Abstract
Helicobacter pylori (H pylori) infection induces a chronic inflammatory response, which promotes gastric carcinogenesis. 15-hydroxyprostaglandin dehydrogenase (15–PGDH) plays a key role as a tumor suppressor in gastrointestinal cancers. The aim of this study was to elucidate the role of 15-PGDH in gastric carcinogenesis associated with H pylori. 15-PGDH expression in gastric biopsies from H pylori-infected (n=25) and non-infected (n=15) subjects was analyzed by quantitative real-time PCR, western blot analysis, and immunohistochemisty. 15-PGDH DNA methylation was evaluated by methylation specific PCR and pyrosequencing. The expression of 15-PGDH, Snail, ERK1/2, TLR4 and MyD88 in response to H pylori infection was assessed by immunoblot analysis. Compared to negative specimens, H pylori positive specimens had 2-fold lower 15-PGDH mRNA levels and significantly less 15-PGDH protein. In four H pylori infected subjects with longitudinal follow-up, the suppression of 15-PGDH expression was reversed by H pylori eradication therapy. In parallel with suppressing 15-PGDH expression, H pylori infection activated expression of TLR4 and MyD88 expression, increased levels of phospho-ERK1/2, and increased expression of epidermal growth factor receptor (EGFR)-Snail. Inhibition of Snail and MyD88 reversed suppression of 15-PGDH expression and small interfering Myd88 reduced phosphorylated ERK1/2. Similarly, treatment with an ERK1/2 and EGFR inhibitor also restored 15-PGDH expression. Heliocobacter pylori appeared to promote gastric carcinogenesis by suppressing15-PGDH. This process is mediated by the TLR4/MyD88 pathway via ERK1/2 or EGFR - Snail transcriptional regulation. 15-PGDH may be a useful marker and a potential therapeutic target in H pylori-induced gastric carcinogenesis.
Keywords: Gastric cancer, Helicobacter pylori, 15-prostaglandin dehydrogenase
Introduction
Although the incidence of gastric cancer is declining, it remains the fourth most common cancer and the second leading cause of cancer-related deaths in the world (1). Gastric carcinogenesis is a multifactorial process that involves complex interactions between host and environmental factors. H pylori is one of the most important environmental risk factor for gastric malignancies (2). The chronic inflammation that develops in response to this organism contributes to tumor cell proliferation and metastasis, and affects survival (3, 4). Prostaglandins (PGs) play an important role in the growth and stimulation of the inflammation associated gastric carcinogenesis that is associated with H pylori infection (5–7). In particular, the expression of cyclooxygenase-2 (COX-2), a rate-limiting enzyme for prostaglandin biosynthesis, is induced in H pylori-associated carcinogenesis and results in the induction of the proinflammatory prostaglandin, PGE2 (8, 9).
PGE2 levels are regulated not only by its synthesis, but also by its degradation (8). The key enzyme responsible for the biological inactivation of prostaglandins is NAD+-dependent 15-hydroxyprostaglandin dehydrogenase (15–PGDH) (10). 15-PGDH inactivates PGE2 by catalyzing the oxidation of its 15(S)-hydroxyl group, which results in the formation of inactive 15-keto metabolites (10, 11). Therefore, 15-PGDH plays an important role in regulating the local concentration of PGE2. Studies by our group and others have demonstrated that while 15-PGDH is highly expressed by normal colonic epithelial cells, transcription of 15-PGDH mRNA is lost in most colon cancers (11, 12). Follow-up research showed that 15-PGDH may have a potential tumor suppressor function in lung, breast, bladder, thyroid, pancreas (13–17) and gastric cancers (18, 19). Previously, we have demonstrated that 15-PGDH expression was lost in 70.1% of malignant human gastric tissues and 83.3% of adenomas, but was preserved in normal and metaplastic gastritis (19). In addition, we found that the repression of 15-PGDH in gastric cancer may be due to the activation of the EGFR-ERK1/2 pathway (19). However, it is not known whether 15-PGDH plays any role in H pylori associated gastric carcinogenesis. We hypothesized that H. pylori infection may suppress 15-PGDH expression, thereby elevating PGE2 levels, which in turn promote gastric carcinogenesis.
Toll-like receptors (TLRs) are surface exposed pattern recognition receptors. The binding of a microbial antigen to the TLR-receptors activates their interaction with MyD88 (myeloid differentiation primary response protein-88) (20), which triggers intracellular signals that induce the expression of inflammatory cytokines, including NF-κB; this in turn augments antiapoptotic proteins and thereby promotes the invasion and metastasis of cancer (21). In addition, TLR4/MyD88 activation by H pylori may promote stromal macrophage activation that induces the COX2/PGE2 pathway (22). Accordingly, it is also possible that the TLR4/MyD88 pathway mediates the suppression of 15-PGDH in H pylori infection-associated gastric carcinogenesis that elevates PGE2 levels.
The present study revealed that H pylori infection suppresses 15-PGDH transcription, which suggests that the infection decreases the degradation of PGE2, the major procarcinogenic prostaglandin, which in turn promotes gastric carcinogenesis. The suppression of 15-PGDH was mediated by the activation of TLR4/MyD88 and Snail transcriptional regulation by ERK1/2 and EGFR. In addition, H pylori-infected gastric specimens exhibited more 15-PGDH DNA promoter methylation than negative specimens, which suggests that epigenetic modulation contributes to the suppression of 15-PGDH transcription. Finally, 15-PGDH expression was restored after H pylori eradication, which suggests that the inhibition of 15-PGDH transcription is a reversible process. These results indicate that the elevated PGE2 levels in H pylori-associated gastric carcinogenesis are likely to be the consequence of both reduced catabolism and increased synthesis.
Materials and Methods
Study population
The study population consisted of 40 participants who underwent upper gastrointestinal endoscopy at Asan Medical Center between June 2011 and November 2011. Their median age was 56 years (range, 21–79 years) and 26 participants were male (65.0%). The exclusion criteria were as follows 1) consumption of a non-steroidal anti-inflammatory drug and drugs that affect gastric acid secretion, such as antacids, bismuth compounds, H2-receptor antagonists, and proton pump inhibitors in 6 weeks, 2) previous gastric surgery, 3) a history of H pylori infection or eradication therapy, and/or 4) a known allergy to antibiotics. Of the 40 participants, 32 lacked symptoms and underwent an endoscopy for a routine check-up, while the remaining eight had gastrointestinal symptoms including dyspepsia, acid regurgitation, or epigastric pain (Table 1). Of the 25 patients who were infected with H pylori, four were treated with the standard triple H pylori eradication therapy for 7 days (proton pump inhibitor 40 mg twice daily, clarithromycin 0.5g twice daily and amoxicillin 1g twice daily). At least 4 weeks later, they underwent follow-up endoscopy and sample collection.
Table 1.
Characteristics of the H pylori-positive and –negative study participants
| Variables |
H pylori negative (n=15) |
H pylori positive (n=25) |
P-value |
|---|---|---|---|
| Age, median (years) | 52 (21–66) | 63 (36–79) | 0.002* |
| Sex, male, no.(%) | 9 (60.0%) | 17 (68.0%) | 0.61 |
| Current smoking, no.(%) | 4 (26.7%) | 6 (24.0%) | 1.00 |
| Alcohol consumption, no.(%) | 4 (26.7%) | 12 (48.0%) | 0.18 |
| Hypertension, no.(%) | 4 (26.7%) | 11 (44.0%) | 0.27 |
| Diabetes, no.(%) | 3 (20.0%) | 7 (28.0%) | 0.72 |
| BMI, mean±SD (Kg/m2) | 24.57±2.81 | 24.50±3.65 | 0.94 |
| Cholesterol, mean±SD (mg/dL) | 181.33±33.35 | 171.00±34.17 | 0.36 |
| Symptom at endoscopy, no.(%) | 0.24 | ||
| No symptoms | 13 (86.7%) | 19 (76.0%) | |
| Dyspepsia | 1 (6.7%) | 2 (8.0%) | |
| Acid regurgitation | 1 (6.7%) | 0 (0.0%) | |
| Epigastric pain | 0 (0.0%) | 4 (16.0%) | |
| Endoscopic diagnosis, no.(%) | 0.3 | ||
| Normal | 3 (20.0%) | 1 (4.0%) | |
| Gastritis | 11 (73.3%) | 20 (80.0%) | |
| Gastric ulcer | 0 (0.0%) | 4 (16.0%) | |
| Duodenal ulcer | 0 (0.0%) | 0 (0.0%) | |
| Others | 1 (6.7%) | 0 (0.0%) | |
BMI: Body Mass Index,
P<0.01
Sample collection and determination of H pylori infection
At baseline, the subjects underwent endoscopy with biopsies for histology (two samples from the antrum) and a rapid urease test (one sample from the antrum) (Hp kit; Chongkundang Pharm. Corp., Seoul, Korea). In addition, three more biopsies were taken for DNA, RNA, and protein analysis. The biopsies were frozen in liquid nitrogen immediately and stored at −70°C until processing. Endoscopy (Endoscopy model: CF-H2160AL; Olympus, Tokyo, Japan) was performed by the same physician (S.J.M) after sedation with intravenous midazolam. Histological examinations were performed by one experienced specialist gastrointestinal pathologist (Y.S.P.), who used H&E staining for the assessment of gastritis and Gram staining for the detection of H pylori. At entry, patients were considered H pylori positive if the results of 2 tests (histologic analysis and rapid urease test) were positive. Follow-up sample collection and processing after H pylori eradication was the same as baseline collection. All processes relating to human tissue sampling were performed under the approval of the Asan Medical Center Institutional Review Board.
Quantitative Real-time PCR
Total RNA was extracted by using the RNeasy kit (Ambion, Austin, TX). The concentration and quality of the RNA samples were determined by using a Nanodrop-1000 spectrophotometer and cDNA was generated by using MultiScribe™ Reverse Transcriptase (Applied Biosystems, CA, USA) and 40 ng of cDNA was used for real-time PCR assays for 15-PGDH and COX-2. All samples had an O.D. 260/280 ratio greater than 1.8. Quantitative real-time PCR was performed with Applied Biosystem® 7900 Real-time PCR systems. Real-time PCR of 15-PGDH and COX-2 was performed by using the human 15-PGDH TaqMan Probe/Primer kit Hs 00168359_m1 and the human COX-2 TaqMan Probe/Primer kit Hs 00153133_m1 (Applied Biosystems, CA, USA). β-ACTIN was amplified by using the human ACTB TaqMan primer/probe kit Hs 99999903_m1 (Applied Biosystems, CA, USA). The relative gene expression was normalized to mRNA of β-ACTIN and calculated by 2−ΔΔCt method.
Western blot analysis
Human tissue lysates were prepared by homogenization using the TissueLyser II system in cell lysis buffer (Cell Signaling Technology, MA, USA) with 1 mM PMSF (phenylmethylsulphonyl fluoride). The protein content was measured by the BCA method using the Bicinchoninic Acid Protein Assay Kit (GenDEPOT, TX, USA) and a SmartSpec 3000 microplate reader (Molecular Devices, CA, USA). Protein samples (30 μg) were separated on 12% polyacrylamide gels, and transferred to Immobilon-P Membrane (Millipore, Billerica, MA, USA). The primary antibodies were polyclonal rabbit antibodies against 15-PGDH (1:5000; Novus Biologicals, CO, USA), Snail (1:1000; Santa Cruz, CA, USA), and GAPDH (1:5000; Cell Signaling, MA, USA). The secondary antibody was a goat anti-rabbit antibody (1:5000; Cell Signaling, MA, USA). Detection was performed by using the ECL Plus Detection System (Amersham Pharmacia, CA, USA) according to the manufacturer’s instructions and then exposing the gel to Kodak CP-BU film (Kodak, New Haven, CT).
The cells were washed with phosphate-buffered saline (PBS) and lysed with cell lysis buffer with 1 mM PMSF. Western blot analysis was performed as described above. The primary antibodies were polyclonal anti-15-PGDH (1:5000; Novus Biologicals, CO, USA), anti-Snail 1 (1:1000; Santa Cruz, CA, USA), anti-Phospho-ERK1/2, anti-ERK1, Phospho-p38, anti-p38, Phospho-JNK 1, anti-JNK (1:1000; Cell Signaling, MA, USA), anti-TLR4, anti-TLR2 and anti-MyD88 (1:1000; Abcam, MA, USA) antibodies. Appropriate horseradish-peroxidase-conjugated secondary antibodies (Cell Signaling, MA, USA) were used. Immunoblot analysis with anti-β-actin served as a loading control.
Immunohistochemistry
The H pylori-negative and –positive tissues were subjected to immunohistochemical staining for 15-PGDH by using a monoclonal anti-15-PGDH antibody (provided by Sanford D. Markowitz) (12). The tissues were fixed in 10% formalin and embedded in praffin. Four-micrometer-thick sections were deparaffinized with xylene and then gradually rehydrated with a graded series of alcohol solutions. 15-PGDH staining was performed as described previously (19). In short, Antigen retrieval was performed (60 min at 96°C) in 10 mM citrate buffer (pH 6.0). The primary anti-15-PGDH (1:5000 dilution) antibody was incubated overnight at 4°C. The slides were incubated with Dako Envision System Kit (DAKO, Glostrup, Denmark) and developed with DAB solution (Dako, Glostrup, Denmark). Finally, the sections were counterstained with hematoxylin. All slides were reviewed by one gastrointestinal pathologist.
Bisulfite modification and methylation-specific PCR (MSP)
Genomic DNA was isolated from gastric tissues by using a DNeasy tissue extraction kit (Qiagen, Maryland, USA) and then subjected to bisulfate modification with an EZ DNA Methylation-Gold kit (Zymo Research, CA, USA). The modified DNA was amplified with primers that were specific for either the unmethylated (U) or methylated (M) sequences of 15-PGDH (18). Methylation and unmethylated human control DNA was purchased from Qiagen (Hilden, Germany). Methylation-specific PCR (MSP) was individually performed in 25 μl reaction volumes by using the EpiTect MSP kit (Qiagen, Hilden, Germany). The products were separated on a 2% agarose gel and detected by ethidium bromide staining.
Pyrosequencing analysis
Each primer was designed by using the PSQ assay design program (Biotage, Uppsala, Sweden). The primer sequences that were used are listed in supplementary Table S1. The amplification was carried out initiated at 95° for 10 min, followed by 45 cycles at 95° for 30 sec, at 55° for 30 sec, at 72° for 30 sec and final extension at 72° for 5 min. The single-stranded DNA template was prepared from biotinylated PCR product by using streptavidin Sepharose® HP beads (Amersham Biosciences, Sweden) as described by the PSQ 96 sample preparation guide. Sequencing was performed on a PyroMark ID system by using the PyroGold reagents kit (Biotage, Uppsala, Sweden) according to the manufacturer’s instructions. The percentage of 15-PGDH methylation was determined by calculating the average methylation at 10 CpG sites in pyrosequencing.
Cell culture and H pylori infection
The human gastric cancer cell line AGS was purchased in the year 2008 from the Korea Cell Line Bank (Seoul, Korea). The AGS cell line was authenticated by Korean Cell Line Bank (Seoul, Korea). The authenticity of this cell line was validated by STR DNA fingerprinting using the AmplFLSTR identifiler PCR Amplification kit (Applied Biosystems, CA, USA). The STR profiles were analyzed using 3730 DNA analyzer (Applied Biosystems, CA, USA) and GeneMapper ID v3.2 (Applied Biosystems, CA, USA). The AGS cell line was last authenticated at the end of this study in the year 2013 by Korean Cell Line Bank. The Korean Cell Line Bank guaranteed the authenticity of cell lines. The AGS cell line was maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin and 100 μg/mL streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. The cells were seeded onto 60 mm culture dishes at 1×106 cells/dish in RPMI-1640 medium and cultured for 24 h before being infected. For AGS cell infections, the H. pylori (ATCC43504, type strain 11637, caAg+ vacA+) was grown under microaerophilic conditions for 48h on Brucella broth agar plates containing 7% sheep blood, counted by using a UV-scanning spectrophotometer (Shimazu), and added to the AGS cell monolayers for 4h at a multiplicity of infection (MOI) of 20. In the another experiment, the cells were pretreated with inhibitors; PD98059 (ERK1/2 inhibitor), SB203580 (p38 inhibitor), SP600125 (JNK inhibitor), AG1478 (EGFR inhibitor), LY294002 (PI3K inhibitor) (Calbiochem, Darmstadt, Germany) for 1hr prior to the infection of with H. pylori for 4hr. For Lipopolysaccharide (LPS) treatments, the AGS cells were cultured for 24 hrs and subsequently treated with LPS (1 μg/ml or 10 μg /ml; Sigma, MO, USA) for 12 hr.
Small interfering RNA
Small interfering RNA (siRNA) for Snail 1 (UUGCAGUUGAAGGCCUUUCGUU), MyD88 (GCUCAUCGAAAAGAGGUGC) (23) and negative control siRNA were purchased from Genolution (Seoul, Korea) and Invitrogen (CA, USA), repectively. The AGS cells were cultured in 60mm cell culture dish for 24 hrs at 37 °C. Before H.pylori infection, The cells were transfected for 48hrs with 50 nmol/L siRNA by using Lipofectamine™ RNAiMAX (Invitrogen Inc., CA, USA) as described by the manufacturer’s instructions. The medium was replaced and the cells were infected with H.pylori.
Statistics
Statistical analysis was performed by using GraphPad PRISM version 5.01 for Window (GraphPad Software, San Diego, USA). Differences between two groups in terms of continuous variables were analyzed by using the Student’s t test and Mann-Whitney test, while differences in terms of categorical variables were evaluated by using the X2 test or Fisher’s exact test. P values of <0.05 were considered to indicate statistically significant differences.
Results
Clinical characteristics of the study population
In total, 40 adult subjects (26 males [60%]; median age, 56 years [range, 21–79 years]) were recruited. Their baseline demographic and clinical characteristics are described in Table 1. Of the 40 subjects, 25 were positive for H pylori infection (63%). The H pylori positive group was older than the negative group (P= 0.002) but did not differ significantly in terms of sex, smoking status, alcohol consumption, hypertension, diabetes, body mass index, or cholesterol level. In addition, the two groups did not differ in terms of the symptoms at the time of endoscopy or the endoscopic diagnosis.
15-PGDH expression is down-regulated in H pylori-positive specimens
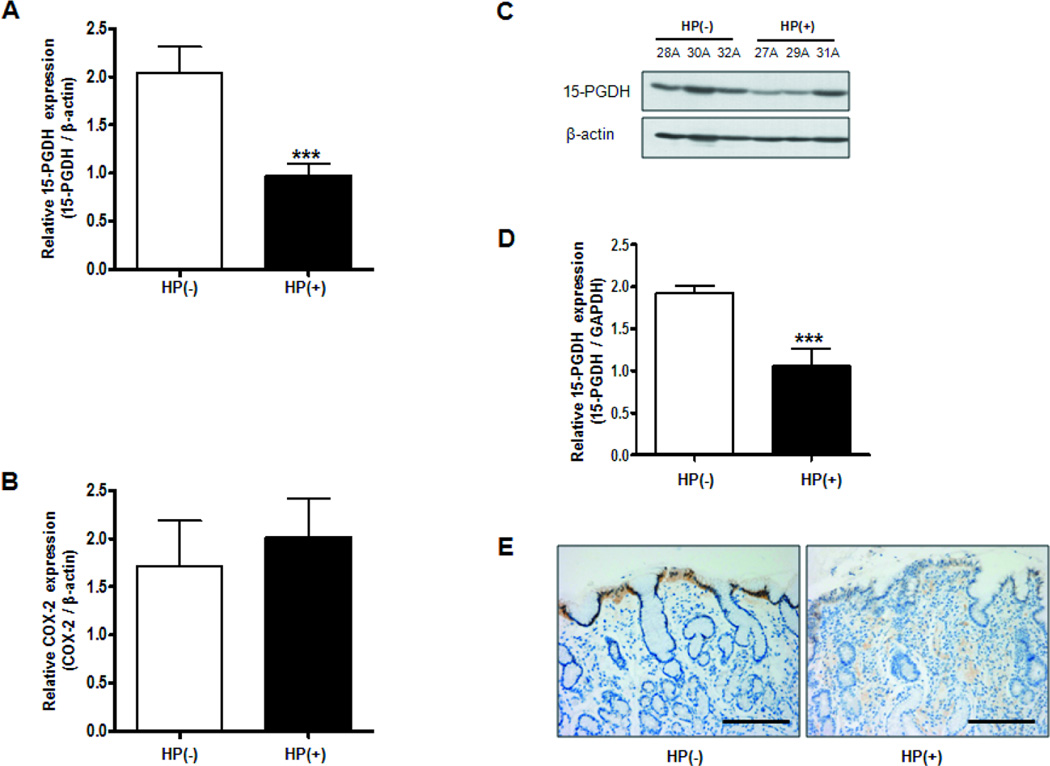
To investigate the regulation of 15-PGDH expression in the infected gastric specimens, the H pylori-positive and -negative gastric specimens taken at baseline were first compared in terms of 15-PGDH mRNA expression. As shown in Fig. 1A, the positive specimens had significantly lower 15-PGDH mRNA expression than the negative specimens (P= 0.0004) but did not differ in terms of COX-2 mRNA expression (P=0.6395) Fig. 1B. The 15-PGDH protein levels in the positive and negative specimens were then determined by western blot analysis. As shown by Fig. 1C, the H pylori-positive specimens expressed less 15-PGDH protein than the negative specimens. As shown in Fig. 1D, quantitation with densitometry and normalization relative to the GAPDH levels revealed that the relative mean intensity of 15-PGDH in the positive specimens (0.41; range 0.00–0.98) was significantly lower than that in the negative specimens (0.75; range 0.56–1.05) (P= 0.0007). To confirm the suppression of 15-PGDH expression in H pylori-positive tissues, immunohistochemisty with a 15-PGDH-specific antibody was performed. The H pylori-negative specimens exhibited strong 15-PGDH expression in epithelial cells located at the upper luminal surface of the gastric proliferative zone. In contrast, the H pylori-positive specimens showed a significant loss of 15-PGDH expression along with higher numbers of mononuclear inflammatory cells in the lamina propria (Fig. 1E).
Figure 1. Expression of 15-PGDH and COX-2 in gastric tissues from H pylori-positive and – negative subjects.
A, Expression of 15-PGDH mRNA. The positive specimens expressed significantly less 15-PGDH than the negative specimens. B, Expression of COX-2 mRNA. The specimens did not differ in terms of COX expression. HP(+), H pylori-positive subjects (n=23); HP(−), H pylori-negative subjects (n=15). The values are the means±SD of three independent experiments. *** indicates statistically significant differences (P< 0.001). C, Representative immunoblots showing 15-PGDH expression in infected and non-infected specimens. D, Densitometry of 15-PGDH protein expression normalized relative to GAPDH levels. The H pylori-positive specimens had a significantly lower relative mean 15-PGDH expression intensity (0.44; range 0.00–0.98) than the H pylori-negative specimens (0.75; range 0.56–1.05). HP(+), H. pylori-positive subjects (n=14); HP(−), H pylori-positive subjects (n=14). The values are the means ±SD of three independent experiments. *** indicates statistically significant differences (P< 0.001). E, Immunohistochemical staining of 15-PGDH. Compared to the non-infected specimens, the infected specimens had significantly lower 15-PGDH expression in epithelial cells located at the upper luminal surface and higher numbers of mononuclear inflammatory cells in the lamina propria. Original magnification, X 200. Scale bars, 500 mm.
15-PGDH down-regulation and Snail up-regulation in H pylori-infected AGS cells and their role in regulation of TLR4-ERK pathway
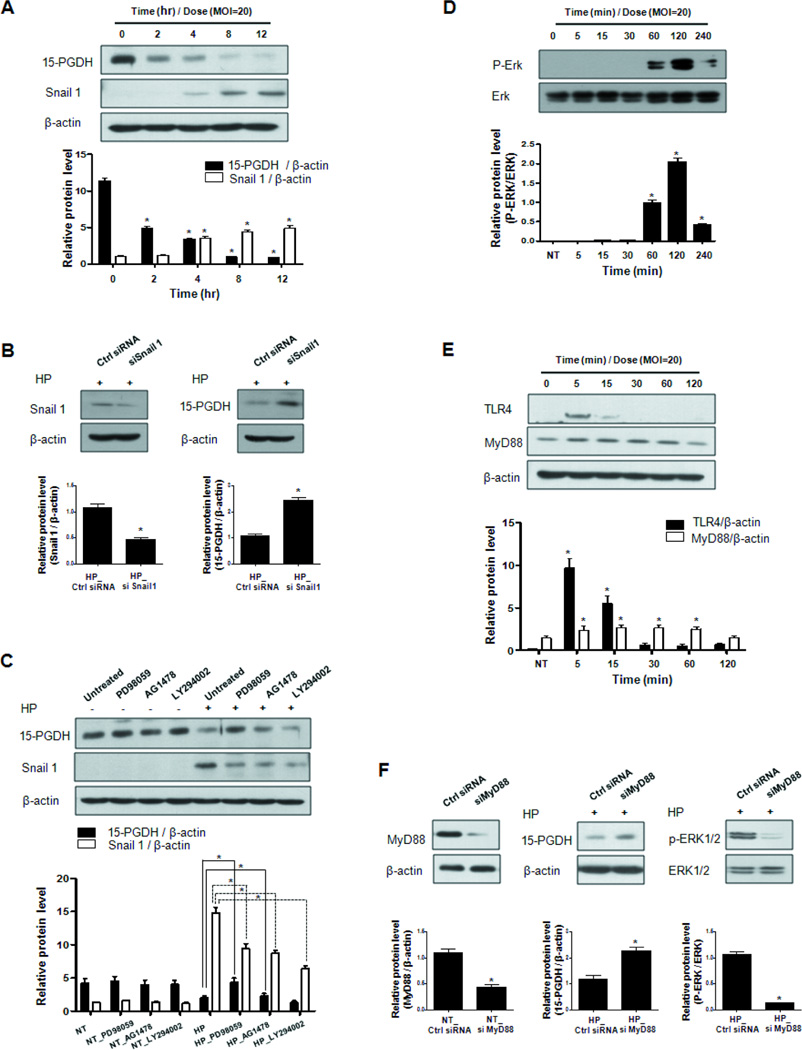
To investigate the mechanism underlying the H pylori associated suppression of 15-PGDH expression, AGS cells (which has a high endogenous level of 15-PGDH) were infected with H pylori (Supplementary Fig. S1A). Western blot analysis revealed that the H pylori infection significantly decreased 15-PGDH protein levels (Fig. 2A, Supplementary Fig. S1B) and increased the transcription factor, Snail, after 4hrs (Fig. 2A). To determine whether inhibition of 15-PGDH was specific for the H pylori, we examined whether 15-PGDH expression was affected by LPS treatment. 15-PGDH expressions did not change after LPS treatment in AGS cells (Supplementary Fig. S2). Since previous reports have demonstrated that the transcription factor Snail represses the transcription of 15-PGDH (24, 25). We confirmed that inhibition of Snail using siRNA against Snail 1 blocked the H pylori-mediated down-regulation of 15-PGDH expression (Fig. 2B). The present results suggest that H pylori suppress 15-PGDH protein levels by activating Snail.
Figure 2. H pylori suppresses 15-PGDH protein levels by activating TLR4/MyD88 and ERK1/2 in a gastric cancer cell line.
A, AGS cells were infected with H pylori for 12 hr and harvested at various time points. The infection decreased 15-PGDH levels while increasing Snail activation. B, AGS cells were transfected with Snail siRNA or control siRNA by using Lipofectamine™ iMAX and infected with H pylori. The inhibition of Snail suppressed H pylori-mediated up-regulation of Snail and restored 15-PGDH inhibition by H pylori infection. HP, H pylori C, AGS cells were pretreated with signaling pathway inhibitors for 1 hr and then infected with H pylori (or left uninfected) for 4hr in serum-free conditions. The inhibitors were the ERK1/2 inhibitor PD98059 (20 μM), the EGFR inhibitor AG1478 (10 μM), and the PI3K inhibitor LY294002 (40 μM). The ERK1/2 inhibitor PD98059 was clearly restored the 15-PGDH expressions. NT, no treat D, The phosphorylated ERK1/2 and total ERK1/2 protein levels in infected and uninfected AGS cells over time were determined by western blot analysis. Infection elevated ERK1/2 phosphorylation. E, The TLR4 protein levels in H pylori- infected and -uninfected AGS cells were examined by western blot analysis. H pylori elevated the TLR4 and MyD88 protein levels. F, AGS cells were transfected with MyD88 siRNA or control siRNA and infected with H pylori. The inhibition of MyD88 blocked the H pylori-mediated down-regulation of 15-PGDH protein levels. It also significantly suppressed the H pylori-induced phosphorylation of ERK1/2. The data shown represent the mean ± SD for one experiment performed in triplicate; * P < 0.05.
Specific chemical inhibitors, namely the ERK1/2 inhibitor PD98059, the EGFR inhibitor AG1478 and the PI3K inhibitor LY294002, were used to elucidate the cell signaling pathway that was involved in the down-regulation of 15-PGDH in H pylori-infected AGS cells. Pretreatment with PD98059, AG1478 and LY294002 partially reversed the stimulatory effect of H pylori on Snail (Fig. 2C). However, blocking with PD98059 and AG1478, an ERK1/2 and EGFR inhibitor, restored the down-regulation of 15-PGDH mediated by H pylori infection (Fig. 2B). To determine if ERK1/2 is activated by H pylori infection, we performed a western blot for active, phosphorylated ERK1/2 (p-ERK1/2) and found that ERK1/2 was activated by H pylori within 1hr after infection commenced, with maximal activity occurring by 2hr (Fig. 2D). We also demonstrated that another MAPK pathway signaling p38 and JNK were activated by H pylori (Supplementary Fig. S3A), however, only blocking with PD98059 restored 15-PGDH expressions and suppressed up-regulated Snail expressions by H pylori (Supplementary Fig. S3B). These results indicated that activation of the ERK1/2 pathway by H pylori is specific for Snail up-regulation and subsequent 15-PGDH suppression by H pylori infection.
TLR4 regulates the response of human gastric epithelial cells to H pylori (26). To test whether it participates in the H pylori-mediated suppression of 15-PGDH, infected AGS cells were subjected to western blot analysis of TLR4 expression. The expression MyD88 was also examined because it is an adaptor molecule that participates in TLR signaling (20). H pylori infection significantly induced TLR4 protein expression after 5 min of exposure, while the expression of MyD88 increased between 5 min and 1 hr of exposure (Fig. 2E). In addition, TLR2 expression was increased by H pylori infection (Supplementary Fig. S4).
To investigate the functional role of MyD88 in H pylori-associated 15-PGDH regulation, an siRNA against MyD88 was used to knock-down MyD88 expression. Interestingly, the inhibition of MyD88 blocked the H pylori-mediated down-regulation of 15-PGDH expression (Fig. 2F). It also significantly suppressed the H pylori-induced phosphorylation of ERK1/2 (Fig. 2F), which suggests that the 15-PGDH suppression was mediated by TLR4/MyD88 and activated ERK1/2.
Promotor Methylation of 15-PGDH DNA in H pylori positive Gastric tissues
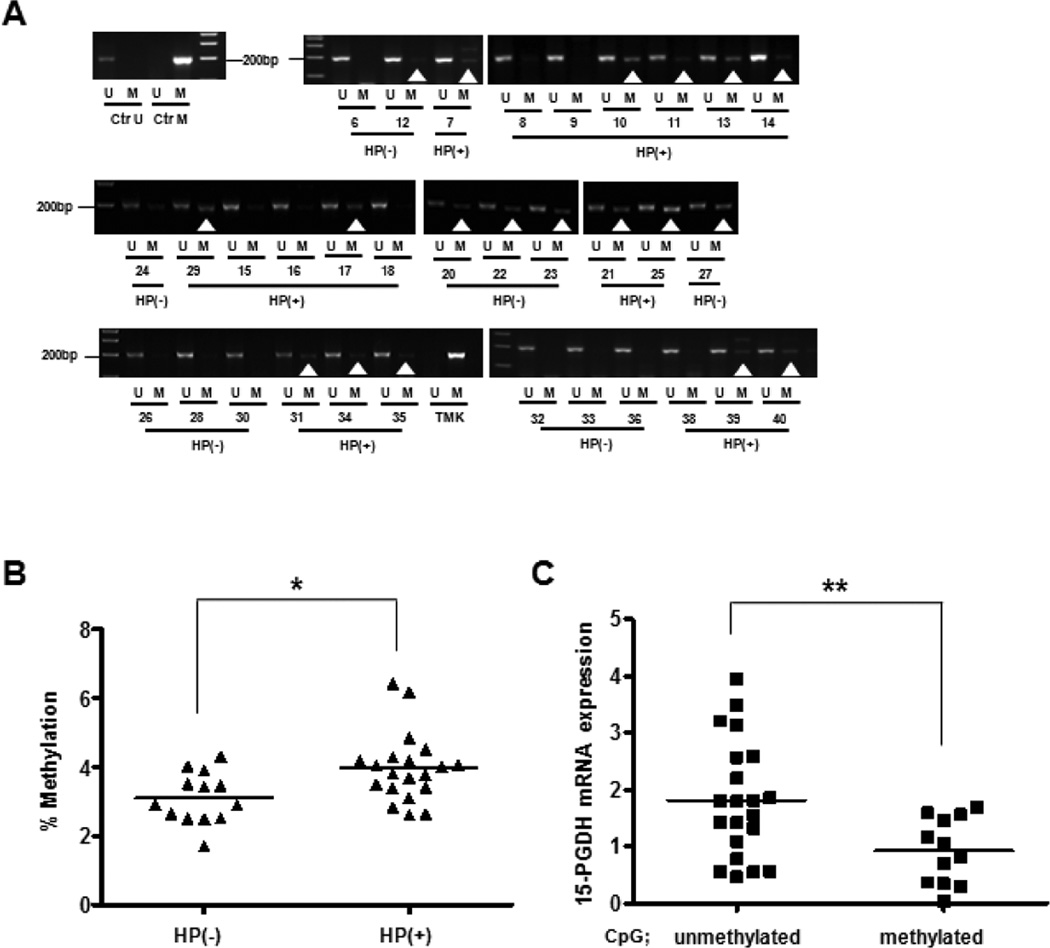
To investigate the significance of 15-PGDH promoter methylation in H pylori-infected gastric specimens, the 15-PGDH promoter methylation status of 13 H pylori-negative and 20 positive specimens was examined. In 14 of the 20 H pylori-positive subjects (70%), the 15-PGDH promoter showed increased methylation (Fig. 3A). In contrast, only five of the 13 H pylori-negative subjects (38%) showed this (Fig. 3A). To assess the precision with which bisulfate-PCR determined the 15-PGDH promoter methylation in gastric specimens, the 20 positive and 13 negative specimens were also subjected to pyrosequencing analysis. The percent methylation for 10 CpG sites analyzed ranged from 2.6 to 6.4 percent methylated in H pylori positive, while only from 1.7 to 4.3 percent methylated in H pylori negative subjects. The levels of 15-PGDH promoter methylation was significantly higher in H pylori positive specimens compared with negative ones (P= 0.0111) (Fig. 3B). When all samples were pooled and divided into two groups that had >4% and ≤4% promoter methylation, respectively, the group with >4% promoter methylation had significantly lower 15-PGDH mRNA levels than the ≤4% group (P= 0.0097) (Fig. 3C) suggesting that the subtle increase in 15-PGDH methylation seen in H pylori infected individuals in vivo may play a role in modulating 15-PGDH mRNA levels.
Figure 3. 15-PGDH gene CpG island Promoter Methylation Status according to H pylori infection.
A, Gastric specimens from H pylori-positive (n=20) and -negative (n=13) subjects were subjected to methylation-specific PCR with unmethylated (U) or methylated (M) 15-PGDH primer sets. Completely unmethylated (Ctr U) or methylated (CtrM) bisulfate-converted human DNA served as a control. The human gastric cancer cell line TMK-1 served as a positive control. The white arrowheads indicate products obtained with the M-primer pair. B, The gastric specimens were analyzed by pyrosequencing. The H pylori-positive samples had a significantly higher percentage of 15-PGDH promoter DNA methylation than the H pylori-negative specimens. *indicates a statistically significant difference (P< 0.05). C, The 33 gastric specimens were divided into two groups according to whether they had >4% or ≤4% 15-PGDH promoter methylation. The >4% group had significantly less 15-PGDH mRNA expression than the ≤4% group. **indicates a statistically significant difference (P< 0.01).
Restoration of 15-PGDH expression after H pylori eradication
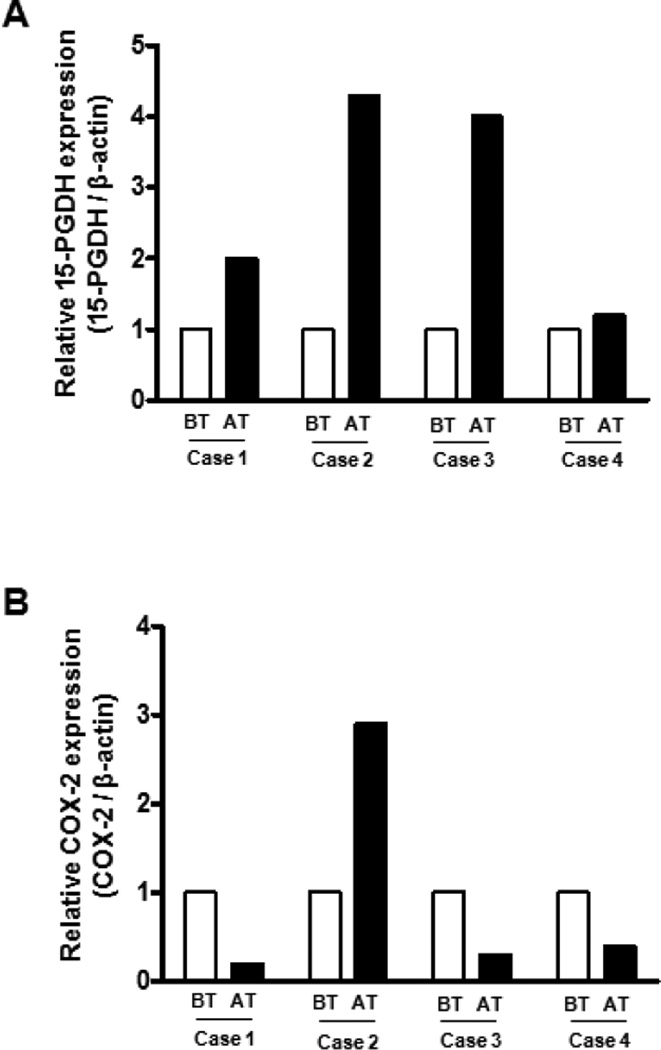
To explore whether H pylori-associated suppression of 15-PGDH expression can change if H pylori is eradicated, gastric samples were collected from four of the H pylori-positive subjects 8 weeks after triple therapy regimen and the 15-PGDH mRNA expression was evaluated. In all four cases, 15-PGDH mRNA expression rose markedly after treatment (Fig. 4A), while the expression of COX-2 mRNA decreased in three of the four cases (Fig. 4B). A follow-up CLO test and histologic examination showed that all four patients had become H pylori-negative after treatment.
Figure 4. 15-PGDH and COX-2 mRNA levels in gastric specimens from four subjects before and after H pylori was eradicated, as measured by quantitative real-time PCR.
A, The gastric 15-PGDH mRNA expression rose significantly in all four subjects after H pylori eradication. BT, before treatment; AT, after treatment. B, The gastric COX-2 mRNA expression dropped in three of the four subjects after eradication.
Discussion
Infection-associated inflammation plays an important role in promoting cancer development (27). Examples of cancers that are linked to infection include bladder cancer (shistosomiasis), hepatocellular carcinoma (hepatitis B and C infection), cervical cancer (human papilloma virus), and gastric cancer (H pylori infection) (27). Such “tumor-promoting inflammation” is now considered to be one of the “hallmarks of cancer” (28). In the pathophysiologic mechanism of inflammation associated carcinogenesis, activated prostaglandins are critical for stimulating cell invasion and promoting angiogenesis (5, 7). The present study showed for the first time that 15-PGDH (a key enzyme that degrades major prostaglandins) was down-regulated by H pylori infection in the human stomach. This suggests that H pylori infection represses the catabolism of prostaglandins, which elevates prostaglandin levels, which in turn may promote gastric carcinogenesis. The present study also showed for the first time that this process was reversible: 15-PGDH expression recovered after H pylori was eradicated. Moreover, an in vitro study revealed that the infection-mediated suppression of 15-PGDH was mediated by TLR4-MyD88 activation and involved ERK1/2 or EGFR-Snail transcriptional regulation and this inhibition of 15-PGDH was specific for the H pylori infection. Previously, it was reported that EGFR signaling induces Snail, which binds conservd E-box elements in the PGDH promoter to repress transcription (25). We elucidated that H pylori associated signaling pathway involving TLR4-MyD88 and ERK1/2 is also related to the 15-PGDH suppression that might be related with gastric carcinogenesis. In addition, a modest elevated CpG island methylation of 15-PGDH DNA was found associated with the down-regulation of 15-PGDH by H pylori.
Recent studies including our own, have suggested that 15-PGDH behaves as a tumor suppressor in several tumor types including colon, lung, breast, bladder, thyroid and pancreas carcinomas (12–17). Previously, we reported that 70.1% of gastric cancer specimens lack 15-PGDH protein expression (19). In addition, exploration of 15-PGDH expression in preneoplastic lesions showed that while 15-PGDH repression was not present in metaplasia, it was markedly reduced in 83.3% of adenomas (19). The present study showed that, 15-PGDH was even repressed in H pylori positive specimens that only showed mild gastritis. This suggests that H pylori infection initiates gastric carcinogenesis by elevating PGE2 levels even before the formation of preneoplastic lesion. The elevated expression of COX-2 is also found in more than 70% of gastric cancers, and this is reversed when H. pylori is eradicated (29). In addition, transgenic mice expressing COX-2 and the PGE2-converting enzyme microsomal prostaglandin E synthase-1 (mPGES-1) develop hyperplastic gastric tumors, which suggests that the activation of prostaglandin synthesis plays a major role in H pylori-associated gastric carcinogenesis (30). The present study also demonstrated that elevated PGE2 levels in gastric carcinogenesis are a consequence of not only increased synthesis but also reduced catabolism.
Murine model studies have indicated that PGE2 suppresses the T helper 1 immune response in the H pylori-infected stomach and that inhibition of COX-2 enzymatic activity accelerates the development of gastritis and premalignant gastric lesions; moreover, systemic administration of synthetic PGE2 prevents premalignant changes (7). These observations suggest that PGE2 may suppress infection-associated carcinogenesis, which appears to be inconsistent with the present results. PGE2 also promotes crypt stem cell survival and proliferation (31) and plays a role in the wound-healing process by enhancing the production of vascular endothelial growth factor (VEGF), a key factor in angiogenesis (32). 15-PGDH is also down-regulated in inflammatory bowel diseases (33). Thus, in the short term, it seems likely that the reduced degradation of PGE2 in infected gastric tissue contributes to the healing of the inflamed mucosa. However, if the elevation of PGE2 in inflamed mucosa is chronic, it may stimulate mitogenesis and inhibit apoptosis, thereby lowering the threshold for the development of cancer. Recent reports suggest that bacterial infection, in cooperation with PGE2 signaling through the EP4 receptor, induces macrophage recruitment and promotes gastric tumorigenesis by TNF-a and Wnt signaling (6). This supports the notion that chronic activation of PGE2 has procarcinogenic effects. The short term effect of PGE2 activation on gastric carcinogenesis should be investigated in future studies.
The 15-PGDH gene has two regions that contain clusters of putative transcription factor-binding sites. The transcription factors that have been reported to regulate these regions are Ets, activating protein-1 (AP-1), Snail, Alug, and ZEB1 (10). Previous studies indicate that EGFR activation suppressed 15-PGDH through Snail (25). In addition, blockade of EGFR signaling induces 15-PGDH while reducing Snail in vitro and PGE2-dependent xenografts model (25). EGFR signaling induces Snail, which binds to conserved E-box elements in the 15-PGDH promoter to repress transcription (25). In our study, analysis of the mechanism by which H. pylori regulated 15-PGDH expression in a gastric cancer cell line revealed that infection with H pylori suppressed 15-PGDH expression and up-regulated Snail expression. H pylori infection also activated the phosphorylation of ERK1/2, and ERK1/2 inhibition clearly prevented the suppression of 15-PGDH and the upregulation of Snail levels by H pylori. Also, the blockade of EGFR signaling restored H pylori-mediated down-regulation of 15-PGDH. This suggests that ERK1/2 and EGFR are necessary for the H pylori-mediated repression of 15-PGDH. This result is consistent with our previous report, which suggests that 15-PGDH is repressed in gastric cancer by the activation of the EGFR-ERK1/2 pathway (19).
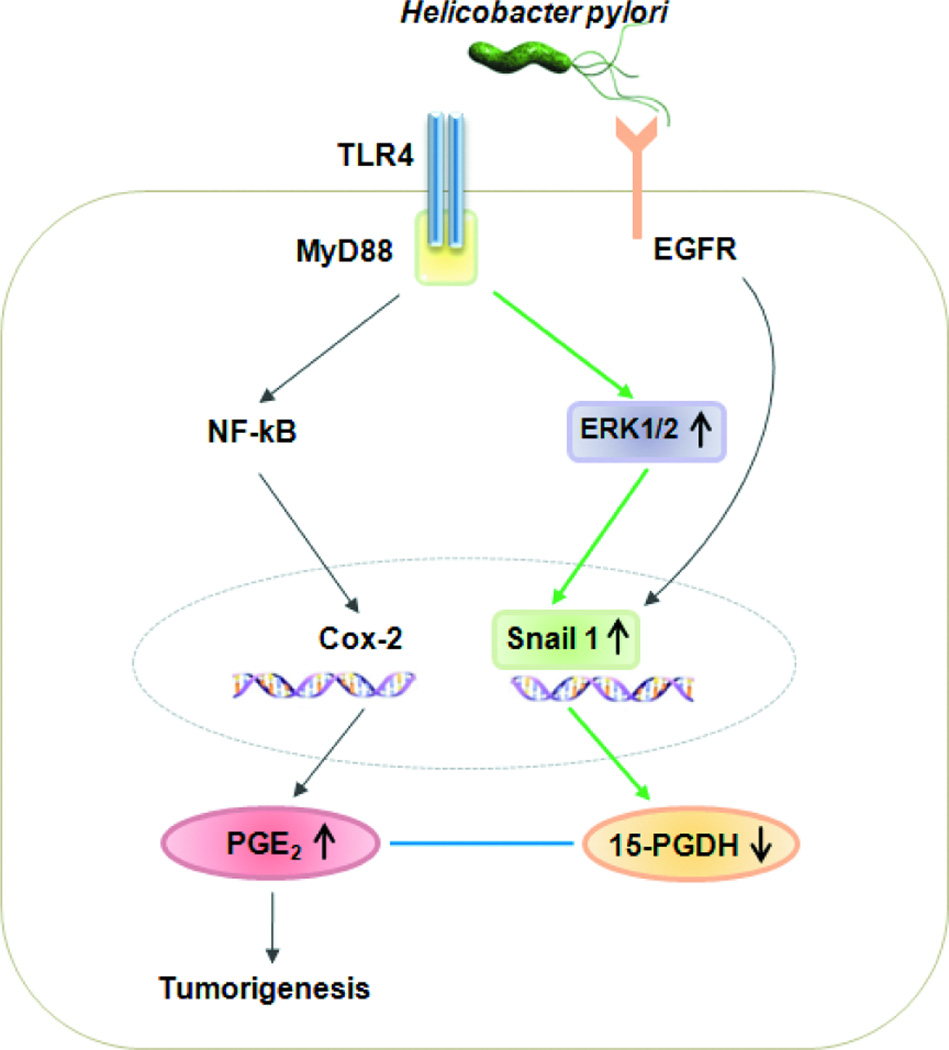
The present study also assessed whether the TLR/MyD88 pathway, which is a well-known bacterial recognition system, plays a role in the H pylori-associated repression of 15-PGDH. It was found that TLR4 and MyD88 were activated by H pylori, as has been reported previously (20). To elucidate whether MyD88 is needed for H pylori-induced 15-PGDH repression, a siRNA against MyD88 was used. Interestingly, this siRNA lifted the repression of 15-PGDH and blocked the phosphorylation of ERK1/2. These observations together suggest that H pylori activate TLR4/MyD88, thereby elevating ERK1/2 phosphorylation, which in turn represses 15-PGDH expression (Diagram 1).
Diagram 1. Schematic representation of H pylori signaling on tumorigenesis through 15-PGDH.
H pylori activates TLR4/MyD88, thereby elevating ERK1/2 phosphorylation, which increases Snail transcriptional regulation, which in turn represses 15-PGDH expression. The activation of EGFR suppressed 15-PGDH through Snail. This 15-PGDH down-regulation activates PGE2 promoting tumorigenesis.
TLR4 signaling via MyD88 activates a signaling cascade that results in the enhanced transcription of COX-2 and the increased production of PGE2 (22, 23). PGE2 activates Wnt and peroxisome proliferators-activated receptor-δ signaling to induce target genes involved in replication and survival; it also triggers cell migration by activating the EGFR pathway (5, 8). The present study identified another pathway that could promote gastric carcinogenesis, namely one where the catalysis of PGE2 by 15-PGDH is suppressed via TLR4/MyD88 signaling. Notably, our analysis of human samples revealed that H. pylori infection clearly down-regulated 15-PGDH but did not significantly activate COX-2. It is unclear why COX-2 activation was not evident in the samples but it is possible that at this stage of H. pylori infection, PGE2 levels are controlled by the degradation of PGE2 rather than the synthesis of PGE2. It is intriguing observation that one case had increased COX-2 after H pylori eradication. It is difficult to explain, however, it might be because the gastric sample obtained from this patient contains large portion of remained lymphocytes resulting in increase of COX-2 expression.
The main strategies of gastric cancer chemoprevention include H pylori eradication and the use of non-steroidal anti-inflammatory drugs that mainly target the inhibition of prostaglandin synthesis (34). Previous trials on drugs that inhibit COX-2 activity and that could decrease the risk of colon carcinogenesis showed that these drugs are problematic because they induce unfavorable cardiovascular side effects (35). Our previous data indicated that these side effects can be reduced by using effective biomarkers such as 15-PGDH to select the subjects who will benefit from these chemopreventive drugs (36). In addition, we hypothesize that gastric neoplasia could be prevented by combining H pylori eradication with compounds that can act in the gastrointestinal tract to re-induce 15-PGDH expression in early neoplastic cells.
We could not recruit enough patients because it was very difficult to get the permission for the sampling in patients before and after H pylori eradication therapy. However, we believe that our preliminary result provide valuable suggestion that 15-PGDH can be restored after H pylori eradication. Further study including long-term follow up measurement will be necessary to prove this hypothesis.
In conclusion, the present study demonstrated that 15-PGDH, a novel tumor suppressor, was significantly down-regulated in H pylori-infected gastric human tissues, which suggests that H pylori infection may repress the catabolism of prostaglandins, thereby elevating prostaglandin levels, which could promote gastric carcinogenesis. An in vitro study revealed that this process is mediated by TLR4-MyD88 activation and ERK1/2 or EGFR-Snail transcriptional activation. In addition, H pylori appeared to suppress 15-PGDH expression by inducing aberrant 15-PGDH promoter methylation. Finally, sequential analysis of 15-PGDH transcription activity after H pylori eradication revealed that the infection-induced suppression of 15-PGDH expression was reversible. The elucidation of this novel mechanism provides new insights that may improve our understanding of H pylori-induced gastric carcinogenesis. Further studies into 15-PGDH inducers that could serve as chemopreventive agents of gastric carcinogenesis are warranted, as are studies that determine the usefulness of this novel enzyme as a biomarker of gastric carcinogenesis.
Supplementary Material
Acknowlegements
We thank Prof. Ki-Up Lee, for helpful discussions.
Grant Support
This work was supported by a grant from the Asan Institute for Life Sciences (2012-261), the National Research Foundation of Korea (2009-0083559), the KRIBB Research Initiative Program (KGM2210911), the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MEST) (2011-0019632), and the Korean Health Technology R&D Project, Ministry of Health & Welfare (A062254). The funders had no role in study design, the data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Polk DB, Peek RM. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. New England Journal of Medicine. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 4.Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, DuBois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oshima H, Hioki K, Popivanova BK, Oguma K, Van Rooijen N, Ishikawa TO, et al. Prostaglandin E2 signaling and bacterial infection recruit tumor-promoting macrophages to mouse gastric tumors. Gastroenterology. 2011;140:596.e7–607.e7. doi: 10.1053/j.gastro.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Toller IM, Hitzler I, Sayi A, Mueller A. Prostaglandin E2 prevents Helicobacter-induced gastric preneoplasia and facilitates persistent infection in a mouse model. Gastroenterology. 2010;138:1455–1467. 67.e1–67.e4. doi: 10.1053/j.gastro.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Oshima H, Popivanova BK, Oguma K, Kong D, Ishikawa TO, Oshima M. Activation of epidermal growth factor receptor signaling by the prostaglandin E(2) receptor EP4 pathway during gastric tumorigenesis. Cancer Sci. 2011;102:713–719. doi: 10.1111/j.1349-7006.2011.01847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong BCY, Zhang L, Ma J, Pan K, Li J, Shen L, et al. Effects of selective COX-2 inhibitor and Helicobacter pylori eradication on precancerous gastric lesions. Gut. doi: 10.1136/gutjnl-2011-300154. [DOI] [PubMed] [Google Scholar]
- 10.Na HK, Park JM, Lee HG, Lee H, Myung SJ, Surh YJ. 15-Hydroxyprostaglandin dehydrogenase as a novel molecular target for cancer chemoprevention and therapy. Biochemical pharmacology. 2011 doi: 10.1016/j.bcp.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Yan M, Rerko RM, Platzer P, Dawson D, Willis J, Tong M, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proc Natl Acad Sci U S A. 2004;101:17468–17473. doi: 10.1073/pnas.0406142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, Dotson A, et al. 15-Hydroxyprostaglandindehydrogenase is an in vivo suppressor of colon tumorigenesis. Proceedings of the National Academy of Sciences. 2006;103:12098–12102. doi: 10.1073/pnas.0603235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding Y, Tong M, Liu S, Moscow JA, Tai HH. NAD+-linked 15-hydroxyprostaglandin dehydrogenase (15-PGDH) behaves as a tumor suppressor in lung cancer. Carcinogenesis. 2005;26:65–72. doi: 10.1093/carcin/bgh277. [DOI] [PubMed] [Google Scholar]
- 14.Wolf I, O'Kelly J, Rubinek T, Tong M, Nguyen A, Lin BT, et al. 15-hydroxyprostaglandin dehydrogenase is a tumor suppressor of human breast cancer. Cancer research. 2006;66:7818. doi: 10.1158/0008-5472.CAN-05-4368. [DOI] [PubMed] [Google Scholar]
- 15.Tseng-Rogenski S, Gee J, Ignatoski KW, Kunju LP, Bucheit A, Kintner HJ, et al. Loss of 15-hydroxyprostaglandin dehydrogenase expression contributes to bladder cancer progression. The American journal of pathology. 2010;176:1462–1468. doi: 10.2353/ajpath.2010.090875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quidville V, Segond N, Lausson S, Frenkian M, Cohen R, Jullienne A. 15-Hydroxyprostaglandin-dehydrogenase is involved in anti-proliferative effect of non-steroidal anti-inflammatory drugs COX-1 inhibitors on a human medullary thyroid carcinoma cell line. Prostaglandins & other lipid mediators. 2006;81:14–30. doi: 10.1016/j.prostaglandins.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Pham H, Chen M, Li A, King J, Angst E, Dawson DW, et al. Loss of 15-hydroxyprostaglandin dehydrogenase increases prostaglandin E2 in pancreatic tumors. Pancreas. 2010;39:332. doi: 10.1097/MPA.0b013e3181baecbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiel A, Ganesan A, Mrena J, Junnila S, Nykänen A, Hemmes A, et al. 15-hydroxyprostaglandin dehydrogenase is down-regulated in gastric cancer. Clinical cancer research. 2009;15:4572–4580. doi: 10.1158/1078-0432.CCR-08-2518. [DOI] [PubMed] [Google Scholar]
- 19.Song HJ, Myung SJ, Kim IW, Jeong JY, Park YS, Lee SM, et al. 15-Hydroxyprostaglandin Dehydrogenase is Downregulated and Exhibits Tumor Suppressor Activity in Gastric Cancer. Cancer Investigation. 2011;29:257–265. doi: 10.3109/07357907.2011.568562. [DOI] [PubMed] [Google Scholar]
- 20.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 21.Lee CC, Avalos AM, Ploegh HL. Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol. 2012;12:168–179. doi: 10.1038/nri3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oshima H, Oshima M. The inflammatory network in the gastrointestinal tumor microenvironment: lessons from mouse models. J Gastroenterol. 2012;47:97–106. doi: 10.1007/s00535-011-0523-6. [DOI] [PubMed] [Google Scholar]
- 23.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, et al. Cox-2 isregulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyaki A, Yang P, Tai HH, Subbaramaiah K, Dannenberg AJ. Bile acids inhibit NAD+-dependent 15-hydroxyprostaglandin dehydrogenase transcription in colonocytes. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2009;297:G559–G566. doi: 10.1152/ajpgi.00133.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann JR, Backlund MG, Buchanan FG, Daikoku T, Holla VR, Rosenberg DW, et al. Repression of prostaglandin dehydrogenase by epidermal growth factor and snail increases prostaglandin E2 and promotes cancer progression. Cancer research. 2006;66:6649–6656. doi: 10.1158/0008-5472.CAN-06-1787. [DOI] [PubMed] [Google Scholar]
- 26.Uno K, Kato K, Atsumi T, Suzuki T, Yoshitake J, Morita H, et al. Toll-like receptor (TLR) 2 induced through TLR4 signaling initiated by Helicobacter pylori cooperatively amplifies iNOS induction in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1004–G1012. doi: 10.1152/ajpgi.00096.2007. [DOI] [PubMed] [Google Scholar]
- 27.Piazuelo MB, Epplein M, Correa P. Gastric cancer: an infectious disease. Infect Dis Clin North Am. 2010;24:853–869. doi: 10.1016/j.idc.2010.07.010. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Sun WH, Yu Q, Shen H, Ou XL, Cao DZ, Yu T, et al. Roles of Helicobacter pylori infection and cyclooxygenase-2 expression in gastric carcinogenesis. World J Gastroenterol. 2004;10:2809–2813. doi: 10.3748/wjg.v10.i19.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oshima H, Oshima M, Inaba K, Taketo MM. Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. EMBO J. 2004;23:1669–1678. doi: 10.1038/sj.emboj.7600170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tessner TG, Muhale F, Riehl TE, Anant S, Stenson WF. Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving AKT activation and bax translocation. J Clin Invest. 2004;114:1676–1685. doi: 10.1172/JCI22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 33.Otani T, Yamaguchi K, Scherl E, Du B, Tai HH, Greifer M, et al. Levels of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase are reduced in inflammatory bowel disease: evidence for involvement of TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2006;290:G361–G368. doi: 10.1152/ajpgi.00348.2005. [DOI] [PubMed] [Google Scholar]
- 34.Ford AC. Chemoprevention for gastric cancer. Best Pract Res Clin Gastroenterol. 2011;25:581–592. doi: 10.1016/j.bpg.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 36.Yan M, Myung SJ, Fink SP, Lawrence E, Lutterbaugh J, Yang P, et al. 15-Hydroxyprostaglandin dehydrogenase inactivation as a mechanism of resistance to celecoxib chemoprevention of colon tumors. Proc Natl Acad Sci U S A. 2009;106:9409–9413. doi: 10.1073/pnas.0902367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.