Abstract
Background
While behavioral studies have established that prenatal alcohol exposure (PAE) can result in diminished arithmetic processing capability, the underlying neural correlates of this deficit is still unclear. The aim of the present study was to use fMRI to determine the effect of PAE on neuronal activation during a subtraction task.
Methods
Participants were young adults from a low socio-economic status population who were identified prenatally; the sample consisted of healthy unexposed controls (n=17) and those with PAE, who were subdivided based on the presence (n=19) or absence of physical dysmorphic signs (n=18). Multiple regression analysis was used to determine extent of activation and percent signal change during arithmetic processing, using a letter-matching task as the baseline. Region of interest analysis of activation was performed in the native space and normalized for each individual to compensate for the considerable variability in head size observed in the alcohol-exposed population.
Results
An exposure-dependent response was observed in task performance and neuronal activation. Dysmorphic PAE individuals showed significantly lower task-related performance and activation in regions known to be associated with arithmetic processing, including left superior and right inferior parietal regions and medial frontal gyrus, while the non-dysmorphic PAE group was generally intermediate but not significantly different from the control group in task performance and activation.
Conclusions
Results indicate that there is a range of effects of PAE on arithmetic processing and that the severity this deficit may be dependent on degree of impairment demonstrated by the exposed individual. Evidence of physical dysmorphia may be indicative of functional damage to regions associated with arithmetic calculation, resulting in markedly impaired neuronal recruitment.
Keywords: prenatal alcohol, fMRI, arithmetic processing
Introduction
The hazards of prenatal alcohol exposure (PAE) have been documented for decades, yet it continues to be a prevalent social and health concern today. It has been estimated that approximately 1 of 500 infants in the United States is born affected by such exposure (Abel, 1995). The teratogenic results for offspring of maternal alcohol consumption during pregnancy are referred to as fetal alcohol spectrum disorders, the most severe of which is fetal alcohol syndrome (FAS). Currently, FAS is clinically characterized by facial dysmorphia, diminished growth, and neurodevelopmental disorders including microcephaly (Jones and Smith, 1973). However, diagnosis of FAS can be challenging since there is no one presenting symptom and often behavioral outcomes appear similar to those associated with other neurocognitive disorders (Coles, 2001; Coles et al., 1997; Nash et al., 2006). Structural and functional effects are reported in individuals exposed prenatally who lack the physical dysmorphia associated with FAS (Mattson et al., 1998).
Behavioral problems in individuals with a range of PAE have been observed for decades and are reported to include both neurocognitive deficits as well as social and adaptive dysfunction (Mattson and Riley, 1998). In general, individuals with FAS have lower IQ, often accompanied by impaired visuo-spatial, attentional, memory recall, and/or language skills (Coles et al., 1997; Conry, 1990; Mattson and Riley, 1998; Olson et al., 1998; Streissguth et al., 1994b). Developmental dyscalculia, the reduced ability to understand and/or apply core mathematical processes due to teratogenic damage, is also widely reported to be associated with prenatal alcohol exposure (Goldschmidt et al., 1996; Streissguth et al., 1994b; Streissguth et al., 1989), perhaps even more often than global and verbal deficits (Streissguth et al., 1994b). In adolescents, math-related deficits range from longer response interval for mental math calculations to an inability to do basic addition and subtraction (Streissguth et al., 1994a). Additionally, a study in adults found dysfunction in a number of math skills including the ability to estimate efficiently (Kopera-Frye et al., 1996). Since math processing appears to be a specific deficit associated with prenatal alcohol exposure, the underlying neurocognitive correlates of this arithmetic processing impairment warrant the closer examination that can be provided through functional neuroimaging.
Neural correlates of developmental and clinical dyscalculia have been extensively documented and can serve as a guide to the current investigation. Several studies have reported systematic activation of bilateral parietal, frontal, and precentral cortices during arithmetic calculation (Fehr et al., 2007; Kazui et al., 2000; Menon et al., 2000; Zhang et al., 2005). Lesion studies and examinations of clinical populations indicate that bilateral parietal and frontal regions are responsible for clinical dyscalculia (Dehaene et al., 2004; Menon et al., 2000). Dehaene, et al. (2003) point specifically to the horizontal interparietal sulcus (HIPS) as a region activated for various types of arithmetic calculation, and Cantlon et al. (2006) have confirmed that the intraparietal sulcus (IPS) is activated in both children and adults during both symbolic and nonsymbolic tests of numerosity. It has further been suggested that medial frontal and bilateral parietal regions are specifically related to the nonverbal, spatial aspects of math processing with other areas of the brain subsuming the verbal (symbolic) functions (Fehr et al., 2007; Hubbard, 2005; Kong et al., 2005). To date, no study has examined the effect of PAE on activation in these brain regions in the context of dyscalculia.
A potential methodological confound in functional neuroimaging of individuals affected by PAE is head size differences. Reduced subregion and overall brain size have been widely reported in alcohol-affected individuals (Riley et al., 2004; Sowell et al., 2001) and is especially prevalent in those with FAS (Archibald et al., 2001). The microcephaly common to affected individuals has the potential to distort results of imaging studies if not taken into account during activation analysis. Previous studies of this clinical group have not addressed this issue formally although it has been acknowledged that normal spatial transformation may affect interpretation of results (Bookheimer and Sowell, 2005).
In the present study we used functional MRI (fMRI) to examine the effects of prenatal alcohol exposure on brain activation during performance of a subtraction task. Previous studies have demonstrated successful use of BOLD signal as an indicator of arithmetic functioning and deficiency (Cantlon et al., 2006; Delazer et al., 2003; Fehr et al., 2007; Kong et al., 2005). Expected outcomes included a significant difference between physically affected (dysmorphic) PAE and control groups in task performance and brain activation patterns in those regions previously associated with general arithmetic calculation and specifically subtraction. In order to deal with the problem of potentially confounding head size differences, we identified regions of interest on an individual basis, and normalized activation volumes based on the size of the identified subregion.
A second focus of the study was to examine the extent to which alcohol-exposed individuals with and without dysmorphic features would demonstrate similar patterns of activation in comparison to socio-economic status-matched, nonexposed controls. If neurodevelopment is equally affected in nondysmorphic individuals, we would anticipate a similar pattern of dysfunction in nondysmorphic, PAE individuals as that seen in the more physically affected dysmorphic group. However, since we hypothesize that there is a relationship between severity of physical effects of PAE and the functional deficit associated with dyscalculia, we expect that exposed but non-dysmorphic individuals should show no deficits or should be intermediate in performance between those with dysmorphic features and nonexposed controls.
Materials and Methods
Participants
Participants were 54 young adults, age 20-26, whose prenatal exposure to alcohol was quantified prenatally through maternal report. All were recruited from a longitudinal cohort, derived from a predominantly African-American, low socio-economic status population first identified between 1979 and 1986 when their mothers applied for prenatal care (Smith et al., 1986). From this cohort, three groups were selected for participation in the current study and recontacted. These included individuals who were: 1) Exposed to alcohol prenatally and exhibiting physical signs of such exposure, specifically facial dysmorphia (n=19); 2) Exposed, without dysmorphia (n=18), but with ability scores (i.e., IQ ≤ 83) consistent with mean scores in Group 1; and 3) Unexposed controls from the same low SES population (n=17). All participants were evaluated using a dysmorphia checklist (Fernhoff, Smith, & Falek, 1980), where characteristics associated with the disorder are listed and weighted based on their saliency for the diagnosis (e.g., hypoplastic philtrum is a “3” while anteverted nares is a “1”). The 30 items on the checklist were assessed either by a pediatric dysmophologist or a nurse trained and supervised by a dysmorphologist who were blind to the participant's exposure status. The weightings of items checked are summed to yield a dysmorphology index. The checklist has been evaluated repeatedly as part of longitudinal research studies from birth to adolescence with individuals prenatally exposed to alcohol receiving higher total scores in comparison to non-exposed controls (Fernhoff et al., 1980; Blackston et al., Poster at Annual David Smith Dysmorphology Meeting, Iowa City, Iowa 2005). The criterion used to define the dysmorphic group was that the the participant score had to be one standard deviation above the group mean at any one of three testing points (birth, age 7, mid-adolescence). Potential participants who were left handed, had some risk during the MRI procedure (e.g., due to pregnancy or metal fragments) or who were uncomfortable with the procedure (e.g., claustrophobia) were not imaged.
Demographic and prenatal exposure characteristics are shown in Table 1. While 74 volunteers were imaged originally, 20 were excluded from data analysis and Table 1 includes information from only participants included in the final analysis. Reasons for exclusion were very poor behavioral task performance suggesting that the individual was not able to perform the task (n=2) and unusable data due to head motion or artifact (n=18). To assure that there was no systematic bias associated with this participant loss, we compared those who did and did not have usable data obtained during the math protocol on the variables shown in Table 1 and found no significant differences on any of these measures. The mean ounces of absolute alcohol consumption per week during pregnancy for the dysmorphic and non-dysmorphic groups were 13.5 (sd=15.9) and 10.4 (sd=18), respectively (Table 1).
Table 1.
Background and Prenatal Exposure Characteristics for Participants by Exposure Group
| Variable | Control (n=17)a | Group Non-Dys (n=18)a | Dys (n=19)a | Statistic | Significance |
|---|---|---|---|---|---|
| Gender – % male | 47.1 | 11.1 | 47.4 | x2(2)=6.86 | p=.032 |
| Age at imaging, M (SD) | 23.0 (1.6) | 23.2 (1.7) | 23.3 (1.9) | F(2,51)=.15 | n.s. |
| Monthly income – $ in past 30 d, M (SD) n=53 | 1504 (2150) | 559 (464) | 961 (851) | F(2,50)=2.20 | n.s. |
| Education completed – years, M (SD) n=53 | 12.1 (1.5) | 11.5 (1.4) | 12.0 (1.5) | F(2,50)=.87 | n.s. |
| Full-scale IQ, M (SD) n=53 | 87 (7.9) | 75.6 (7.0) | 76.4 (12.3) | F(2,50)=7.54 | p=.001 |
| Dysmorphia rating at adult visit, M (SD) | 2.6 (3.0) | 3.6 (2.4) | 8.6 (8.3) | F(2,51)=6.61 | p=.003 |
| Adult head circumference – cm, M (SD) n=53 | F(2,47)=8.28 | p=.001 | |||
| Male | 60.6 (1.1) | 58.3 (2.5) | 55.4 (3.0) | ||
| Female | 58.8 (3.8) | 57.5 (2.7) | 55.7 (3.7) | ||
| Amount of alcohol exposure during pregnancy – AA per week, M (SD) | 0 (0) | 10.4 (18.0) | 13.5 (15.9) | F(2,51)=4.46 | p=.016 |
| Cigarettes during pregnancy – % using, n=53 | 17.6 | 55.6 | 83.3 | x2(2)=15.22 | p<.001 |
| Marijuana during pregnancy – % using, n=49 | 5.9 | 35.3 | 26.7 | x2(2)=4.44 | n.s. |
| Cocaine during pregnancy – % using, n=44 | 0 | 12.5 | 9.1 | x2(2)=2.15 | n.s. |
If data for a variable are not available for some participants, the n used for the analysis is noted next to the variable name.
b Two-way Group X Gender analysis of variance was completed for head circumference. No gender or interaction effects were significant.
Participants had been seen during adolescence (Coles et al., 2002) and when recontacted as adults, gave informed consent to continue to participate in the research. To protect the confidentiality of their mothers, who had originally given informed consent, no information about exposure group status or maternal substance use was provided. The informed consent procedure was consistent with the Declaration of Helsinki and was approved by the School of Medicine's Institutional Review Board. Study personnel provided transportation to and from the University research site for data collection and imaging. Experimental procedures, including neuropsychological testing and functional neuroimaging, were carried out by staff blind to group status. Participants were reimbursed for their time and effort.
Experimental paradigm
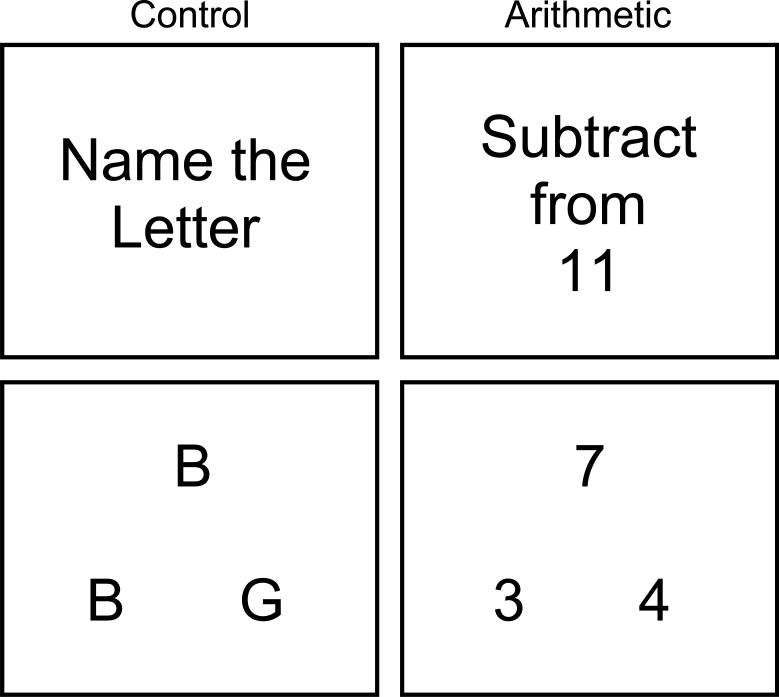
The experimental paradigm, used previously by Connor (personal communication, 2004) with adults affected by alcohol exposure, allowed the evaluation of subtraction performance while using a letter-matching task to control for baseline cognitive and motor activity. The task was presented in blocks, alternating between the letter-matching control task (10 consecutive presentations) and a subtraction task (10 consecutive presentations). Although problems were repeated across blocks, the order of the problems was randomized. Five blocks of each type of task were presented, with instructions stating either “Name the letter” or “Subtract from 11” being shown before each block. Both tasks had a similar visual presentation (Figure 1). Participants were asked to choose between the two letters or numbers on the bottom half of the screen by pressing the left or right button on a button response box (http://www.curdes.com). Paradigm presentation and response collection, including accuracy and reaction time, were done using E-prime (http://www.pstnet.com). It should be noted that the subtraction task was of a type that requires estimation (Klahr, 1973) of quantity and the technique known as “borrowing,” (McCarthy and Warrington, 1987) rendering it “complex” rather than “simple” by the standards of previous studies of arithmetic operation correlates (Fehr et al., 2007; Kong et al., 2005).
Figure 1.
Experimental Paradigm
Image Acquisition
All fMRI data was acquired on a 3T Siemens Trio scanner (Siemens Medical, Erlangen, Germany). The arithmetic study was only one of several functional paradigms implemented, with a total scan time of 39:59 min. For the arithmetic task, single-shot T2*-weighted EPI images were acquired, consisting of 34 contiguous axial slices of with 3mm slice thickness. Pulse sequence parameters, designed to minimize susceptibility to signal loss, were TR/TE/FA/FOV of 3000ms/32ms/90°/22cm. The scan time was 5:06 min, with 102 time points collected. High-resolution, T1-weighted, three-dimensional (3D) anatomical images were also acquired with a 3D MPRAGE (magnetization prepared rapid gradient echo) sequence for all participants. The scan protocol, optimized at 3T, used TR/TI/TE of 2600ms/900ms/3.93ms, flip angle of 8°, field of view of 256 × 224 × 176 mm3, matrix of 256 × 224 × 176, corresponding to an isotropic resolution of 1 mm. Scan time was 7:18 min.
Image Analysis
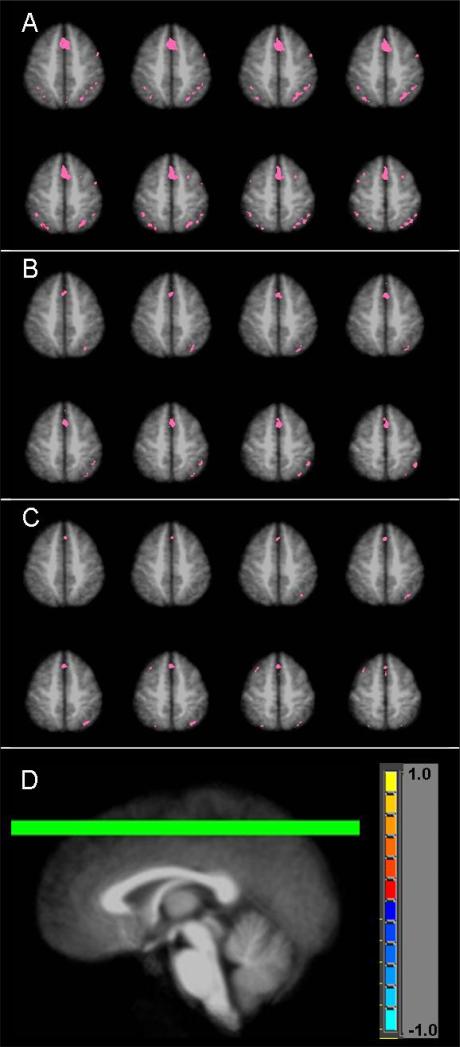
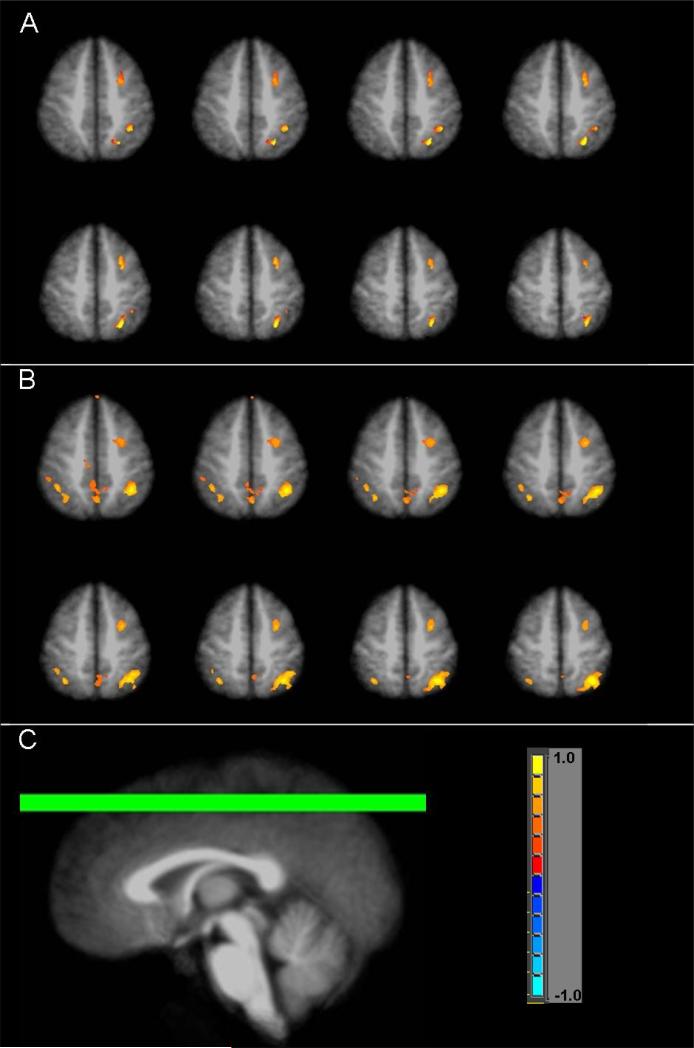
AFNI (http://afni.nimh.nih.gov) was used to perform imaging data analysis. After the data preprocessing steps (slice timing correction, volume registration, signal normalization to percent change, and 5mm FWHM Gaussian blur), 3D+time fMRI datasets for each individual were submitted to a multiple regression analysis. Using the letter task as baseline, the main regressor was generated by convolving the boxcar stimulation functions with a standard impulse response function (y=t^b×exp(−t/c), where b and c are constants) (Cohen, 1997). In order to achieve a better modeling of the motion-related signal variation, the rigid body head motion parameters (x, y, z displacements and roll, pitch, yaw rotations) were included as 6 additional regressors as well. The outcome of this multiple regression analysis included statistical parametric maps, which show voxels with a significant task effect (partial F-statistic), and regression coefficients, which are least squares estimates of the linear model and are proportional to the BOLD (blood oxygenation level-dependent) signal increase level in the arithmetic task from the letter task (baseline). For each group, the statistical parametric maps of individuals were averaged after transforming the dataset into the Talairach space (Talairach and Tournoux, 1988) and normalizing the F values into Z-scores. Voxels that followed the general linear model of letter task baseline and arithmetic block activation were considered to reflect the so-called “arithmetic effect.” Thresholded activation maps of this arithmetic effect, averaged from individual datasets, are shown for PAE and control groups in Figure 2. Voxel-wise group t-test maps were also created to determine activation difference between control and both exposure groups (Figure 3). To account for multiple comparisons, voxel-wise thresholding (p<0.05 for Figure 2; p<0.01 for Figure 3) with cluster thresholding of 4 contiguous voxels was applied. Monte Carlo simulation revealed that these thresholds corresponded to a false-positive discovery rate (alpha) of less than 1% for Figure 2 and less than 0.1% for Figure 3.
Figure 2.
Arithmetic effect for (A) controls, (B) non-dysmorphic PAE, and (C) dysmorphic PAE groups. (D) indicates location of chosen axial slices (z= +46 to +53).
Figure 3.
Subtraction map of the arithmetic effect in control subjects minus arithmetic effect in the (A) non-dysmorphic PAE subjects or (B) dysmorphic PAE subjects. (C) indicates location of chosen axial slices (z= +46 to +53).
In order to quantitatively compare brain activities between groups, we defined regions of interests (ROI) in the Talairach space based on an atlas provided by AFNI. ROIs were chosen based on activation maps of arithmetic effect for the sample as a whole (regions with higher activation in the arithmetic task versus control task). The following brain areas were identified: left and right superior and inferior parietal regions, superior frontal, medial frontal, middle frontal, and inferior frontal gyri. It was noted that all these ROIs were also implicated in previous studies of arithmetic processing. ROI associated functional activation volumes and corresponding regression coefficients were then calculated in native space for each individual. The location and extent of each ROI was defined anatomically in native space by applying the inverse nonlinear warping from the Talairach transformation onto the ROI mask and using the functional dataset as a template. Activation extent (number of active voxels) was subsequently determined for each ROI and then normalized to the voxel size of the entire ROI for each individual. Normalized activation volumes were compared between exposure groups by t-test. Additionally, the unthresholded regression coefficient was calculated for each ROI in native space, converted to percent BOLD signal change, and compared between exposure groups by t-test.
Results
Task Performance
Using “number correct” as the outcome measure, there was a significant difference in performance on the arithmetic task between control subjects and dysmorphic PAE subjects (Table 2), with lower accuracy in the dysmorphic group. When participants gave no response (so-called “skips”), it was counted as an incorrect response in determining accuracy. Participants who skipped more than 50% of arithmetic responses were excluded from analysis (n=2), and of the remaining participants, the number of skips was comparable among groups. Accuracy was at or near 100% on the letter-matching control task for all groups, and there was not a significant difference in reaction time for either task between any of the groups.
Table 2.
Accuracy on the arithmetic task for each exposure group, determined as percent of questions correctly answered (out of 60) and including skipped questions as incorrect.
| Exposure group | Accuracy (%) ± SEM | p-value | Reaction Time (ms) ± SEM | p-value |
|---|---|---|---|---|
| Control | 72.6 ± 3.8 | 1174 ± 46 | ||
| Non-Dys | 65.3 ± 4.2 | 0.104 | 1161 ± 38 | 0.413 |
| Dys | 60.1 ± 4.4 | 0.022* | 1121 ± 48 | 0.218 |
SEM = standard error of mean.
indicates significantly different from control group
fMRI results
Figure 2 shows the arithmetic effect (arithmetic task minus control task) in selected slices for each group. Specifically, robust activation is seen in bilateral parietal lobe, medial frontal gyrus, and bilateral middle frontal gyrus in the control group, while activation in the exposed individuals is sparser and primarily on the right side of the middle frontal and parietal regions. Figure 2 also indicates more overall activation in the control group as compared to the two PAE groups. Regions of interest (ROI), listed in Table 3, were identified based on these activation maps. Selected slices from group difference maps of arithmetic effect-related activation are shown in Figure 3. Greater activation in controls as compared to non-dysmorphic PAE in the middle frontal and parietal regions is notable in Figure 3A. Figure 3B shows significantly more activation in control subjects as compared to dysmorphic PAE subjects in bilateral parietal, middle frontal, and medial frontal gyri.
Table 3.
Comparison of average activation volume and percent signal change in selected regions of interest
| Region of Interest | TAL Coordinatea [x, y, z] | Brodmann Area | Normalized Cluster Volume | Percent Signal Change | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Non-Dys | Non-Dys (ExLS)b | Dys | Dys (ExLS)b | Control | Non-Dys | Non-Dys (ExLS)b | Dys | Dys (ExLS)b | ||||
| Superior parietal | L | [27,57,53] | 7 | 25.1 | 18.7 | 18.7 | 16.1* | 15.4* | 0.30 | 0.24 | 0.24 | 0.22 | 0.20 |
| R | [−27,57,53] | 7 | 19.3 | 15.8 | 15.9 | 15.1 | 16.7 | 0.23 | 0.17 | 0.17 | 0.16 | 0.16 | |
| Inferior parietal | L | [48,41,39] | 7, 40 | 11.8 | 12.2 | 11.4 | 8.23 | 5.74 | 0.12 | 0.12 | 0.11 | 0.09 | 0.07 |
| R | [−48,41,39] | 7, 40 | 11.3 | 10.9 | 12.4 | 6.75* | 7.27* | 0.10 | 0.11 | 0.12 | 0.06+ | 0.05+ | |
| Superior frontal | [±19,−40,27] | 9 | 8.83 | 8.23 | 8.36 | 9.01 | 8.85 | 0.09 | 0.09 | 0.09 | 0.09 | 0.08 | |
| Medial frontal | [±9,−24,35] | 6, 9 | 7.16 | 7.12 | 7.37 | 4.64* | 4.34* | 0.07 | 0.07 | 0.08 | 0.05+ | 0.04+ | |
| Middle frontal | [±37,−29,26] | 9 | 18.1 | 15.3 | 15.7 | 16.7 | 16.6 | 0.20 | 0.16 | 0.17 | 0.16 | 0.14 | |
| Inferior frontal | [±44,−24,2] | 47 | 9.31 | 10.3 | 10.8 | 9.00 | 7.80 | 0.10 | 0.09 | 0.10 | 0.11 | 0.07 | |
TAL coordinate is center coordinate in Talairach space for each ROI
ExLS indicates exposure group excluding low-scoring subjects (below chance)
indicates significantly different from control group (p<0.05)
indicates marginally significant difference from control group (p<0.10)
Activation volumes and percent signal change for each ROI are indicated in Table 3. To verify that activation differences were reflective of impairment and not lack of task engagement, activation was also examined excluding subjects performing below chance (50%) on the arithmetic task (ExLS: n=17 for non-dysmorphic PAE group; n=13 for dysmorphic PAE group). None in the control group scored below chance. Left and right superior and right inferior parietal regions and medial frontal gyrus showed an exposure-dependent response, with the dysmorphic PAE group having the lowest amount of activation. Furthermore, dysmorphic PAE subjects had significantly less (p<0.05) activation as compared to the control group in all of these regions except the right superior parietal area. When low-scoring subjects were excluded, the same ROIs remained significantly different from controls, and no other ROIs had significantly different activation volumes. The percent BOLD signal change is also indicated in Table 3 for each ROI. Average percent signal change trended in the same direction as activation volumes (correlation with average activation volume was r=0.97), with marginally significant differences (p<0.10) between the control and dysmorphic groups in the right inferior parietal and medial frontal gyri. With the exclusion of the low-scoring subjects, these ROI differences were still marginally significant.
No significant correlation was found between task performances and either activation volume or percent signal change (r<0.5 for all groups and all ROIs).
Discussion
Given that prenatal alcohol exposure has been reported to cause deficits in arithmetic processing, we expected an exposure-dependent response in task performance and in brain regions previously associated with arithmetic calculation, with significantly different activation patterns between the dysmorphic PAE group and controls. As predicted, in the present study, dysmorphic PAE individuals showed significantly diminished ability to perform a subtraction task while activation differences were noted in regions known to be associated with arithmetic processing. Activation in the left superior parietal regions, right inferior parietal region, and medial frontal gyrus during the task reflected an exposure-dependent response, with dysmorphic PAE individuals having significantly less activity. It should be noted that excluding those subjects with task performance below chance level still resulted in less activation in the dysmorphic PAE group in the same ROIs which verifies that reduced activation volume was reflective of exposure-based deficit as opposed to lack of engagement in the task. In general, the non-dysmorphic PAE group had both intermediate activation and task performance although they were not significantly different in performance from either group. Furthermore, the trend of less activation in exposed groups than controls by volume measure was also reflected in percent signal change.
It should be noted that the control group had greater activation volume in all ROIs as compared to the dysmorphic PAE group, with the exception of superior frontal gyrus, though the difference was not always significant. Additionally, activation was not significantly impaired in the non-dysmorphic group, and was actually comparable or higher as compared with controls in the inferior parietal region and medial/inferior frontal gyri. While activation volume differences may appear sizeable for some ROIs, they were not always significant. This lack of significance may be due to the considerable inter-subject variability within each group. We also note that while percent signal change correlated with activation volume in this study, it was only marginally statistically significant between groups. The finding of poorer performance on the subtraction task by alcohol-affected individuals is consistent with previous reports that PAE is associated with diminished arithmetic processing in children and adolescents. As noted in the introduction, a number of studies have reported such effects. Streissguth, et al. (1994b; 1989) showed significant effects in children asked to perform arithmetic-based tasks at several stages of academic development. This longitudinal study additionally noted that 91% of the PAE children who showed arithmetic deficiency at 7 years of age, continued to show deficits at 14 years of age as opposed to only 45% in the control group (Streissguth et al., 1994b).
The control and PAE groups in this study were not IQ-matched, raising the question of whether task performance was influenced by IQ differences. However, it should be noted that while both PAE groups had significantly lower IQ as compared to the controls, only the dysmorphic PAE group had significantly poorer task performance. Furthermore, a study of learning deficits in this cohort (Howell, et al., 2006) revealed that while PAE groups specifically demonstrated arithmetic deficits, a low-IQ “special-education” contrast group had deficits in reading and spelling in addition to arithmetic. This finding suggests that the contrast group may have global damage more closely tied to their low IQ whereas the PAE groups have specific problems with math resulting from exposure.
This fMRI study found significant differences in activation in bilateral parietal regions as well as the medial frontal region, which are known to be associated with arithmetic processing (Dehaene et al., 2004; Dehaene et al., 2003; Menon et al., 2000). Recently, Fehr, et al. (2007) used fMRI to comprehensively identify brain areas related to a number of simple arithmetic operation (e.g., addition, subtraction, etc). One specific finding was that, among other regions, medial frontal and bilateral inferior parietal regions were significantly more activated during a “complex” subtraction task as compared to a “simple” arithmetic task. Kong, et al. (2005) also recently examined the neural correlates associated with simple and complex arithmetic operations using fMRI. Complex subtraction was defined by the authors as involving “borrowing,” using tasks similar to those in the present study. They too found involvement of medial frontal gyrus, among other regions, for the complex arithmetic tasks. Furthermore, left superior and right inferior parietal cortices were identified as the two subregions of the parietal lobe specifically associated with subtraction. It was further shown that all regions recruited in performing addition tasks were also required for subtraction. The association of these two subregions with subtraction calculation specifically supports our finding that the dysmorphic PAE group has less activation during the subtraction task in the left superior and right inferior parietal cortices. In the current study, differences in activation in these regions could reflect a deficiency on the part of the dysmorphic PAE group in recruiting the neuronal arithmetic network. Specifically, bilateral parietal region differences could indicate dyscalculia or the inability to perform the subtraction itself, while medial frontal gyrus differences could signify poor recruitment of a region needed for complexity (“borrowing”). This component is believed to be involved in the working memory aspect of the task (Hampson et al., 2006). Dysmorphic alcohol-affected individuals may therefore have neuronal recruitment problems in both the regions activated by all types of arithmetic function and those unique to subtraction operation calculation. Such a deficiency could also account for the poorer task performance by the dysmorphic group.
There have been few other studies that utilize fMRI to examine neurocognitive deficits associated with PAE. Malisza, et al. (2005) reported functional differences in brain regions in individuals with fetal alcohol spectrum disorder (FASD) during a spatial working memory task. In both children and adults, the authors found increased activation in FASD individuals in inferior-middle frontal lobe and greater activation in control individuals in superior frontal and parietal lobes. Additionally, adults had less overall activation as compared to children and FASD groups had lower activation overall versus controls.
Another very recent fMRI study on FASD children (Meintjes et al., Abstract #232, Organization for Human Brain Mapping, Chicago, IL, USA, 2007) reported increased activity in controls as compared to FASD in the left HIPS and left superior frontal region during an exact addition task. The children also performed a proximity judgment task, in which increased activation in controls in left and right HIPS and frontal areas was noted, along with greater activation in FASD in the anterior cingulate and left angular gyrus. As the task in the present study mirrors exact addition more than proximity judgment, our findings are consistent with the report that FASD children have diminished neuronal activation.
As we have noted, one challenge when using the fMRI method on a prenatally alcohol exposed population is the smaller head size that results from perturbed neurodevelopment and characterizes this group. In the present study, for example, whole brain size was found to be significantly different between both PAE groups and the control group (p=0.0048 for non-dysmorphic and p=0.0007 for dysmorphic). Bookheimer and Sowell (2005) point out that because of microcephaly, apparent increases in activation volume in the FASD population could be a result of structural abnormality or improper image registration. Therefore, studies in which anatomical images are normalized to common space may be distorting the activation differences. In this study, we wished to control for this potential methodological issue. We verified that whole brain activation differences between non-dysmorphic and dysmorphic PAE groups and controls were not significant when normalized to whole brain anatomical size (p=0.35 and p=0.39, respectively). Therefore, for our activation volume measurements, we utilized a warping method in which regions of interest were chosen by Talairach atlas in common space and their masks were warped with the inverse matrix back into original space for each individual. The activation volumes in each ROI were then normalized to the size of the whole ROI for each individual. In this way the regions of interest analyzed were uniquely sized and standardized for each individual, making the number of active voxels in the region more accurate.
As noted in the Results section, while the dysmorphic PAE group performed more poorly overall on the subtraction task, no correlation was found between this behavioral performance and activation. The use of different strategies by different subjects (e.g., rote memorization, counting) is a possible explanation for the general lack of association between activation and task performance. However, several studies have shown activation patterns in the parietal lobe varying with arithmetic competency (Delazer et al., 2003; Fehr et al., 2007; Grabner et al., 2007), including degree of automaticity and efficient functioning with task (Ischebeck et al., 2006) and these results suggest that further research is needed to evaluate the relationship between performance and activation.
A next step in understanding the relationship between structural damage induced by PAE exposure and its effects on the functional brain activation is to obtain a more direct correlation between performance and brain activity for cognitive tasks. Using a simpler task could decrease the high variance in activation measures and elucidate a quantifiable relationship between arithmetic calculation and neuronal activation in alcohol affected and exposed individuals. It should also be noted that since the brain regions affected in the present study are associated specifically with subtraction, a paradigm consisting of several different arithmetic operations could elucidate the extent of dyscalculia in the affected population.
The behavioral and imaging results of this study suggest that prenatal alcohol exposure is associated with diminished arithmetic processing capabilities and that such deficits are the result of functional damage to regions known to be associated with mathematical calculation. Specifically, the dysmorphic PAE group appears to have marked impairment in recruiting neurons from bilateral parietal and medial frontal regions for arithmetic processing. Given prior characterization of the neural correlates of arithmetic operations, more heavily exposed alcohol-affected individuals may have difficulty with both the operation itself and its complexity. Furthermore, that the non-dysmorphic PAE group did not have significant activation or performance problems implies a range of responses to the teratogenic exposure that require further study to delineate. Overall, the findings of this study further support the direct relationship between prenatal alcohol exposure and functional brain damage, specifically elucidating a neurological basis for observed arithmetic deficit.
Acknowledgement
This work was supported by Georgia Research Alliance and by the National Institute of Alcohol and Alcoholism (NIAAA) RO1 AA014373. We thank Sharron Paige-Whitaker for her work corresponding with and recruiting participants. We would also like to thank the study participants and their families for their continuing cooperation with this research.
Footnotes
There are no conflicts of interest, including specific financial interests and relationships and affiliations relevant to this manuscript.
References
- Abel EL. An update on incidence of FAS: FAS is not an equal opportunity birth defect. Neurotoxicol Teratol. 1995;17(4):437–43. doi: 10.1016/0892-0362(95)00005-c. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43(3):148–54. [PubMed] [Google Scholar]
- Bookheimer SY, Sowell ER. Brain imaging in FAS: commentary on the article by Malisza et al. Pediatr Res. 2005;58(6):1148–9. doi: 10.1203/01.pdr.0000188720.59781.b3. [DOI] [PubMed] [Google Scholar]
- Cantlon JF, Brannon EM, Carter EJ, Pelphrey KA. Functional imaging of numerical processing in adults and 4-y-old children. PLoS Biol. 2006;4(5):e125. doi: 10.1371/journal.pbio.0040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Coles CD. Fetal alcohol exposure and attention: moving beyond ADHD. Alcohol Res Health. 2001;25(3):199–203. [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Brown RT, Smith IE, Platzman KA, Erickson S, Falek A. Effects of prenatal alcohol exposure at school age. I. Physical and cognitive development. Neurotoxicol Teratol. 1991;13(4):357–67. doi: 10.1016/0892-0362(91)90084-a. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Lynch ME, Freides D. Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcohol Clin Exp Res. 2002;26(2):263–71. [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res. 1997;21(1):150–61. [PubMed] [Google Scholar]
- Conry J. Neuropsychological deficits in fetal alcohol syndrome and fetal alcohol effects. Alcohol Clin Exp Res. 1990;14(5):650–5. doi: 10.1111/j.1530-0277.1990.tb01222.x. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Molko N, Cohen L, Wilson AJ. Arithmetic and the brain. Curr Opin Neurobiol. 2004;14(2):218–24. doi: 10.1016/j.conb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cognitive Neuropsychology. 2003;20(3/4/5/6):487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- Delazer M, Domahs F, Bartha L, Brenneis C, Lochy A, Trieb T, Benke T. Learning complex arithmetic--an fMRI study. Brain Res Cogn Brain Res. 2003;18(1):76–88. doi: 10.1016/j.cogbrainres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Fehr T, Code C, Herrmann M. Common brain regions underlying different arithmetic operations as revealed by conjunct fMRI-BOLD activation. Brain Res. 2007;1172:93–102. doi: 10.1016/j.brainres.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Fernhoff PM, Smith IE, Falek A. Document available through the Maternal Substance Abuse and Child Development Project. Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine; 1980. Dysmorphia Checklist. [Google Scholar]
- Goldschmidt L, Richardson GA, Stoffer DS, Geva D, Day NL. Prenatal alcohol exposure and academic achievement at age six: a nonlinear fit. Alcohol Clin Exp Res. 1996;20(4):763–70. doi: 10.1111/j.1530-0277.1996.tb01684.x. [DOI] [PubMed] [Google Scholar]
- Grabner RH, Ansari D, Reishofer G, Stern E, Ebner F, Neuper C. Individual differences in mathematical competence predict parietal brain activation during mental calculation. Neuroimage. 2007;38(2):346–56. doi: 10.1016/j.neuroimage.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26(51):13338–43. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell KK, Lynch ME, Platzman KA, Smith GH, Coles CD. Prenatal alcohol exposure and ability, academic achievement, and school functioning in adolescence: a longitudinal follow-up. J Pediatr Psychol. 2006;31(1):116–26. doi: 10.1093/jpepsy/jsj029. [DOI] [PubMed] [Google Scholar]
- Hubbard TL. Representational momentum and related displacements in spatial memory: A review of the findings. Psychon Bull Rev. 2005;12(5):822–51. doi: 10.3758/bf03196775. [DOI] [PubMed] [Google Scholar]
- Ischebeck A, Zamarian L, Siedentopf C, Koppelstatter F, Benke T, Felber S, Delazer M. How specifically do we learn? Imaging the learning of multiplication and subtraction. Neuroimage. 2006;30(4):1365–75. doi: 10.1016/j.neuroimage.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2(7836):999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kazui H, Kitagaki H, Mori E. Cortical activation during retrieval of arithmetical facts and actual calculation: a functional magnetic resonance imaging study. Psychiatry Clin Neurosci. 2000;54(4):479–85. doi: 10.1046/j.1440-1819.2000.00739.x. [DOI] [PubMed] [Google Scholar]
- Klahr D. Quantification processes. Academic Press, Inc.; 111 Fifth Ave., New York, New York: 1973. 10003. [Google Scholar]
- Kong J, Wang C, Kwong K, Vangel M, Chua E, Gollub R. The neural substrate of arithmetic operations and procedure complexity. Brain Res Cogn Brain Res. 2005;22(3):397–405. doi: 10.1016/j.cogbrainres.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Kopera-Frye K, Dehaene S, Streissguth AP. Impairments of number processing induced by prenatal alcohol exposure. Neuropsychologia. 1996;34(12):1187–96. doi: 10.1016/0028-3932(96)00043-7. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Allman AA, Shiloff D, Jakobson L, Longstaffe S, Chudley AE. Evaluation of spatial working memory function in children and adults with fetal alcohol spectrum disorders: a functional magnetic resonance imaging study. Pediatr Res. 2005;58(6):1150–7. doi: 10.1203/01.pdr.0000185479.92484.a1. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22(2):279–94. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12(1):146–53. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- McCarthy RA, Warrington E. Deloche G, Seron X, editors. Cognitive mechanism in normal and impaired number processing. Mathematical Disabilities: Cognitive Neuropsychological Perspective. 1987 [Google Scholar]
- Menon V, Rivera SM, White CD, Glover GH, Reiss AL. Dissociating prefrontal and parietal cortex activation during arithmetic processing. Neuroimage. 2000;12(4):357–65. doi: 10.1006/nimg.2000.0613. [DOI] [PubMed] [Google Scholar]
- Nash K, Rovet J, Greenbaum R, Fantus E, Nulman I, Koren G. Identifying the behavioural phenotype in Fetal Alcohol Spectrum Disorder: sensitivity, specificity and screening potential. Arch Womens Ment Health. 2006;9(4):181–6. doi: 10.1007/s00737-006-0130-3. [DOI] [PubMed] [Google Scholar]
- Olson HC, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychological deficits in adolescents with fetal alcohol syndrome: clinical findings. Alcohol Clin Exp Res. 1998;22(9):1998–2012. [PubMed] [Google Scholar]
- Riley EP, McGee CL, Sowell ER. Teratogenic effects of alcohol: a decade of brain imaging. Am J Med Genet C Semin Med Genet. 2004;127(1):35–41. doi: 10.1002/ajmg.c.30014. [DOI] [PubMed] [Google Scholar]
- Smith IE, Coles CD, Lancaster J, Fernhoff PM, Falek A. The effect of volume and duration of prenatal ethanol exposure on neonatal physical and behavioral development. Neurobehav Toxicol Teratol. 1986;8(4):375–81. [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. Neuroreport. 2001;12(3):515–23. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- Spadoni AD, McGee CL, Fryer SL, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Neurosci Biobehav Rev. 2007;31(2):239–45. doi: 10.1016/j.neubiorev.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Olson HC, Sampson PD, Bookstein FL, Burgess DM. Drinking during pregnancy decreases word attack and arithmetic scores on standardized tests: adolescent data from a population-based prospective study. Alcohol Clin Exp Res. 1994a;18(2):248–54. doi: 10.1111/j.1530-0277.1994.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD, Bookstein FL. Prenatal alcohol and offspring development: the first fourteen years. Drug Alcohol Depend. 1994b;36(2):89–99. doi: 10.1016/0376-8716(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Sampson PD, Barr HM. Neurobehavioral effects of prenatal alcohol: Part III. PLS analyses of neuropsychologic tests. Neurotoxicol Teratol. 1989;11(5):493–507. doi: 10.1016/0892-0362(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical Publishers, Inc; New York: 1988. [Google Scholar]
- Zhang YT, Zhang Q, Zhang J, Li W. Laterality of brain areas associated with arithmetic calculations revealed by functional magnetic resonance imaging. Chin Med J (Engl) 2005;118(8):633–8. [PubMed] [Google Scholar]