Abstract
Objective
To examine whether hospitals where patients obtain care explain racial/ethnic differences in treatment delay.
Data Source
Surveillance, Epidemiology, and End Results data linked with Medicare claims.
Study Design
We examined delays in adjuvant chemotherapy or radiation for women diagnosed with stage I–III breast cancer during 1992–2007. We used multivariable logistic regression to assess the probability of delay by race/ethnicity and included hospital fixed effects to assess whether hospitals explained disparities.
Principal Findings
Among 54,592 women, black (11.9 percent) and Hispanic (9.9 percent) women had more delays than whites (7.8 percent, p < .0001). After adjustment, black (vs. white) women had higher odds of delay (odds ratio = 1.25, 95 percent confidence interval = 1.10–1.42), attenuated somewhat by including hospital fixed effects (OR = 1.17, 95 percent CI = 1.02–1.33).
Conclusions
Hospitals are the important contributors to racial disparities in treatment delay.
Keywords: Breast cancer, delays, disparities
Racial disparities in the receipt of breast cancer care are well documented and likely contribute to the worse outcomes of minority women (Bickell et al. 2006; Griggs et al. 2007; Gross et al. 2008; Freedman et al. 2009, 2010). In addition to differences in treatment receipt, black and Hispanic women are more likely to have delays in treatment initiation (Gwyn et al. 2004; Gorin et al. 2006; Lund et al. 2007; Fedewa et al. 2010), which have been associated with poorer outcomes and increased mortality (Richards et al. 1999a,b; Hershman et al. 2006a,b). Although patient factors such as treatment preferences and provider mistrust (Bickell et al. 2009) may lead to racial disparities in treatment, provider and institutional factors likely also play an important role.
Growing evidence suggests that the hospitals where patients receive care may influence racial disparities in rates of surgery and outcomes for cardiovascular disease and other conditions (Barnato et al. 2005; Jha et al. 2005; Skinner et al. 2005; Liu et al. 2006; Lucas et al. 2006; Lathan, Neville, and Earle 2008; Regenbogen et al. 2009). Data also suggest that the hospital factors are important contributors to racial disparities in receipt of definitive local therapy for breast cancer (Keating et al. 2009) and in mortality for breast cancer and other cancers (Morris et al. 2006; Zhang, Ayanian, and Zaslavsky 2007; Breslin et al. 2009).
In this analysis, we assessed adjuvant treatment delays by race/ethnicity for older women with breast cancer and further assessed whether hospitals where women receive care explained such disparities.
Patients and Methods
Data Source
We used Surveillance, Epidemiology, and End Results (SEER)-Medicare data for this analysis. The SEER program of the National Cancer Institute reports information from population-based registries in areas representing 28 percent of the U.S. population (Howlader et al. 2011). SEER registrars uniformly report information from medical records on patient demographics, tumor characteristics, treatment utilization, and mortality for all incident cancers. Since 1991, SEER data have been linked with Medicare administrative data for Medicare-eligible patients, successfully matching over 93 percent of persons aged ≥65 in the SEER registry (Warren et al. 2002; SEER-Medicare n.d.). These data were also linked with hospital characteristics from Medicare Cost Reports. Because this study used previously collected, de-identified data, this study was deemed exempt for review by the Harvard Medical School Committee on Human Studies and the Dana-Farber Cancer Institute Office for Human Research Studies.
Study Cohort
We identified women aged ≥66 with a first invasive breast cancer (with histology likely to be treated by routine guidelines) diagnosed during 1992–2007 and who were enrolled in Parts A and B fee-for-service Medicare and not a health maintenance organization (HMO, excluded because claims for HMO patients are not available) during the 12 months before diagnosis (n = 173,824). We excluded women diagnosed at autopsy, with bilateral breast cancer, and those not continuously enrolled in Parts A and B of fee-for-service Medicare or otherwise missing claims around the time of diagnosis, and we focused on women with stage I-III cancers who underwent cancer-directed surgery, including mastectomy, and breast conserving surgery (Figure 1). We created a cohort of non-Hispanic white, non-Hispanic black, and Hispanic women with stage I–III cancers who underwent breast surgery in hospitals located within SEER areas. We included only women who had an initial chemotherapy claim within 1 year of diagnosis or a first radiation claim within 270 days after surgery or both to focus on women already selected for adjuvant therapy. We then examined treatment delays within this final cohort of 54,592 patients treated at 685 hospitals.
Figure 1.
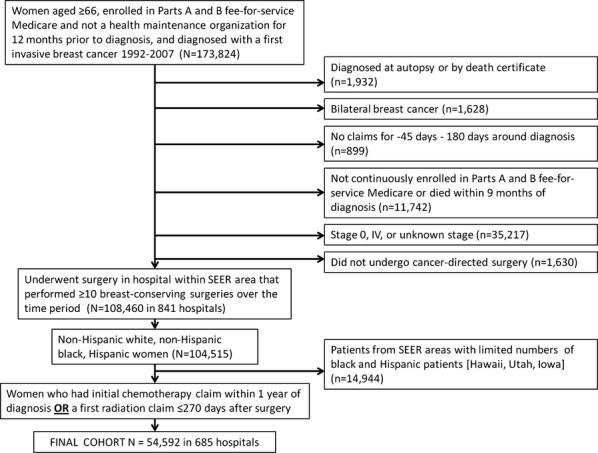
Study Inclusions and Exclusions
Definition of Variables
Dependent Variable
Delay in adjuvant therapy. We defined delay in adjuvant chemotherapy or radiation therapy (in sensitivity analyses we assessed each separately). We defined chemotherapy delay as a first chemotherapy claim >90 days after breast surgery (Hershman et al. 2006b; Fedewa et al. 2010). Women who received preoperative chemotherapy (n = 1,424) were considered to have received timely therapy. Claims for chemotherapy were determined if patients had an inpatient code for chemotherapy (99.25, International Classification of Diseases, 9th edition) or at least one J Code for a chemotherapeutic agent used in the adjuvant breast cancer setting: cyclophosphamide, anthracyclines, carboplatin, docetaxel, 5-fluorouracil, methotrexate, paclitaxel, and trastuzumab. Radiation delay was defined as a first radiation claim (after BCS or mastectomy) >90 days after BCS if no chemotherapy was administered and >90 days after the final chemotherapy claim if chemotherapy was administered (Richards et al. 1999b).
Independent Variables
Our independent variable of interest was race/ethnicity, defined based on the medical record information with Hispanic ethnicity information supplemented with information on birthplace and Hispanic surname (NAACCR Latino Research Group 2005) and categorized as non-Hispanic white, non-Hispanic black, or Hispanic. Control variables included age (66–70, 71–75, 76–80, ≥81), socioeconomic status (SES) (percent with high school diploma and median income based on Census tract of residence from U.S. Census data, in quartiles), marital status (unmarried, married, unknown), comorbidity (Charlson index, categorized as 0, 1, ≥2) (Charlson et al. 1994; Klabunde et al. 2000), year of diagnosis (1992–1995, 1996–1999, 2000–2003, 2004–2007), tumor size (≤2 cm, 2.1–3 cm, >3 cm, unknown), number of positive lymph nodes (0, 1–3, 4–9, ≥10, unknown), disease stage (I, II, III), tumor grade (well differentiated, moderately differentiated, poorly differentiated, unknown), hormone receptor status (positive if estrogen receptor [ER] or progesterone receptor [PR] positive, negative if ER and PR negative, and unknown if ER/PR unknown, ER-negative/PR unknown, or ER-unknown/PR-negative), surgery received (BCS or mastectomy, defined by registry data and Medicare claims) (2002; Cooper et al. 2002; Keating et al. 2009), SEER registry, and location of residence (major metropolitan area, metropolitan county, urban, less urban, rural).
Statistical Analyses
We first used chi-square tests to compare rates of adjuvant treatment delay by patient characteristics. We then performed a logistic regression model for treatment delay with generalized estimating equations (GEE) to account for clustering at the level of the hospital where patients underwent surgery. We examined the association of race/ethnicity with treatment delay, including the patient, and tumor variables described above. To determine whether hospital effects explained racial/ethnic differences in delay, we then performed a second model where we also included hospital fixed effects. We included an individual hospital fixed effect for each hospital with 30 or more patients; hospitals with fewer patients were categorized as less than <10, 10–19, and 20–29 eligible patients. In exploratory analyses, we then ranked hospitals by their t-statistics for treatment delay (categorized in quartiles) and assessed whether hospitals with greater delays differed from other hospitals based on breast cancer surgical volume, bed size, profit status, urban/rural location, American College of Surgeons, or Radiation Therapy Oncology Group affiliations using the chi-square test.
We next performed several sensitivity analyses for the GEE and fixed effects models. First, because older women are less likely to receive chemotherapy (Giordano et al. 2006; Buist et al. 2009) and radiation (Freedman et al. 2009) than younger women and because higher stage patients may arguably benefit from timely treatments more than lower stage patients, we repeated analyses after restricting to patients aged ≤70 (n = 16,128) and after restricting to stage II-III patients only (n = 23,482). To assess the independent contributions of radiation and chemotherapy delays, we also performed a separate set of models for (1) delay in chemotherapy and (2) delay in radiation among separate cohorts of women who received each treatment modality.
Results
Patient Characteristics
Among 54,592 women with breast cancer treated at 685 hospitals, 7 percent were black, 4 percent were Hispanic, and 89 percent were white. Black and Hispanic women were more likely to be unmarried and to live in areas with lower high school graduation rates and lower household median income (Table 1). White women had more favorable-grade cancers, smaller tumors, and less nodal involvement. Charlson comorbidity scores were generally highest for black women.
Table 1.
Patient Characteristics for Treatment Cohort [n = 54,592] (n, %)*
| Characteristic | White (n = 48,626) | Black (n = 3,623) | Hispanic (n = 2,343) | p-Value |
|---|---|---|---|---|
| Age | ||||
| 66–70 | 14,027 (29) | 1,253 (35) | 848 (36) | <.0001 |
| 71–75 | 14,145 (29) | 1,096 (30) | 735 (31) | |
| 76–80 | 11,747 (24) | 751 (21) | 464 (20) | |
| 81–85 | 6,404 (13) | 375 (10) | 222 (9) | |
| >85 | 2,303 (5) | 148 (4) | 74 (3) | |
| Socioeconomic status | ||||
| Median income | ||||
| Quartile 1 (lowest) | 8,646 (18) | 2,142 (59) | 931 (40) | <.0001 |
| Quartile 2 | 11,573 (24) | 806 (22) | 618 (26) | |
| Quartile 3 | 12,948 (27) | 409 (11) | 467 (20) | |
| Quartile 4 (highest) | 15,089 (31) | 246 (7) | 314 (13) | |
| High school (HS) diploma rates | ||||
| Quartile 1 (areas w/lowest HS graduation rates) | 8,219 (17) | 2,018 (56) | 1,225 (52) | <.0001 |
| Quartile 2 | 11,705 (24) | 887 (24) | 515 (22) | |
| Quartile 3 | 13,231 (27) | 471 (13) | 350 (15) | |
| Quartile 4 (highest HS graduation rates) | 15,101 (31) | 227 (6) | 240 (10) | |
| Unknown socioeconomic status (zip code) | 370 (0.8) | 20 (0.6) | 13 (0.6) | |
| Marital status | ||||
| Married | 23,342 (48) | 999 (28) | 964 (41) | <.0001 |
| Single | 23,772 (49) | 2,484 (69) | 1,322 (56) | |
| Unknown | 1,512 (3) | 140 (4) | 57 (2) | |
| Charlson comorbidity | ||||
| 0 | 43,213 (89) | 2,807 (77) | 1,979 (84) | <.0001 |
| 1 | 3,652 (8) | 486 (13) | 236 (10) | |
| ≥2 | 1,761 (4) | 330 (9) | 128 (5) | |
| Year of diagnosis | ||||
| 1992 | 1,623 (3) | 137 (4) | 80 (3) | <.0001 |
| 1993 | 1,618 (3) | 129 (4) | 46 (2) | |
| 1994 | 1,665 (3) | 121 (3) | 73 (3) | |
| 1995 | 1,746 (4) | 147 (4) | 69 (3) | |
| 1996 | 1,758 (4) | 122 (3) | 85 (4) | |
| 1997 | 1,878 (4) | 139 (4) | 82 (4) | |
| 1998 | 1,887 (4) | 141 (4) | 79 (3) | |
| 1999 | 2,001 (4) | 146 (4) | 93 (4) | |
| 2000 | 4,257 (9) | 313 (9) | 199 (8) | |
| 2001 | 4,439 (9) | 290 (8) | 182 (8) | |
| 2002 | 4,519 (9) | 304 (8) | 179 (8) | |
| 2003 | 4,358 (9) | 343 (9) | 223 (10) | |
| 2004 | 4,418 (9) | 347 (10) | 212 (9) | |
| 2005 | 4,139 (9) | 324 (9) | 269 (11) | |
| 2006 | 4,183 (9) | 303 (8) | 241 (10) | |
| 2007 | 4,137 (9) | 317 (9) | 231 (10) | |
| Tumor size | ||||
| ≤2 cm | 22,455 (46) | 1,323 (37) | 857 (37) | <.0001 |
| 2.1–3.0 cm | 5,013 (10) | 447 (12) | 273 (12) | |
| >3 cm | 3,934 (8) | 518 (14) | 245 (10) | |
| Unknown | 17,224 (35) | 1,335 (37) | 968 (41) | |
| Number of nodes positive | ||||
| 0 | 29,037 (60) | 1,857 (51) | 1,288 (55) | <.0001 |
| 1–3 | 8,255 (17) | 764 (21) | 474 (20) | |
| 4–9 | 2,972 (6) | 362 (10) | 209 (9) | |
| ≥ 10 | 1,578 (3) | 157 (4) | 95 (4) | |
| Unknown | 6,784 (14) | 483 (13) | 277 (12) | |
| Stage at diagnosis | ||||
| I | 28,382 (58) | 1,598 (44) | 1,130 (48) | <.0001 |
| II | 16,491 (34) | 1,549 (43) | 963 (41) | |
| III | 3,753 (8) | 476 (13) | 250 (11) | |
| Tumor grade | ||||
| Well differentiated | 10,931 (22) | 499 (14) | 462 (20) | <.0001 |
| Moderately differentiated | 19,899 (41) | 1,201 (33) | 893 (38) | |
| Poorly differentiated/undifferentiated | 13,146 (27) | 1,466 (40) | 756 (33) | |
| Unknown | 4,650 (10) | 457 (13) | 232 (10) | |
| Hormone receptor status | ||||
| Hormone receptor-positive | 23,004 (47) | 1,369 (38) | 899 (38) | <.0001 |
| Hormone receptor-negative | 3,864 (8) | 494 (14) | 208 (9) | |
| Unknown receptor status | 21,758 (45) | 1,760 (49) | 1,236 (53) | |
| Surgery received | ||||
| Mastectomy | 13,036 (27) | 1,426 (39) | 818 (35) | <.0001 |
| Breast conserving surgery | 35,590 (73) | 2,197 (61) | 1,525 (65) | |
| SEER registry | ||||
| Connecticut | 6,392 (13) | 200 (6) | 131 (6) | <.0001 |
| Detroit | 5,348 (11) | 1,028 (28) | 57 (2) | |
| New Mexico | 1,154 (2) | † | 264 (11) | |
| Seattle | 4,728 (10) | 58 (2) | 24 (1) | |
| Atlanta and rural Georgia | 1,966 (4) | 430 (12) | 28 (1) | |
| Kentucky | 2,835 (6) | † | † | |
| Louisiana | 2,227 (5) | 461 (13) | 20 (9) | |
| New Jersey | 6,401 (13) | 515 (14) | 211 (9) | |
| California | 17,575 (36) | 796 (22) | 1,599 (68) | |
| County | ||||
| Major metropolitan | 32,499 (67) | 2,887 (80) | 1,560 (67) | <.0001 |
| Metropolitan | 11,747 (24) | 570 (16) | 618 (26) | |
| Urban | 2,474 (5) | 81 (2) | 60 (3) | |
| Less urban | 1,623 (3) | † | † | |
| Rural | 283 (0.6) | † | † | |
Differences examined using chi-square testing. Percentages within categories may not sum to 100% due to rounding.
Cell sizes suppressed for reasons of confidentiality if at least one column and/or row had sample sizes <11.
Treatment Delay
Overall, 8.1 percent of women experienced a treatment delay, and this occurred more frequently for black (11.9 percent) and Hispanic women (9.9 percent) compared with white women (7.8 percent) (p < .0001). In hospitals with <10 eligible patients, 13.5 percent experienced delay compared with 7.8 percent of women in hospitals with ≥30 patients (p < .0001). In adjusted analyses, compared with white women, black women had higher odds of treatment delay (adjusted odds ratio [OR] = 1.25, 95 percent confidence interval [CI] = 1.10–1.42, p = .001). Hispanic women also had higher odds for delay (OR = 1.14, 95 percent CI = .98–1.33, p = .09), although this finding was not statistically significant (Table 2). After inclusion of hospital fixed effects, the findings for race were attenuated (OR = 1.17, 95 percent CI = 1.02–1.33), with hospitals explaining 32 percent of the differences in black versus white women.
Table 2.
Adjusted Odds for Treatment Delay by Race/Ethnicity in Generalized Estimating Equations (GEE) GEE and Fixed Effects Models
| Characteristic | Model 1 (GEE, Patient Characteristics)* | Fixed Effects (Patient Characteristics + Hospitals)† |
|---|---|---|
| Race/ethnicity | ||
| White | 1.00 | 1.00 |
| Black | 1.25 (1.10, 1.42) | 1.17 (1.02, 1.33) |
| Hispanic | 1.14 (0.98, 1.33) | 1.07 (0.92, 1.25) |
Using logistic regression with GEE to account for clustering at hospital level with inclusion of variables above and adjusted for age, income, percent without high school diploma, marital status, Charlson score, year of diagnosis, tumor size, number of nodes positive, stage, tumor grade, hormone receptor status, surgery, SEER registry, and county.
Using fixed effects model with the variables above in addition to hospitals categorized by number of patients eligible for radiation (<10, 10–19, 20–29, and individual hospitals for those with ≥30 patients).
When we explored characteristics of hospitals with more or fewer treatment delays, the hospitals in the quartile with the highest rates of delay more often were smaller, had lower breast cancer surgical volume, were not-for-profit, located in rural areas, and were less likely to be American College of Surgeons–approved cancer centers. They also had fewer cooperative group affiliations (all p < .05). Hospitals with higher probability of delay also had a higher proportion of black women treated for breast cancer compared with hospitals that had fewer delays (p < .0001).
Several other factors were significantly associated with treatment delay in the base model. Married (vs. unmarried) women had fewer treatment delays (OR = 0.82, 95 percent CI = .77–.88). Factors associated with higher odds of treatment delays included Charlson comorbidity scores 1 and ≥2 (vs. score 0, OR = 1.42, 95 percent CI = 1.30–1.56 and OR = 1.70, 95 percent CI = 1.51–1.92, respectively), rural residence (vs. major metropolitan, OR = 1.79, 95 percent CI = 1.26–2.54), tumors >3 cm (vs. ≤2 cm, OR = 1.36, 95 percent CI = 1.20–1.55), high-grade cancers (vs. low grade, OR = 1.24, 95 percent CI = 1.12–1.36), stage II or III disease (vs. stage I, OR = 1.40, 95 percent CI = 1.26–1.56 and OR = 1.44, 95 percent CI = 1.21–1.71, respectively), and older age (vs. 66–70, OR = 1.10, 95 percent CI = 1.01–1.21 [age 71–75]; OR = 1.30, 95 percent CI = 1.17–1.44 [age 76–80]; OR = 1.92, 95 percent CI = 1.73–2.14 [age >80]). Year of diagnosis was also associated with treatment delay. Compared with women diagnosed in 1992–1995, women diagnosed in 1996–1999 (OR = .81, 95 percent CI = .72–.91) and 2000–2003 (OR = .69, 95 percent CI = .61–.78) had lower odds of treatment delay; however, odds of delay were similar to 1992–1995 for women diagnosed in 2004–2007 (OR = 1.15, 95 percent CI = .73–1.81).
In sensitivity analyses, results for race/ethnicity were similar after restriction to women aged ≤70 and after restriction to stage II–III patients only, black (vs. white) women had nonsignificantly higher odds of delay. In the models where we examined radiation delay and chemotherapy delay separately, results were similar (black vs. white women had higher odds of radiation delay [OR = 1.30, 95 percent CI = 1.11–1.52] and chemotherapy delay [OR = 1.28, 95 percent CI = .97–1.70]. In these sensitivity models, all associations were attenuated when accounting for hospital fixed effects (results not shown).
Discussion
In this population-based analysis of older women with breast cancer, we found differences in the likelihood of treatment delay by race/ethnicity, particularly for black women compared with white women. Inclusion of fixed effects for the hospitals where patients obtained surgery partially attenuated the association of race with receipt of timely therapy, suggesting that hospitals where patients are treated are important contributors to the observed disparities.
Although understanding the barriers to cancer care delivery is crucial, how to optimally characterize hospitals and the quality of care they provide has proved challenging, because of the complexity that comes with heterogeneous patient populations, cancer treatments, and health care settings. Prior studies have highlighted the impact of hospital factors on receipt of cancer care and long-term outcomes, including mortality, although differing definitions of hospital quality make generalizing results difficult. One study has suggested that hospital breast cancer quality, defined predominantly by surgical volume, was associated with improved 5-year survival for patients with breast cancer (Breslin et al. 2009). This analysis also reported that hospitals treating lower proportions of black breast cancer patients have lower mortality rates. Studies in patients with colon cancer have also observed that black patients, poor patients, and those with more comorbidities are more likely to receive care at lower volume centers, and that these centers have higher risk-adjusted 30-day mortality (Zhang, Ayanian, and Zaslavsky 2007). There is also some suggestion that differences in mortality for patients with colon cancer substantially diminish when analyses are adjusted for hospital volume (Rhoads et al. 2008; Breslin et al. 2009). We observed that hospitals were important contributors to treatment delays, and also observed that lower volume centers, those who serve a higher proportion of black patients, and those in rural areas tended to have higher rates of treatment delays.
Our results suggest that interventions directed at the hospitals where patients obtain surgery, which is often the initial point-of-contact for cancer-directed treatments, could improve the timely receipt of recommended care. To accomplish this, efforts will likely require multifaceted and tailored approaches for hospitals in need rather than broad programs across all hospitals (Trivedi et al. 2005; Sequist et al. 2006) and could include interventions such as tracking the time from surgery to initiation of treatment or triggering providers and/or patients when delays in care are impending. Nevertheless, the increased risk of delay even after accounting for hospital fixed effects suggests that within-hospital disparities remain important as well.
Our findings of higher odds of treatment delay in older patients, those with more comorbidities, and those from rural areas were not surprising given the challenges associated with competing medical problems and long travel distances for such patients. Furthermore, rural, lower volume centers may have fewer resources directed to the specialized care required for patients with breast cancer, necessitating centers to prioritize treatment of “higher risk” or possibly younger patients and postpone treatment for others. Our observations that women with larger tumors and more advanced stage disease have higher odds of delay may be related to unmeasured confounders that also predispose women to delays in diagnosis, such as competing stressors or poor access to care because of physical, financial, or cultural barriers. Further study will be required to understand the relationship between disease stage and treatment delay. Over time, the odds of delay declined in the late 1990s and early 2000s but were back to levels of the early 1990s during 2004–2007, despite increasing national attention focused on reducing disparities in care.
We acknowledge several study limitations. First, our study was limited to older women insured by fee-for-service Medicare who resided in SEER areas only, which may limit generalizability. However, our findings were consistent after restricting the cohort to the youngest Medicare beneficiaries. Furthermore, our analysis captured care for over 50,000 women treated nationally using a population-based registry. Second, we did not have information on individualized SES, patient preferences, or reasons for treatment delay. In addition, information on potential confounders such as comorbidity was ascertained based on administrative data. Third, there may be unmeasured confounding factors in hospitals that may influence our findings. Finally, we studied delays in adjuvant therapies that are typically delivered in the outpatient setting, yet we attributed the care to the hospitals where women underwent surgery. However, other research suggests that physicians cluster around hospitals and that outpatient care is often impacted by initial cancer management (and surgery) (Zhang, Ayanian, and Zaslavsky 2007), such as the observations of associations of hospital surgical volume with 5-year survival (Breslin et al. 2009).
In conclusion, hospitals where patients underwent surgery were associated with treatment delay in our analysis and impacted the observed disparities in treatment delay for black women. In addition to further defining patient and provider factors that contribute to disparities in breast cancer care, specific efforts to better understand and improve upon the delivery of timely, high-quality care will ultimately lead to optimized cancer-related outcomes.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: The authors would like to thank Yang Xu, MS, and Huichuan Lii, MS, MA, for their expert programming assistance. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries m the creation of the SEER-Medicare database. The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC45105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Disclosures: None.
Disclaimer: None.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
References
- Barnato AE, Lucas FL, Staiger D, Wennberg DE, Chandra A. “Hospital-Level Racial Disparities in Acute Myocardial Infarction Treatment and Outcomes”. Medical Care. 2005;43(4):308–19. doi: 10.1097/01.mlr.0000156848.62086.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickell NA, Wang JJ, Oluwole S, Schrag D, Godfrey H, Hiotis K, Mendez J, Guth AA. “Missed Opportunities: Racial Disparities in Adjuvant Breast Cancer Treatment”. Journal of Clinical Oncology. 2006;24(9):1357–62. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- Bickell NA, Weidmann J, Fei K, Lin JJ, Leventhal H. “Underuse of Breast Cancer Adjuvant Treatment: Patient Knowledge, Beliefs, and Medical Mistrust”. Journal of Clinical Oncology. 2009;27(31):5160–7. doi: 10.1200/JCO.2009.22.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin TM, Morris AM, Gu N, Wong SL, Finlayson EV, Banerjee M, Birkmeyer JD. “Hospital Factors and Racial Disparities in Mortality after Surgery for Breast and Colon Cancer”. Journal of Clinical Oncology. 2009;27(24):3945–50. doi: 10.1200/JCO.2008.20.8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buist DS, Chubak J, Prout M, Yood MU, Bosco JL, Thwin SS, Gold HT, Owusu C, Field TS, Quinn VP, Wei F, Silliman RA. “Referral, Receipt, and Completion of Chemotherapy in Patients with Early-Stage Breast Cancer Older Than 65 Years and at High Risk of Breast Cancer Recurrence”. Journal of Clinical Oncology. 2009;27(27):4508–14. doi: 10.1200/JCO.2008.18.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson M, Szatrowski TP, Peterson J, Gold J. “Validation of a Combined Comorbidity Index”. Journal of Clinical Epidemiology. 1994;47(11):1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Virnig B, Klabunde CN, Schussler N, Freeman J, Warren JL. “Use of SEER-Medicare Data for Measuring Cancer Surgery”. Medical Care. 2002;40(8 suppl) doi: 10.1097/00005650-200208001-00006. IV–43–8. [DOI] [PubMed] [Google Scholar]
- Fedewa SA, Ward EM, Stewart AK, Edge SB. “Delays in Adjuvant Chemotherapy Treatment among Patients with Breast Cancer are More Likely in African American and Hispanic Populations: A National Cohort Study 2004–2006”. Journal of Clinical Oncology. 2010;28(27):4135–41. doi: 10.1200/JCO.2009.27.2427. [DOI] [PubMed] [Google Scholar]
- Freedman RA, He Y, Winer EP, Keating NL. “Trends in Racial and Age Disparities in Definitive Local Therapy of Early-Stage Breast Cancer”. Journal of Clinical Oncology. 2009;27(5):713–9. doi: 10.1200/JCO.2008.17.9234. [DOI] [PubMed] [Google Scholar]
- Freedman RA, Virgo KS, He Y, Pavluck AL, Winer EP, Ward EM, Keating NL. “The Association of Race/Ethnicity, Insurance Status, and Socioeconomic Factors with Breast Cancer Care”. Cancer. 2010;117((1)):180–9. doi: 10.1002/cncr.25542. [DOI] [PubMed] [Google Scholar]
- Giordano SH, Duan Z, Kuo YF, Hortobagyi GN, Goodwin JS. “Use and Outcomes of Adjuvant Chemotherapy in Older Women with Breast Cancer”. Journal of Clinical Oncology. 2006;24(18):2750–6. doi: 10.1200/JCO.2005.02.3028. [DOI] [PubMed] [Google Scholar]
- Gorin SS, Heck JE, Cheng B, Smith SJ. “Delays in Breast Cancer Diagnosis and Treatment by Racial/Ethnic Group”. Archives of Internal Medicine. 2006;166(20):2244–52. doi: 10.1001/archinte.166.20.2244. [DOI] [PubMed] [Google Scholar]
- Griggs JJ, Culakova E, Sorbero ME, Poniewierski MS, Wolff DA, Crawford J, Dale DC, Lyman GH. “Social and Racial Differences in Selection of Breast Cancer Adjuvant Chemotherapy Regimens”. Journal of Clinical Oncology. 2007;25(18):2522–7. doi: 10.1200/JCO.2006.10.2749. [DOI] [PubMed] [Google Scholar]
- Gross CP, Smith BD, Wolf E, Andersen M. “Racial Disparities in Cancer Therapy: Did the Gap Narrow between 1992 and 2002?”. Cancer. 2008;112(4):900–8. doi: 10.1002/cncr.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwyn K, Bondy ML, Cohen DS, Lund MJ, Liff JM, Flagg EW, Brinton LA, Eley JW, Coates RJ. “Racial Differences in Diagnosis, Treatment, and Clinical Delays in a Population-Based Study of Patients with Newly Diagnosed Breast Carcinoma”. Cancer. 2004;100(8):1595–604. doi: 10.1002/cncr.20169. [DOI] [PubMed] [Google Scholar]
- Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. “Delay in Initiating Adjuvant Radiotherapy Following Breast Conservation Surgery and Its Impact on Survival”. International Journal of Radiation Oncology, Biology, Physics. 2006a;65(5):1353–60. doi: 10.1016/j.ijrobp.2006.03.048. [DOI] [PubMed] [Google Scholar]
- Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. “Delay of Adjuvant Chemotherapy Initiation Following Breast Cancer Surgery among Elderly Women”. Breast Cancer Research and Treatment. 2006b;99(3):313–21. doi: 10.1007/s10549-006-9206-z. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK. SEER Cancer Statistics Review, 1975–2008. Bethesda, MD: National Cancer Institute; 2011. [accessed on December 15, 2012]. Available at http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- Jha AK, Fisher ES, Li Z, Orav EJ, Epstein AM. “Racial Trends in the Use of Major Procedures among the Elderly”. New England Journal of Medicine. 2005;353(7):683–91. doi: 10.1056/NEJMsa050672. [DOI] [PubMed] [Google Scholar]
- Keating NL, Kouri E, He Y, Weeks JC, Winer EP. “Racial Differences in Definitive Breast Cancer Therapy in Older Women: Are They Explained by the Hospitals Where Patients Undergo Surgery?”. Medical Care. 2009;47(7):765–73. doi: 10.1097/MLR.0b013e31819e1fe7. [DOI] [PubMed] [Google Scholar]
- Klabunde CN, Potosky AL, Legler JM, Warren JL. “Development of a Comorbidity Index Using Physician Claims Data”. Journal of Clinical Epidemiology. 2000;53(12):1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- Lathan CS, Neville BA, Earle CC. “Racial Composition of Hospitals: Effects on Surgery for Early-Stage Non-Small-Cell Lung Cancer”. Journal of Clinical Oncology. 2008;26(26):4347–52. doi: 10.1200/JCO.2007.15.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JH, Zingmond DS, McGory ML, SooHoo NF, Ettner SL, Brook RH, Ko CY. “Disparities in the Utilization of High-Volume Hospitals for Complex Surgery”. Journal of the American Medical Association. 2006;296(16):1973–80. doi: 10.1001/jama.296.16.1973. [DOI] [PubMed] [Google Scholar]
- Lucas FL, Stukel TA, Morris AM, Siewers AE, Birkmeyer JD. “Race and Surgical Mortality in the United States”. Annals of Surgery. 2006;243(2):281–6. doi: 10.1097/01.sla.0000197560.92456.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund MJ, Brawley OP, Ward KC, Young JL, Gabram SS, Eley JW. “Parity and Disparity in First Course Treatment of Invasive Breast Cancer”. Breast Cancer Research and Treatment. 2007;109:545–57. doi: 10.1007/s10549-007-9675-8. [DOI] [PubMed] [Google Scholar]
- Morris AM, Wei Y, Birkmeyer NJ, Birkmeyer JD. “Racial Disparities in Late Survival after Rectal Cancer Surgery”. Journal of the American College of Surgeons. 2006;203(6):787–94. doi: 10.1016/j.jamcollsurg.2006.08.005. [DOI] [PubMed] [Google Scholar]
- NAACCR Latino Research Group. NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2] Springfield, IL: North American Association of Central Cancer Registries; 2005. [Google Scholar]
- Regenbogen SE, Gawande AA, Lipsitz SR, Greenberg CC, Jha AK. “Do Differences in Hospital and Surgeon Quality Explain Racial Disparities in Lower-Extremity Vascular Amputations?”. Annals of Surgery. 2009;250(3):424–31. doi: 10.1097/SLA.0b013e3181b41d53. [DOI] [PubMed] [Google Scholar]
- Rhoads KF, Ackerson LK, Jha AK, Dudley RA. “Quality of Colon Cancer Outcomes in Hospitals with a High Percentage of Medicaid Patients”. Journal of the American College of Surgeons. 2008;207(2):197–204. doi: 10.1016/j.jamcollsurg.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Richards MA, Smith P, Ramirez AJ, Fentiman IS, Rubens RD. “The Influence on Survival of Delay in the Presentation and Treatment of Symptomatic Breast Cancer”. British Journal of Cancer. 1999a;79(5–6):858–64. doi: 10.1038/sj.bjc.6690137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. “Influence of Delay on Survival in Patients with Breast Cancer: A Systematic Review”. Lancet. 1999b;353(9159):1119–26. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- SEER-Medicare. How the SEER & Medicare Data Are Linked [accessed on October 20, 2009]. Available at http://healthservices.cancer.gov/seermedicare/overview/linked.html.
- Sequist TD, Adams A, Zhang F, Ross-Degnan D, Ayanian JZ. “Effect of Quality Improvement on Racial Disparities in Diabetes Care”. Archives of Internal Medicine. 2006;166(6):675–81. doi: 10.1001/archinte.166.6.675. [DOI] [PubMed] [Google Scholar]
- Skinner J, Chandra A, Staiger D, Lee J, McClellan M. “Mortality after Acute Myocardial Infarction in Hospitals That Disproportionately Treat Black Patients”. Circulation. 2005;112(17):2634–41. doi: 10.1161/CIRCULATIONAHA.105.543231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ. “Trends in the Quality of Care and Racial Disparities in Medicare Managed Care”. New England Journal of Medicine. 2005;353(7):692–700. doi: 10.1056/NEJMsa051207. [DOI] [PubMed] [Google Scholar]
- Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. “Overview of the SEER-Medicare Data: Content, Research Applications, and Generalizability to the United States Elderly Population”. Medical Care. 2002;40(8 suppl):IV–3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ayanian JZ, Zaslavsky AM. “Patient Characteristics and Hospital Quality for Colorectal Cancer Surgery”. International Journal for Quality in Health Care. 2007;19(1):11–20. doi: 10.1093/intqhc/mzl047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


