Abstract
Goal
To evaluate whether the association between obesity and Barrett's esophagus (BE) is due to total body fatness, abdominal obesity, or both.
Background
BE risk appears more strongly related to central obesity than total obesity. However, no studies have investigated the association between total obesity and BE using direct measures of total body fatness.
Study
We conducted a case-control study among patients scheduled for elective esophagogastroduodenoscopy (EGD), and a sample of patients eligible for screening colonoscopy recruited from primary care clinics. BE cases were patients with specialized intestinal metaplasia; while controls had no endoscopic or histopathologic BE. All patients underwent a study EGD and had body measurements taken. Fat mass and fat-free mass were estimated from bioelectrical impedance analysis (BIA). We calculated odds ratios (OR) and 95% confidence intervals (95%CI) using multivariable logistic regression.
Results
There were 70 BO cases, 229 endoscopy controls and 118 primary care controls. BMI and BIA derived fat mass were highly correlated; however we found no association between BMI, fat mass and BE (vs. all controls: BMI, OR per 1 standard deviation [s.d.] = 1.01, 95%CI 0.76–1.34; fat mass, OR=1.02, 95%CI 0.77–1.36). WHR was significantly associated with increased BE risk (vs. all controls: OR=1.45, 95%CI 1.03–2.04). We found similar results when we analyzed the control groups separately.
Conclusion
WHR, but not fat mass or BMI, was associated with increased BE risk. This study provides strong evidence that BE is related to body size and composition via central adiposity and not via total body fatness.
INTRODUCTION
Barrett's esophagus (BE), a metaplastic change of the normal mucosal lining of the lower esophagus, is the precursor lesion to esophageal adenocarcinoma (EAC). The incidence rates for both BE and EAC are increasing in Western populations, especially among white men.1, 2 Gastroesophageal reflux disease (GERD) is the primary risk factor underlying most cases of EAC and BE.3, 4 However, while epidemiological studies have consistently found that obesity is independently associated with an increased risk of EAC (reviewed by Lagergren5), those examining the potential effects of body mass and composition on BE risk have reported conflicting results. Results from two meta-analyses suggest that persons with a high body mass index (BMI ≥ 30 kg/m2) may have a modest increased risk of BE, but whether the increased risk reflects solely their GERD remains an open question.6, 7 More recently, we and others have shown that measures of abdominal obesity (such as waist-to-hip ratio [WHR] and waist circumference) are more strongly associated with increased risk of BE than BMI and that this effect is independent of GERD.8–11
It is possible however that the lack of association between BMI and BE risk may be due to poor correlation between BMI and total body fatness and/or BMI not directly reflecting abdominal obesity. While BMI is commonly used as an index of obesity, it may fail to distinguish between fat mass and fat-free mass, may not reflect abdominal fat and may not be reliable for older men who constitute the population at greatest risk for BE.12–14 On the other hand, bioelectrical impedance analysis (BIA) is a noninvasive, inexpensive method of accurately measuring body fatness. To follow-up on our prior investigation of abdominal obesity, BMI and BE risk,8 we used in this study estimates of body composition from BIA and direct anthropometric measurements for fat distribution to assess whether the association between obesity and BE is due to overall body fatness, abdominal obesity, or both.
MATERIALS AND METHODS
Study population
We used data from study participants in a case-control study of BE conducted at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas. Details of this study population were previously described.8 Briefly, participants were recruited either before an elective esophagogastroduodenoscopy (EGD) for any indication at MEDVAMC or from among patients eligible for screening colonoscopy who attended one of seven selected MEDVAMC primary care clinics. None of primary care patients were primarily referred for EGD and, if they agreed to participate in the study, underwent the study EGD during the same clinical visit as their colonoscopy.
Cases were patients from either the elective EGD group or primary care group with both endoscopically-suspected and histologically confirmed BE (i.e., specialized intestinal metaplasia on biopsy). We excluded patients with only endoscopically-suspected BE from the analysis. We compared the BE cases with two control groups: participants without endoscopically-suspected BE who underwent an elective EGD (“endoscopy controls”) and participants recruited from primary care without endoscopically-suspected BE on their study EGD (“primary care controls”). The primary care controls represent patients, who, if they had BE, would be diagnosed with BE among a less symptomatic population at the MEDVAMC. The minimum age limit for the EGD group (40 years) was lower than that in the primary care group (50 years). However, exclusion of patients aged < 50 years did not change the results. Patients with a previous history of gastroesophageal surgery or diagnosis of cancer, currently taking anticoagulants, with significant liver disease (as indicated by platelet count < 70,000, ascites, or known gastroesophageal varices), or a history of major stroke or mental disorder were ineligible for the study.
For this study, we had BIA data and direct anthropometric measurements from 284 patients in the elective EGD group (229 endoscopy controls and 55 BE cases) and 133 patients in the primary care group (118 primary care controls and 15 BE cases) recruited between September 1, 2008 and October 10, 2012. Participants in the BIA study included 23% of all patients from the EGD group and 27% of all patients from the primary care group that were eligible for the overall case-control study. The participation percentage increased to approximately 42% overall when we excluded patients attending the MEDVAMC when the BIA device was not consistently being used. Among the 70 cases, 9 had a prior diagnosis of BE (prevalent cases) while 61 were newly diagnosed and considered incident cases. The characteristics of participants in this study (those with BIA data) were similar to non-participants (those recruited into the case-control study but excluded from this study as they did not have bioimpedance measurements taken).
Anthropometric measurements and body composition
A flexible tape measure was used to measure waist (at umbilicus level at the narrowest part of the waist) and hip (over the participant's right side at greatest buttock protrusion) circumferences to the nearest half inch over light clothing and we calculated WHR by dividing waist circumference by hip circumference. Body composition and weight were assessed in bare feet by using the InBody 520 Direct Segmental 8-point Multi-frequency BIA device (Biospace, Los Aneles, CA), which has 98% correlation with dual-energy X-ray absorptiometry (DEXA) and 99% reproducibility.15, 16 Fat mass, fat-free mass and percent body fat (BF%) were estimated using the device's standard built in prediction equations and were displayed on the machine and printed out. Height in inches was also obtained using a study designated stadiometer, and was entered directly into the BIA device for calculation of BMI using the Quetlet index formula (weight in pounds × 703 / height in inches squared).
Questionnaire measures
Prior to the study EGD, all participants completed a computer assisted survey with guidance from a trained research assistant. The survey elicited information about race and ethnicity, social background, frequency and severity of GERD symptoms, cigarette smoking, alcohol use, medical history, ever use of acid-suppressant medications (e.g., proton pump inhibitors and H2-receptor antagonists), and use of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDS) in the last year.
Statistical analysis
The participants' characteristics were compared between cases and controls using Student's t tests or chi-square tests. We fitted unconditional multivariable logistic regression models to calculate adjusted odds ratios (ORs) and 95% confidence intervals (95% CI) for the association between each anthropometric measure (such as BMI, fat mass, BF% and WHR) and the risk of BE. All anthropometric measures were fitted as continuous terms in the model to estimate linear trends on the log-odds scale, and we presented ORs per 1-standard deviation (s.d.) increase in the respective anthropometric measure. Generalized additive logistic models showed no evidence for departures from linearity (P>0.10 for all anthropometric measures). Potential confounders were included in the final models if they changed the β coefficient for the anthropometric measure by 10% or more or improved the fit of the model. Analyses are shown adjusted for age (years; continuous), sex, and race (White, Other). Further adjustment was made for GERD symptoms (Never, Ever) where appropriate. Terms for tobacco smoking, alcohol intake, NSAID use and use of acid-suppressant medications were not included in the final model as adjustment for any of these variables did not influence the risk estimates. Statistical significance was determined at α = 0.05 and all tests for statistical significance were two-sided. All statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
A total of 70 BE cases, 229 endoscopy controls and 118 primary care controls were included in the analyses. Participants had an average age of 58.7 years (s.d. = 8.1 years), and were predominately male (86%) and White (60%). BE cases were significantly older and more likely to be male than endoscopy controls, and more likely to be White than both endoscopy controls and primary care controls. BE cases were significantly more likely to have ever experienced GERD symptoms than primary care controls (83% vs. 40%, P<0.001), but not endoscopy controls (81%, P=0.70). The participants' characteristics are shown in Table 1.
Table 1.
Characteristics of controls and cases
| All controls (n=347) |
Primary care controls (n=118) |
Endoscopy controls (n=229) |
BE cases (n=70) |
|
|---|---|---|---|---|
| Variable | n (%) | n (%) | n (%) | n (%) |
| Age, Mean years (s.d.) | 58.4 (8.1) | 60.4 (5.7) | 57.4 (8.9) | 59.9 (8.1) |
| Males | 292 (84.1) | 110 (93.2) | 182 (79.5) | 68 (97.1) |
| White | 187 (53.9) | 53 (44.9) | 134 (58.5) | 63 (90.0) |
| GERD ever | 211 (67.8) | 40 (40.4) | 171 (80.7) | 53 (82.8) |
| Smoking status | ||||
| Never | 95 (32.0) | 28 (29.5) | 67 (33.2) | 13 (20.3) |
| Ex-smoker | 115 (38.7) | 38 (40.0) | 77 (38.1) | 32 (50.0) |
| Current smoker | 87 (29.3) | 29 (30.5) | 58 (28.7) | 19 (29.7) |
| Ever used NSAIDs | 173 (57.7) | 59 (61.5) | 114 (55.9) | 42 (65.6) |
| Ever used PPIs | 152 (50.7) | 21 (21.9) | 131 (64.2) | 45 (70.3) |
GERD, gastroesophageal reflux disease; NSAIDs, non-steroidal anti-inflammatory drugs; PPI, proton pump inhibitor.
Average BMI was similar between controls and BE cases (Table 2). The scatter plots of BMI versus body fat assessed by BIA are presented in Figure 1. BMI was highly correlated with fat mass and BF% among all controls (fat mass, Spearman's r=0.91, P<0.001; BF%, r=0.70, P<0.001) and BE cases (fat mass, r=0.90, P<0.001; BF%, r=0.73, P<0.001). However, we found no associations between BIA derived measures of fat mass, BF%, BMI and the risk of BE (Table 3). Similarly, when only White men or only participants with a history of GERD symptoms were used there were no associations between fat mass, BF%, BMI and BE (Table 4). When we examined short-segment (n=45) and long-segment (n=25) cases separately, the two case groups had similar average fat mass, BF% and BMI. The ORs for short- and long-segment BE were the same as those for all cases combined but less precise due to the small sample sizes (data not shown). Finally, when 9 prevalent BE cases were excluded, the ORs for the various measures of total body fatness did not change (data not shown).
Table 2.
Mean values and standard deviations for each anthropometric measurement among controls and cases
| All controls (n=347) |
Primary care controls (n=118) |
Endoscopy controls (n=229) |
BE cases (n=70) |
|
|---|---|---|---|---|
| Variable | Mean (s.d.) | Mean (s.d.) | Mean (s.d.) | Mean (s.d.) |
| Height (cm) | 173.5 (8.3) | 174.7 (7.2) | 172.9 (8.7) | 175.3 (6.6) |
| Weight (kg) | 89.6 (19.2) | 92.3 (20.4) | 88.2 (18.5) | 92.4 (17.7) |
| Body mass index (kg/m2) | 29.8 (6.2) | 30.2 (6.5) | 29.5 (6.0) | 30.0 (5.2) |
| Fat mass (kg) | 26.9 (12.0) | 27.4 (12.8) | 26.6 (11.6) | 26.4 (11.2) |
| Percent fat (%) | 29.1 (8.8) | 28.5 (8.1) | 29.3 (9.1) | 27.6 (8.2) |
| Fat-free mass (kg) | 62.6 (11.2) | 65.0 (10.9) | 61.4 (11.2) | 66.2 (10.2) |
| Waist circumference (cm) | 106.9 (13.8) | 108.6 (13.8) | 106.0 (13.8) | 110.5 (12.5) |
| Hip circumference (cm) | 113.1 (11.5) | 114.6 (12.2) | 112.4 (11.1) | 112.7 (10.9) |
| Waist-to-hip ratio | 0.94 (0.07) | 0.95 (0.06) | 0.94 (0.07) | 0.98 (0.06) |
Figure 1.
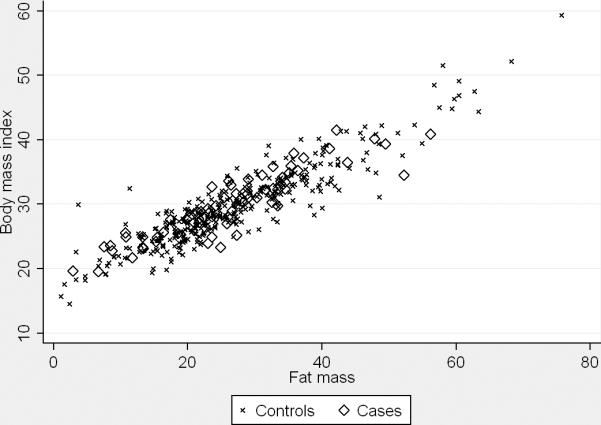
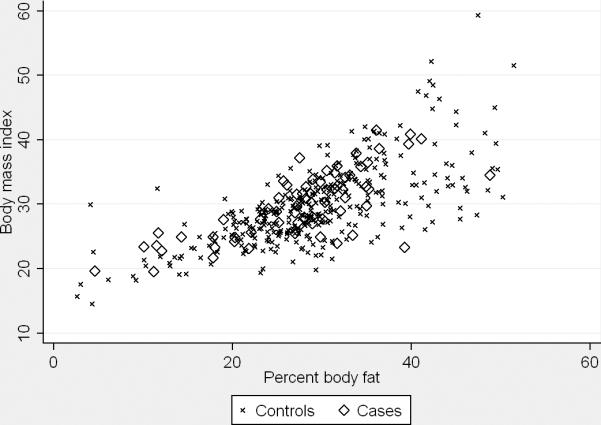
Scatter plots show univariate correlations between body mass index and fat mass (left panel; All controls, Spearman's r=0.91, P<0.001; BE cases, r=0.90, P<0.001) and body mass index and total body fat percentage (right panel; All controls, r=0.70, P<0.001; BE cases, r=0.73, P<0.001), by case status.
Table 3.
BE risk in relation to anthropometric measurements (per 1-standard deviation increase)
| BE cases vs. All controls (n=347) |
BE cases vs. Primary care controls (n=118) |
BE cases vs. Endoscopy controls (n=229) |
|
|---|---|---|---|
| Variable | ORa (95% CI) | ORa (95% CI) | ORa (95% CI) |
| Height | 1.19 (0.85–1.66) | 1.19 (0.81–1.75) | 1.24 (0.87–1.78) |
| Weight | 1.08 (0.82–1.42) | 0.91 (0.64–1.30) | 1.19 (0.89–1.61) |
| Body mass index | 1.01 (0.76–1.34) | 0.84 (0.58–1.22) | 1.10 (0.81–1.50) |
| Fat mass | 1.02 (0.77–1.36) | 0.87 (0.60–1.26) | 1.10 (0.81–1.50) |
| Percent fat | 0.98 (0.71–1.36) | 0.87 (0.60–1.27) | 1.00 (0.70–1.45) |
| Fat-free mass | 1.20 (0.86–1.68) | 1.02 (0.69–1.50) | 1.37 (0.95–1.99) |
| Waist circumference | 1.13 (0.85–1.49) | 0.96 (0.68–1.37) | 1.24 (0.92–1.69) |
| Hip circumference | 0.97 (0.74–1.28) | 0.78 (0.54–1.13) | 1.10 (0.82–1.48) |
| Waist-to-hip ratio | 1.45 (1.03–2.04) | 1.48 (1.02–2.16) | 1.40 (0.99–1.98) |
All models are adjusted for age, sex, and race.
Table 4.
BE risk in relation to total and abdominal obesity (per 1-standard deviation increase)
| BE cases vs. All controls |
BE cases vs. Primary care controls |
BE cases vs. Endoscopy controls |
|
|---|---|---|---|
| Variable | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| White men only a | |||
| Body mass index | 0.99 (0.72–1.35) | 0.79 (0.50–1.27) | 1.07 (0.77–1.49) |
| Fat mass | 1.03 (0.76–1.41) | 0.87 (0.55–1.38) | 1.10 (0.80–1.52) |
| Percent fat | 1.06 (0.77–1.46) | 1.00 (0.64–1.54) | 1.08 (0.77–1.51) |
| Waist-to-hip ratio | 1.44 (1.03–2.00) | 1.54 (1.01–2.35) | 1.32 (0.99–1.78) |
| Participants with ever GERD symptoms only b | |||
| Body mass index | 0.85 (0.60–1.21) | 0.62 (0.34–1.14) | 0.88 (0.60–1.28) |
| Fat mass | 0.85 (0.60–1.22) | 0.65 (0.37–1.15) | 0.87 (0.60–1.27) |
| Percent fat | 0.82 (0.56–1.21) | 0.65 (0.36–1.17) | 0.81 (0.53–1.22) |
| Waist-to-hip ratio | 1.25 (0.84–1.86) | 1.49 (0.81–2.72) | 1.20 (0.79–1.80) |
Adjusted for age.
Adjusted for age, sex, and race.
On the other hand, we observed a statistically significant association between WHR and BE risk (vs. all controls, OR per 1 s.d. increase in WHR = 1.45, 95%CI 1.03–2.04), and the risk of BE remained elevated after adjusting for fat mass (OR per 1 s.d. increase in WHR = 1.40, 95%CI 0.97–2.04) or BMI (OR per 1 s.d. increase in WHR = 1.51, 95%CI 1.05–2.18). Adjustment for GERD symptoms did not appreciably change the OR for WHR (OR per 1 s.d. increase in WHR = 1.42, 95%CI 1.00–2.03). We found similar results when we analyzed the control groups separately (Table 3).
Waist circumference and hip circumference were not associated with BE when modeled separately (Table 3); however, after mutual adjustment, waist circumference was positively associated with BE (OR per 1 s.d. increase in waist circumference = 1.87, 95%CI 1.03–3.40) and hip circumference was inversely associated with BE (OR per 1 s.d. increase in hip circumference = 0.57, 95%CI 0.31–1.03). The associations were stronger when we compared BE cases with only the primary care control group (waist circumference, OR=2.20, 95%CI 1.00–4.82; hip circumference, OR=0.38, 0.17–0.88).
DISCUSSION
In our previous study of WHR, BMI and the risk of BE, we found that WHR but not BMI was associated with an increased risk of BE.8 In follow-up, we used in this study BIA to examine further the association between total body fatness and BE risk. Consistent with our findings for BMI, there was no association between BE and BIA measured fat mass or BF%.
A number of studies have examined the association between BMI and the risk of BE. In their meta-analysis, Cook et al.6 reported a pooled OR (all ORs were unadjusted) of 0.99 per 1 kg/m2 (95%CI 0.96–1.01) for six studies that compared BE cases with GERD controls and a pooled OR of 1.02 per 1 kg/m2 (95%CI 1.01–1.04) for three studies that compared BE cases with population controls. The unadjusted ORs for BMI in our study (vs. primary care controls, OR per 1kg/m2 = 0.99, 95%CI 0.95–1.04; vs. endoscopy controls, OR = 1.02, 95%CI 0.97–1.06) are consistent with those in the meta-analysis. Taken together, these data suggest that total obesity has a limited effect, if any, on the risk of developing BE.
In contrast, the significant positive associations between WHR, waist circumference and BE observed here and reported previously indicate that central obesity is an important, independent predictor of increased BE risk.8, 9, 11 Consistent with findings from a similarly conducted study among veterans,17 we found lower risk of BE associated with higher hip circumference after controlling for waist circumference. Thus, for two men with similar, large waist circumferences (i.e., with high abdominal obesity), the one with a smaller hip circumference and lower ratio of gluteofemoral to abdominal obesity has a higher risk of BE. These results suggest that, while abdominal obesity is likely to play an important role in the pathogenesis of BE through mechanical (e.g., promoting gastroesophageal reflux) as well as humoral effects,8,18 the humoral effect on BE may be mediated by gluteofemoral fat.
Although BMI-based categorizations are the most commonly employed measures of adiposity, they do not provide information on body composition, including on relative abdominal or visceral adiposity, may erroneously suggest increased adiposity in those with increased muscle or skeletal mass, and have been shown to be unreliable or biased predictors or discriminators of adiposity in some populations.12, 13, 19, 20 On the other hand, body fatness or BF% have been shown to be independent, and in some instances also much better predictors of disease risk than BMI.12 Using BMI as a proxy for adiposity may have led to incorrect assumptions about the relationship between total obesity and BE, whereas use of these direct measures may result in a more accurate assessment of BE risk. However, these have not been systematically evaluated in BE studies.
There are several gold-standard methods like DEXA and MRI that could be used to assess important aspects of adiposity, including body composition in terms of fat mass and fat-free mass. However, their cost and accessibility limit their use in large-scale epidemiological research. Recent advancements in BIA technology along with validation data against DEXA in diverse populations including adolescents,21 Hispanic diabetics,22 general population adults,23, 24 and morbidly obese bariatric surgery candidates 25 support the use of BIA in epidemiological research.
In contrast to our original hypothesis, we found that BMI was a relatively good predictor of total body fatness. In this study, the BIA derived measures of fat mass and BF% were in fact highly correlated with BMI, and the unadjusted ORs for fat mass (vs. primary care controls, OR = 0.99, 95%CI 0.97–1.02; vs. endoscopy controls, OR = 1.00, 95%CI 0.98–1.02) and BF% (vs. primary care controls, OR = 0.99, 95%CI 0.95–1.02; vs. endoscopy controls, OR = 0.98, 95%CI 0.95–1.01) were almost identical to the unadjusted OR for BMI. Therefore, in our relatively homogeneous study population, BMI does appear to adequately reflect adiposity and the lack of association between BMI and BE in this study and in previous studies of a similar population are unlikely due to its reliability as a measure of total adiposity. On the other hand, WHR was significantly associated with BE, thereby further highlighting the importance of abdominal obesity in causing BE.
In our previous study, the effect of WHR on BE varied by ethnicity where high WHR was associated with increased BE risk in Caucasians but not in African-Americans.8 There are recent data suggesting that there are interethnic differences in adipose tissue distribution and partitioning and metabolic syndrome presentation, including for insulin resistance and hepatic steatosis.26–28 Overall, Caucasians and Hispanics have significantly higher abdominal or visceral fat, extremity fat, and liver fat than African-Americans in these reports. However, among primary care controls in this analysis, we found no significant differences in mean BMI, fat mass or BF% between Caucasians and African-Americans. Furthermore, when we considered only White men, there was still no association between BMI, fat mass or BF% and BE. Because we studied predominantly White male veterans, our study included only 7 African-American BE cases and we were unable to assess these relationships in non-White subgroups.
The major strengths of this study include the direct assessment of total body fatness via BIA, the prospective enrolment of study participants which reduced the potential for recall bias, and the use of a standardized diagnostic definition for BE. Furthermore, by recruiting patients from both the elective EGD group (patients more likely to be symptomatic) and from primary care clinics (patients less likely to be symptomatic) we captured a representative sample of all patients likely to be diagnosed with BE at MEDVAMC. A limitation of our study however was the small number of cases available, leading to relatively imprecise estimates. However, the concordance of the ORs for each BIA derived measure with that for BMI suggests that the findings of previous studies (using BMI to assess the relationship between obesity and BE) accurately quantify the total obesity-related risk for BE. That is, the lack of association between BMI and BE in these studies reflects a true lack of effect for total obesity on BE risk.
In summary, we found an increased risk of BE associated abdominal obesity, but no association between total body fatness and BE. When we modeled measures of abdominal obesity and total obesity together, the risk estimate for abdominal obesity did not appreciably change and remained statistically significant. Our results provide strong additional evidence that the etiology of BE is related to body size and composition via central adiposity and not via total body fatness.
Acknowledgments
Grant support: This work is funded in part by NIH grant NCI R01 116845, the Houston VA HSR&D Center of Excellence (HFP90-020), and the Texas Digestive Disease Center NIH DK58338. HES is also supported by NIDDK K24-04-107.
Abbreviations
- BE
Barrett's esophagus
- BF%
percent body fat
- BIA
bioelectrical impedance analysis
- BMI
body mass index
- CI
confidence interval
- EAC
esophageal adenocarcinoma
- EGD
esophagogastroduodenoscopy
- GERD
gastroesophageal reflux disease
- OR
odds ratio
- WHR
waist-to-hip ratio
Footnotes
Disclosure of potential conflicts of interest: The authors disclose no conflicts.
Publisher's Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Author contributions: APT wrote the statistical analysis plan, cleaned and analyzed the data, interpreted the data, and drafted and revised the manuscript. JRK contributed to study design, monitored data collection, and drafted and revised the manuscript. AA designed data collection tools, enrolled subjects and participated in study design and coordination. HES conceived the study and participated in its design, acquired the funding, and drafted and revised the manuscript. All authors read and approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.van Soest EM, Dieleman JP, Siersema PD, et al. Increasing incidence of Barrett's oesophagus in the general population. Gut. 2005;54:1062–1066. doi: 10.1136/gut.2004.063685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol. 2012;23:3155–3162. doi: 10.1093/annonc/mds181. [DOI] [PubMed] [Google Scholar]
- 3.Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman DA, Oehlke M, Helfand M. Risk factors for Barrett's esophagus in community-based practice. Am J Gastroenterol. 1997;92:1293–1297. [PubMed] [Google Scholar]
- 5.Lagergren J. Influence of obesity on the risk of esophageal disorders. Nat Rev Gastroenterol Hepatol. 2011;8:340–347. doi: 10.1038/nrgastro.2011.73. [DOI] [PubMed] [Google Scholar]
- 6.Cook MB, Greenwood DC, Hardie LJ, et al. A systematic review and meta-analysis of the risk of increasing adiposity on Barrett's esophagus. Am J Gastroenterol. 2008;103:292–300. doi: 10.1111/j.1572-0241.2007.01621.x. [DOI] [PubMed] [Google Scholar]
- 7.Kamat P, Wen SJ, Morris J, et al. Exploring the association between elevated body mass index and Barrett's esophagus: a systematic review and meta-analysis. Ann Thorac Surg. 2009;87:655–662. doi: 10.1016/j.athoracsur.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Kramer JR, Fischbach LA, Richardson P, et al. Waist-to-hip ratio, but not body mass index, is associated with an increased risk of Barrett's esophagus in white men. Clin Gastroenterol Hepatol. 2012 doi: 10.1016/j.cgh.2012.11.028. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendall BJ, Macdonald GA, Hayward NK, et al. The risk of Barrett's esophagus associated with abdominal obesity in males and females. Int J Cancer. 2012 doi: 10.1002/ijc.27887. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett's esophagus. Gastroenterology. 2007;133:34–41. doi: 10.1053/j.gastro.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 11.Edelstein ZR, Farrow DC, Bronner MP, et al. Central adiposity and risk of Barrett's esophagus. Gastroenterology. 2007;133:403–411. doi: 10.1053/j.gastro.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Frankenfield DC, Rowe WA, Cooney RN, et al. Limits of body mass index to detect obesity and predict body composition. Nutrition. 2001;17:26–30. doi: 10.1016/s0899-9007(00)00471-8. [DOI] [PubMed] [Google Scholar]
- 13.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes. 2008;32:959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roubenoff R, Dallal GE, Wilson PW. Predicting body fatnessthe body mass index vs. estimation by bioelectrical impedance. Am J Public Health. 1995;85:726–728. doi: 10.2105/ajph.85.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malavolti M, Mussi C, Poli M, et al. Cross-calibration of eight-polar bioelectrical impedance analysis versus dual-energy X-ray absorptiometry for the assessment of total and appendicular body composition in healthy subjects aged 21–82 years. Ann Hum Biol. 2003;30:380–391. doi: 10.1080/0301446031000095211. [DOI] [PubMed] [Google Scholar]
- 16.Medici G, Mussi C, Fantuzzi AL, et al. Accuracy of eight-polar bioelectrical impedance analysis for the assessment of total and appendicular body composition in peritoneal dialysis patients. Eur J Clin Nutr. 2005;59:932–937. doi: 10.1038/sj.ejcn.1602165. [DOI] [PubMed] [Google Scholar]
- 17.Rubenstein JH, Morgenstern H, Chey WD, et al. Protective role of gluteofemoral obesity in erosive oesophagitis and Barrett's oesophagus. Gut. 2013 doi: 10.1136/gutjnl-2012-304103. doi:10.1136/gutjnl-2012-304103. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duggan C, Onstad L, Hardikar S, et al. Association between markers of obesity and progression from Barrett's esophagus to esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.02.017. doi:10.1016/j.cgh.2013.02.017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson AS, Ellis KJ, McFarlin BK, et al. Body mass index bias in defining obesity of diverse young adults: the Training Intervention and Genetics of Exercise Response (TIGER) Study. Br J Nutr. 2009;102:1084–1090. doi: 10.1017/S0007114509325738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nevill AM, Stewart AD, Olds T, et al. Relationship between adiposity and body size reveals limitations of BMI. Am J Phys Anthropol. 2006;129:151–156. doi: 10.1002/ajpa.20262. [DOI] [PubMed] [Google Scholar]
- 21.Zamrazilova H, Hlavaty P, Dusatkova L, et al. A new simple method for estimating trunk and visceral fat by bioelectrical impedance: comparison with magnetic resonance imaging and dual X-ray absorptiometry in Czech adolescents. Cas Lek Cesk. 2010;149:417–422. [PubMed] [Google Scholar]
- 22.Beeson WL, Batech M, Schultz E, et al. Comparison of body composition by bioelectrical impedance analysis and dual-energy X-ray absorptiometry in Hispanic diabetics. Int J Body Compos Res. 2010;8:45–50. [PMC free article] [PubMed] [Google Scholar]
- 23.Ling CH, de Craen AJ, Slagboom PE, et al. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin Nutr. 2011;30:610–615. doi: 10.1016/j.clnu.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Kim H, Kim CH, Kim DW, et al. External cross-validation of bioelectrical impedance analysis for the assessment of body composition in Korean adults. Nutr Res Pract. 2011;5:246–252. doi: 10.4162/nrp.2011.5.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leal AA, Faintuch J, Morais AA, et al. Bioimpedance analysis: should it be used in morbid obesity? Am J Hum Biol. 2011;23:420–422. doi: 10.1002/ajhb.21143. [DOI] [PubMed] [Google Scholar]
- 26.Guerrero R, Vega GL, Grundy SM, et al. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49:791–801. doi: 10.1002/hep.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liska D, Dufour S, Zern TL, et al. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS ONE. 2007;2:e569. doi: 10.1371/journal.pone.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ingram KH, Lara-Castro C, Gower BA, et al. Intramyocellular lipid and insulin resistance: differential relationships in European and African Americans. Obesity. 2011;19:1469–1475. doi: 10.1038/oby.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]


