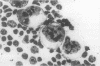
Abstract
AIM: To evaluate the morphofunctional characteristics of lymph node cells from patients with Hodgkin's disease by measuring silver stained nucleolar organiser regions (AgNORs). METHODS: Nucleoli in Hodgkin's and Reed-Sternberg (HRS) cells, lymphocytes and prolymphocytes were investigated in cytological smears and histological sections of lymph nodes from 32 patients with Hodgkin's disease, and from 34 patients with reactive lymphadenopathy. According to the Rye histological classification of Hodgkin's disease, three cases were the lymphocyte predominant (LP) type, 14 the nodular sclerosing (NS) type, and 15 the mixed cellularity (MC) type. The investigation was done before treatment, by means of a one step silver staining method. In each case, 50 to 100 HRS cells, lymphocytes, and prolymphocytes were evaluated to determine the mean numbers of nucleoli and AgNORs per nucleus. The nonparametric Wilcoxon Mann-Whitney test was used to compare the groups. RESULTS: The mean numbers of nucleoli and AgNORs were higher in lymphocytes and prolymphocytes compared with those from reactive lymph nodes used as controls. Numbers of nucleoli and AgNORs were higher (not significant) in the NS type of Hodgkin's disease than in the MC type. There was a significant increase in numbers of nucleoli in HRS cells, and their AgNOR counts were increased. The greatest number of nucleoli in HRS cells was found in the NS type. Furthermore, the nucleolar activity of HRS cells was greater in the NS type compared with the MC type (50.2 (SEM 3.9) v 37.7 (2.9) AgNORs per nucleus (p = 0.025)). Comparative analysis of cytological and histological samples showed that the AgNOR score was significantly higher in touch imprints than in tissue sections with tumours of the same histological type. CONCLUSIONS: Assessment of cell activity in Hodgkin's disease patients by silver staining is more convenient and informative in lymph node imprints than in histological sections. The highest expression of interphase ribosomal RNA cistrons found in NS HRS cells is probably explained by their high proliferative activity and elevated production of transforming growth factor 1 which is known to be the most potent cytokine present in the NS subtype of Hodgkin's disease.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crocker J., Nar P. Nucleolar organizer regions in lymphomas. J Pathol. 1987 Feb;151(2):111–118. doi: 10.1002/path.1711510203. [DOI] [PubMed] [Google Scholar]
- Crocker J., Nar P. Nucleolar organizer regions in lymphomas. J Pathol. 1987 Feb;151(2):111–118. doi: 10.1002/path.1711510203. [DOI] [PubMed] [Google Scholar]
- Crocker J. Nucleolar organiser regions. Curr Top Pathol. 1990;82:91–149. doi: 10.1007/978-3-642-74668-0_3. [DOI] [PubMed] [Google Scholar]
- Derenzini M., Pession A., Trerè D. Quantity of nucleolar silver-stained proteins is related to proliferating activity in cancer cells. Lab Invest. 1990 Jul;63(1):137–140. [PubMed] [Google Scholar]
- Freeman J., Kellock D. B., Yu C. C., Crocker J., Levison D. A., Hall P. A. Proliferating cell nuclear antigen (PCNA) and nucleolar organiser regions in Hodgkin's disease: correlation with morphology. J Clin Pathol. 1993 May;46(5):446–449. doi: 10.1136/jcp.46.5.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gause A., Keymis S., Scholz R., Schobert I., Jung W., Diehl V., Pohl C., Pfreundschuh M. Increased levels of circulating cytokines in patients with untreated Hodgkin's disease. Lymphokine Cytokine Res. 1992 Apr;11(2):109–113. [PubMed] [Google Scholar]
- Howell W. M., Black D. A. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia. 1980 Aug 15;36(8):1014–1015. doi: 10.1007/BF01953855. [DOI] [PubMed] [Google Scholar]
- Hsu P. L., Hsu S. M. Production of tumor necrosis factor-alpha and lymphotoxin by cells of Hodgkin's neoplastic cell lines HDLM-1 and KM-H2. Am J Pathol. 1989 Oct;135(4):735–745. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Lin J., Xie S. S., Hsu P. L., Rich S. Abundant expression of transforming growth factor-beta 1 and -beta 2 by Hodgkin's Reed-Sternberg cells and by reactive T lymphocytes in Hodgkin's disease. Hum Pathol. 1993 Mar;24(3):249–255. doi: 10.1016/0046-8177(93)90034-e. [DOI] [PubMed] [Google Scholar]
- Jakić-Razumović J., Tentor D., Petrovecki M., Radman I. Nucleolar organiser regions and survival in patients with non-Hodgkin's lymphomas classified by the working formulation. J Clin Pathol. 1993 Oct;46(10):943–947. doi: 10.1136/jcp.46.10.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadin M. E., Agnarsson B. A., Ellingsworth L. R., Newcom S. R. Immunohistochemical evidence of a role for transforming growth factor beta in the pathogenesis of nodular sclerosing Hodgkin's disease. Am J Pathol. 1990 Jun;136(6):1209–1214. [PMC free article] [PubMed] [Google Scholar]
- Korkolopoulou P., Patsouris E., Pangalis G., Tsenga A., Elemenoglou J., Thomas-Tsangli E., Spandidos D., Kittas C. A comparative assessment of proliferating cell nuclear antigen, c-myc p62, and nucleolar organizer region staining in non-Hodgkin's lymphomas: a histochemical and immunohistochemical study of 200 cases. Hum Pathol. 1993 Apr;24(4):371–377. doi: 10.1016/0046-8177(93)90084-t. [DOI] [PubMed] [Google Scholar]
- Mamaev N. N., Grichanova T. I., Shandlorenko D. S., Koloskov A. V. Morfofunktsional'naia kharakteristika megakariotsitov cheloveka v norme i pri patologii po dannym izbiratel'nogo serebreniia iadrushek. Gematol Transfuziol. 1990 Nov;35(11):17–20. [PubMed] [Google Scholar]
- Mamaev N. N., Mamaeva S. E. Nucleolar organizer region activity in human chromosomes and interphase nuclei of normal, leukemic, and tumor cells as evaluated by silver staining. Int Rev Cytol. 1990;121:233–266. doi: 10.1016/s0074-7696(08)60661-0. [DOI] [PubMed] [Google Scholar]
- Mamaev N. N., Mamaeva S. E. Struktura i funktsiia iadryshkoobrazuiushchikh raionov khromosom: molekuliarnye, tsitologicheskie i klinicheskie aspekty. Tsitologiia. 1992;34(10):3–25. [PubMed] [Google Scholar]
- Mamaev N. N., Salogub G. N., Koloskov A. V. Interphase ribosomal RNA cistron staining in chronic myeloid leukaemia. Clin Mol Pathol. 1995 Oct;48(5):M260–M263. doi: 10.1136/mp.48.5.m260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata S., Hendricks J. B. Morphometric analysis of AgNORs in imprints and sections from non-Hodgkin's lymphomas. An approach to standardization. Anal Quant Cytol Histol. 1993 Oct;15(5):329–334. [PubMed] [Google Scholar]
- Newcom S. R., Gu L. Transforming growth factor beta 1 messenger RNA in Reed-Sternberg cells in nodular sclerosing Hodgkin's disease. J Clin Pathol. 1995 Feb;48(2):160–163. doi: 10.1136/jcp.48.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcom S. R., Kadin M. E., Ansari A. A., Diehl V. L-428 nodular sclerosing Hodgkin's cell secretes a unique transforming growth factor-beta active at physiologic pH. J Clin Invest. 1988 Dec;82(6):1915–1921. doi: 10.1172/JCI113810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcom S. R., Tagra K. K. High molecular weight transforming growth factor beta is excreted in the urine in active nodular sclerosing Hodgkin's disease. Cancer Res. 1992 Dec 15;52(24):6768–6773. [PubMed] [Google Scholar]
- Smith F. G., Murray P. G., Crocker J. Correlation between PCNA and AgNOR scores in non-Hodgkin's lymphomas using sequential staining technique. J Clin Pathol. 1993 Jan;46(1):28–31. doi: 10.1136/jcp.46.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina D., Leoncini L., Close P., Megha T., Pacenti L., Tosi P., Pileri S., Sabattini E., Kraft R., Laissue J. Growth vs. DNA strand breaks in Hodgkin's disease: impaired proliferative ability of Hodgkin and Reed-Sternberg cells. Int J Cancer. 1996 Apr 10;66(2):179–183. doi: 10.1002/(SICI)1097-0215(19960410)66:2<179::AID-IJC7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Xu L. Z., Wang L. F. Nucleolar organizer regions in aspirates of malignant lymphomas and benign disorders of the lymph nodes. Anal Quant Cytol Histol. 1992 Apr;14(2):148–152. [PubMed] [Google Scholar]
- Yekeler H., Ozercan M. R., Yumbul A. Z., Ağan M., Ozercan I. H. Nucleolar organizer regions in lymphomas: a quantitative study. Pathologica. 1993 May-Jun;85(1097):353–360. [PubMed] [Google Scholar]