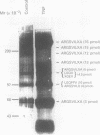
Abstract
AIMS: To develop an in vitro assay for the putative glutamyl endopeptidase, "aggrecanase", which is thought to degrade cartilage aggrecan, and to examine the role of the enzyme in tumour necrosis factor stimulated aggrecan cleavage. METHODS: Aggrecan fragments released by bovine nasal cartilage explants, with and without exposure to tumour necrosis factor alpha, were purified and analysed by western blotting and N-terminal sequencing. Intact bovine aggrecan was incubated with extracts of cartilage, lysed chondrocytes, or cartilage explant conditioned culture medium under a variety of conditions. Deglycosylated aggrecan was incubated with nasal cartilage explants. Proteoglycan breakdown was assessed by metachromatic assay of fragments in culture media, and cleavage of the substrate at the aggrecanase cleavage site was detected and measured using the antibody BC3, which recognises a neoepitope produced by aggrecanase cleavage of aggrecan. RESULTS: Aggrecan fragments generated from explants treated with tumour necrosis factor had N-terminal sequences consistent with cleavage of aggrecan at a restricted number of glutamyl bonds. Aggrecanase generated fragments were found in cartilage explant culture medium and chondrocyte monolayers. However, no aggrecanase activity could be detected in extracts of cartilage, or chondrocytes from which endogenous aggrecan fragments had been removed, under a variety of assay conditions. Deglycosylated aggrecan, added to explant cultures, efficiently inhibited endogenous aggrecan breakdown. CONCLUSIONS: Aggrecanase is active in cartilage and in chondrocyte monolayers, and its action is stimulated by tumour necrosis factor alpha. However, activity due to this enzyme could not be detected in vitro under our assay conditions, although a deglycosylated version of the substrate inhibited aggrecan breakdown in explant cultures.
Full text
PDF


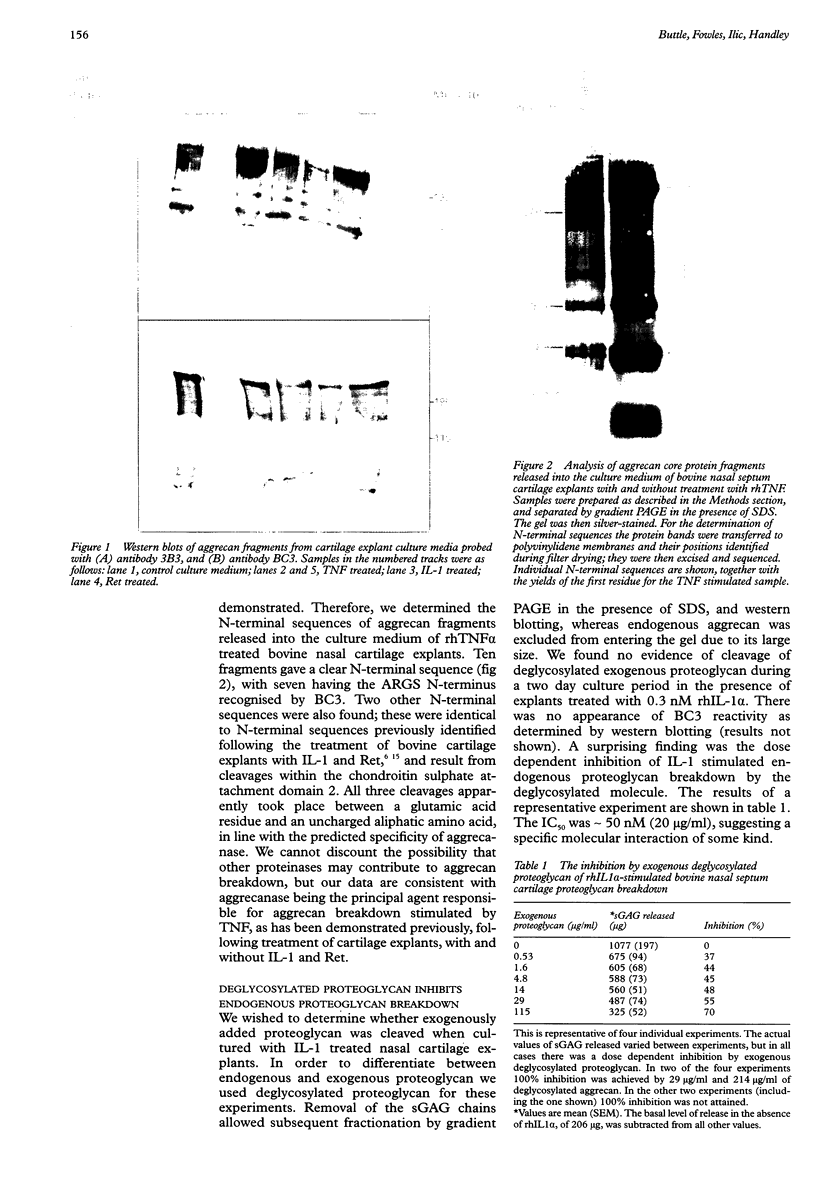



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews H. J., Plumpton T. A., Harper G. P., Cawston T. E. A synthetic peptide metalloproteinase inhibitor, but not TIMP, prevents the breakdown of proteoglycan within articular cartilage in vitro. Agents Actions. 1992 Sep;37(1-2):147–154. doi: 10.1007/BF01987904. [DOI] [PubMed] [Google Scholar]
- Bunning R. A., Crawford A., Richardson H. J., Opdenakker G., Van Damme J., Russell R. G. Interleukin 1 preferentially stimulates the production of tissue-type plasminogen activator by human articular chondrocytes. Biochim Biophys Acta. 1987 Jun 22;924(3):473–482. doi: 10.1016/0304-4165(87)90163-2. [DOI] [PubMed] [Google Scholar]
- Buttle D. J., Handley C. J., Ilic M. Z., Saklatvala J., Murata M., Barrett A. J. Inhibition of cartilage proteoglycan release by a specific inactivator of cathepsin B and an inhibitor of matrix metalloproteinases. Evidence for two converging pathways of chondrocyte-mediated proteoglycan degradation. Arthritis Rheum. 1993 Dec;36(12):1709–1717. doi: 10.1002/art.1780361210. [DOI] [PubMed] [Google Scholar]
- Buttle D. J., Saklatvala J., Tamai M., Barrett A. J. Inhibition of interleukin 1-stimulated cartilage proteoglycan degradation by a lipophilic inactivator of cysteine endopeptidases. Biochem J. 1992 Jan 1;281(Pt 1):175–177. doi: 10.1042/bj2810175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow G., Knudson C. B., Homandberg G., Knudson W. Increased expression of CD44 in bovine articular chondrocytes by catabolic cellular mediators. J Biol Chem. 1995 Nov 17;270(46):27734–27741. doi: 10.1074/jbc.270.46.27734. [DOI] [PubMed] [Google Scholar]
- Couchman J. R., Caterson B., Christner J. E., Baker J. R. Mapping by monoclonal antibody detection of glycosaminoglycans in connective tissues. Nature. 1984 Feb 16;307(5952):650–652. doi: 10.1038/307650a0. [DOI] [PubMed] [Google Scholar]
- Culty M., Nguyen H. A., Underhill C. B. The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J Cell Biol. 1992 Feb;116(4):1055–1062. doi: 10.1083/jcb.116.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndale R. W., Buttle D. J., Barrett A. J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986 Sep 4;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Fell H. B., Jubb R. W. The effect of synovial tissue on the breakdown of articular cartilage in organ culture. Arthritis Rheum. 1977 Sep-Oct;20(7):1359–1371. doi: 10.1002/art.1780200710. [DOI] [PubMed] [Google Scholar]
- Handley C. J., Buttle D. J. Assay of proteoglycan degradation. Methods Enzymol. 1995;248:47–58. doi: 10.1016/0076-6879(95)48006-4. [DOI] [PubMed] [Google Scholar]
- Hollander A. P., Atkins R. M., Eastwood D. M., Dieppe P. A., Elson C. J. Human cartilage is degraded by rheumatoid arthritis synovial fluid but not by recombinant cytokines in vitro. Clin Exp Immunol. 1991 Jan;83(1):52–57. doi: 10.1111/j.1365-2249.1991.tb05587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer H., Abbott J., Lash J., Holtzer S. THE LOSS OF PHENOTYPIC TRAITS BY DIFFERENTIATED CELLS IN VITRO, I. DEDIFFERENTIATION OF CARTILAGE CELLS. Proc Natl Acad Sci U S A. 1960 Dec;46(12):1533–1542. doi: 10.1073/pnas.46.12.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L., Glynn P. Membrane-associated metalloproteinase recognized by characteristic cleavage of myelin basic protein: assay and isolation. Methods Enzymol. 1995;248:388–395. doi: 10.1016/0076-6879(95)48025-0. [DOI] [PubMed] [Google Scholar]
- Hughes C. E., Caterson B., Fosang A. J., Roughley P. J., Mort J. S. Monoclonal antibodies that specifically recognize neoepitope sequences generated by 'aggrecanase' and matrix metalloproteinase cleavage of aggrecan: application to catabolism in situ and in vitro. Biochem J. 1995 Feb 1;305(Pt 3):799–804. doi: 10.1042/bj3050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic M. Z., Handley C. J., Robinson H. C., Mok M. T. Mechanism of catabolism of aggrecan by articular cartilage. Arch Biochem Biophys. 1992 Apr;294(1):115–122. doi: 10.1016/0003-9861(92)90144-l. [DOI] [PubMed] [Google Scholar]
- Kempson G. E., Tuke M. A., Dingle J. T., Barrett A. J., Horsfield P. H. The effects of proteolytic enzymes on the mechanical properties of adult human articular cartilage. Biochim Biophys Acta. 1976 May 28;428(3):741–760. doi: 10.1016/0304-4165(76)90205-1. [DOI] [PubMed] [Google Scholar]
- Kozaci L. D., Buttle D. J., Hollander A. P. Degradation of type II collagen, but not proteoglycan, correlates with matrix metalloproteinase activity in cartilage explant cultures. Arthritis Rheum. 1997 Jan;40(1):164–174. doi: 10.1002/art.1780400121. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lark M. W., Gordy J. T., Weidner J. R., Ayala J., Kimura J. H., Williams H. R., Mumford R. A., Flannery C. R., Carlson S. S., Iwata M. Cell-mediated catabolism of aggrecan. Evidence that cleavage at the "aggrecanase" site (Glu373-Ala374) is a primary event in proteolysis of the interglobular domain. J Biol Chem. 1995 Feb 10;270(6):2550–2556. doi: 10.1074/jbc.270.6.2550. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., Neame P. J., Sandy J. D. The structure of aggrecan fragments in human synovial fluid. Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arthritis Rheum. 1993 Sep;36(9):1214–1222. doi: 10.1002/art.1780360906. [DOI] [PubMed] [Google Scholar]
- Loulakis P., Shrikhande A., Davis G., Maniglia C. A. N-terminal sequence of proteoglycan fragments isolated from medium of interleukin-1-treated articular-cartilage cultures. Putative site(s) of enzymic cleavage. Biochem J. 1992 Jun 1;284(Pt 2):589–593. doi: 10.1042/bj2840589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKie N., Dallas D. J., Edwards T., Apperley J. F., Russell R. G., Croucher P. I. Cloning of a novel membrane-linked metalloproteinase from human myeloma cells. Biochem J. 1996 Sep 1;318(Pt 2):459–462. doi: 10.1042/bj3180459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris E. A., Wilcon S., Treadwell B. V. Inhibition of interleukin 1-mediated proteoglycan degradation in bovine articular cartilage explants by addition of sodium hyaluronate. Am J Vet Res. 1992 Nov;53(11):1977–1982. [PubMed] [Google Scholar]
- Ng C. K., Handley C. J., Preston B. N., Robinson H. C., Bolis S., Parker G. Effect of exogenous hyaluronan and hyaluronan oligosaccharides on hyaluronan and aggrecan synthesis and catabolism in adult articular cartilage explants. Arch Biochem Biophys. 1995 Jan 10;316(1):596–606. doi: 10.1006/abbi.1995.1079. [DOI] [PubMed] [Google Scholar]
- Ng C. K., Handley C. J., Preston B. N., Robinson H. C. The extracellular processing and catabolism of hyaluronan in cultured adult articular cartilage explants. Arch Biochem Biophys. 1992 Oct;298(1):70–79. doi: 10.1016/0003-9861(92)90095-e. [DOI] [PubMed] [Google Scholar]
- Nixon J. S., Bottomley K. M., Broadhurst M. J., Brown P. A., Johnson W. H., Lawton G., Marley J., Sedgwick A. D., Wilkinson S. E. Potent collagenase inhibitors prevent interleukin-1-induced cartilage degradation in vitro. Int J Tissue React. 1991;13(5):237–241. [PubMed] [Google Scholar]
- Plaas A. H., Sandy J. D. A cartilage explant system for studies on aggrecan structure, biosynthesis and catabolism in discrete zones of the mammalian growth plate. Matrix. 1993 Mar;13(2):135–147. doi: 10.1016/s0934-8832(11)80072-7. [DOI] [PubMed] [Google Scholar]
- Rawlings N. D., Barrett A. J. Evolutionary families of metallopeptidases. Methods Enzymol. 1995;248:183–228. doi: 10.1016/0076-6879(95)48015-3. [DOI] [PubMed] [Google Scholar]
- Saklatvala J., Pilsworth L. M., Sarsfield S. J., Gavrilovic J., Heath J. K. Pig catabolin is a form of interleukin 1. Cartilage and bone resorb, fibroblasts make prostaglandin and collagenase, and thymocyte proliferation is augmented in response to one protein. Biochem J. 1984 Dec 1;224(2):461–466. doi: 10.1042/bj2240461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986 Aug 7;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter D. M., Godolphin J. L., Gourlay M. S., Lawson M. F., Hughes D. E., Dunne E. Analysis of human articular chondrocyte CD44 isoform expression and function in health and disease. J Pathol. 1996 Aug;179(4):396–402. doi: 10.1002/(SICI)1096-9896(199608)179:4<396::AID-PATH606>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Sandy J. D., Flannery C. R., Neame P. J., Lohmander L. S. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J Clin Invest. 1992 May;89(5):1512–1516. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Neame P. J., Boynton R. E., Flannery C. R. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991 May 15;266(14):8683–8685. [PubMed] [Google Scholar]
- Seed M. P., Ismaiel S., Cheung C. Y., Thomson T. A., Gardner C. R., Atkins R. M., Elson C. J. Inhibition of interleukin 1 beta induced rat and human cartilage degradation in vitro by the metalloproteinase inhibitor U27391. Ann Rheum Dis. 1993 Jan;52(1):37–43. doi: 10.1136/ard.52.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]