Abstract
P300 amplitude in childhood predicts substance use disorders by young adulthood. Trajectories of visual P300 amplitude show an association between low amplitude P300 and familial risk for alcohol dependence (AD). Variation in the cholinergic muscarinic receptor gene (CHRM2) has previously been associated with P300 amplitude and AD. The present study used group based trajectory modeling of auditory P300 data collected longitudinally from offspring in families with and without familial loading for AD to determine if specific trajectories would be associated with familial risk and CHRM2 variation. Trajectory modeling confirms previous reports of an association between the low visual P300 trajectory with high familial risk in male offspring. This association was detected in offspring in the 8–12 age range, but not in 13–18 or 19–29 year olds or in high-risk female offspring. CHRM2 association analysis with P300 finds 8–12 year olds who are homozygous for the T allele of rsl824024 are 2.6 times more likely to follow a P300 trajectory characterized by lower and slower change regardless of familial loading. Combining the odds for being male and having a TT genotype results in odds of 6.5 that individuals will follow the low P300 trajectory.
Keywords: P300, CHRM2, High-risk, Alcohol dependence, Offspring, ERP, Childhood
1. Introduction
The P300 component of the event-related potential (ERP) was first identified by Sutton et al (1965). This component is a scalp positive wave that occurs approximately 300 ms after an informative stimulus occurs. The informative nature of a stimulus is defined by the experimental design in which the subject is typically instructed to attend to one stimulus (target) while ignoring another in the often used “oddball” paradigm. The frequency of the target stimulus presentation or the probability it will occur, along with the task relevance to the subject affects the amplitude of P300 component so that early studies concluded that P300 was thought to be an index of capacity to attend to and process stimulus information (Donchin, 1979; Pritchard, 1981). Currently, we know that amplitude is affected by the stimulus paradigms used, the age and gender of subjects, and host of other variables including genetic factors (Polich, 2007; Euser et al 2012; Iacono and Malone, 2011).
Many early cross-sectional studies have shown that P300 is reduced in children from alcoholic families in comparison to offspring from control families (Begleiter et al., 1984; Hill et al., 1990; Hill and Steinhauer, 1993; Steinhauer and Hill, 1993). Several studies have shown that P300 response is primarily the outcome of delta (1.0–3.0Hz) and theta (3.5–7.5 Hz) oscillations during cognitive processing of stimuli (see Porjesz et al., 2005 for review). Studies reporting variation in delta and theta oscillations in the high risk relatives of alcohol dependent individuals suggest that these oscillations are not state dependent effects seen only in alcohol dependent individuals but rather a familial trait (Jones et al., 2004; Jones et al., 2006; Porjesz and Rangaswamy et al., 2007).
Additionally, P300 obtained at one point in time has been used to predict substance use disorders at a later time (Carlson et al., 2007; Habeych et al., 2005; Hill et al., 2009). These studies have assessed P300 and determined substance use disorder outcome at 3, 7 and 11 years post assessment, respectively, finding those with lower P300 were more likely to develop a substance use disorder at follow-up. The conclusion of a recent meta-analysis is that P300 does index risk for SUD (Euser et al 2012).
Studies using a pre/post design are not technically considered trajectory studies because trajectory analyses were not applied to the data, based as they were on two measures. In discrete mathematics, a trajectory is based on a sequences of values. Accordingly, trajectory analysis typically requires inclusion of data for individuals with three or more observations. Two previous studies did involve the analysis of visual P300 collected at up to three points in time at ages 17, 20, and 23 (Carlson and Iacono, 2006, 2008). These studies found a decrease in P300 over time and reported that P300 amplitude was most related to the severity of externalizing disorders in parents (Carlson and Iacono, 2008), though parents with internalizing disorder were not included. In the other study, biometrical modeling in twins was used to assess the genetic contribution to intercept and slope finding significant heritability for both though specific genes were not tested (Carlson and Iacono, 2006).
A large scale follow-up using repeated ERP assessments of child and adolescent offspring from alcohol dependent and control families revealed that trajectories of visual P300 for the offspring, most of whom were tested four or more times, differed by familial risk group (Hill et al., 1999; Hill and Shen, 2002). These studies suggested that while amplitude of P300 at one point in time may reveal risk for later development of substance use disorders, age-related change in P300 amplitude may provide a more salient biological marker of AD risk in youth than single point measures.
Although long-term longitudinal studies are in agreement that P300 amplitude is associated with familial risk for alcohol dependence, there is some variation in the emphasis that has been placed on whether reduction in P300 amplitude is an endophenotype exclusively or predominantly representing an externalizing behavioral dimension that, in turn, increases the risk for a variety of antisocial behaviors (Carlson et al., 2007; Carlson and Iacono, 2008; Krueger et al., 2002; Kruger et al., 2005; Patrick et al., 2006) including alcohol dependence and substance use disorder. Alternatively, P300 reduction may be an endophenotype that reflects an overall greater likelihood of experiencing child/adolescent psychopathology which, in turn, elevates the risk for subsequent alcohol dependence or substance use disorders. Longitudinal follow up of offspring at ultra high risk for alcohol dependence through their membership in multiplex families has demonstrated an increase in both externalizing and internalizing disorders in comparison to low risk controls (Hill et al., 2008).
These studies also found that childhood psychopathology whether externalizing or internalizing is associated with lower P300 amplitude (Hill et al., 1999) with any childhood psychopathology associated with greater likelihood of being in the low amplitude trajectory class (Hill and Shen 2002). Interestingly, children with internalizing disorder had lower P300 amplitude, and those with both internalizing and externalizing disorders had the lowest P300 amplitude (Hill et al.,1999). Similarly, Van Der Stelt (1999) found both internalizing and externalizing disorders moderated the effect of family history on P300 amplitude.
Overall, the internalizing dimension has received less attention in studies of children and adolescents with familial risk for alcohol dependence, with most studies assessing the externalizing dimension exclusively (Krueger et al., 2002; Krueger et al., 2005; Hicks et al., 2007; Carlson and Iacono, 2008). Inclusion of assessment for internalizing disorders would appear warranted in view of the clear indication that the presence of depression is associated with reduction of P300 amplitude in adults (Bruder et al., 1995; Blackwood et al., 1987; Diner et al., 1985; Yanai et al., 1997; Gangadhar et al., 1993; Roschke and Wagner, 2003).
The usefulness of P300 amplitude as an endophenotype for AD risk is strengthened by observations that P300 amplitude is heritable (O’Connor et al., 1994; Katsanis et al., 1997; van Beijsterveldt, 2002; Carlson and Iacono, 2006; Perlman et al., 2009). A search for specific genes influencing these oscillations has been of considerable interest and has resulted in reports of positive associations for visual P300 and a dopamine D2 receptor locus on chromosome 11 (Hill et al., 1998), SNPs within the CHRM2 gene on chromosome 7 (Jones et al., 2004; Jones et al., 2006), and GABRA2 variation on chromosome 4 (Porjesz and Rangaswamy, 2007).
The CHRM2 gene shows particular promise because both association and linkage analyses point to a significant relationship between the muscarinic 2 receptor (CHRM2) gene and alcohol dependence even in the presence of comorbid conditions such as depression and drug dependence (Wang et al., 2004; Dick et al., 2007). Several SNPs within the CHRM2 gene have been studied to determine their possible association with event-related oscillations (Jones et al., 2006; Jones et al., 2004). These findings support the role of muscarinic receptors in EEG oscillations and P300 generation. Although there are undoubtedly many genes that affect neurochemical processes that may, in turn, influence the generation of P300 (Frodl-Bauch et al., 1999), the CHRM2 gene appears to be an important candidate gene for better understanding developmental changes in P300 amplitude changes.
Because developmental trajectories of P300 have been shown to differ by familial risk group status (Hill et al., 1999) and are related to the presence of child/adolescent psychiatric status (Hill and Shen, 2002), it was of interest to determine if variation in developmental trajectories of P300 obtained from repeated assessments would be related to variation in the CHRM2 gene, a gene that has been shown to be associated with both AD risk and cross-sectionally obtained P300 variation.
2. Methods
2.1. Description of Family Studies
The high and low-risk (control) children/adolescents are participants in one of two ongoing family studies (Cognitive and Personality Factors in Relatives of Alcoholics family study [CPFFS] and the Biological Risk Factors in Relatives of Alcoholic Women family study [BRFFS]). The high-risk families had been identified through a proband pair of alcohol dependent siblings. One member of the pair was in a substance abuse treatment facility in the Pittsburgh area at the time of recruitment (late 1980’s and early 1990’s). Probands were screened (Diagnostic Interview Schedule [DIS]) (Robins et al., 1981) for the presence of alcohol dependence (AD) and other Axis I (DSM-III) psychopathology (Feighner Criteria for AD was also obtained). Family history information for biological relatives provided screening information to determine if the proband might have a same-sex sibling meeting criteria for alcohol dependence. If this appeared to be the case, the proband assisted in the recruitment of his/her sibling who then completed the same diagnostic assessments.
2.2. Inclusion Criteria for High-Risk Families
Probands and their families were selected if a pair of same-sexed adult siblings with an alcohol dependence diagnosis was present (sister pairs for the BRFFS study and brother pairs for the CPFFS study). Each multiplex family required the screening of approximately 100 families to meet the present goals, and for the broader goals of the family studies that included a search for developmental neurobiological markers (Hill et al., 1999) and gene finding efforts (Hill et al., 2004).
2.3. Exclusion Criteria for High-Risk Families
The DIS was administered to all available relatives (adult probands, their siblings and parents [>90% of first degree relatives]). Unavailable or deceased relatives were diagnosed using a minimum of two family-history reports. Targeted families were excluded if the proband or his or her first-degree relatives showed evidence of primary recurrent Major Depressive Disorder (MDD), Bipolar Disorder (BD), Primary Drug Dependence (PDD) (i.e., drug dependence preceded alcohol dependence by 1 or more years) or Schizophrenia by DSM-III criteria, the diagnostic system in place at the time the studies were initiated. Presence of Axis II disorders was not used as either an exclusionary or inclusionary condition. No attempt was made to limit the psychiatric disorders in “marrying in” spouses who represent the parents of the children/adolescents reported here. However, available spouses were diagnosed using the same methods (DIS) as members of the “target” families.
2.4. Selection of Control Families
Selection of control families was based on availability of a pair of same sex adult siblings. Selection of families was based on one of two methods. In the first method (Control Group I – CPFFS Study), volunteers were screened for Axis I psychopathology including alcohol and drug dependence using the DIS. Control families were selected if the volunteer’s first-degree relatives (parents and siblings) were similarly free of psychopathology. In the second method (Control Group II – BRFFS Study), volunteers from the same census tract who indicated they had children between the ages of 8–18 years were screened as a potential control family in order to match the family to a high-risk family using census tract information. The control parents of these offspring were screened for parental alcohol or drug dependence. Previous comparison of control groups I and II did not reveal significant differences in socioeconomic or in offspring rates of psychopathology in the two control groups (See Hill et al., 2008 for details), allowing the control groups to be merged for the present analyses.
2.5. Participants
The minor offspring of HR proband pairs and their adult siblings along with control offspring are currently being followed in longitudinal initiatives and are the subject of this report. Three generation pedigree information for the HR offspring reveals an average of 4 first and second-degree relatives with alcohol dependence.
Young adult subjects in the study gave written consent after the nature and purpose of the study was fully explained to them (consent forms approved by the University of Pittsburgh Institutional Review Board). Minor children provided assent with accompanying permission from parents.
2.6. Auditory Event-Related Potential Recordings and analysis of ERP
Each subject performed two blocks (80 trials) of a Choice Reaction auditory task using high (1500 Hz) and low pitched (800 Hz) tones, presented every 3 seconds (70 dB; 40 ms duration with an abrupt [2 3s rise time]) in a modified oddball paradigm in which subjects are asked to press a button (right or left) corresponding to the presence of a high or low tone as previously described (Hill et al., 1990; Hill et al., 1995; Steinhauer and Hill, 1993). P300 was evaluated for the infrequent high-pitched tones which occurred on 25% of the trials.
To provide exactly the same recording conditions throughout the longitudinal study, a PDP-11/23 has been continuously maintained for stimulus presentation. Electrophysiological data were amplified by 20k using a Grass Model 12 Neurodata system set to a bandpass of .01 to 30 Hz. Each trial was sampled for 1200 ms at 8 ms intervals beginning with a 200 ms prestimulus baseline. Each participant performed an auditory (Choice Reaction Time) task with Ag/AgCl electrodes placed at frontal, vertex, parietal, and occipital locations (Fz, Cz, Pz, Oz, P3, P4). An additional electrode was placed under the left eye and referred to linked ears for recording eye movement and blink artifacts. All active electrodes were referred to linked ears with a forehead ground. Eye blinks were tracked online using an oscilloscope. Any trial affected by eye artifact (blinks or eye movements greater than 50μV) were excluded online. Average ERPs were computed from artifact-free trials.
A computer algorithm was used to search for the maximum peak amplitude for each component within predefined latency ranges (80–136 ms at Cz for N100, 136–240 ms at Cz for P200, 200–320 ms at Cz for N250, and 264–424 sec at Pz for P300). Peaks were verified offline by two trained raters blind to diagnosis of the subject. Through this interactive algorithm, P300 and other ERP components were checked. The peak amplitude was computed as the deviation from the median voltage during the 200 ms prestimulus baseline.
2.6.1 Task Performance Data
Trials were discarded for the presence of eye blinks or incorrect performance. Results were analyzed within each group to determine if the number of discarded trials differed between the genotype groups. The total number of discarded trials (performance errors and eye blinks) as a proportion of the160 trials did not differ by genotype (see Table 1).
Table 1.
Number of trials discarded and performance errors by age group.
| Genotype | Ages 8–12 | Ages 13–18 | Ages 19–29 | |||
|---|---|---|---|---|---|---|
| Discarded Trials (%) | Performance Errors | Discarded Trials (%) | Performance Errors | Discarded Trials (%) | Performance Errors | |
| 1,1 | 23.3 | 3.93 | 16.7 | 1.79 | 9.0 | 0.59 |
| 1,2 or 2,2 | 24.1 | 2.11 | 13.0 | 0.93 | 8.9 | 0.67 |
2.6.2 P300 Trajectories
ERP data were collected at approximately annual intervals during childhood and biennially during young adulthood (ages 19 and greater) for third generation offspring at high or low familial risk for developing alcohol dependence. Amplitude of the P300 component (at the midline parietal location [Pz electrode]) of the ERP wave was available for 496 subjects with at least one P300 recording obtained during the 8– 29 age span representing 2774 separate ERP recordings. The present analysis focused on 455 subjects with at least two recordings between 8–29 years. Data were analyzed using subsets by age range (8–12, 13–18, and 19–29). A total of 424 subjects provided data that included at least two recordings within an age range.
2.7. SNP Selection, DNA Isolation, and Genotyping
SNPs were chosen with a minor allele frequency (MAF) > 0.15 and pair-wise linkage disequilibrium (LD) of r2 < 0.8 using the HapMap CEU population. Seven SNPs within a 132 kb region of CHRM2 were investigated. Two SNPs are located in intron 3 (rs1424569, rs1424387), two in intron 4 (rs1824024, rs2061174), one in intron 5 (rs324650) and two in Exon 8 (rs8191992, rs8191993) (Entrez gene). The CHRM2 genotyping was completed using a Biotage PSQ 96MA Pyrosequencer (Biotage AB, Uppsala, Sweden). An amplimer containing the polymorphism was generated by PCR in 96-well plates in a 50 uL total reaction volume, containing 10 ng of human genomic DNA; 1X GeneAmp® PCR Gold Buffer; 2.5 mM magnesium chloride; 200 uM dNTPs; 1 unit of AmpliTaq Gold™ taq polymerase; 1pmol each of the unmodified forward primer and the biotinylated reverse primer.
For SNP rs1824024, the SNP showing statistical significance for its association with the P300 trajectories in the present analysis, a 1 pmol of each of the unmodified forward primer 5′-GTGGGCCTCAGAGAGACCATA-3′ and the biotinylated reverse primer 5′-GGCAGTAGGAAGTAAATGGATCA-3′. (primers for the other six SNPs are available on request) was used. Thermal cycling included 45 cycles at an annealing temperature of 60 degrees. The Biotage workstation was used to isolate the biotinylated single strand from the double strand PCR products. The isolated product was then sequenced using the complimentary sequencing primer 5′-AGACCATACCGAGGG-3′. At the polymorphic site, the minor allele was detected by the presence of a G nucleotide whereas the major allele was detected by the presence of a T nucleotide.
2.7.1. SNP Quality Control
SNP genotyping quality control involved ongoing monitoring of SNP signals provided by Qiagen software. Output is provided using three categories for each SNP: pass, fail and check. Data analysis was performed for only those signals meeting the “pass” criterion. Signals that failed or were returned as needing further checking were rerun. If after 3 attempts the SNP did not meet the “pass” criterion, it was eliminated from the analysis and another SNP chosen as a replacement.
2.8. Statistical Methods
2.8.1. Group Based Trajectory Modeling
Group based trajectory modeling (GBTM) was used to characterize the development of auditory P300 amplitude over the ages of 8 through 29 and to investigate the possible association of P300 developmental trajectory with familial risk for alcohol dependence. Analyses were conducted using SAS PROC TRAJ (Jones et al., 2001; Jones and Nagin, 2007) using a semi-parametric group-based approach for modeling of developmental trajectories. GBTM provides a flexible method for identifying clusters of individual trajectories following a similar developmental path and characterizing individuals within those clusters. The group-based approach uses a discrete distribution and multinomial model to characterize population heterogeneity in development. The multinomial model allows for the estimation and testing of potential covariate associations with group membership. This semi-parametric approach contrasts with hierarchical and latent curve methodologies that model population variability in development with continuous distributions.
Trajectory model selection involves the determination of an appropriate number of trajectory groups to fit as well as the estimation of parameters describing a polynomial relationship of P300 with age that will vary by trajectory group. Bayesian Information Criterion (BIC) was used as a goodness of fit measure to select the number of trajectory groups for each of the six gender – age range trajectory models. As an individual pattern of P300 measurements will not generally follow any identified group trajectory path perfectly, probabilities of individual membership in each group are provided by the model to reflect the inherent uncertainty in group membership.
Because P300 amplitude is known to differ for males and females, analyses were conducted separately by gender (Hoffman and Polich, 1999; Euser et al., 2012). Analyses were conducted for age ranges consisting of ages 8–12, 13–18, and 19–29 to illustrate the development and stabilization of P300 amplitude as well as test for possible age dependence for any association of P300 amplitude development with familial risk status. Within each age range, subjects were included if at least two P300 recordings were available (Table 2). Approximately 10% of cases within an age range were excluded from analysis because a single recording was available within that range. Missing P300 recordings were assumed to be missing at random. With the missing at random assumption, the maximum-likelihood parameter estimation approach used in PROC TRAJ provided unbiased parameter and error estimates. Robust estimates of error in the parameter estimates were obtained using a clustered sandwich estimator that corrects for correlations in measurements from offspring within families.
Table 2.
Number of ERP Assessments by Age and Gender.
| Ages 8–12, Number of P300 Assessments | |||||
|---|---|---|---|---|---|
| Number of Assessments | High-risk | Low-risk | Total Subjects | ||
| Male | Female | Male | Female | ||
| 2 | 24 | 19 | 24 | 19 | 86 |
| 3 | 11 | 12 | 10 | 5 | 38 |
| 4 | 8 | 16 | 11 | 10 | 45 |
| 5 | 6 | 3 | 3 | 4 | 16 |
| Total Subjects: | 49 | 50 | 48 | 38 | 185 |
| Ages 13–18, Number of P300 Assessments | |||||
|---|---|---|---|---|---|
| Number of Assessments | High-risk | Low-risk | Total Subjects | ||
| Male | Female | Male | Female | ||
| 2 | 17 | 24 | 14 | 15 | 70 |
| 3 | 8 | 15 | 9 | 11 | 43 |
| 4 | 14 | 15 | 19 | 12 | 60 |
| 5 | 19 | 22 | 25 | 17 | 83 |
| 6 | 20 | 12 | 22 | 11 | 65 |
| Total Subjects: | 78 | 88 | 89 | 66 | 321 |
| Ages 19–29, Number of P300 Assessments | |||||
|---|---|---|---|---|---|
| Number of Assessments | High-risk | Low-risk | Total Subjects | ||
| Male | Female | Male | Female | ||
| 2 | 19 | 21 | 37 | 23 | 100 |
| 3 | 16 | 23 | 19 | 10 | 68 |
| 4 | 8 | 12 | 13 | 7 | 40 |
| 5 | 5 | 6 | 2 | 4 | 17 |
| 6 | 0 | 0 | 1 | 1 | 2 |
| Total Subjects: | 48 | 62 | 72 | 45 | 227 |
2.8.2. Familial Risk Status and P300 Development
Membership in a particular P300 developmental group was tested for its association with familial risk status for each age group (8–12, 13–18, and 19–29) and gender combination using Wald tests. The Wald tests, which are provided by PROC TRAJ, account for uncertainty in trajectory group assignment. Neglecting group assignment uncertainty can lead to biased parameter estimates and biased standard error estimates (Roeder et al., 1999). Robust estimates of variance were used to account for family grouping.
2.8.3. CHRM2 and P300 Developmental Trajectories
The seven SNPs located within a 132 kb region of CHRM2 were investigated for association with P300 group phenotype using logistic regression. Genetic Map Interpolator (GMI) software (Mukhopadhyay et al., 2010) was used to retrieve current physical map positions from Ensembl (Ensembl 65); these physical positions were then used to linearly interpolate genetic map positions based on the Rutgers Combined Linkage-Physical Map (Kong et al., 2004; Matise et al., 2007). Tests for Hardy-Weinberg equilibrium (HWE), linkage disequilibrium measures, and allele frequencies were computed in Haploview (Barrett et al., 2005). No SNP was found to exhibit significant Hardy-Weinberg departure. SNP associations were tested using logistic regression with robust estimates of variance calculated to account for family clustering of offspring.
3.0. Results
3.1. Trajectory Models
Constant and linear functions were found to be sufficient for modeling of the developmental changes in P300 amplitude within the age ranges considered. Based on the BIC goodness of fit measure, trajectory groups were identified. Two groups characterized the majority of subjects in the 8–12 age range, though a small third group (N=9) with the highest P300 was seen. Three groups characterized the majority of subjects in the 13–18, and 19–29 age ranges. BIC values supporting the resulting trajectory classes studied are shown in Table 3 with the trajectory models illustrated in Figures 1 and 2.
Table 3.
BIC values used to determine the correct number of trajectory groups for analyses by gender and age range.
| #Groups | Males Ages 8 – 12 | Females Ages 8 – 12 | Males Ages 13 – 18 | Females Ages 13 – 18 | Males Ages 19 – 29 | Females Ages 19 – 29 |
|---|---|---|---|---|---|---|
| 1 | −1014.3 | −976.0 | −2534.3 | −2134.0 | −1149.1 | −1117.2 |
| 2 | −988.5 | −958.3 | −2409.7 | −2044.0 | −1111.3 | −1083.2 |
| 3 | −980.8 | −955.7 | −2386.7 | −2012.8 | −1088.7 | −1066.8 |
| 4 | −989.4 | −962.0 | −2361.3 | −2011.4 | −1085.4 | −1059.0 |
| 5 | -- | -- | −2370.3 | −2021.2 | −1091.6 | −1065.1 |
Figure 1.
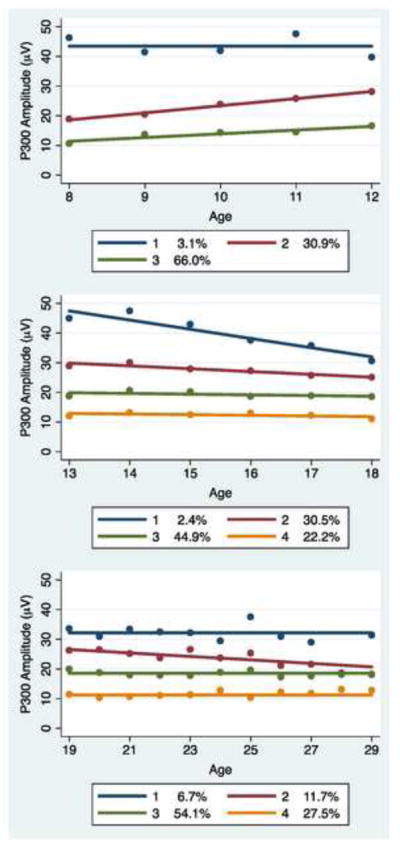
Trajectory models for P300 amplitude by age range for males. Three trajectory classes characterize individuals between the ages of 8–12, while four classes shows the best fit for those between the ages of 13–18 and 19–29.
Figure 2.
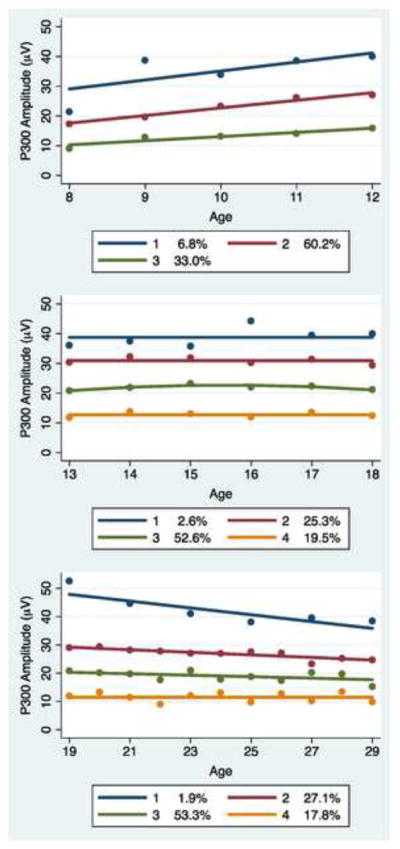
Trajectory models for P300 amplitude by age range for females. The same number of trajectory classes were identified in females as in males. For ages 8–12 years of age three classes provide the best fit to the data, while four classes characterize those between the ages of 13–18 and 19–29. It may be noted that in comparison to males, fewer females follow the low P300 trajectory (class 3 pattern) particularly during the ages of 8–12 years.
Gender differences in P300 development were most apparent as a divergence of group proportions. Male subjects were consistently more prevalent than female subjects in the low-level P300 groups identified by the trajectory models, especially for the 8–12 year old group where 66% of male subjects but only 33% of female subjects were in the low-level P300 group. As may be seen in Figures 1 and 2, the male and female 8–12 year old trajectory groups show linearly increasing P300 amplitude. The rate of increase was lower in the P300 group having the lowest P300 compared to those in the higher level P300 group (p = 0.045). The 13–18 and 19–29 trajectory groups exhibit a mix of stable and slightly declining P300 patterns from age 13 through age 29.
The stability of trajectory group membership across developmental age groups is of potential importance with respect to utilization of P300 as a reliable predictor of later psychopathology. We note that only 33% of subjects classified as being in the low-level P300 group in the age range 8–12 were subsequently identified as in the low-level P300 group in the age range 13–18 or 19–29.
3.2. Familial Risk Status and P300 Development
Based on the model results and the Wald test from PROC TRAJ, low-level P300 developmental trajectory group for 8–12 year olds was found to be significantly associated with high-risk status in male subjects. The high-risk male subjects in the 8–12 age range were 4.3 times more likely (p =0.021) to be found in the low-level P300 group compared to the mid-level P300 group; however, no association of group membership with risk status was evident in male subjects in the 13–18 or 19–29 age ranges. No association of group membership with familial risk status was found for female subjects in any of the three age ranges. Tabulations of trajectory group classifications as well as trajectory group compositions by risk status are summarized in Table 4, along with the number of subjects that were available for analysis in each age range.
Table 4.
P300 trajectory group size and group composition by gender, age-range, and familial risk status.
| Males | Age 8 – 12 | Age 13 – 18 | Age 19 – 29 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P300 Group | High-Risk | Low-Risk | Total | P300 Group | High-Risk | Low-Risk | Total | High-Risk | Low-Risk | Total |
| -- | -- | -- | -- | Highest (G1) | 0 (0.0%) | 4 (4.5%) | 4 (2.4%) | 0 ( 0.0%) | 8 (11.1%) | 8 (6.7%) |
| Highest Group 1 | 1 (2.0%) | 2 (4.2%) | 3 (3.1%) | High (G2) | 28 (35.9%) | 23 (25.8%) | 51 (30.5%) | 13 (27.1%) | 1 (1.4%) | 14 (11.7%) |
| Medium Group 2 | 7 (14.3%) | 23 (47.9%) | 30 (30.9%) | Medium (G3) | 30 (38.5%) | 45 (50.6%) | 75 (44.9%) | 23 (47.9%) | 42 (58.3%) | 65 (54.1%) |
| Low Group 3 | 41(83.7%) | 23 (47.9%) | 64(66.0%) | Low (G4) | 20 (25.6%) | 17 (19.1%) | 37 (22.2%) | 12 (25.0%) | 21(29.2%) | 33 (27.5%) |
| Total | 49 (50.5%) | 48 (49.5%) | 97 | Total | 78 (46.7%) | 89 (53.3%) | 167 | 48(60.0%) | 72 (40.0%) | 120 |
| Females | Age 8 – 12 | Age 13 – 18 | Age 19 – 29 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P300 Group | High-Risk | Low-Risk | Total | P300 Group | High-Risk | Low-Risk | Total | High-Risk | Low-Risk | Total |
| -- | -- | -- | -- | Highest (G1) | 3 (3.4%) | 1 (1.5%) | 4 (2.6%) | 1 (1.6%) | 1 (2.2%) | 2 (1.9%) |
| Highest Group 1 | 5 (10.0%) | 1 (2.6%) | 6 (6.8%) | High (G2) | 23 (26.1%) | 16 (24.2%) | 39 (25.3%) | 13 (21.0%) | 16 (35.6%) | 29 (27.1%) |
| Medium Group 2 | 34 (68.0%) | 19 (50.0%) | 53 (60.2%) | Medium (G3) | 45 (51.2%) | 36 (54.6%) | 81 (52.6%) | 38 (61.3%) | 19 (42.2%) | 57 (53.3%) |
| Low Group 3 | 11(22.0%) | 18 (47.4%) | 29 (33.0%) | Low (G4) | 17 (19.3%) | 13 (19.7%) | 30 (19.5%) | 10 (16.1%) | 9 (20.0%) | 19 (17.8%) |
| Total | 50 (56.8%) | 38 (43.2%) | 88 | Total | 88 (57.1%) | 66 (42.9%) | 154 | 62(57.9%) | 45 (42.1%) | 107 |
3.3. CHRM2 and P300 Development
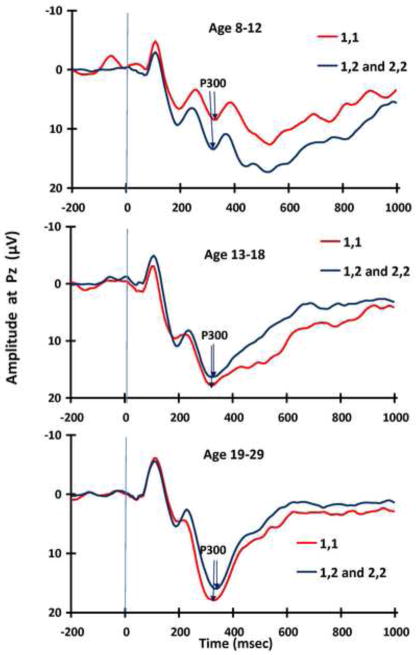
One SNP, rs1824024 located in intron 4 of the CHRM2 gene showed variation that was significantly associated with the P300 trajectory pattern of interest (Figure 3). In the 8–12 year old group, those having two copies of the T nucleotide CHRM2 rs1824024 were 2.60 times more likely (p = 0.032, 95% CI = [1.08, 6.24]) than those with either 1 or 0 copies to exhibit the low and slow developmental P300 trajectory pattern. This relationship did not persist beyond the 8–12 age range. ERP waves by genotype within each age group may be seen in Figure 3.
Figure 3.
ERP wave forms are shown for the CHRM2 rs1824024 SNP by age group. Presence of one or two copies (1,2 or 2,2) of the minor allele, G, is significantly associated with greater P300 amplitude in the youngest age group (8–12 years) suggesting a possible protective effect with respect to later development of substance use.
3.4. Multiplicative Effects of CHRM2 and Gender
When genotype and gender are considered in an additive model, we find the CHRM2 TT genotype confers an increase in odds for having a low P300 trajectory of 2.32 (p = 0.054), while being male increases the odds to 2.84 (p=0.036) with the multiplicative effect of having a TT genotype and being male resulting in an increased odds of 6.58 (p=0.004) of having a low P300 trajectory. This relationship was seen only in the 8–12 age range.
3.5. CHRM2 and Risk
The relationship between CHRM2 and P300 trajectory class membership could potentially be the result of a significant association between CHRM2 and familial risk status. Therefore, an association analysis was performed for SNPs within the CHRM2 gene and familial risk status. All p values were greater than 0.18 in this sample.
4.0. Discussion
The present results find auditory P300 trajectories associated with high familial risk for AD in males but not females in the 8–12 year old group. High-risk males in the 8–12 age range were 4.3 times more likely to be found in the low-level P300 group. The role of CHRM2 in the association between P300 developmental trajectories and familial risk was assessed. One SNP in this gene, rs1824024, was found to be associated with the trajectory characterized by lower P300 amplitude. Males were more likely to follow the low P300 trajectory, and if they were homozygous for the TT allele were much more likely to have a lower P300 trajectory during the 8–12 year old time period.
Using electrophysiological measures, a previous linkage study of SNPs within the CHRM2 gene reported highly significant differences for the frontal [Fz] electrode; (p= 0.000009) and p=0.01 at the parietal [Pz] electrode) for the rs1824024 SNP (Jones et al, 2006). Among the 16 SNPs tested within the CHRM2 gene, six showed significance between p = 0.04–0.0003. These data were based on 462 alcohol dependent and 582 non-dependent individuals from the Collaborative Study on the Genetics of Alcoholism (COGA) study. Families included in that report had been selected for multiple cases of alcohol dependence. The present report tested seven SNPs finding only one with statistical significance. However, the importance of the finding may lie in the convergence of evidence across studies.
In addition to electrophysiological findings, two studies examined associations between SNPs in the CHRM2 gene and alcohol dependence, drug dependence and affective disorders (Luo et al., 2005). Wang et al., (2004) studied 11 SNPs within the CHRM2 gene, relating them to four phenotypes (alcohol dependence, major depressive syndrome, either disorder or both) finding at least one significant finding for all SNPs. Interestingly, SNP rs1824024 showed significance across all phenotypes. This suggests that CHRM2 variation may be a risk factor for disorders typically characterized as externalizing such as alcohol dependence but also may be associated with more internalizing pathologies such as major depression. If so, the association of rs1824024 with P300 amplitude during childhood may suggest that those with the TT allele may be a greater risk for all types of psychopathology and not just substance use disorders. Future follow up and analysis of this question would be of interest in determining whether CHRM2 and P300 are specific predictors of SUD or, alternatively, reflect a common genetic/endophenotypic effect on future psychiatric outcome.
The present findings provide information on stability and change across development for the P300 component of the ERP. Group based trajectory modeling of approximately annual P300 amplitude measurements obtained for child and adolescent offspring and biennially for young adults with high familial risk for AD revealed heterogeneity of early P300 amplitude development among the subjects. Auditory P300 development for the youngest age group (8–12 year olds) was characterized by two groups of linearly increasing P300 amplitude with slower linear increase in an attenuated P300 group and a faster increase in a higher-level P300 group. For the adolescent subjects (13–18) and the young adult subjects (19–29) three groups with stable P300 levels were found. These findings illustrate the important role that brain development has on the elicited P300.
Two previous reports from this laboratory suggest that risk status, presence of psychopathology, and age are critical determinants of P300 amplitude. Hill et al (1999) found high risk offspring to show a delay in acquiring age-appropriate P300. Presence of either an externalizing or internalizing disorder of childhood/adolescence altered the trajectory with lower amplitudes being seen across the trajectory of change in those having a childhood disorder. In a later report from this laboratory trajectories of visual P300 (P3b) were analyzed using data from annual assessments of 126 children and adolesents who were either at high risk for alcohol dependence because of their family loading of alcohol dependence or control subjects with a low risk because of absence of AD in first and second degree relatives (Hill and Shen, 2002). Using a mixture analysis approach, trajectory analysis of children with 5 or more assessments during childhood and adolescence revealed three patterns of visual P3b during development. Group I with the highest P300 amplitude showed relative stability over the 8–18 year age range (26%). Those in Group II exhibited higher amplitude in the earlier years of observation but showed a decrease in P3b amplitude. This pattern was the most frequently occurring pattern (54%). Group III had a distinctly lower visual P300 amplitude throughout the observation period. This pattern occurred much less frequently (20%). Importantly, the latter pattern was highly associated with risk for child/adolescent disorders, some of which are considered prodromal for substance use disorder. Also, a much greater proportion of high risk males followed the Group III pattern.
The present findings based on auditory P300 confirm our previous analyses showing an association between lower visual P300 amplitude and familial risk for alcohol dependence. The present report found an association between genetic variation of the CHRM2 rs1824024 SNP in the 8–12 year old group and not in the older age groups. This may represent a greater influence that muscarinic receptors might have in younger children. Alternatively, environmental effects on P300 amplitude should be considered. Van Beijsterveldt et al., (1999) have noted that biometrical modeling of ERP data for adolescent twins reveals both genetic and environmental variance with approximately 50% of the variance in P300 amplitude explained by genetic factors while the other proportion appears to be environmental. Possibly environmental influences that accrue with advancing age may dampen the impact of specific genetic variation, here variation in intron 4 of the CHRM2 gene.
The present results based on auditory P300 are in accord with two previous studies from our laboratory involving multiple measures of visual P300 in finding predictable age related effects (Hill et al 1999; Hill and Shen, 2002) and confirm age- related visual P300 findings obtained with up to three repeated assessments from the Minnesota group (Carlson and Iacono, 2006; Iacono and Malone, 2011).
4.1. Limitations of the Study
Longitudinal follow up of offspring from families presents a number of challenges for data analysis. The present analysis was based on offspring from either high or low risk families who were invited to participate if they were between the ages of 8–18 years at initial assessment. Although the majority of subjects were between the ages of 8–12 years at initial assessment, nevertheless all cohorts were not at the same age at each wave. The choice of analytic methods for trajectory analysis can either be based on repeated observations (waves) or by age. If one uses a wave approach in which all offspring are studied by wave irrespective of age, considerable age variation is introduced but the number of previous times experiencing the assessment paradigm is controlled. For the present analyses, all individuals of a given age were analyzed with repeated measures increasing by age. A limitation of this method is that some individuals might have varying number of previous assessments so that previous experience doing the ERP tasks might influence the measured results. Although this is a limitation, it may not have a major impact because the tasks used are simple and straightforward requiring no previous experience to accomplish with a high degree of accuracy.
Another limitation of the age-based approach is that a varying number of assessments were available within each age range. This might be of concern if widely varying numbers of assessments were present so that power issues might affect one age range and not another. Examination of the number of assessments by age shows remarkable uniformity in available data up to the age of 22. Therefore, our conclusion that the 8–12 age range was associated with risk status but the other age range (13–18) was not could not have been based on insufficient cases in the older age ranges under age 22.
It should be mentioned that not all subjects were followed an equal number of years and not all were followed across the age range studied (8–29). Subjects entered the study at different ages and had their last ERP assessment at differing ages. Although these limitations are present, the overall conclusion that P300 trajectories are related to risk status and male gender in early development appears to be solid. The analyses support previous findings for the effects of the CHRM2 gene and P300 amplitude. Because previous reports find a relationship between CHRM2 and alcohol dependence, it was somewhat surprising that the CHRM2 variation was not associated with familial risk. However, the absence of risk status association with the CHRM2 gene may indicate that CHRM2 variation is only manifest when those with high risk status actually become alcohol dependent. Nevertheless, the present results suggest that both high risk status and CHRM2 variation are associated with a low amplitude trajectory in early development that from other investigations (Hill et al., 2009) appears to be related to SUD outcome.
4.2. Conclusion
The present results using repeated measures of auditory P300 amplitude in the same individuals confirm previous findings using visual P300 amplitude by identifying three trajectory classes during childhood and adolescence. The present findings extend our previous results by showing that trajectories obtained at the earliest ages (8–12) are most likely to be the most robust predictors of familial risk, especially in males, and are additionally related to muscarinic receptor variation. Because low amplitude P300 in childhood predicts substance use outcome by young adulthood, identifying the factors associated with presence of the lower P300 amplitude trajectory in childhood provides important clues regarding possible mechanisms of susceptibility.
Acknowledgments
This research was supported by NIAAA Grants AA018289, AA005909, AA 008082, and AA015168 to SYH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Association; 1982. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Association; 1987. revised. [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Blackwood DHR, Whalley LJ, Christie JE, Blackburn IM, St Clair DM, McInnes A. Changes in auditory P3 event-related potential in schizophrenia and depression. British Journal of Psychiatry. 1987;150:154–160. doi: 10.1192/bjp.150.2.154. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Stewart JW, Towey JP, Leite P, Voglmaier M, Quitkin FM. Brain event-related potentials to complex tones in depressed patients: Relations to perceptual asymmetry and clinical features. Psychophysiology. 1995;32:373–381. doi: 10.1111/j.1469-8986.1995.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG. Heritability of P300 amplitude development from adolescence to adulthood. Psychophysiology. 2006;43:470–480. doi: 10.1111/j.1469-8986.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG. Deviant P300 amplitude development in males is associated with paternal externalizing psychopathology. Journal of Abnormal Psychology. 2008;117:910–923. doi: 10.1037/a0013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SR, McLarmon ME, Iacono WG. P300 amplitude, externalizing psychopathology, and earlier- versus later-onset substance-use disorder. Journal of Abnormal Psychology. 2007;116:565–577. doi: 10.1037/0021-843X.116.3.565. [DOI] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Wang JC, Hinrichs A, Bertelsen S, Bucholz KK, Schuckit M, Kramer J, Nurnberger J, Jr, Tischfield J, Edenberg HJ, Goate A, Bierut LJ. Alcohol dependence with comorbid drug dependence: genetic and phenotypic associations suggest a more severe form of the disorder with stronger genetic contributions to risk. Addiction. 2007;102:1131–1139. doi: 10.1111/j.1360-0443.2007.01871.x. [DOI] [PubMed] [Google Scholar]
- Diner BC, Holcomb PJ, Dykman RA. P300 in major depressive disorder. Psychiatry Research. 1985;15:175–184. doi: 10.1016/0165-1781(85)90074-5. [DOI] [PubMed] [Google Scholar]
- Donchin E. Event-related brain potentials: A tool in the study of human information processing. In: Begleiter H, editor. Evoked Brain Potentials and Behavior. Vol. 2. Plenum Press; New York: 1979. pp. 13–88. [Google Scholar]
- Euser AS, Arends LR, Evans BE, Greaves-Lord K, Huizink AC, Franken HA. The P300 event-related brain potential as a neurobiological endophenotype for substance use disorders: A meta-analytic investigation. Neuroscience and Biobehavioral Reviews. 2012;36:572–603. doi: 10.1016/j.neubiorev.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatry research. Archives of General Psychiatry. 1972;28:238–243. doi: 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- Frodl-Bauch T, Bottlender R, Hegerl U. Neurochemical substrates and neuroanatomical generators of the event-related P300. Neuropsychobiology. 1999;40:86–94. doi: 10.1159/000026603. [DOI] [PubMed] [Google Scholar]
- Gangadhar BN, Ancy J, Janakiramaiah N, Umapathy C. P300 amplitude in non-bipolar, melancholic depression. Journal of Affective Disorders. 1993;28:57–60. doi: 10.1016/0165-0327(93)90077-w. [DOI] [PubMed] [Google Scholar]
- Habeych ME, Charles PJ, Sclabassi RJ, Kirisci L, Tarter RE. Direct and mediated associations between P300 amplitude in childhood and substance use disorders outcome in young adult outcome. Biological Psychiatry. 2005;57:76–82. doi: 10.1016/j.biopsych.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Helzer JE, Robins LN, McEvoy LT, Spitznagel EL, Stoltzman RK, Farmer A, Brockington IF. A comparison of clinical and diagnostic interview schedule diagnoses. Physician reexamination of lay-interviewed cases in the general population. Archives of General Psychiatry. 1985;42:657–666. doi: 10.1001/archpsyc.1985.01790300019003. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Bernat E, Malone SM, Iacono WG, Patrick CJ, Krueger RF, McGue M. Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology. 2007;44:98–105. doi: 10.1111/j.1469-8986.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Locke J, Zezza N, Kaplan B, Neiswanger K, Steinhauer S, Wipprecht G, Xu J. Genetic association between reduced P300 amplitude and the DRD2 dopamine receptor A1 allele in children at high risk for alcoholism. Biological Psychiatry. 1998;43:40–51. doi: 10.1016/s0006-3223(97)00203-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S. Neurodevelopmental patterns of visual P3b in association with familial risk for alcohol dependence and childhood diagnosis. Biological Psychiatry. 2002;51:621–631. doi: 10.1016/s0006-3223(01)01301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke-Wellman J, Matthews AG, McDermott M. Psychopathology in offspring from multiplex alcohol dependence families with and without parental alcohol dependence: A prospective study during childhood and adolescence. Psychiatry Research. 2008;160:155–166. doi: 10.1016/j.psychres.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Zezza N, Hoffman EK, Perlin M, Allan W. A genome wide search for alcoholism susceptibility genes. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics. 2004;128B:102–113. doi: 10.1002/ajmg.b.30013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Steinhauer SR, Konicky C, Lowers L, Connolly J. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biological Psychiatry. 1999;46:970–981. doi: 10.1016/s0006-3223(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Park J, Zubin J. Event-related potential characteristics in children of alcoholics from high density families. Alcoholism: Clinical and Experimental Research. 1990;14:6–16. doi: 10.1111/j.1530-0277.1990.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR. Assessment of prepubertal and postpubertal boys and girls at risk for developing alcoholism with P300 from a visual discrimination task. Journal of Studies on Alcohol. 1993;54:350–358. doi: 10.15288/jsa.1993.54.350. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Locke J. Event-related potentials in alcoholic men, their high-risk male relatives and low-risk male controls. Alcoholism: Clinical and Experimental Research. 1995;9:567–576. doi: 10.1111/j.1530-0277.1995.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Locke-Wellman J, Ulrich R. Childhood risk factors for young adult substance dependence outcome in offspring from multiplex alcohol dependence families: A propspective study. Biological Psychiatry. 2009;66:750–757. doi: 10.1016/j.biopsych.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman LD, Polich J. P300, handedness, and corpus callosal size: gender, modality, and task. Int J Psychophysiol. 1999;31(2):163–174. doi: 10.1016/s0167-8760(98)00050-6. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM. Developmental endophenotypes: Indexing genetic risk for substance abuse with the P300 brain event-related potential. Child Dev Perspect. 2011;5:239–247. doi: 10.1111/j.1750-8606.2011.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods and Research. 2001;29:374–393. [Google Scholar]
- Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociological Methods and Research. 2007;5:542–571. [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut LJ, Goate A, Wang JC, Hinrichs T, Kwon J, Rice JP, Rohrbaugh J, Stock H, Wu W, Bauer LO, Chorlian DB, Crowe RR, Edenberg HJ, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O’Connor SJ, Schuckit MA, Stimus AT, Tischfield JA, Reich T, Begleiter H. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. International Journal of Psychophysiology. 2004;53:75–90. doi: 10.1016/j.ijpsycho.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Dick D, Goate A, Hinrichs A, Rice JP, Wang JC, Bauer LO, Crowe R, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O’Connor SJ, Rohrbaugh J, Schuckit MA, Tischfield J, Edenberg HJ, Begleiter H. A cholinergic receptor gene (CHRM2) affects event-related oscillations. Behavioral Genetics. 2006;36:627–639. doi: 10.1007/s10519-006-9075-6. [DOI] [PubMed] [Google Scholar]
- Jung MH, Park BL, Lee BC, Ro Y, Park R, Shin HD, Bae JS, Kang TC, Choi IG. Association of CHRM2 polymorphisms with severity of alcohol dependence. Genes, Brain, and Behavior. 2011;10:253–256. doi: 10.1111/j.1601-183X.2010.00663.x. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, McGue MK, Carlson SR. P300 event-related potential heritability in monozygotic and dizygotic twins. Psychophysiology. 1997;34:47–58. doi: 10.1111/j.1469-8986.1997.tb02415.x. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Iacono WG. Externalizing psychopathology in adulthood, A dimensional-spectrum conceptualization and its implications for DSM-V. Journal of Abnormal Psychology. 2005;114:537–550. doi: 10.1037/0021-843X.114.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Murphy K, Raj T, He C, White PS, Matise TC. A combined linkage-physical map of the human genome. American Journal of Human Genetics. 2004;75:1143–1148. doi: 10.1086/426405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Kranzler HR, Zuo L, Wang S, Blumberg HP, Gelernter J. CHRM2 gene predisposes to alcohol dependence, drug dependence and affective disorders: results from an extended case-control structured association study. Human and Molecular Genetics. 2005;14:2421–2434. doi: 10.1093/hmg/ddi244. [DOI] [PubMed] [Google Scholar]
- Matise TC, Chen F, Chen W, De La Vega FM, Hansen M, He C, Hyland FC, Kennedy GC, Kong X, Murray SS, Ziegle JS, Stewart WC, Buyske S. A second-generation combined linkage physical map of the human genome. Genome Research. 2007;17:1783–1786. doi: 10.1101/gr.7156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay N, Tang X, Weeks DE. Genetic Map Interpolator. Paper presented at the annual meeting of the American Society of Human Genetics; Washington D.C. 2010. [Google Scholar]
- O’Connor S, Morzorati S, Christian J, Li T. Heritable features of the auditory oddball event-related potential peaks, latencies, morphology and topography. Electroencephalography and Clinical Neurophysiology. 1994;92:115–125. doi: 10.1016/0168-5597(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bernat EM, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman G, Johnson W, Iacono WG. The heritability of P300 amplitude in 18 year-olds is robust to adolescent alcohol use. Psychophysiology. 2009;46:962–969. doi: 10.1111/j.1469-8986.2009.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M. Neurophysiological endopnenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. Scientific World Journal. 2007;7:131–141. doi: 10.1100/tsw.2007.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clinical Neurophysiology. 2005;116:933–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Pritchard WS. Psychophysiology of P300. Psychological Bulletin. 1981;89:506–540. [PubMed] [Google Scholar]
- Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, Kuperman S, Rohrbaugh J, O’Connor SJ, Bauer LO, Schuckit MA, Begleiter H. Delta and theta oscillations as risk markers in adolescent offspring of alcooholics. International Journal of Psychophysiology. 2007;63:3–15. doi: 10.1016/j.ijpsycho.2006.10.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder K, Lynch KG, Nagin DS. Modeling uncertainty in latent class membership: a case study in criminology. Journal of the American Statistical Association. 1999;94:766–776. [Google Scholar]
- Roschke J, Wagner P. A confirmatory study of the mechanisms behind reduced P300 waves in depression. Neuropsychopharmacology. 2003;28:S9–S12. doi: 10.1038/sj.npp.1300139. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Hill SY. Auditory event-related potentials in children at high risk for alcoholism. Journal of Studies on Alcohol. 1993;54:408–421. doi: 10.15288/jsa.1993.54.408. [DOI] [PubMed] [Google Scholar]
- Sutton S, Barren M, Zubin J, John ER. Evoked potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt T, VanBaal GCM. Twin and family studies of the human electroencphalogram: a review and a meta-analysis. Biological Psychology. 2002;61:111–138. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- Van Der Stelt O. Visual P3 as a potential vulnerability marker of alcoholism : Evidence from the Amsterdam study of children of alcoholics. Alcohol & Alcoholism. 1999;34:267–282. doi: 10.1093/alcalc/34.3.267. [DOI] [PubMed] [Google Scholar]
- Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, Bertelsen S, Kwon JM, Wu W, Dick DM, Rice J, Jones K, Nurnberger JL, Jr, Tischfield J, Porjesz B, Edenberg HJ, Hesselbrock V, Crowe R, Schuckit M, Begleiter H, Reich T, Goate AM, Bierut LJ. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Human Molecular Genetics. 2004;13:1903–1911. doi: 10.1093/hmg/ddh194. [DOI] [PubMed] [Google Scholar]
- Yanai I, Fujikawa T, Osada M, Yamawaki S, Touhouda Y. Changes in auditory P300 in patients with major depression and silent cerebral infarction. Journal of Affective Disorders. 1997;46:263–271. doi: 10.1016/s0165-0327(97)00100-6. [DOI] [PubMed] [Google Scholar]