFC between dACC and subcortical regions.34
Meta-analysis of worldwide data showed that smoking rates in schizophrenia are significantly higher than in depression and anxiety.2 In a previous study, we found decreases in rsFC in a similar circuit between the dACC and limbic regions in a cohort of psychiatrically ill subjects that also included patients with depression and anxiety disorders.13 Decreased rsFC in this circuit may be a common final pathway for smoking in psychiatric illness in general. Further studies are needed to see if the even greater smoking prevalence in schizophrenia is associated with relatively larger decreases in dACC-right limbic rsFC compared with other psychiatric disorders.
The mechanisms behind shared rsFC reduction in schizophrenia and nicotine addiction are unclear. Hyperdopaminergic striatal function is one of the most consistent dopaminergic findings in schizophrenia.35 Psychosis has been proposed to be due to the attribution of aberrant salience, in which individuals attribute enhanced salience to innocuous stimuli. This process, mediated by striatal dopaminergic release, may lead to the development of paranoid delusions in which neutral cues are misinterpreted as being salient or threatening.36 Similarly, the attribution of incentive salience to previously neutral cues associated with nicotine use is critical in the establishment of nicotine dependence37 and is mediated by phasic dopaminergic release induced by nicotinic actions on nAChRs in the mesoaccumbens system. Such drug-related cues acquire the ability to independently evoke phasic dopaminergic release in the striatum,38 associated with craving and drug-seeking behavior in dependent individuals.37 The identified dACC rsFC network involving dACC, insula, and multiple subcortical areas including the VS and sublenticular extended amygdala, closely matches the salience network,39 which is thought to mediate the selection of important internal/environmental signals. We therefore speculate that because of the inherent vulnerability of aberrant salience attribution in schizophrenia mediated through the dACC-right limbic network identified in this study, patients with schizophrenia may have an increased propensity to develop incentive salience to drug-related cues, leading to a predilection toward developing nicotine dependence. Consistent with the notion of a unitary mechanism of abnormal salience attribution underlying psychosis and nicotine dependence, rsFC of the dACC-right limbic network was not only significantly associated with nicotine addiction but also with psychosis.
This study has several limitations. Our findings were restricted to dACC circuits, and other regions may contribute to nicotine addiction that are independent of or interact with the dACC-limbic circuit. In addition, nicotine levels or withdrawal may have contributed to reduced rsFC in dACC circuits in smoking severity analyses. Previous fMRI studies of nicotine withdrawal have identified increased cerebral blood flow in similar brain regions to those identified in this study, such as the ACC, VS, amygdala, and hippocampus.40 Although we cannot rule out the possibility that nicotine craving or withdrawal contributed to decreased connectivity in these circuits, the finding of decreased connectivity in nonsmokers with schizophrenia comparable with that of heavy smokers is more consistent with shared neural circuitry of nicotine dependence and schizophrenia. Furthermore, we found no significant correlations between rsFC and CO, a measure of recent nicotine exposure. Given the small sample size of first-degree relatives, the analysis in relatives was underpowered to perform circuit identification by comparing whole brain dACC seed correlation maps between relatives and controls. We therefore instead extracted mean rsFC from candidate circuits identified from control vs schizophrenia analyses to test the hypothesis that reduced rsFC was also present in relatives. Decreased rsFC of the dACC-right limbic circuit in relative nonsmokers did not survive Bonferroni correction. Thus, our conclusion that the dACC-right limbic circuit reflects the genetic liability for schizophrenia should be considered preliminary and requires replication in a larger sample of relatives.
To summarize, results of this study support a shared brain circuit theory to explain the high risk of smoking in schizophrenia. Each condition may share a common pathway at the gross brain circuit level captured by resting state synchronization measures. Such a neural circuit mechanism may provide a theoretical alternative or complement to the prevailing self-medication hypothesis, by explaining the biology of comorbidity using concrete, falsifiable brain circuit biomarkers suitable for subsequent animal and human research.
Supplementary Material
Supplementary material is available at http:// schizophreniabulletin.oxfordjournals.org.
Funding
National Institutes of Health (T32MH067533, R01DA027680, R01MH085646, R21DA033817); National Institute on Drug Abuse Intramural Research Program; Maryland Cigarette Restitution Fund Program—Other Tobacco-Related Diseases Research Grant.
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Chapman S, Ragg M, McGeechan K. Citation bias in reported smoking prevalence in people with schizophrenia. Aust N Z J Psychiatry, 2009;43:277–282 [DOI] [PubMed] [Google Scholar]
- 2. de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res, 2005;76:135–157 [DOI] [PubMed] [Google Scholar]
- 3. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry, 2000;177:212–217 [DOI] [PubMed] [Google Scholar]
- 4. McEvoy JP, Freudenreich O, Levin ED, Rose JE. Haloperidol increases smoking in patients with schizophrenia. Psychopharmacology (Berl), 1995;119:124–126 [DOI] [PubMed] [Google Scholar]
- 5. Kelly C, McCreadie RG. Smoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Am J Psychiatry, 1999;156:1751–1757 [DOI] [PubMed] [Google Scholar]
- 6. Lyons MJ, Bar JL, Kremen WS, et al. Nicotine and familial vulnerability to schizophrenia: a discordant twin study. J Abnorm Psychol, 2002;111:687–693 [DOI] [PubMed] [Google Scholar]
- 7. de Leon J, Becoña E, Gurpegui M, Gonzalez-Pinto A, Diaz FJ. The association between high nicotine dependence and severe mental illness may be consistent across countries. J Clin Psychiatry, 2002;63:812–816 [DOI] [PubMed] [Google Scholar]
- 8. Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA, 2009;106:13040–13045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vincent JL, Patel GH, Fox MD, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature, 2007;447:83–86 [DOI] [PubMed] [Google Scholar]
- 10. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci, 2007;8:700–711 [DOI] [PubMed] [Google Scholar]
- 11. Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron, 2009;62:42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hong LE, Gu H, Yang Y, et al. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry, 2009;66:431–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hong LE, Hodgkinson CA, Yang Y, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci USA, 2010;107:13509– 13514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carter CS, MacDonald AW, III, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry, 2001;158:1423–1428 [DOI] [PubMed] [Google Scholar]
- 15. Morey RA, Inan S, Mitchell TV, et al. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Arch Gen Psychiatry, 2005;62:254–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sim K, DeWitt I, Ditman T, et al. Hippocampal and parahippocampal volumes in schizophrenia: a structural MRI study. Schizophr Bull, 2006;32:332–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Job DE, Whalley HC, McConnell S, et al. Structural gray matter differences between first-episode schizophrenics and normal controls using voxel-based morphometry. Neuroimage, 2002;17:880–889 [PubMed] [Google Scholar]
- 18. Gallinat J, Meisenzahl E, Jacobsen LK, et al. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci, 2006;24:1744–1750 [DOI] [PubMed] [Google Scholar]
- 19. Polli FE, Barton JJ, Thakkar KN, et al. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain, 2008;131:971–986 [DOI] [PubMed] [Google Scholar]
- 20. First M, Gibbon M, Spitzer R, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV), Washington, DC:: American Psychiatric Press; 1997. [Google Scholar]
- 21. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict, 1991;86:1119–1127 [DOI] [PubMed] [Google Scholar]
- 22. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res, 1996;29:162–173 [DOI] [PubMed] [Google Scholar]
- 23. Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA, 2005;102:9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage, 2012;59:431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 2012;59:2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jones TB, Bandettini PA, Kenworthy L, et al. Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. Neuroimage, 2010;49:401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Leon J, Diaz FJ, Becoña E, et al. Exploring brief measures of nicotine dependence for epidemiological surveys. Addict Behav, 2003;28:1481–1486 [DOI] [PubMed] [Google Scholar]
- 28. Heimer L. A new anatomical framework for neuropsychiatric disorders and drug abuse. Am J Psychiatry, 2003;160:1726–1739 [DOI] [PubMed] [Google Scholar]
- 29. Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci, 2009;12:535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leonard S, Mexal S, Freedman R. Smoking, genetics and schizophrenia: evidence for self medication. J Dual Diagn, 2007;3:43–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goff DC, Henderson DC, Amico E. Cigarette smoking in schizophrenia: relationship to psychopathology and medication side effects. Am J Psychiatry, 1992;149:1189–1194 [DOI] [PubMed] [Google Scholar]
- 32. Lencz T, Smith CW, McLaughlin D, et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry, 2006;59:863–871 [DOI] [PubMed] [Google Scholar]
- 33. Snitz BE, Macdonald AW, III, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull, 2006;32:179–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jacobsen LK, D’Souza DC, Mencl WE, et al. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry, 2004;55:850–858 [DOI] [PubMed] [Google Scholar]
- 35. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull, 2009;35:549–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry, 2003;160:13–23 [DOI] [PubMed] [Google Scholar]
- 37. Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl), 2007;191:391–431 [DOI] [PubMed] [Google Scholar]
- 38. Volkow ND, Wang GJ, Tomasi D, et al. Methylphenidate attenuates limbic brain inhibition after cocaine-cues exposure in cocaine abusers. PLoS ONE, 2010;5:e11509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci, 2007;27:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Z, Faith M, Patterson F, et al. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci, 2007;27:14035–14040 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.