Abstract
The use of nano-carriers has been shown to improve the delivery and efficacy of chemotherapeutic agents in cancer patients. Recent studies suggest that decoration of the surface of nano-carriers with various targeting moieties may further improve the overall therapeutic efficacy. In this study, we compared the therapeutic efficacy of Doxil® (commercial doxorubicin-loaded liposomes) and that of Doxil® conjugated with anti-CD30 antibodies (CD30-targeted Doxil®) in treating anaplastic large cell lymphoma (ALCL), a type of T-cell lymphoma characterized by a high CD30 expression. Compared to Doxil®, the CD30-targeted Doxil® showed a significantly higher binding affinity to ALCL cells (5.3% versus 27%, p=0.005) and a lower inhibitory concentration at 50% (IC50) in-vitro (32.6 μg/mL versus 12.6 μg/mL, p=0.006). In a SCID mouse xenograft model, CD30-targeted Doxil® inhibited tumor growth more significantly than the unconjugated formulation; specifically, tumors in mice treated with CD30-targeted Doxil® were significantly smaller than those in mice treated with Doxil® (average, 117 mm3 vs. 270 mm3, p=0.001) at 18 days after the tumors were inoculated. Our findings have provided the proof-of-principle of using CD30-targeted nano-carriers to treat cancers that are characterized by a high level of CD30 expression, such as ALCL.
1. Introduction
In recent years, accumulating evidence has suggested that the use of nano-carriers can be more effective than free drugs in delivering anti-cancer agents [1-5]. The most important feature of nano-carriers that accounts for their superior drug delivery is their size range. Specifically, since intra-tumoral blood vessels are often more leaky as compared to the healthy blood vessels, the unique size range of nano-carriers allows them to diffuse into tumors while they are too large to diffuse into normal tissues [6, 7]. Furthermore, since the intra-tumoral lymphatics are often poorly formed, nano-carriers that have diffused into tumors cannot be effectively drained out, again resulting in their preferential accumulation in tumors [6-8]. Liposomal carriers, which were first described in 1966, have been extensively investigated as a tool to improve the efficacy of drug delivery [4]. A number of liposomal formulations have already been approved for clinical use, as exemplified by liposomal doxorubicin (Doxil® or Caelyx®). As compared to free doxorubicin, this liposomal formulation has been shown to be superior in drug delivery and the overall therapeutic efficacy. The liposomal formulation of doxorubicin has been used widely in treating various types of cancer [4, 9, 10].
More recent studies have suggested that the efficiency of nano-carriers as a drug delivery tool can be further improved through modification of nanocarrier surface with various moieties designed for active targeting of cancer cells, thereby increasing drug delivery to tumor cells [11-14]. To date, various ligands including antibodies (or their fragments), small organic molecules, carbohydrates, and peptides have been successfully coupled to various forms of nano-carriers [14]. Among these moieties, antibodies have been studied most extensively. Nevertheless, the use of antibody-conjugated nano-carriers to treat cancer remains to be in its infancy; to our knowledge, none of these nanoparticles have been approved for clinical use, and only a small number of these agents are being tested in clinical trials [15, 16]. One of the potential limitations associated with the use of antibodies for drug delivery is the absorption of these antibodies by circulating free soluble antigens, leading to the neutralization of these antibodies before reaching to their targets. To overcome this problem, one strategy is to generate antibodies which only recognize the cell membrane-bound epitopes, thereby avoiding the neutralization of these antibodies by soluble antigens present in the circulation [17].
Anaplastic large cell lymphoma (ALCL) is a type of T-cell malignant neoplasm characterized by a strong and uniform expression of CD30, which is a member of the tumor necrosis factor receptor superfamily [18, 19]. Importantly, CD30 expression is highly restricted in normal tissues, being found largely in a small subset of activated T-lymphocytes [19, 20]. Thus, CD30 may serve as a potential target specific for CD30-expressing malignancies such as ALCL. To further justify that CD30 is an excellent target, it has been previously reported that ligation of CD30 in ALCL cells can directly induce both cell cycle arrest and apoptosis [21, 22]. A previous study also has shown that anti-CD30-conjugated drugs have the propensity of being internalized by cells through clathrin-mediated endocytosis [23], a feature associated with a high efficiency of drug delivery [24]. Taken together, CD30 appears to be an excellent candidate for serving as a tumor-seeking molecule for nano-carriers used to treat CD30-positive malignancies. Based on this concept, SGN-30, a chimeric anti-CD30 monoclonal antibody, was developed. Clinical trial studies have found the clinical benefit of SGN-30 in patients with primary cutaneous ALCL; importantly, SGN-30 was well tolerated by patients included in these studies [25, 26]. The safety and modest clinical activity of SGN-30 was also reported in patients with systemic ALCL [27]. Brentuximab vedotin (SGN-35), an intravenously administered CD30-specific antibody-drug conjugate, has recently been clinically approved for the therapy of Hodgkin’s lymphoma and ALCL [28]. Nevertheless, the combination of anti-CD30 and nanomedicines has not yet been attempted.
In this study, we aimed to test if the conjugation of anti-CD30 monoclonal antibody to Doxil®, a liposomal formulation of doxorubicin, which is a component of the current standard frontline regimen for ALCL [29], can result in higher therapeutic efficacy against these cancer cells. The design of these nano-carriers is based on the benefits associated with the use of nanoparticles and anti-CD30, as described here. One additional experimental consideration in this study is that we employed a unique anti-CD30 antibody that recognizes an epitope found only on CD30 expressed on the cell surface but not circulating soluble CD30 fragments.
2. Materials and Methods
2.1. Reagents
Methoxypoly(ethylene glycol) (Mw 2000 Da), covalently linked to distearoyphosphadidylethanoloamine (mPEG2000-DSPE) were from ALZA Corporation. 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[succinimidyl (polyethylene glycol)] (DSPE-PEG3400-NHS) was purchased from Nanocs Inc. (USA). 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethyelen glycol)2000] (DSPE-PEG2000-NH2) was purchased from Avanti Polar Lipids, Inc (USA). Sephadex G-50, Sepharose CL-4B and Cy5.5-monofunctional reactive dye were purchased from GE Healthcare (Baie D’Urfe, PQ). Bio-Rad Protein Assay Reagent was purchased from Bio-Rad Laboratories (Mississaguga, ON). FITC and Cy5.5 dyes were obtained from Invitrogen (Eugene, Oregon, USA).
2.2. Cell lines and tissue culture
Two ALCL cell lines, KARPAS 299 and SUP-M2 which over-express CD30 [30, 31] were grown and expanded in RPMI 1640 (Sigma-Aldrich, St Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich, St, Louis, MO) and 2 mM L-glutamine (Gibco, Grand Island, NY, USA) in 5% CO2 atmosphere at 37°C.
2.3. Modification of Doxil® with anti-CD30 monoclonal antibody
Doxil®, a commercially available preparation of doxorubicin in PEGylated liposomes was from Schering Plough (Kirkland QC, Canada). The anti-CD30 monoclonal antibody was purified from a hybridoma cell line (T408) [17]. Anti-CD30 antibody was purified using a rapid antibody purification kit from Cell Biolab Inc (San Diego, CA, USA). Cy5.5 labeled anti-CD30 antibody was prepared by adding 1 mg anti-CD30 antibody in PBS to 1 mg of Cy5.5 monofunctional reactive dye, followed by incubation with stirring at room temperature in the dark for 1 h. Cy5.5 labeled anti-CD30 antibodies were purified by a G50 column using PBS (pH7.2) as the mobile phase. The conjugation efficiency of Cy5.5 to anti-CD30 antibodies was determined by a UV/visible spectrophotometer measuring the extinction coefficient at 675 nm, as described by the manufacturer. Stealth immunoliposomes were prepared using a modification of the post-insertion method. First anti-CD30 antibody (unlabeled or labeled with Cy5.5) was conjugated to PEG3400-DSPE by adding 2 mg of DSPE-PEG3400-NHS to anti-CD30 antibody solution (1 mg/mL) in PBS. The mixture was kept stirring for 4 h at room temperature. Then, excess amount of glycine (5 mg) was added to the solution to quench the reaction. The resulted micelle solution was purified by Sepharose CL 4B using PBS buffer (pH7.2) as the mobile phase. Then CD30-targeted and non-targeted mixed micelles were prepared from lipid compositions of Cy5.5 or anti-CD30 antibody or Cy5.5 labeled anti-CD30 antibody conjugated to PEG3400-DSPE and mPEG2000-DSPE (1:3, weight ratio). To prepare CD30-targeted Doxil®, CD30-targeted or non-targeted mixed PEG3400-DSPE micelles (~2 mg equivalent lipid in 0.5 mL PBS) were incubated with 1 mL of Doxil® (2 mg doxorubicin) for 4 h at 60 C in a water bath with continuous stirring. Un-conjugated antibody and antibody-conjugated lipid micelles were separated from CD30-targeted Doxil® by chromatography on Sepharose CL-4B columns in PBS (pH7.2). The purified Cy5.5 labeled Doxil® and Cy5.5 labeled CD30-targeted Doxil® formulations were visualized by doxorubicin fluorescence and near infrared fluorescence (NIRF) using appropriate filters with a Kodak image station (Imaging Station IS4000-MM, Kodak). Formulations were placed into a the imaging system equipped with band-pass excitation and long-pass emission filters at 630 and 700 nm for NIRF and 465 and 570 for doxorubicin fluorescence imaging (Chroma Technology Corporation, Rockingham, VT). Mean diameter and polydispersity of liposomes were defined by light scattering (3000 HSA Zetasizer Malvern Zeta-Plus™ zeta potential analyzer, Malvern Instrument Ltd., UK). The anti-CD30 density on the liposomes was determined by protein assay [32]. The concentration of doxorubicin in antibody-conjugated liposomes was determined by measuring its absorbance at 485 nm using FLUOstar Omega microplate reader (BMG-LABTECH Cary, NC, USA).
2.4. In vitro cellular binding
To evaluate binding of CD30-targeted nano-carriers to ALCL cell lines, 1 × 106 of SUP-M2 cells (a CD30 positive ALCL cell line)[31] suspended in media were incubated with either of the following formulations for 30 minute at 4°C: Cy5.5 labeled PEG2000-DSPE micelles (non-targeted) or Cy5.5 labeled CD30-targeted PEG-DSPE micelles at a lipid concentration of 20 ug/mL, Cy5.5 labeled Doxil® (non-targeted) or Cy5.5 labeled CD30-targeted Doxil® at doxorubicin concentration of 2.5 ug/mL. Cells were then washed twice with cold PBS buffer (pH7.2) and the cell-associated fluorescence was quantified by flow cytometry (Becton-Dickinson Instruments, Franklin lakes, NJ). Flow cytometry was performed using a 488 nm argon laser and FL4 band-pass emission (at 695 nm) for the near infrared Cy5.5. Fluorescence histograms and dot plots were generated using Cell Quest software (for figures, histograms were recreated using FCSexpress software).
2.5. Cytotoxicity study
In vitro cytotoxicity was determined by the MTT tetrazolium assay as previously described [33]. SUP-M2 cells were incubated with Doxil® and CD30-targeted Doxil® in media for 48 h. 20 uL of MTT (5 mg/mL) was added to each well and incubated for another 4 h to develop the color. After centrifugation, the media was replaced by 200 uL of DMSO. The absorbance was read by a microplate reader. Data are expressed as concentration of doxorubicin that gives a 50% inhibition of cell growth compared to untreated control cells (IC50)
2.6. Animal Study
Male C.B-17 SCID-beige mice were purchased from Taconic Farms, Inc. at 3-4 weeks of age and housed in our core animal facility (U of Alberta). To establish local tumors, 0.5 million KARPAS 299 cells in Matrigel were injected (s.c) to each SCID mouse. The mice were monitored for tumor growth and total body weight. When the tumors became palpable, the mice were divided to 4 groups. The first group was kept with no treatment and the other three groups received 4 doses (every other day) of free doxorubicin, Doxil®, or CD30-targeted Doxil® through intravenous route (tail vein). The dose of doxorubicin was 1 mg/kg in all 4 doses given to the animals. The mice were monitored for tumor growth by measuring the tumor size during the treatment. Tumor dimensions were measured daily and the following formula was used for calculating tumor volume: tumor volume (mm3) = length (cm)×[width(cm)]2/2. All the mice were sacrificed 4 days after the last dose (the endpoint) and tumors were collected. Tumor size and weight were plotted versus time.
2.7. Statistical analysis
The results are expressed as mean plus or minus standard derivation for each experimental group. The significance of differences among groups was analyzed by the Student t test or one-way analysis of variance (ANOVA) for parametric data, followed by the Student-Newman-Keuls post-hoc test for multiple comparisons. A p-value of ≤ 0.05 was set for the significance of difference among groups. The statistical analysis was performed with SigmaStat software (Systat Software Inc. San Jose, California, USA).
3. Results
3.1. Preparation and characterization of CD30-targeted Doxil®
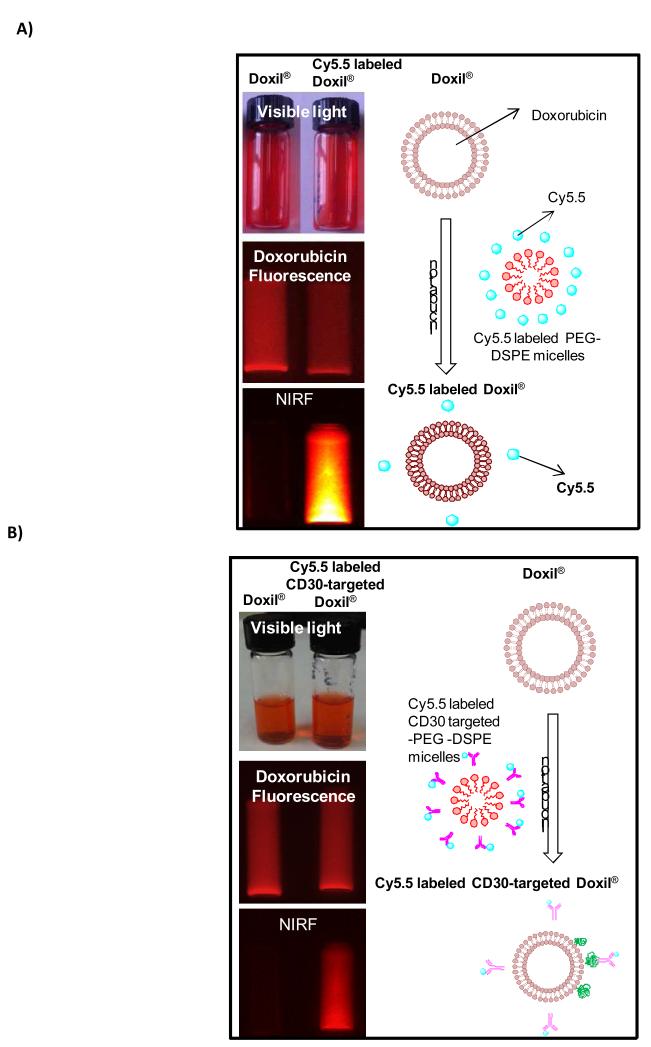
To prepare Doxil® conjugated with anti-CD30 monoclonal antibodies (CD30-targeted Doxil®), first anti-CD30 antibodies unlabeled or labeled with Cy5.5 were chemically conjugated to DSPE-PEG micelles (Scheme 1). Then we generated CD30-targeted Doxil® by mixing CD30-targeted DSPE-PEG micelles with Doxil® using the post insertion method as illustrated in scheme 2. To evaluate the efficiency of our post insertion method, we measured the fluorescence intensity of doxorubicin and Cy5.5 in Doxil®, Cy5.5 labeled Doxil®, and Cy5.5 labeled CD30-targeted Doxil®. All of these three types of Doxil® liposomes were imaged by using a Kodak Imaging Station (Chroma Technology Corporation, Rockingham, VT ) equipped with band-pass excitation and long-pass emission filters at 630 and 700 nm for near-infrared fluorescent imaging. The results of imaging of liposomes have been summarized in Fig. 1 which shows the fluorescence emission of Cy5.5 in Cy5.5 labeled Doxil®, and Cy5.5 labeled CD30-targeted Doxil® formulations at 700 nm indicating the presence of Cy5.5 or Cy5.5-labeled anti-CD30 antibody on Doxil® liposomes.
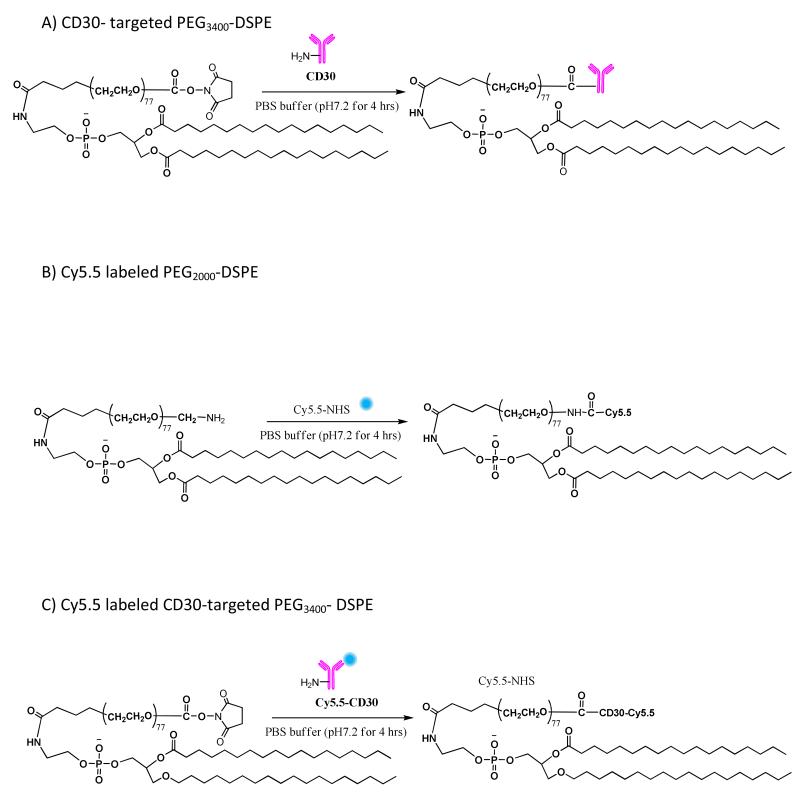
Scheme 1.
Synthesis of A) CD30 targeted PEG-DSPE, B) Cy5.5 labeled PEG-DSPE, and C) Cy5.5 labeled CD30-targeted PEG-DSPE.
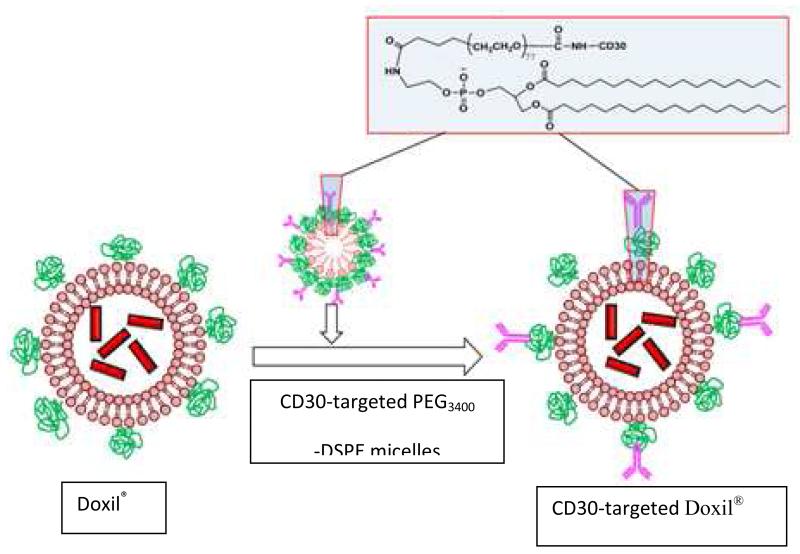
Scheme 2.
Preparation of CD30-targeted Doxil® using post-insertion method.
Fig.1.
Fluorescent images of Doxil® , Cy5.5 labeled Doxil® , and Cy5.5 labeled CD30-targeted Doxil® by Kodak Imaging Station. The liposomes were visualized by doxorubicin fluorescence and NIRF (near infrared fluorescence) with a Kodak image station.
The generated CD30-targeted Doxil® was then characterized for their biochemical properties. As summarized in Table 1, we found that CD30-targeted Doxil® was 87.4 ± 2.6 nm in size with a polydispersity index of 0.11 ± 0.01. The final concentration of doxorubicin in CD30-targeted Doxil® was 0.26 mg/mL. In comparison, the concentration of doxorubicin in Doxil® was 2.0 mg/mL. Anti-CD30 monoclonal antibody density on the liposomes was estimated to be 0.30 nmol/umol (molar ratio of antibody to lipid).
Table 1.
Characteristics of CD30-targeted and non-targeted Doxil® (n = 3)
| Liposomes | Particle size (nm) | polydispersity |
|---|---|---|
| Doxil® | 82.7 ± 2.2 | 0.10 ± 0.01 |
| CD30-targeted Doxil® | 87.4 ± 2.6 | 0.11 ± 0.01 |
3.2. The binding of CD30-targeted Doxil® to ALCL cells
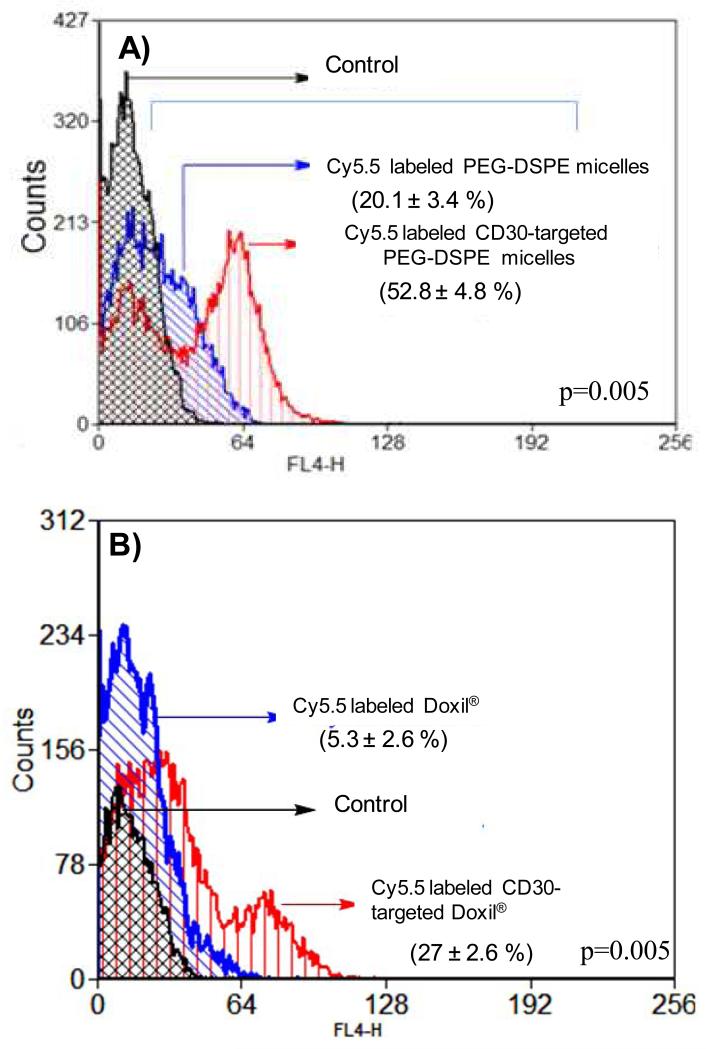
To evaluate the binding of CD30-targeted Doxil® to CD30-expressing cells, SUP-M2 which is a CD30 positive ALCL cell line, was used [31]. We assessed if the conjugation of anti-CD30 to PEG-DSPE exerts a significant effect on its binding to ALCL cells. As shown in Figure 2A, with the use of SUP-M2 cells, we found that CD30-targeted PEG-DSPE micelles bound to ALCL cells significantly higher than non-targeted PEG-DSPE micelles did. We then assessed if CD30-targeted Doxil® showed to be more efficient in binding to ALCL cells, as compared to non-targeted Doxil®. As shown in Figure 2B, CD30-targeted Doxil® bound to ALCL cells significantly higher than Doxil®.
Fig. 2.
Enhanced binding of nano-carriers decorated with anti-CD30 antibody to CD30 positive SupM2 cells. A) The binding of Cy5.5 labeled CD30-targeted PEG-DSPE micelles to SUP-M2 cells. Cy5.5 labeled PEG-DSPE micelles were used as the non-targeted control. B) Cy5.5 labeled CD30-targeted Doxil® binding to SUP-M2 cells. Cy5.5 labeled Doxil® was used as the non-targeted control. These results are representative of three independent experiments
3.3. Cytotoxicity of CD30-targeted Doxil® against ALCL in vitro
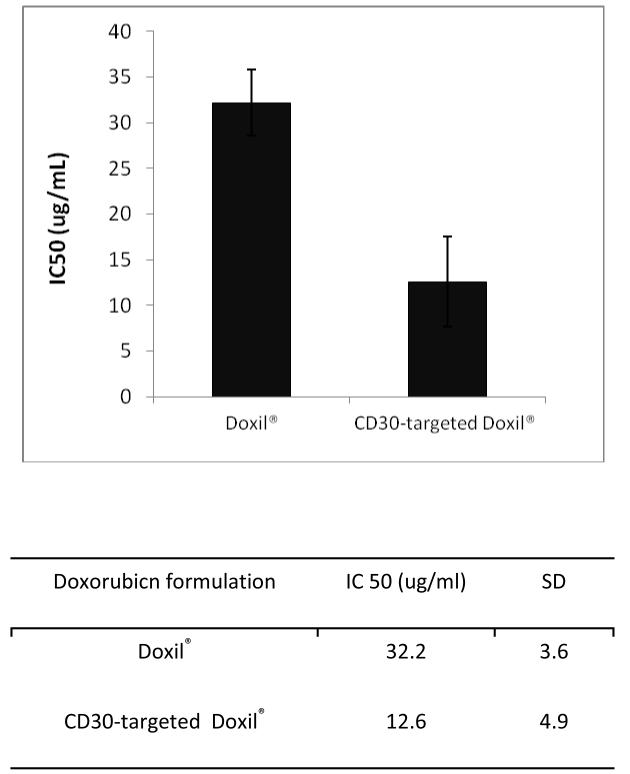
We then assesses the cell killing effect of CD30-targeted Doxil® against ALCL compared to non-targeted Doxil®. As shown in Fig. 3, CD30-targeted Doxil® significantly reduced the IC50 of Doxil® against SUP-M2 cells. Specifically, the IC50 of Doxil® is approximately three times higher than that of CD30-targeted Doxil® (p<0.0001).
Fig. 3.
Cytotoxicity of Doxil® and CD30-targeted Doxil® against SUP-M2 cells (n=3).
3.4. The in vivo therapeutic efficacy of CD30-targeted Doxil®
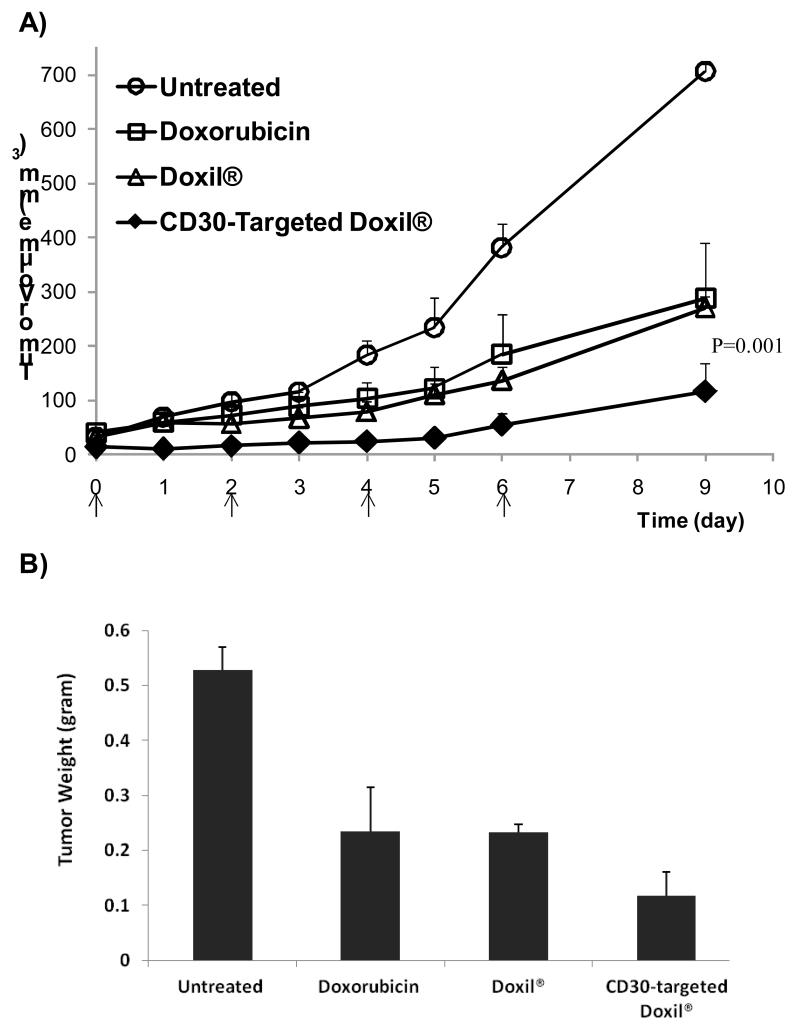
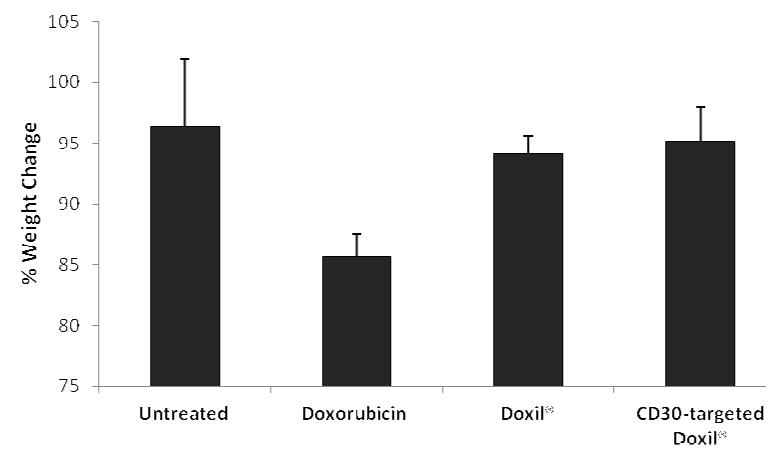
Next we evaluated the therapeutic efficacy of CD30-targeted Doxil® in comparison with Doxil® in a SCID mouse xenograft model. SCID mice were implanted with KARPAS 299, a CD30 positive ALCL cell line [30]. After the xenografts became palpable (0.1 cm3), the mice were treated with 4 doses of either the CD30-targeted Doxil® or Doxil®, as illustrated in Fig. 4A. Nine days after tumor implementation, the average tumor size was found to be significantly lower in the mice treated with CD30-targeted Doxil® compared to those treated with Doxil® (Fig. 4A). Correlating with these results, the average tumor weight at the endpoint, which was 4 days after the last dose of treatment, was significantly lower in mice treated with CD30-targeted Doxil® than those treated with Doxil® (Fig. 4B). We also evaluated the toxicity of CD30-targeted Doxil® in comparison to that of Doxil® or free doxorubicin, using a reduction in the body weight as the criterion. As shown in Fig. 5, we found that treatment with free doxorubicin resulted in 14.3 ± 1.9% decrease in the mouse body weight, Doxil® and CD30-targeted Doxil® did not show a significant change in the body weight as compared to untreated animals. As shown in Fig. 4 and 5, Doxil® significantly reduced the toxicity of doxorubicin while it did not significantly improve the overall therapeutic efficacy, as compared to free drug.
Fig.4.
Therapeutic efficacy of CD30-targeted Doxil® liposomes in SCID mice xenografted with KARPAS 299, a ALK+ALCL cell line. A) Average tumor volume in the SCID mice carrying subcutaneous ALCL tumors; arrows indicate the time of therapeutic injections; B) Average tumor weight at the endpoint of study (n=4-5, mean± SE.)
Fig.5.
Changes in the mice body weight during the treatment (n=4-5, mean± SE.)
4. Discussion
Although there has been great expectations regarding the use of active targeting of drug-loaded nano-carriers to treat cancer, this strategy has been met with several challenges [14]. One of the important challenges is related to the choice of tumor specific antigens for active targeting, which is believed to be a key determinant of the success of these therapies. In this study, we carefully selected CD30 as the tumor specific antigen for ALCL for a number of reasons. First, while CD30 is strongly expressed on the surface of ALCL cells as well as Reed Sternberg cells in Classical Hodgkin lymphomas, its expression in normal cells is highly restricted to a few cell types, such as activated T-cells [19, 20]. This unique expression pattern of CD30 is also the very basis of why we selected ALCL as the study model. Moreover, as mentioned in the introduction, CD30 is a receptor that can be internalized [18, 23], and this property can theoretically facilitate the uptake of nano-carriers conjugated with anti-CD30 and lead to higher accumulation of drugs inside the targeted cells. Lastly, ligation of CD30 by monoclonal antibodies has been previously shown to directly inhibit tumor growth and induce apoptosis in ALCL cell lines in-vitro as well as in a SCID mouse model [21, 22]. Taken together, we believe that we have selected an excellent study model to test the clinical utility of active targeted nano-particles.
In this study, we successfully decorated Doxil®, a commercial preparation of doxorubicin in long-circulating PEGylated liposomes, with anti-CD30 monoclonal antibodies. We found that CD30-targeted Doxil® can bind to ALCL cells more efficiently, and lead to significantly higher anti-cancer effects, compared to non-targeted liposomes (i.e. Doxil®). Furthermore, the therapeutic efficacy of CD30-targeted Doxil® was superior to unmodified Doxil® and free doxorubicin in a SCID mouse xenograft model. These observations are consistent with the results of a small number of previous studies, which also showed that the conjugation of antibodies with drug-loaded nano-carriers can improve their therapeutic efficacy [34-38]. These studies have attempted to target nano-carriers to cell surface receptors overexpressed on cancer cells (i.e growth factor receptors). One of the important limitations for this kind of targeting strategy is that shedding of overexpressed receptor from cancer cells can significantly reduce the effectiveness of targeting agents due to their neutralization by soluble fragments[39]. One of the unique properties of CD30-targeted nano-carries reported in the present paper is that they have been conjugated to an anti-CD30 monoclonal antibody specific to membrane bound CD30 antigen overexpressed on ALCL [18]. The extracellular domain of CD30 has been shown to be shed from cancer cells in Hodgkin’s lymphoma and anaplastic large cell lymphoma so these patients show a high concentration of soluble CD30 in their blood stream [40, 41]. The antibodies recognizing the membrane bound CD30 epitopes will have the advantage of evading the neutralization by soluble CD30 in blood and can potentially result in a relatively high efficiency of active targeting. The effectiveness of this anti-CD30 antibody for active targeting of a clinically approved liposomal formulation of doxorubicin, Doxil® in ALCL was evaluated here. For this purpose, more importantly in comparison to Doxil®, CD30-targeted Doxil® showed better therapeutic efficacy in SICD mice carrying ALCL.
Doxil® is a liposomal formulation which has an ability to accumulate in solid tumors due to Enhanced Permeation and Retention (EPR) effect [42]. Indeed both CD30-targeted and non-targeted Doxil® have ability to accumulate in tumor by passive targeting. However the conjugation of anti-CD30 antibody to Doxil® is expected to increase the homing of these nano-carriers in tumor and prevent the removal of targeted carriers from tumor thereby providing better retention of the carriers in tumor as compared to non-targeted Doxil®. Beside in comparison to Doxil®, CD30-targeted Doxil is expected to deliver a higher payload of drug to cancer cells due to better uptake by cancer cells. Previous studies suggest that the path of delivering drug to cancer cells by targeted doxorubicin loaded liposomes is different than that of non-targeted liposomes. In case of non-targeted Doxil®, drug delivery is mainly dependent on the release of free doxorubicin from the liposomes into the extracellular space and further diffusion of doxorubicin to cancer cells and target intracellular compartments [43, 44]. Endocytosis of the carrier and release of encapsulated doxorubicin inside the cells can take place but with a slower kinetics and a lesser extent at any given time point. On the other hand, actively targeted anti-CD30 antibody conjugated Doxil® can be taken up by cells as a result of the receptor mediated endocytosis after binding to the tumor cell surface. This can lead to the delivery of a higher load of doxorubicin inside cancer cells, which translates into significantly increased cytotoxicity of targeted Doxil against SUP-M2 cell line compared to Doxil® in vitro and better anti-cancer activity in vivo.
5. Conclusion
In this study we have evaluated the potential of a newly developed monoclonal antibody recognizing membrane bond CD30 for targeting a commercial liposomal formulation loaded with doxorubicin (Doxil®) to ALCL, a human malignancy which highly express CD30. Our findings confirm the developed anti-CD30 antibody can be effectively conjugated to a liposomal drug formulation and provide the targeted delivery of functional drug loaded in liposomes to human cancer expressing CD30. Our findings provide the proof-of-principle regarding the feasibility of developing targeted nano-carriers to treat cancer. Further studies are underway in our laboratory to increase the selectivity and specificity of our CD30-targeted formulation by encapsulating anticancer agents which block key oncogenic molecules in cancer cells. Success in these studies can provide a framework with which specific targeted therapy can be designed for other cancer types
Acknowledgements
This work was supported by a research operating grant from the Alberta Cancer Foundation awarded to RL and AL. OM was supported by a fellowship grant from the Canadian Institute of Health Research. SN was supported by a NIH COBRE grant (1P20RR024219-01A2). This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mahmud A, Xiong XB, Aliabadi HM, Lavasanifar A. Polymeric micelles for drug targeting. J Drug Target. 2007;15(9):553–84. doi: 10.1080/10611860701538586. [DOI] [PubMed] [Google Scholar]
- [2].Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–71. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- [3].Jain KK. Nanotechnology-based drug delivery for cancer. Technol Cancer Res Treat. 2005;4(4):407–16. doi: 10.1177/153303460500400408. [DOI] [PubMed] [Google Scholar]
- [4].Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009 doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- [5].Aliabadi HM, Shahin M, Brocks DR, Lavasanifar A. Disposition of drugs in block copolymer micelle delivery systems: from discovery to recovery. Clin Pharmacokinet. 2008;47(10):619–34. doi: 10.2165/00003088-200847100-00001. [DOI] [PubMed] [Google Scholar]
- [6].Noguchi Y, Wu J, Duncan R, Strohalm J, Ulbrich K, Akaike T, et al. Early phase tumor accumulation of macromolecules: a great difference in clearance rate between tumor and normal tissues. Jpn J Cancer Res. 1998;89(3):307–14. doi: 10.1111/j.1349-7006.1998.tb00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–92. [PubMed] [Google Scholar]
- [8].Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- [9].Dass CR. Drug delivery in cancer using liposomes. Methods Mol Biol. 2008;437:177–82. doi: 10.1007/978-1-59745-210-6_9. [DOI] [PubMed] [Google Scholar]
- [10].Ning YM, He K, Dagher R, Sridhara R, Farrell AT, Justice R, et al. Liposomal doxorubicin in combination with bortezomib for relapsed or refractory multiple myeloma. Oncology (Williston Park) 2007;21(12):1503–8. discussion 11, 13, 16 passim. [PubMed] [Google Scholar]
- [11].Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66(13):6732–40. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- [12].Hatakeyama H, Akita H, Ishida E, Hashimoto K, Kobayashi H, Aoki T, et al. Tumor targeting of doxorubicin by anti-MT1-MMP antibody-modified PEG liposomes. Int J Pharm. 2007;342(1-2):194–200. doi: 10.1016/j.ijpharm.2007.04.037. [DOI] [PubMed] [Google Scholar]
- [13].Drummond DC, Hong K, Park JW, Benz CC, Kirpotin DB. Liposome targeting to tumors using vitamin and growth factor receptors. Vitam Horm. 2000;60:285–332. doi: 10.1016/s0083-6729(00)60022-5. [DOI] [PubMed] [Google Scholar]
- [14].Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev. 2008;60(15):1615–26. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- [15].Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161(2):175–87. doi: 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- [16].Debbage P. Targeted drugs and nanomedicine: present and future. Curr Pharm Des. 2009;15(2):153–72. doi: 10.2174/138161209787002870. [DOI] [PubMed] [Google Scholar]
- [17].Nagata S, Ise T, Onda M, Nakamura K, Ho M, Raubitschek A, et al. Cell membrane-specific epitopes on CD30: Potentially superior targets for immunotherapy. Proc Natl Acad Sci U S A. 2005;102(22):7946–51. doi: 10.1073/pnas.0502975102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stein H, Foss HD, Durkop H, Marafioti T, Delsol G, Pulford K, et al. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96(12):3681–95. [PubMed] [Google Scholar]
- [19].Stein H, Mason DY, Gerdes J, O’Connor N, Wainscoat J, Pallesen G, et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66(4):848–58. [PubMed] [Google Scholar]
- [20].Schwab U, Stein H, Gerdes J, Lemke H, Kirchner H, Schaadt M, et al. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin’s disease and a subset of normal lymphoid cells. Nature. 1982;299(5878):65–7. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- [21].Hubinger G, Muller E, Scheffrahn I, Schneider C, Hildt E, Singer BB, et al. CD30-mediated cell cycle arrest associated with induced expression of p21(CIP1/WAF1) in the anaplastic large cell lymphoma cell line Karpas 299. Oncogene. 2001;20(5):590–8. doi: 10.1038/sj.onc.1204128. [DOI] [PubMed] [Google Scholar]
- [22].Tian ZG, Longo DL, Funakoshi S, Asai O, Ferris DK, Widmer M, et al. In vivo antitumor effects of unconjugated CD30 monoclonal antibodies on human anaplastic large-cell lymphoma xenografts. Cancer Res. 1995;55(22):5335–41. [PubMed] [Google Scholar]
- [23].Sutherland MS, Sanderson RJ, Gordon KA, Andreyka J, Cerveny CG, Yu C, et al. Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J Biol Chem. 2006;281(15):10540–7. doi: 10.1074/jbc.M510026200. [DOI] [PubMed] [Google Scholar]
- [24].Mahmud A, Lavasanifar A. The effect of block copolymer structure on the internalization of polymeric micelles by human breast cancer cells. Colloids Surf B Biointerfaces. 2005;45(2):82–9. doi: 10.1016/j.colsurfb.2005.07.008. [DOI] [PubMed] [Google Scholar]
- [25].Pinter-Brown LC. SGN-30: a basis for the effective treatment of CD30 positive hematopoietic malignancies. Expert Opin Investig Drugs. 2008;17(12):1883–7. doi: 10.1517/13543780802493440. [DOI] [PubMed] [Google Scholar]
- [26].Duvic M, Reddy SA, Pinter-Brown L, Korman NJ, Zic J, Kennedy DA, et al. A phase II study of SGN-30 in cutaneous anaplastic large cell lymphoma and related lymphoproliferative disorders. Clin Cancer Res. 2009;15(19):6217–24. doi: 10.1158/1078-0432.CCR-09-0162. [DOI] [PubMed] [Google Scholar]
- [27].Forero-Torres A, Leonard JP, Younes A, Rosenblatt JD, Brice P, Bartlett NL, et al. A Phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol. 2009;146(2):171–9. doi: 10.1111/j.1365-2141.2009.07740.x. [DOI] [PubMed] [Google Scholar]
- [28].Deng C, Pan B, O’Connor OA. Brentuximab vedotin. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-12-0290. [DOI] [PubMed] [Google Scholar]
- [29].Vadakara J, Pro B. Targeting CD30 in anaplastic large cell lymphoma. Curr Hematol Malig Rep. 2012;7(4):285–91. doi: 10.1007/s11899-012-0137-y. [DOI] [PubMed] [Google Scholar]
- [30].Fischer P, Nacheva E, Mason DY, Sherrington PD, Hoyle C, Hayhoe FG, et al. A Ki-1 (CD30)-positive human cell line (Karpas 299) established from a high-grade non-Hodgkin’s lymphoma, showing a 2;5 translocation and rearrangement of the T-cell receptor beta-chain gene. Blood. 1988;72(1):234–40. [PubMed] [Google Scholar]
- [31].Morgan R, Smith SD, Hecht BK, Christy V, Mellentin JD, Warnke R, et al. Lack of involvement of the c-fms and N-myc genes by chromosomal translocation t(2;5)(p23;q35) common to malignancies with features of so-called malignant histiocytosis. Blood. 1989;73(8):2155–64. [PubMed] [Google Scholar]
- [32].Adrian JE, Kamps JA, Scherphof GL, Meijer DK, van Loenen-Weemaes AM, Reker-Smit C, et al. A novel lipid-based drug carrier targeted to the non-parenchymal cells, including hepatic stellate cells, in the fibrotic livers of bile duct ligated rats. Biochim Biophys Acta. 2007;1768(6):1430–9. doi: 10.1016/j.bbamem.2007.03.027. [DOI] [PubMed] [Google Scholar]
- [33].Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- [34].Gupta B, Torchilin VP. Monoclonal antibody 2C5-modified doxorubicin-loaded liposomes with significantly enhanced therapeutic activity against intracranial human brain U-87 MG tumor xenografts in nude mice. Cancer Immunol Immunother. 2007;56(8):1215–23. doi: 10.1007/s00262-006-0273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Siwak DR, Tari AM, Lopez-Berestein G. The potential of drug-carrying immunoliposomes as anticancer agents. Clin. Cancer Res. 2002;8:1172–1181. Commentary re: J. W. Park et al., Anti-HER2 immunoliposomes: enhanced efficacy due to targeted delivery. [PubMed] [Google Scholar]; Clin Cancer Res. 2002;8(4):955–6. [PubMed] [Google Scholar]
- [36].Hosokawa S, Tagawa T, Niki H, Hirakawa Y, Ito N, Nohga K, et al. Establishment and evaluation of cancer-specific human monoclonal antibody GAH for targeting chemotherapy using immunoliposomes. Hybrid Hybridomics. 2004;23(2):109–20. doi: 10.1089/153685904774129711. [DOI] [PubMed] [Google Scholar]
- [37].Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, et al. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8(4):1172–81. [PubMed] [Google Scholar]
- [38].Nishikawa K, Asai T, Shigematsu H, Shimizu K, Kato H, Asano Y, et al. Development of anti-HB-EGF immunoliposomes for the treatment of breast cancer. J Control Release. 2012;160(2):274–80. doi: 10.1016/j.jconrel.2011.10.010. [DOI] [PubMed] [Google Scholar]
- [39].Perez-Torres M, Valle BL, Maihle NJ, Negron-Vega L, Nieves-Alicea R, Cora EM. Shedding of epidermal growth factor receptor is a regulated process that occurs with overexpression in malignant cells. Exp Cell Res. 2008;314(16):2907–18. doi: 10.1016/j.yexcr.2008.07.013. [DOI] [PubMed] [Google Scholar]
- [40].Nadali G, Vinante F, Stein H, Todeschini G, Tecchio C, Morosato L, et al. Serum levels of the soluble form of CD30 molecule as a tumor marker in CD30+ anaplastic large-cell lymphoma. J Clin Oncol. 1995;13(6):1355–60. doi: 10.1200/JCO.1995.13.6.1355. [DOI] [PubMed] [Google Scholar]
- [41].Josimovic-Alasevic O, Durkop H, Schwarting R, Backe E, Stein H, Diamantstein T. Ki-1 (CD30) antigen is released by Ki-1-positive tumor cells in vitro and in vivo. I. Partial characterization of soluble Ki-1 antigen and detection of the antigen in cell culture supernatants and in serum by an enzyme-linked immunosorbent assay. Eur J Immunol. 1989;19(1):157–62. doi: 10.1002/eji.1830190125. [DOI] [PubMed] [Google Scholar]
- [42].Northfelt DW, Martin FJ, Working P, Volberding PA, Russell J, Newman M, et al. Doxorubicin encapsulated in liposomes containing surface-bound polyethylene glycol: pharmacokinetics, tumor localization, and safety in patients with AIDS-related Kaposi’s sarcoma. J Clin Pharmacol. 1996;36(1):55–63. doi: 10.1002/j.1552-4604.1996.tb04152.x. [DOI] [PubMed] [Google Scholar]
- [43].Horowitz AT, Barenholz Y, Gabizon AA. In vitro cytotoxicity of liposome-encapsulated doxorubicin: dependence on liposome composition and drug release. Biochim Biophys Acta. 1992;1109(2):203–9. doi: 10.1016/0005-2736(92)90084-y. [DOI] [PubMed] [Google Scholar]
- [44].Carrion C, de Madariaga MA, Domingo JC. In vitro cytotoxic study of immunoliposomal doxorubicin targeted to human CD34(+) leukemic cells. Life Sci. 2004;75(3):313–28. doi: 10.1016/j.lfs.2003.12.020. [DOI] [PubMed] [Google Scholar]