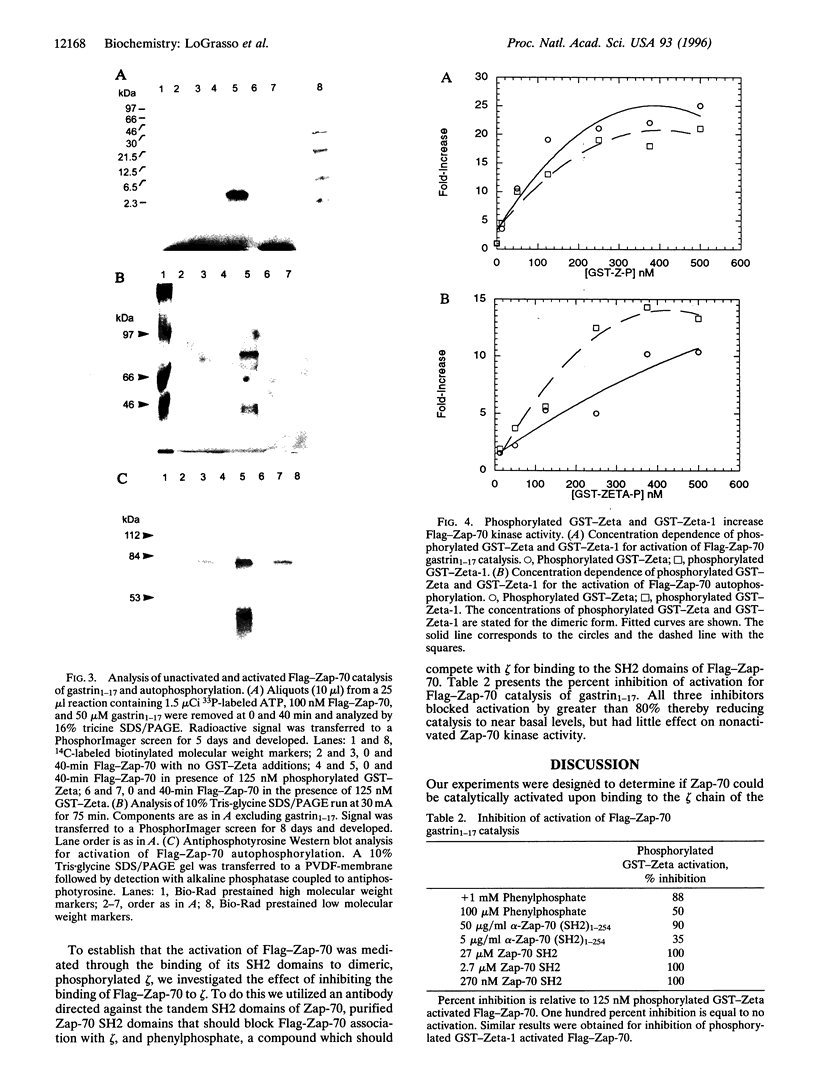
Abstract
There is a growing body of evidence, including data from human genetic and T-cell receptor function studies, which implicate a zeta-associated protein of M(r) 70,000 (Zap-70) as a critical protein tyrosine kinase in T-cell activation and development. During T-cell activation, Zap-70 becomes associated via its src homology type 2 (SH2) domains with tyrosine-phosphorylated immune-receptor tyrosine activating motif (ITAM) sequences in the cytoplasmic zeta chain of the T-cell receptor. An intriguing conundrum is how Zap-70 is catalytically activated for downstream phosphorylation events. To address this question, we have used purified Zap-70, tyrosine phosphorylated glutathione S-transferase (GST)-Zeta, and GST-Zeta-1 cytoplasmic domains, and various forms of ITAM-containing peptides to see what effect binding of zeta had upon Zap-70 tyrosine kinase activity. The catalytic activity of Zap-70 with respect to autophosphorylation increased approximately 5-fold in the presence of 125 nM phosphorylated GST-Zeta or GST-Zeta-1 cytoplasmic domain. A 20-fold activity increase was observed for phosphorylation of an exogenous substrate. Both activity increases showed a GST-Zeta concentration dependence. The increase in activity was not produced with nonphosphorylated GST-Zeta, phosphorylated zeta, or phosphorylated ITAM-containing peptides. The increase in Zap-70 activity was SH2 mediated and was inhibited by phenylphosphate, Zap-70 SH2, and an antibody specific for Zap-70 SH2 domains. Since GST-Zeta and GST-Zeta-1 exist as dimers, the data suggest Zap-70 is activated upon binding a dimeric form of phosphorylated zeta and not by peptide fragments containing a single phosphorylated ITAM. Taken together, these data indicate that the catalytic activity of Zap-70 is most likely activated by a trans-phosphorylation mechanism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arpaia E., Shahar M., Dadi H., Cohen A., Roifman C. M. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking zap-70 kinase. Cell. 1994 Mar 11;76(5):947–958. doi: 10.1016/0092-8674(94)90368-9. [DOI] [PubMed] [Google Scholar]
- Barker S. C., Kassel D. B., Weigl D., Huang X., Luther M. A., Knight W. B. Characterization of pp60c-src tyrosine kinase activities using a continuous assay: autoactivation of the enzyme is an intermolecular autophosphorylation process. Biochemistry. 1995 Nov 14;34(45):14843–14851. doi: 10.1021/bi00045a027. [DOI] [PubMed] [Google Scholar]
- Bu J. Y., Shaw A. S., Chan A. C. Analysis of the interaction of ZAP-70 and syk protein-tyrosine kinases with the T-cell antigen receptor by plasmon resonance. Proc Natl Acad Sci U S A. 1995 May 23;92(11):5106–5110. doi: 10.1073/pnas.92.11.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. C., Dalton M., Johnson R., Kong G. H., Wang T., Thoma R., Kurosaki T. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995 Jun 1;14(11):2499–2508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. C., Desai D. M., Weiss A. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu Rev Immunol. 1994;12:555–592. doi: 10.1146/annurev.iy.12.040194.003011. [DOI] [PubMed] [Google Scholar]
- Chan A. C., Irving B. A., Fraser J. D., Weiss A. The zeta chain is associated with a tyrosine kinase and upon T-cell antigen receptor stimulation associates with ZAP-70, a 70-kDa tyrosine phosphoprotein. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9166–9170. doi: 10.1073/pnas.88.20.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. C., Iwashima M., Turck C. W., Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992 Nov 13;71(4):649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- Chan A. C., Kadlecek T. A., Elder M. E., Filipovich A. H., Kuo W. L., Iwashima M., Parslow T. G., Weiss A. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994 Jun 10;264(5165):1599–1601. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Johnson S. A., Cambier J. C. Analysis of Ig-alpha-tyrosine kinase interaction reveals two levels of binding specificity and tyrosine phosphorylated Ig-alpha stimulation of Fyn activity. EMBO J. 1994 Apr 15;13(8):1911–1919. doi: 10.1002/j.1460-2075.1994.tb06460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb M. H., Sang B. C., Gonzalez R., Goldsmith E., Ellis L. Autophosphorylation activates the soluble cytoplasmic domain of the insulin receptor in an intermolecular reaction. J Biol Chem. 1989 Nov 5;264(31):18701–18706. [PubMed] [Google Scholar]
- Cooper J. A., MacAuley A. Potential positive and negative autoregulation of p60c-src by intermolecular autophosphorylation. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4232–4236. doi: 10.1073/pnas.85.12.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder M. E., Hope T. J., Parslow T. G., Umetsu D. T., Wara D. W., Cowan M. J. Severe combined immunodeficiency with absence of peripheral blood CD8+ T cells due to ZAP-70 deficiency. Cell Immunol. 1995 Oct 1;165(1):110–117. doi: 10.1006/cimm.1995.1193. [DOI] [PubMed] [Google Scholar]
- Elder M. E., Lin D., Clever J., Chan A. C., Hope T. J., Weiss A., Parslow T. G. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 1994 Jun 10;264(5165):1596–1599. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- Exley M., Varticovski L., Peter M., Sancho J., Terhorst C. Association of phosphatidylinositol 3-kinase with a specific sequence of the T cell receptor zeta chain is dependent on T cell activation. J Biol Chem. 1994 May 27;269(21):15140–15146. [PubMed] [Google Scholar]
- Frangioni J. V., Neel B. G. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal Biochem. 1993 Apr;210(1):179–187. doi: 10.1006/abio.1993.1170. [DOI] [PubMed] [Google Scholar]
- Gauen L. K., Zhu Y., Letourneur F., Hu Q., Bolen J. B., Matis L. A., Klausner R. D., Shaw A. S. Interactions of p59fyn and ZAP-70 with T-cell receptor activation motifs: defining the nature of a signalling motif. Mol Cell Biol. 1994 Jun;14(6):3729–3741. doi: 10.1128/mcb.14.6.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatada M. H., Lu X., Laird E. R., Green J., Morgenstern J. P., Lou M., Marr C. S., Phillips T. B., Ram M. K., Theriault K. Molecular basis for interaction of the protein tyrosine kinase ZAP-70 with the T-cell receptor. Nature. 1995 Sep 7;377(6544):32–38. doi: 10.1038/377032a0. [DOI] [PubMed] [Google Scholar]
- Hubbard S. R., Wei L., Ellis L., Hendrickson W. A. Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature. 1994 Dec 22;372(6508):746–754. doi: 10.1038/372746a0. [DOI] [PubMed] [Google Scholar]
- Irving B. A., Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991 Mar 8;64(5):891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Iwashima M., Irving B. A., van Oers N. S., Chan A. C., Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994 Feb 25;263(5150):1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- Johnson S. A., Pleiman C. M., Pao L., Schneringer J., Hippen K., Cambier J. C. Phosphorylated immunoreceptor signaling motifs (ITAMs) exhibit unique abilities to bind and activate Lyn and Syk tyrosine kinases. J Immunol. 1995 Nov 15;155(10):4596–4603. [PubMed] [Google Scholar]
- Kurosaki T., Johnson S. A., Pao L., Sada K., Yamamura H., Cambier J. C. Role of the Syk autophosphorylation site and SH2 domains in B cell antigen receptor signaling. J Exp Med. 1995 Dec 1;182(6):1815–1823. doi: 10.1084/jem.182.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrenas J., Wange R. L., Wang J. L., Isakov N., Samelson L. E., Germain R. N. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science. 1995 Jan 27;267(5197):515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- McTigue M. A., Williams D. R., Tainer J. A. Crystal structures of a schistosomal drug and vaccine target: glutathione S-transferase from Schistosoma japonica and its complex with the leading antischistosomal drug praziquantel. J Mol Biol. 1995 Feb 10;246(1):21–27. doi: 10.1006/jmbi.1994.0061. [DOI] [PubMed] [Google Scholar]
- Negishi I., Motoyama N., Nakayama K., Nakayama K., Senju S., Hatakeyama S., Zhang Q., Chan A. C., Loh D. Y. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995 Aug 3;376(6539):435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- Neumeister E. N., Zhu Y., Richard S., Terhorst C., Chan A. C., Shaw A. S. Binding of ZAP-70 to phosphorylated T-cell receptor zeta and eta enhances its autophosphorylation and generates specific binding sites for SH2 domain-containing proteins. Mol Cell Biol. 1995 Jun;15(6):3171–3178. doi: 10.1128/mcb.15.6.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotou G., Gish G., End P., Truong O., Gout I., Dhand R., Fry M. J., Hiles I., Pawson T., Waterfield M. D. Interactions between SH2 domains and tyrosine-phosphorylated platelet-derived growth factor beta-receptor sequences: analysis of kinetic parameters by a novel biosensor-based approach. Mol Cell Biol. 1993 Jun;13(6):3567–3576. doi: 10.1128/mcb.13.6.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas D. R., Johnson R., Pingel J. T., Matthews R. J., Dalton M., Roy G., Chan A. C., Thomas M. L. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science. 1996 May 24;272(5265):1173–1176. doi: 10.1126/science.272.5265.1173. [DOI] [PubMed] [Google Scholar]
- Pleiman C. M., Abrams C., Gauen L. T., Bedzyk W., Jongstra J., Shaw A. S., Cambier J. C. Distinct p53/56lyn and p59fyn domains associate with nonphosphorylated and phosphorylated Ig-alpha. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4268–4272. doi: 10.1073/pnas.91.10.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo C., Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell. 1991 Mar 8;64(5):1037–1046. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- Rowley R. B., Burkhardt A. L., Chao H. G., Matsueda G. R., Bolen J. B. Syk protein-tyrosine kinase is regulated by tyrosine-phosphorylated Ig alpha/Ig beta immunoreceptor tyrosine activation motif binding and autophosphorylation. J Biol Chem. 1995 May 12;270(19):11590–11594. doi: 10.1074/jbc.270.19.11590. [DOI] [PubMed] [Google Scholar]
- Shiue L., Zoller M. J., Brugge J. S. Syk is activated by phosphotyrosine-containing peptides representing the tyrosine-based activation motifs of the high affinity receptor for IgE. J Biol Chem. 1995 May 5;270(18):10498–10502. doi: 10.1074/jbc.270.18.10498. [DOI] [PubMed] [Google Scholar]
- Shoelson S. E., Boni-Schnetzler M., Pilch P. F., Kahn C. R. Autophosphorylation within insulin receptor beta-subunits can occur as an intramolecular process. Biochemistry. 1991 Aug 6;30(31):7740–7746. doi: 10.1021/bi00245a010. [DOI] [PubMed] [Google Scholar]
- Sotirellis N., Johnson T. M., Hibbs M. L., Stanley I. J., Stanley E., Dunn A. R., Cheng H. C. Autophosphorylation induces autoactivation and a decrease in the Src homology 2 domain accessibility of the Lyn protein kinase. J Biol Chem. 1995 Dec 15;270(50):29773–29780. doi: 10.1074/jbc.270.50.29773. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wange R. L., Guitián R., Isakov N., Watts J. D., Aebersold R., Samelson L. E. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP-70. J Biol Chem. 1995 Aug 11;270(32):18730–18733. doi: 10.1074/jbc.270.32.18730. [DOI] [PubMed] [Google Scholar]
- Wange R. L., Isakov N., Burke T. R., Jr, Otaka A., Roller P. P., Watts J. D., Aebersold R., Samelson L. E. F2(Pmp)2-TAM zeta 3, a novel competitive inhibitor of the binding of ZAP-70 to the T cell antigen receptor, blocks early T cell signaling. J Biol Chem. 1995 Jan 13;270(2):944–948. doi: 10.1074/jbc.270.2.944. [DOI] [PubMed] [Google Scholar]
- Wange R. L., Malek S. N., Desiderio S., Samelson L. E. Tandem SH2 domains of ZAP-70 bind to T cell antigen receptor zeta and CD3 epsilon from activated Jurkat T cells. J Biol Chem. 1993 Sep 15;268(26):19797–19801. [PubMed] [Google Scholar]
- Wei L., Hubbard S. R., Hendrickson W. A., Ellis L. Expression, characterization, and crystallization of the catalytic core of the human insulin receptor protein-tyrosine kinase domain. J Biol Chem. 1995 Apr 7;270(14):8122–8130. doi: 10.1074/jbc.270.14.8122. [DOI] [PubMed] [Google Scholar]
- Weissman A. M., Hou D., Orloff D. G., Modi W. S., Seuanez H., O'Brien S. J., Klausner R. D. Molecular cloning and chromosomal localization of the human T-cell receptor zeta chain: distinction from the molecular CD3 complex. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9709–9713. doi: 10.1073/pnas.85.24.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers N. S., Killeen N., Weiss A. ZAP-70 is constitutively associated with tyrosine-phosphorylated TCR zeta in murine thymocytes and lymph node T cells. Immunity. 1994 Nov;1(8):675–685. doi: 10.1016/1074-7613(94)90038-8. [DOI] [PubMed] [Google Scholar]