Abstract
Objectives
Clinical isolates of Pseudomonas aeruginosa can be characterized as cytotoxic or invasive, based on their differing effects on host cells. Previous studies have shown that strain type influences pathology in animal models. The aim of this study was to determine if invasive and cytotoxic strains differentially impact clinical presentation, outcome, or therapeutic response in bacterial keratitis.
Methods
P.aeruginosa isolates from the NEI-funded Steroids for Corneal Ulcers Trial (SCUT) were subtyped as invasive or cytotoxic strains. The main outcome measure compared between the two subtypes was change in visual acuity at three months using Huber robust regression, adjusting for topical corticosteroid treatment.
Results
Of the 101 confirmed P.aeruginosa isolates from the SCUT, 74 had a classically invasive or cytotoxic genotype. While corneal ulcers caused by genotypically invasive P.aeruginosa strains presented with significantly better visual acuity than those caused by genotypically cytotoxic P.aeruginosa strains when adjusting for the effect of ulcer location (p=0.008), invasive ulcers improved significantly less than cytotoxic ulcers at three months (0.35 logMAR (three and a half line difference), 95% CI 0.04 to 0.66 p=0.03). When compared with topical moxifloxacin alone, adjunctive treatment with topical corticosteroids was associated with significantly more improvement in visual acuity in the invasive subgroup (p=0.04), but was associated with less improvement in vision in the cytotoxic subgroup (p=0.07).
Conclusions
The results of this study suggest that rational profiling of differentially expressed virulence determinants, e.g. cytotoxicity and invasiveness for P.aeruginosa, could be used as a tool for decision making in management of infections to optimize outcome.
Keywords: Pseudomonas aeruginosa, bacterial keratitis, virulence, type III secretion
Introduction
Pseudomonas aeruginosa is a significant cause of bacterial keratitis in both the United States and South India, accounting for between 8–29% and 11–48% of cases, respectively.1–7 However, not all P. aeruginosa strains impact host cells in the same manner. Two important virulence determinants are invasiveness and cytotoxicity.8–12 Invasive strains encode exoS, and therefore can sequester themselves intracellularly, replicating and stimulating membrane bleb formation within host cells.13–15 Cytotoxic strains lack exoS, and instead encode the acute cytotoxin exoU, which can quickly kill cells.16 Both ExoS and ExoU are effectors of the P. aeruginosa type III secretion system (T3SS). Effector proteins are injected into eukaryotic cells via the T3SS apparatus, activated within the targeted cell, and then trafficked to specific organelles, where they mediate a variety of phenotypic changes that ultimately result in cell death.17
Studies in cultured cells and in mouse models have compared invasive and cytotoxic strains. Cell culture studies show an inverse relationship between capacity for cell killing and capacity for invasion, while mouse models show different effects on pathology, immune responses, and response to antibiotics.8–10, 12, 18, 19 Although there have been some studies showing differences in ulcer presentation between these two subtypes of P. aeruginosa, a larger study in humans investigating differences in functional outcomes and therapeutic response is lacking.11, 20–24
The Steroids for Corneal Ulcers Trial (SCUT) was an NIH-funded, randomized, placebo-controlled trial investigating the effect of topical corticosteroids as adjunctive treatment to antibiotics for bacterial keratitis.25 The use of topical corticosteroids for bacterial keratitis has been controversial, and prior to this trial, there was insufficient evidence to guide clinical practice. The SCUT found no overall benefit or harm with adjunctive topical corticosteroid treatment across all types of bacterial corneal ulcers but suggested a benefit for patients with the most severe ulcers. The large sample size from the SCUT allows for further investigation by subgroup, including by organism type. The purpose of this pre-specified study was to determine whether the invasive and cytotoxic subtypes of P. aeruginosa have different clinical signs at baseline and result in a different response to therapy, which may allow for a more tailored treatment approach.
Methods
In the SCUT, patients with culture-confirmed bacterial keratitis were randomized to receive adjunctive treatment with either topical 1% prednisolone phosphate or placebo after 48 hours of topical 0.5% moxifloxacin treatment. Specific methods of the trial, including inclusion and exclusion criteria as well as examination and treatment protocol, are described in detail elsewhere.26
Patients were evaluated at enrollment, three weeks, and three months by certified refractionists and ophthalmologists who performed visual acuity and slit-lamp examinations, respectively. Best spectacle-corrected visual acuity (BSCVA) was measured in logMAR units using a tumbling “E” chart at four meters, with 0.1 logMAR being approximately one line of acuity. Slit lamp examination was used to measure infiltrate/scar size, epithelial defect size, depth of the ulcer, and size of hypopyon, if present, as well as to assess for ocular adverse events. Infiltrate/scar size and epithelial defect size were evaluated to the nearest 0.1 millimeter by taking the geometric mean of the longest diameter and the longest perpendicular to the first measurement. Re-epithelialization was defined as the absence of an epithelial defect with administration of fluorescein. Depth of the ulcer was measured in thirds (>0–33%, >33–67%, or >67–100%). Size of hypopyon was measured to the nearest 0.5 mm. Ulcer location was assessed using photographs with an artificial 4mm pupil superimposed by cornea-specific software. Location was graded as completely central, partially central, or peripheral. Further details of how these assessments were done have been previously described.26
Microbiological Methods
All corneal isolates from the SCUT with growth morphology and Gram stain characteristics consistent with P. aeruginosa were strain typed after confirming speciation by growth on centrimide agar. Lab personnel were masked to clinical data, both baseline data and treatment outcomes, until strain typing was completed. Bacteria were grown on Trypticase soy agar overnight, and rabbit corneal epithelial cells were exposed to bacterial strain suspensions. Corneal epithelial cells were washed and treated with gentamicin to kill extracellular bacteria. Isolates which were resistant to gentamicin were also treated with amikacin. Rabbit corneal epithelial cells were washed and lysed. Lysate was plated on MacConkey agar and incubated. Percent invasiveness was determined by comparing growth from the intracellular lysate of each isolate to growth from P. aeruginosa clinical isolate 6294, which was used as a positive control. Since the assay for invasion depends on sensitivity to either gentamicin or amikacin, percent invasiveness was not measured for gentamicin and amikacin resistant strains. However, P. aeruginosa speciation was confirmed for these strains with an Analytical Profile Index kit.
Percent cytotoxicity for each corneal isolate was determined by measuring the amount of lactate dehydrogenase released into the media by dead or dying host cells after bacterial exposure using a cytotoxicity detection kit (Roche Diagnostics, Indianapolis, IN). Values were compared to a positive control, P. aeruginosa clinical isolate 6206. In some experiments, invasive control 6294 had slightly lower LDH values than the media (baseline) control. Negative values for percent cytotoxicity corresponded to strains that were not cytotoxic and had negative LDH values after the media control had been included in the calculation. Values greater than one corresponded to invasiveness or cytotoxicity greater than the respective positive control.
Each bacterial isolate was genotyped using polymerase chain reaction (PCR). PCR was specifically performed on target loci for the four effectors, exoU, S, T, and Y, of the type III secretion system for P. aeruginosa. Strains 6206 and 6294 were used as positive controls for exoU and exoS amplification, respectively. Both strains were used as positive controls for exoY and exoT amplification. Negative controls were also used for amplification of each effector protein sequence. Strains which were positive for either exoU or exoS, but not both, were considered typical strains. Within this group, exoU+/exoS− strains were classified as classically cytotoxic while exoU−/exoS+ strains were classified classically invasive on the basis of genotype. Strains which were either positive or negative for both exoU and exoS were classified as atypical strains. More detailed methodology for the invasion and cytotoxicity assays as well as the PCR protocol with specific primers are described elsewhere.9, 24, 27
Statistical Methods
Univariate analyses for genotype were performed using either Fisher’s exact test or a two- mean-comparison t-test for categorical and continuous variables, respectively. Multivariate analyses were performed using Huber robust regression to assess the relationship between strain type, either genotype or phenotype, and clinical outcome. In these models, strain genotype was used as a dichotomous predictor variable, either classically invasive or classically cytotoxic. Strains with atypical genotypes were not used. Phenotype was used as a continuous predictor variable, measured as either percent invasiveness or percent cytotoxicity. All confirmed isolates were used in phenotype analyses except when percent invasiveness could not be measured. Baseline characteristics used were BSCVA enrollment, location of ulcer, epithelial defect size at presentation, infiltrate/scar size at enrollment, depth of ulcer at enrollment, size of hypopyon, age, and contact lens wear. Clinical outcome was measured using change in BSCVA at 3 months. Ulcer location was added as a covariate in analyses involving BSCVA to control for possible confounding. Additionally, treatment arm was added as a covariate to control for the possible effect of steroids on change in visual acuity at 3 months. An interaction term (treatment arm×strain genotype) was added to the model assessing genotype and clinical outcome to test if a differential effect of steroids on vision was present for cytotoxic versus invasive P. aeruginosa ulcers. Analyses were performed using STATA 11.0 (StataCorp, College Station, TX). P-values reported for all analyses are nominal values and have not been adjusted for multiple comparisons.
Informed consent was obtained for all subjects enrolled in the SCUT. Institutional Review Board approval was granted by the Aravind Eye Care System Institutional Review Board, the University of California, San Francisco Committee on Human Research, and the Dartmouth-Hitchcock Medical Center Committee for Protection of Human Subjects.
Results
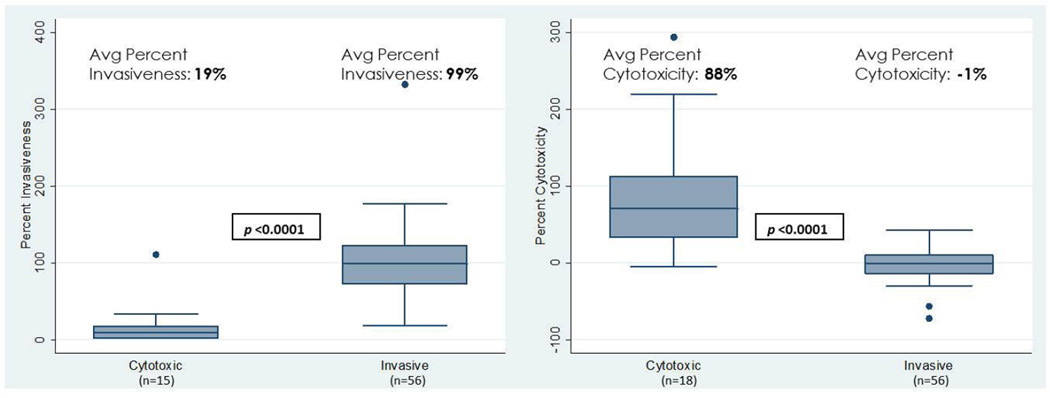
Of the 500 patients in the SCUT enrolled between September 1, 2006 and February 22, 2010, 111 corneal bacterial isolates were strain typed based on growth morphology and Gram stain characteristics consistent with P. aeruginosa. One hundred one of these isolates were confirmed P. aeruginosa based on the methodology described above. Of the 101 confirmed P. aeruginosa isolates, 27 were determined to have atypical genotypes defined as exoU+/exoS+ or exoU−/exoS−. The remaining 74 isolates had typical genotypes; 56 were classically invasive and 18 were classically cytotoxic. Percent invasiveness, expressed as a decimal, ranged from 0.03 to 3.32. Percent cytotoxicity ranged from −0.72 to 2.94. A significant difference was present between the genotypically cytotoxic and invasive strains for both percent invasiveness and percent cytotoxicity (Figure 1).
Figure 1. Percent Invasiveness and Percent Cytotoxicity by Strain Type for Genotypically Classically Cytotoxic and Invasive P. aeruginosa Strains.
The left panel of this figure displays the spread, as well as the average value, of percent invasiveness for genotypically invasive and cytotoxic strains. Three of the genotypically cytotoxic strains were gentamicin and amikacin resistant; thus, percent invasiveness was not measured for these three strains. The right panel displays the spread, as well as the average value, of percent cytotoxicity for genotypically invasive and cytotoxic strains. The median for each group is represented by the line in the middle of each box. The interquartile range(IQR), the span of the 25th to 75th percentiles, is denoted by the lower and upper bounds of each box, respectively. The whiskers extend from the smallest percent invasiveness or percent cytotoxicity within 1.5×IQR below the 25th percentile to the largest percent invasiveness or percent cytotoxicity within 1.5×IQR above the 75th percentile. Individual data points denote values outside this range. P-values displayed in the figure were obtained by two-group mean-comparison t-test.
Baseline demographic features and clinical exam findings were compared between the two genotype groups (Table 1). The mean infiltrate/scar sizes of 4.66 and 3.61 mm for invasive and cytotoxic isolates, respectively, were significantly different (p=0.049, Table 1). The difference in enrollment BSCVA between the two genotype groups was not statistically significant (p=0.80, Table 1). Phenotypically, there was no significant association between percent cytotoxicity and enrollment BSCVA (0.10 logMAR, 95% CI −0.18 to 0.38 p=0.47). However, there was a statistically significant correlation between percent invasiveness and enrollment BSCVA; regression analysis showed that one hundred percent invasiveness was associated with an approximately two and a half line better visual acuity at enrollment compared zero percent invasiveness (−0.26 logMAR, 95%CI −0.51 to −0.01 p=0.04).
Table 1.
Baseline Characteristics: Comparison Between Cytotoxic and Invasive Strains
| Baseline Characteristic | Invasive (N=56) | Cytotoxic (N=18) | P-Value |
|---|---|---|---|
| Enrollment BSCVA (logMAR)a | 1.15 | 1.19 | 0.80b |
| Location | 0.17c | ||
| Completely Central | 22 | 3 | |
| Partially Central | 30 | 13 | |
| Completely Peripheral | 4 | 2 | |
| Enrollment Infiltrate/Scar Size (mm)a | 4.66 | 3.61 | 0.049b |
| Size of Hypopyon (mm)a | 0.62 | 0.26 | 0.13b |
| Epithelial Defect Size (mm)a | 3.59 | 3.16 | 0.42b |
| Depth at Enrollmentd | 0.83c | ||
| 0–33% | 16 | 6 | |
| >33–67% | 13 | 5 | |
| >67–100% | 27 | 7 | |
| Age (years)a | 45.5 | 45.2 | 0.96b |
| Contact Lens Wear | 0.59c | ||
| Yes | 3 | 2 | |
| No | 53 | 16 | |
Values listed are the mean for the invasive and cytotoxic subgroups
P-value obtained by two-group mean-comparison t-test
P-value obtained by Fisher’s exact test
Depth at enrollment measured in thirds, as a percentage of the thickness of the cornea
When controlling for location, P. aeruginosa ulcers caused by genotypically invasive strains had significantly better visual acuity at enrollment, approximately three and a half lines, than those caused by genotypically cytotoxic strains (−0.36 logMAR, 95%CI −0.63 to −0.10 p=0.008, Table 2). Phenotypically, increasing percent invasiveness was associated with a significantly better visual acuity at enrollment when controlling for location. One hundred percent invasiveness was associated with an approximately three line better visual acuity at enrollment than zero percent invasiveness (−0.32 logMAR, 95%CI −0.49 to −0.15 p<0.001, Table 2). Increasing percent cytotoxicity was associated with worse visual acuity at enrollment; however, this relationship was not significant (0.19 logMAR, 95%CI −0.002 to 0.39 p=0.052, Table 2).
Table 2.
Multivariate Linear Regressi on Models Predicting Enrollment Visual Acuity (logMAR BSCVA)
| Covariate | Coefficient | Standard Error | 95% Confidence Interval |
P-Valuea |
|---|---|---|---|---|
| Association between Genotype and Enrollment BSCVA (N=74)b | ||||
| Invasive (vs. Cytotoxic) | −0.363 | 0.134 | −0.630 to −0.096 | 0.008 |
| Completely Central Location | 1.626 | 0.221 | 1.184 to 2.067 | <0.001 |
| Partially Central Location | 0.782 | 0.211 | 0.363 to 1.202 | <0.001 |
| Association between Percent Invasiveness and Enrollment BSCVA (N=96)c | ||||
| Percent Invasivenessd | −0.322 | 0.087 | −0.494 to −0.149 | <0.001 |
| Completely Central Location | 1.636 | 0.201 | 1.237 to 2.035 | <0.001 |
| Partially Central Location | 0.812 | 0.195 | 0.426 to 1.198 | <0.001 |
| Association between Percent Cytotoxicity and Enrollment BSCVA (N=101)e | ||||
| Percent Cytotoxicityd | 0.194 | 0.098 | −0.002 to 0.389 | 0.05 |
| Completely Central Location | 1.538 | 0.205 | 1.132 to 1.945 | <0.001 |
| Partially Central Location | 0.699 | 0.198 | 0.306 to 1.093 | 0.001 |
P-values obtained using Huber robust regression
Atypical genotypes were excluded
Gentamicin and amikacin resistant strains, for which percent invasiveness could not be determined, were excluded
Percent invasiveness and cytotoxicity expressed as a decimal
All confirmed P. aeruginosa isolates included
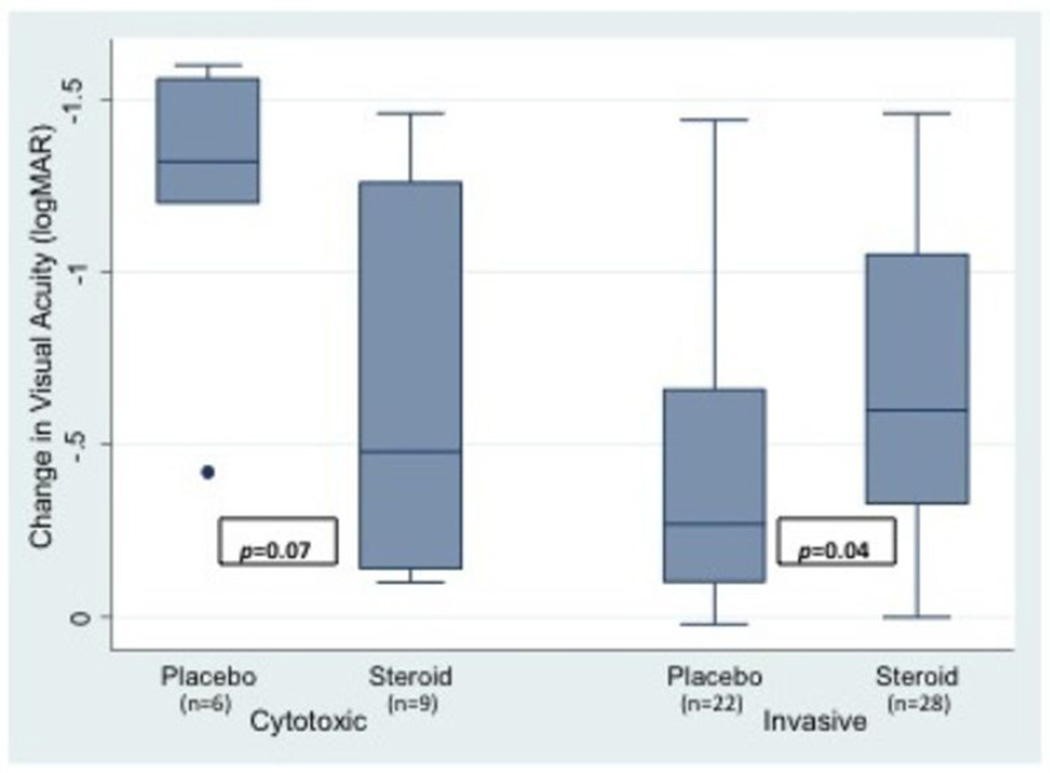
Change in BSCVA at three months was compared between patients with ulcers caused by invasive and cytotoxic P. aeruginosa strains. Genotypically invasive strains were associated with an approximately three and a half line less improvement in visual acuity compared to genotypically cytotoxic strains (0.35 logMAR, 95%CI 0.04 to 0.66 p=0.027, Table 3). When controlling for the effect of location on change in BSCVA at three months, genotypically invasive strains were associated with an approximately four line less improvement in visual acuity compared to genotypically cytotoxic strains (0.40 logMAR, 95%CI 0.09 to 0.70 p=0.013, Table 4). Sensitivity analyses demonstrated that treatment arm, either corticosteroid or placebo, did not affect these results; the corticosteroid treatment difference, approximately one line, was not significant (−0.09 logMAR, 95%CI −0.36 to 0.17 p=0.49). However, an interaction term between treatment arm and strain type added to the model was significant (p=0.005, Table 5), suggesting a differential effect of corticosteroids on ulcers caused by invasive and cytotoxic P. aeruginosa strains. (Figure 2)
Table 3.
Univariate analyses predicting change in BSCVA (logMAR) at 3 months
| Coefficient | Standard Error | 95% Confidence Interval |
P-Valuea | |
|---|---|---|---|---|
| Invasive (vs. Cytotoxic)b | 0.350 | 0.155 | 0.041 to 0.659 | 0.03 |
| Percent Invasivenessc | 0.216 | 0.094 | 0.030 to 0.402 | 0.02 |
| Percent Cytotoxicityd | −0.292 | 0.105 | −0.501 to −0.083 | 0.007 |
P-value obtained using Huber robust regression
Atypical strains excluded (N=74)
Percent invasiveness expressed as a decimal. Gentamicin and amikacin resistant strains excluded (N=96)
Percent cytotoxicity expressed as a decimal. All confirmed P. aeruginosa isolates included (N=101)
Table 4.
Multivariate analyses predicting change in BSCVA (logMAR) at 3 months, controlling for location of ulcer
| Covariate | Coefficient | Standard Error | 95% Confidence Interval |
P-Valuea |
|---|---|---|---|---|
| Association between Genotype and Enrollment BSCVA (N=74)b | ||||
| Invasive (vs. Cytotoxic) | 0.395 | 0.155 | 0.086 to 0.704 | 0.01 |
| Completely Central Location | −0.580 | 0.279 | −1.138 to −0.023 | 0.04 |
| Partially Central Location | −0.423 | 0.271 | −0.965 to 0.119 | 0.12 |
| Association between Percent Invasiveness and Enrollment BSCVA (N=96)c | ||||
| Percent Invasivenessd | 0.236 | 0.092 | 0.054 to 0.419 | 0.01 |
| Completely Central Location | −0.572 | 0.236 | −1.041 to −0.103 | 0.02 |
| Partially Central Location | −0.481 | 0.231 | −0.940 to −0.022 | 0.04 |
| Association between Percent Cytotoxicity and Enrollment BSCVA (N=101)e | ||||
| Percent Cytotoxicityd | −0.296 | 0.104 | −0.503 to −0.089 | 0.006 |
| Completely Central Location | −0.521 | 0.238 | −0.994 to −0.047 | 0.03 |
| Partially Central Location | −0.329 | 0.234 | −0.793 to 0.135 | 0.16 |
P-values obtained using Huber robust regression
Atypical genotypes were excluded
Gentamicin and amikacin resistant strains, for which percent invasiveness could not be determined, were excluded
Percent invasiveness and cytotoxicity expressed as a decimal
All confirmed P. aeruginosa isolates included
Table 5.
Multivariate analyses predicting change in BSCVA (logMAR) at 3 months, controlling for treatment arm
| Covariate | Coefficient | Standard Error | 95% Confidence Interval |
P-Valuea |
|---|---|---|---|---|
| Association between Genotype and Enrollment BSCVA (N=74)b | ||||
| Invasive (vs. Cytotoxic) | 0.850 | 0.229 | 0.392 to 1.309 | <0.001 |
| Steroid (vs. Placebo) | 0.594 | 0.262 | 0.070 to 1.119 | 0.03 |
| Interaction (strain × treatment arm) | −0.876 | 0.298 | −1.472 to −0.280 | 0.005 |
| Association between Percent Invasiveness and Enrollment BSCVA (N=96)c | ||||
| Percent Invasivenessd | 0.378 | 0.131 | 0.116 to 0.639 | 0.005 |
| Steroid (vs. Placebo) | 0.315 | 0.202 | −0.086 to 0.716 | 0.12 |
| Interaction (strain × percent invasiveness1) | −0.374 | 0.186 | −0.744 to −0.004 | 0.047 |
| Association between Percent Cytotoxicity and Enrollment BSCVA (N=101)e | ||||
| Percent Cytotoxicityd | −0.317 | 0.107 | −0.529 to −0.105 | 0.004 |
| Steroid (vs. Placebo) | −0.182 | 0.109 | −0.399 to 0.034 | 0.10 |
P-values obtained using Huber robust regression
Atypical genotypes were excluded
Gentamicin and amikacin resistant strains, for which percent invasiveness could not be determined, were excluded
Percent invasiveness and cytotoxicity expressed as a decimal
All confirmed P. aeruginosa isolates included
Figure 2. Change in logMAR BSCVA at 3 months by treatment arm (placebo vs. steroid) for each P. aeruginosa strain type.
The median for each group is represented by the line in the middle of each box. The interquartile range(IQR), the span of the 25th to 75th percentiles, is denoted by the lower and upper bounds of each box, respectively. The whiskers extend from the smallest visual acuity change within 1.5×IQR below the 25th percentile to the largest visual acuity change within 1.5×IQR above the 75th percentile. Individual data points denote values outside this range. P-values displayed in the figure were obtained by two-group mean-comparison t-test. P-value for the interaction term between strain type and treatment arm was 0.005 and was obtained using Huber robust regression.
Analyses of phenotypic properties of each corneal isolate supported these findings. One hundred percent invasiveness was associated with an approximately two line smaller improvement in BSCVA at three months versus zero percent invasiveness (0.22 logMAR, 95%CI 0.03 to 0.41 p=0.023, Table 3). Similarly, one hundred percent cytotoxicity was associated with an approximately three greater improvement in BSCVA at three months compared to zero percent cytotoxicity(−0.29 logMAR, 95%CI −0.50 to −0.08 p=0.007, Table 3). Treatment arm was added to these models as a covariate, was not significant. However, an interaction term between percent invasiveness and treatment significant (−0.37 logMAR, 95% CI −0.74 to −0.004 p=0.047, Table 5), supporting a differential effect corticosteroids on change in visual acuity as percent invasiveness varied.
Discussion
Several studies have compared the effect of cytotoxicity and invasiveness of P. aeruginosa the cornea in mouse models and have shown a difference in pathogenesis.8, 9, 19 The few studies done in humans with corneal infection have demonstrated an association between strain type and age, as contact lens wear, but the effect on clinical correlates, such as visual acuity, is unknown.20, 23 In this study, we found that ulcers caused by invasive strains presented with better visual acuity than caused by cytotoxic strains, but they showed less improvement at three months compared to cytotoxic strain ulcers. Additionally, ulcers in the corticosteroid treatment arm improved more than those placebo arm within the invasive subgroup but not within the cytotoxic subgroup.
At presentation, we found that patients with genotypically invasive strain ulcers had significantly better visual acuity compared to patients with genotypically cytotoxic ulcers when controlling for ulcer location. Similarly, analyses based on phenotypic rather than genotypic characteristics, i.e. percent invasiveness and percent cytotoxicity, supported these findings. In a mouse model, cytotoxic P. aeruginosa strains have been previously associated with more may be severe corneal edema, which may be expected to cause decreased visual acuity.19
The SCUT demonstrated no overall difference in visual acuity at three months with adjunctive topical corticosteroid treatment.25 Analysis of the data from all patients with confirmed P. aeruginosa infection demonstrated the same result.28 In the current study, we found a statistically significant difference between the two treatment arms in the invasive P. aeruginosa subgroup. On average, patients with invasive ulcers in the steroid treatment arm had a two and a half line greater improvement in BSCVA from enrollment to three month follow-up compared to the placebo treatment arm. Phenotypic analyses supported this finding; increasing percent invasiveness was associated with a greater difference in visual acuity improvement between the steroid and placebo arms.
Prior studies have showed that neutrophilic infiltration into the center of the cornea, not just damage caused by bacteria, is the hallmark of a pathologic response to invasive P. aeruginosa corneal infection in mouse models.9, 19, 29 In contrast, cytotoxic strains suppress neutrophil infiltration as a direct result of exoU expression.29 The improved outcomes we observed in the steroid treatment arm of the invasive subgroup may reflect the local inhibition of PMN infiltration due to topical corticosteroid exposure. However, modification of other host responses may also play a role in the effect of topical corticosteroids on P. aeruginosa corneal ulcers. We found that patients with cytotoxic ulcers in the corticosteroid arm had approximately five and a half lines less improvement in visual acuity at three month follow-up compared to those in the placebo arm; however, this result did not quite reach significance. One prior study showed delayed clearance of cytotoxic, but not invasive, P. aeruginosa strains from the ocular surface in mice deficient in surfactant protein D, an immunologic protein present in the tear film.30 Systemic literature has shown that inhaled corticosteroids decrease serum surfactant protein D levels.31 This raises the question of whether there is a negative effect of topical corticosteroids on immunologic proteins, such as surfactant protein D, that are important for clearance of cytotoxic P. aeruginosa at the level of the ocular surface.
We examined differences in several other clinical characteristics between these two P. aeruginosa subtypes. Specifically, we found that cytotoxic ulcers presented with an approximately one millimeter smaller infiltrate size on average compared to invasive ulcers in our sample. While previous studies have found that ulcers caused by cytotoxic strains are more common in patients younger than 50 years old, we did not find a significant difference in the age of patients with cytotoxic and invasive ulcers.20, 24 However, the relatively young age of patients in our sample may have made it hard to assess the association between strain type and older age. Additionally, one prior study found that corneal isolates from contact lens wearers were more likely to be cytotoxic.23 We were not able to find this relationship, but there were only five contact lens wearers included in the study.
There are a few potential limitations to consider. This study focused on two specific pathogen virulence determinants of P. aeruginosa without considering differences in host factors or other pathogen virulence determinants that might also impact corneal ulcer healing.29 For instance, factors such as tear film composition and presence of specific surfactant proteins at the ocular surface have been shown to affect the capacity to recover from P. aeruginosa keratitis.27, 32–34 Additionally, almost of the patients in the sample studied were enrolled at the Aravind Eye Care System in India. Differences in demographics, contact lens wear, and mechanism of injury may affect the generalizability of the findings to other populations. It is also possible that geographic location may have had an effect on pathogen genotype distribution. For example, there were 27 atypical isolates, which were neither genotypically classically invasive nor cytotoxic. In comparison, a study of 55 human corneal P. aeruginosa isolates from Australia reported only two atypical genotypes.23 Moreover, previous studies reported an approximately even distribution between the cytotoxic and invasive genotypes.20–22 In this study, only 18 of the 74 corneal isolates with typical genotypes were cytotoxic. This difference in distribution may be attributed to the fact that cytotoxic strains have been reported more commonly among infections associated with contact lens wear, which was rare among the SCUT patient population.23 Of interest is the finding that the proportion of cytotoxic strains isolated from the SCUT study participants resembled the distribution found among canines with P. aeruginosa keratitis.24 Additionally, a large sample of clinical nonocular P. aeruginosa isolates had a similar proportion of cytotoxic strains.35 While the small sample of cytotoxic strains could have affected the findings, particularly for subgroup analyses of the cytotoxic strains, the clinical data was collected prospectively a standardized manner at set time points, increasing the ability to detect relationships.
Currently, the microbiological methods used in this study have been performed in a basic science laboratory setting. However, the distinction between cytotoxic and invasive P. aeruginosa genotypes can be made using PCR, which is inexpensive and available in many clinical microbiology laboratory settings. Additionally, the time needed to obtain PCR results may be acceptable given that corticosteroid treatment could be added after 48 hours of antibiotic treatment, as was done in SCUT.
In summary, the results of this study showed that P. aeruginosa bacterial keratitis caused by genotypically invasive strains presented with significantly better visual acuity than genotypically cytotoxic ulcers but had less improvement in visual acuity at three months. They also revealed that adjunctive treatment with topical corticosteroids had a differential effect on invasive and cytotoxic ulcers. The results of this study suggest the potential to guide management decisions using existing therapies, such as corticosteroid treatment, based on these specific virulence determinants. Additionally, they illustrate the concept that not all infections caused by pathogens of a single species present or respond to treatment similarly. Further studies to elucidate differences in clinical presentation and therapeutic response based on specific virulence determinants for other pathogens may be warranted.
Acknowledgements
The Steroids for Corneal Ulcers Trial was funded by National Eye Institute grant U10 EY015114. Dr. Acharya is supported by National Eye Institute grant K23 EY017897 and a Research to Prevent Blindness Career Development Award. The UCSF Department of Ophthalmology is supported by National Eye Institute core grant, EY02162, an unrestricted grant from Research to Prevent Blindness, the South Asia Research Fund, and That Man May See, Inc. Dr. Fleiszig is supported by National Institutes of Health grants RO1 EY011221 and RO1 AI079192. Dr. Ghanekar, Dr. Li, and Chelsia Leong were supported by National Institutes of Health grant T35 EY07139-18. Alcon provided moxifloxacin (Vigamox) for the trial but did not play a role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, and approval of this manuscript. Dr. Acharya had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflicts of Interest: None of the authors have any financial or personal disclosures to report.
References
- 1.Schein OD, Ormerod LD, Barraquer E, et al. Microbiology of contact lens-related keratitis. Cornea. 1989 Dec;8(4):281–285. [PubMed] [Google Scholar]
- 2.Liesegang TJ, Forster RK. Spectrum of Microbial Keratitis in South Florida. Am J Ophthalmol. 1980;90(1):38–47. doi: 10.1016/s0002-9394(14)75075-5. [DOI] [PubMed] [Google Scholar]
- 3.Koidoutsiligianni A, Alfonso E, Forster RK. Ulcerative Keratitis Associated with Contact-Lens Wear. Am J Ophthalmol. 1989 Jul 15;108(1):64–67. doi: 10.1016/s0002-9394(14)73262-3. [DOI] [PubMed] [Google Scholar]
- 4.Gudmundsson OG, Ormerod LD, Kenyon KR, et al. Factors influencing predilection and outcome in bacterial keratitis. Cornea. 1989;8(2):115–121. [PubMed] [Google Scholar]
- 5.Asbell P, Stenson S. Ulcerative keratitis. Survey of 30 years' laboratory experience. Arch Ophthalmol. 1982 Jan;100(1):77–80. doi: 10.1001/archopht.1982.01030030079005. [DOI] [PubMed] [Google Scholar]
- 6.Ormerod LD, Hertzmark E, Gomez DS, Stabiner RG, Schanzlin DJ, Smith RE. Epidemiology of microbial keratitis in southern California. A multivariate analysis. Ophthalmology. 1987 Oct;94(10):1322–1333. doi: 10.1016/s0161-6420(87)80019-2. [DOI] [PubMed] [Google Scholar]
- 7.Varaprasathan G, Miller K, Lietman T, et al. Trends in the etiology of infectious corneal ulcers at the FI Proctor Foundation. Cornea. 2004 May;23(4):360–364. doi: 10.1097/00003226-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Fleiszig SM, Lee EJ, Wu C, et al. Cytotoxic strains of Pseudomonas aeruginosa can damage the intact corneal surface in vitro. CLAO J. 1998 Jan;24(1):41–47. [PubMed] [Google Scholar]
- 9.Fleiszig SMJ, Zaidi TS, Preston MJ, Grout M, Evans DJ, Pier GB. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect Immun. 1996 Jun;64(6):2288–2294. doi: 10.1128/iai.64.6.2288-2294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee EJ, Cowell BA, Evans DH, Fleiszig SMJ. Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Invest Ophthalmol Vis Sci. 2003 Sep;44(9):3892–3898. doi: 10.1167/iovs.02-1302. [DOI] [PubMed] [Google Scholar]
- 11.Winstanley C, Kaye SB, Neal TJ, et al. Genotypic and phenotypic characteristics of Pseudomonas aeruginosa isolates associated with ulcerative keratitis. J Med Microbiol. 2005 Jun;54(6):519–526. doi: 10.1099/jmm.0.46005-0. [DOI] [PubMed] [Google Scholar]
- 12.Fleiszig SM, Wiener-Kronish JP, Miyazaki H, et al. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997 Feb;65(2):579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleiszig SM, Zaidi TS, Fletcher EL, Preston MJ, Pier GB. Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect Immun. 1994 Aug;62(8):3485–3493. doi: 10.1128/iai.62.8.3485-3493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleiszig SM, Zaidi TS, Pier GB. Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect Immun. 1995 Oct;63(10):4072–4077. doi: 10.1128/iai.63.10.4072-4077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angus AA, Lee AA, Augustin DK, Lee EJ, Evans DJ, Fleiszig SM. Pseudomonas aeruginosa induces membrane blebs in epithelial cells, which are utilized as a niche for intracellular replication and motility. Infect Immun. 2008 May;76(5):1992–2001. doi: 10.1128/IAI.01221-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finck-Barbancon V, Goranson J, Zhu L, et al. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997 Aug;25(3):547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 17.Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol. 2009 Sep;7(9):654–665. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee EJ, Truong TN, Mendoza MN, Fleiszig SM. A comparison of invasive and cytotoxic Pseudomonas aeruginosa strain-induced corneal disease responses to therapeutics. Curr Eye Res. 2003 Nov;27(5):289–299. doi: 10.1076/ceyr.27.5.289.17220. [DOI] [PubMed] [Google Scholar]
- 19.Cole N, Willcox MD, Fleiszig SM, et al. Different strains of Pseudomonas aeruginosa isolated from ocular infections or inflammation display distinct corneal pathologies in an animal model. Curr Eye Res. 1998 Jul;17(7):730–735. [PubMed] [Google Scholar]
- 20.Cowell BA, Weissman BA, Yeung KK, et al. Phenotype of Pseudomonas aeruginosa isolates causing corneal infection between 1997 and 2000. Cornea. 2003 Mar;22(2):131–134. doi: 10.1097/00003226-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H, Conibear TC, Bandara R, Aliwarga Y, Stapleton F, Willcox MD. Type III secretion system-352 associated toxins, proteases, serotypes, and antibiotic resistance of Pseudomonas aeruginosa isolates associated with keratitis. Curr Eye Res. 2006 Apr;31(4):297–306. doi: 10.1080/02713680500536746. [DOI] [PubMed] [Google Scholar]
- 22.Lomholt JA, Poulsen K, Kilian M. Epidemic population structure of Pseudomonas aeruginosa: evidence for a clone that is pathogenic to the eye and that has a distinct combination of virulence factors. Infect Immun. 2001 Oct;69(10):6284–6295. doi: 10.1128/IAI.69.10.6284-6295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choy MH, Stapleton F, Willcox MD, Zhu H. Comparison of virulence factors in Pseudomonas aeruginosa strains isolated from contact lens- and non-contact lens-related keratitis. J Med Microbiol. 2008 Dec;57(Pt 12):1539–1546. doi: 10.1099/jmm.0.2008/003723-0. [DOI] [PubMed] [Google Scholar]
- 24.Ledbetter EC, Mun JJ, Kowbel D, Fleiszig SM. Pathogenic phenotype and genotype of Pseudomonas aeruginosa isolates from spontaneous canine ocular infections. Invest Ophthalmol Vis Sci. 2009 Feb;50(2):729–736. doi: 10.1167/iovs.08-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srinivasan M, Mascarenhas J, Rajaraman R, et al. Corticosteroids for Bacterial Keratitis: The Steroids for Corneal Ulcers Trial (SCUT) Arch Ophthalmol. 2011 Oct 10; doi: 10.1001/archophthalmol.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srinivasan M, Mascarenhas J, Rajaraman R, et al. The Steroids for Corneal Ulcers Trial: Study Design and Baseline Characteristics. Arch Ophthalmol. 2011 Oct 10; doi: 10.1001/archophthalmol.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleiszig SM, Kwong MS, Evans DJ. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect Immun. 2003 Jul;71(7):3866–3874. doi: 10.1128/IAI.71.7.3866-3874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sy A, Srinivasan M, Mascarenhas J, et al. Pseudomonas aeruginosa Keratitis: Outcomes and Response to Corticosteroid Treatment. Invest Ophthalmol Vis Sci. 2011 Dec 9; doi: 10.1167/iovs.11-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zolfaghar I, Evans DJ, Ronaghi R, Fleiszig SM. Type III secretion-dependent modulation of innate immunity as one of multiple factors regulated by Pseudomonas aeruginosa RetS. Infect Immun. 2006 Jul;74(7):3880–3889. doi: 10.1128/IAI.01891-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mun JJ, Tam C, Kowbel D, et al. Clearance of Pseudomonas aeruginosa from a healthy ocular surface involves surfactant protein D and is compromised by bacterial elastase in a murine null-infection model. Infect Immun. 2009 Jun;77(6):2392–2398. doi: 10.1128/IAI.00173-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sin DD, Man SF, Marciniuk DD, et al. The effects of fluticasone with or without salmeterol on systemic biomarkers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008 Jun 1;177(11):1207–1214. doi: 10.1164/rccm.200709-1356OC. [DOI] [PubMed] [Google Scholar]
- 32.Ni M, Evans DJ, Hawgood S, Anders EM, Sack RA, Fleiszig SM. Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by Pseudomonas aeruginosa. Infect Immun. 2005 Apr;73(4):2147–2156. doi: 10.1128/IAI.73.4.2147-2156.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ni M, Tam C, Verma A, et al. Expression of surfactant protein D in human corneal epithelial cells is upregulated by Pseudomonas aeruginosa. FEMS Immunol Med Microbiol. 2008 Nov;54(2):177–184. doi: 10.1111/j.1574-695X.2008.00461.x. [DOI] [PubMed] [Google Scholar]
- 34.McCormick CC, Hobden JA, Balzli CL, et al. Surfactant protein D in Pseudomonas aeruginosa keratitis. Ocul Immunol Inflamm. 2007 Sep-Oct;15(5):371–379. doi: 10.1080/09273940701486423. [DOI] [PubMed] [Google Scholar]
- 35.Bradbury RS, Roddam LF, Merritt A, Reid DW, Champion AC. Virulence gene distribution in clinical, nosocomial and environmental isolates of Pseudomonas aeruginosa. J Med Microbiol. 2010 Aug;59(Pt 8):881–890. doi: 10.1099/jmm.0.018283-0. [DOI] [PubMed] [Google Scholar]