Abstract
Itch and pain are closely related but distinct sensations. They share largely overlapping mediators and receptors, and itch-responding neurons are also sensitive to pain stimuli. Itch-mediating primary sensory neurons are equipped with distinct receptors and ion channels for itch transduction, including Mas-related G protein-coupled receptors (Mrgprs), protease-activated receptors (PARs), histamine receptors, bile acid receptor (TGR5), toll-like receptors (TLRs), and transient receptor potential subfamily V1/A1 (TRPV1/A1). Recent progress has indicated the existence of an itch-specific neuronal circuitry. The MrgprA3-expressing primary sensory neurons exclusively innervate the epidermis of skin and their central axons connect with gastrin-releasing peptide receptor (GRPR)-expressing neurons in the superficial spinal cord. Notably, ablation of MrgprA3-expressing primary sensory neurons or GRPR-expressing spinal cord neurons results in selective reduction in itch but not pain. Chronic itch results from dysfunction of the immune and nervous system and can manifest as neural plasticity, despite the fact that chronic itch is often treated by dermatologists. While differences between acute pain and acute itch are striking, chronic itch and chronic pain share many similar mechanisms, including peripheral sensitization (increased responses of primary sensory neurons to itch and pain mediators), central sensitization (hyperactivity of spinal projection neurons and excitatory interneurons), loss of inhibitory control in the spinal cord, and neuro-immune and neuro-glial interactions. Notably, painful stimuli can elicit itch in some chronic conditions (e.g., atopic dermatitis) and some drugs for treating chronic pain are also effective in chronic itch. Thus, itch and pain have more similarities in pathological and chronic conditions.
Keywords: Central sensitization, neuro-immune interaction, nociceptor, peripheral sensitization, pruritus, pruriceptor
Introduction
Itch (pruritus) was first defined by a German physician Samuel Hafenreffer as “an unpleasant sensation that elicits the desire or reflex to scratch” [61]. Although this definition is still valid today, it also reflected an incomplete understanding of the underlying mechanisms of itch. Itch can be induced acutely, such as mosquito bite, and this itch only lasts minutes and is inhibited by scratching the affected area [34]. Itch was considered as a sub-modality of pain, because two sensations share many similarities (Box 1). Like acute pain, acute itch is protective and serves as a warning signal for potential tissue damage caused by invader such as parasite [126]. However, chronic itch leads to sleep disruption and severe skin lesions [75]. Chronic itch commonly occurs in patients with skin diseases, and also is a comorbidity of other systemic disorders, such as liver and kidney diseases, HIV/AIDS, as well as metabolic disorders [151]. Notably, chronic itch is underappreciated and difficult to manage. Although antihistamines are the first choice for anti-itch treatment, most chronic itch conditions are resistant to antihistamines, possibly due to dominant contributions of the histamine-independent mechanisms. In chronic itch patients, scratching-evoked sensation can be perceived as itch and produce an “itch-scratch-itch” cycle to exacerbate itch. In some chronic itch conditions such as atopic dermatitis, even painful stimuli could be perceived as itch [57;60;86]. Continuous scratching not only increases the risk of skin infection, but also disrupts the social life of patients [75;119;151].
Box 1. Similarities and differences between itch and pain.
| Similarities | Differences | |
|---|---|---|
| Acute states |
|
|
| Chronic states |
|
|
To date, our understanding for chronic itch associated with systemic diseases (e.g., renal, liver or metabolic diseases) is still incomplete, due to the lack of suitable animal models of chronic itch. Several animal models were developed for dermatitis-induced chronic itch, including dry skin model induced by a mixture of acetone/ether (1:1) and water (AEW) [3;4;106], toxic contact dermatitis model induced by diphenylcyclopropenone (DCP) [134;145], and allergic contact dermatitis model induced by 2,4-Dinitro-1-fluorobenzene (DNFB) [130]. In addition, an inbred mouse line (NC/Nga mice) was used to study atopic dermatitis-related itch [50;116;146]. Development of animal models to mimic clinical itch conditions is essential to elucidate the molecular mechanisms of chronic itch [80], which are different from that of acute itch (Box-2)
Box 2. Outstanding questions.
What are the neurotransmitters for itch transmission? What are the roles of GRP, SP, CRGP, and glutamate in spinal cord itch transmission?
Are GRPR-expressing neurons projection neurons or interneurons?
What are the neurochemical properties of the Bhlhb5-expressing inhibitory neurons in the spinal cord?
What are the endogenous ligands for MrgprA3/C11/D and TLR3/7? How are Mrgprs and TLRs regulated under chronic itch conditions?
Are there itch-specific fibers in human that are equivalent to MrgprA3+ itch fibers in mice?
How do glial cells control itch? Do glial cells release different mediators to regulate pain and itch transmission in the spinal cord?
Are there itch mediators that are only produced in chronic conditions and act when pruroceptors are up-regulated?
Are there suitable animal models for clinical itch? Although acute itch models are useful for studying itch physiology, the chronic itch models that recapitulate clinical itch conditions are urgently needed for studying pathological itch.
In this review, we provide new insights into distinct molecular mechanisms of itch, especially nonhistaminergic itch signaling, mediated by Mas-related G protein-coupled receptor (Mrgprs), protease-activated receptors (PARs), toll-like receptors (TLRs), oxidative stress, transient receptor potential cation channel subfamily V1/A1 (TRPV1/A1), and interleukin-31 (IL-31). We also discuss recent evidence for the existence of an itch-specific neuronal pathway consisting of MrgprA3-expressing primary sensory neurons and GRPR-expressing spinal dorsal horn neurons. We further discuss the mechanisms underlying chronic itch [33], especially the crosstalk of the itch and pain pathways (labeled lines) in physiological and pathological conditions. While the differences of itch and pain are striking in acute conditions, itch and pain share many similar mechanisms in chronic conditions. Pain stimuli could potentiate itch in pathological conditions as a result of neural plasticity.
Characterization of itch
Itch is exclusively sensed as an irritation in the skin, while pain can be experienced from almost all the body parts [157]. Base on the distinct origin, itch is classified into 4 categories: (1) pruriceptive itch, defined as itch that originates from the skin. Itch is perhaps the most common symptom in dermatology, as a result of dry skin, atopic eczema, psoriasis, urticaria, scabies or other inflammatory skin diseases [105]; (2) neuropathic itch, caused by nerve damage, such as itch associated with post-herpes zoster neuropathy, brain tumors and multiple sclerosis [115]; (3) neurogenic itch, caused by dysfunction of the nervous system in the absence of peripheral neuropathy, such as itch related to cholestasis [21]; and (4) psychological or psychiatric itch, such as itch related to psychological abnormalities (e.g., obsessive-compulsive disorders [157]. For comparison, pain also can be divided into 4 categories: 1) nociceptive pain, caused by activation of peripheral nerve fibers by noxious stimulation; 2) inflammatory pain, pain associated with tissue damage and inflammation; 3) neuropathic pain, caused by damage or disorder affecting of the nervous system; 4) Psychogenic pain, caused by mental, emotional or behavioral factors [155]. Depending on the duration, itch and pain can also be characterized as acute and chronic itch and pain in humans. Acute itch lasts minutes to days, whereas chronic itch lasts more than 6 weeks. On the other hand, acute pain lasts less than 30 days, but chronic pain can last more than six months [61]. Notably, the duration of chronic itch and pain could be much shorter in experimental animals (e.g., rodents), in part due to short life expectancy of rodents.
Peripheral mechanisms of itch
Itch mediators and receptors
Itch and pain sensations are mediated by primary sensory neurons, whose cell bodies are located in dorsal root ganglia (DRG) or trigeminal ganglia (TG) [61]. Traditionally, noxious chemicals can be classified as algogens or proritogens. However, increasing evidence shows that most noxious chemicals are not specific for itch or pain, except for the allyl isothiocyanate (AITC) that only elicits pain [126;139]. Many itch-inducing mediators (Figure 1), including histamine, serotonin (5-HT), and endothelin-1 (ET-1) also induce pain. On the other hand, many receptors or ion channels such as TRPV1 and TRPA1 that are involved in pain transduction also play pivotal roles in itch transduction [22].
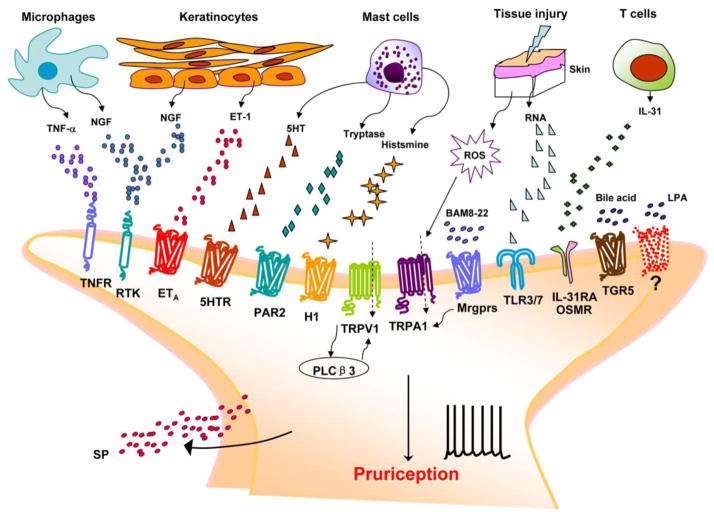
Figure 1. Peripheral itch mediators and their respective receptors in skin nerve terminal.
Upon tissue damage or inflammation, skin cells (keratinocytes, fibroblasts) and residential and infiltrating immune cells (macrophages, mast cells, neutrophils) release itch mediators that can activate pruriceptors in nerve terminal of C-fiber nociceptor. These itch mediators (pruritogens), such as ET-1, serotonin, histamine, tryptase, thromboxanes, leukotrienes, bile acids, LPA, nerve growth factor (NGF), tumor necrosis factor α (TNF-α) and RNAs can directly bind their cognate receptors such as GPCRs (ETA, 5HTR, H1R or H4R, PAR2/4, Mrgprs, LPAR, TGR5), TLRs (TLR3/7), and cytokines receptors (IL-31R/OSMR), expressed by pruriceptors. Activation of these itch receptors results in activation of PLCβ3 and increases in intracellular calcium through TRPV1 and/or TRPA1 to elicit itch transduction and sensation (pruriception). Activation of pruriceptors also releases additional itch mediators such as substance P to induce neurogenic inflammation and potentiate itch signaling.
Histamine
Histamine is one of the best known itch mediators. However, antihistamines are ineffective in many chronic itch conditions, such as itch associated with atopic dermatitis, kidney and liver diseases, suggesting the existence of histamine-independent mechanism in chronic conditions. Thus, itch is often characterized as histamine-dependent (histaminergic) and histamine-independent (nonhistaminergic) itch [63]. Histamine is naturally synthesized from the amino acid histidine and is released from mast cells in skin when these cells are activated by allergens or inflammatory mediators [136]. Free nerve fibers in the skin are responsible for detecting pruritogens [125]. A subgroup of mechano-insensitive unmyelinated C-fibers are responsible for histamine-induced itch in humans [132]. Histamine receptors are members of the G-protein-coupled receptors (GPCRs), and four subtypes of histamine receptors (H1–H4) have been identified to date [58;136]. Both H1R and H4R have been detected on DRG neurons and are involved in histamine-induced itch [52]. H1R is coupled with Gq/G11 to induce activation of phospholipase Cβ3 (PLCβ3), leading to increase of intracellular Ca2+ in DRG neurons via TRPV1 [20;38]. Histamine also activates the phospholipase A2 (PLA2), lipoxygenase, and TRPV1 in primary sensory neurons to induce itch [70;136;137;150] (Figure 1). H1R antagonists have been widely used to relieve itching and reduce size of flares. H4R may also play a role in itch. In mouse model of atopic dermatitis, histamine activates H4R to cause itching, and H4R antagonists decrease itching and inflammation [30]. However, the H4R-mediated signaling in primary sensory neurons still needs to be determined.
5-HT
The role of 5-HT in itch may be species-dependent. In rats, 5-HT induces pure itch behavior, while in mouse and human it may evoke mixed pain and itch sensations [103]. Skin mast cells are likely a primary source of 5-HT. 5-HT induces itch mainly via 5-HT2 receptors [156], which are coupled to Gq/11 to activate PLC, especially PLCβ3 [62], leading to the activation of the mitogen-activated protein kinase (MAPK) and PKC in sensory neurons [103].
ET-1
ET-1 is a 21 amino acids peptide from skin keratinocytes and endothelial cells. Intradermal application of ET-1 causes mixed pain and itch behaviors in mice [45]. ET-1 binds to two subtypes of GPCRs, ETA and ETB, and ETA was thought to regulate both itch and pain [104]. ETA couples to Gs to raise cAMP levels and also activates PLC. Interestingly, PLCβ3 and TRPV1 are not required for ET-1-induced itch in mice [62].
Bile acids
A recent study indicated that bile acids, which are elevated in the plasma and also accumulated in skin in patients with cholestasis [21], cause itch by activating the GPCR TGR5 (also called GPR131 or GPBAR1) [8]. TGR5 was detected in a subpopulation of DRG neurons that co-express gastrin-releasing peptide (GRP), TRPA1 and TRPV1. Intradermal injection of bile acids and other TGR5 agonist evoked TGR5-dependent scratching behavior in mice. Interestingly, this behavior also depends on GRP and opioid [8]. Bile acids cause hyperexcitability of DRG neurons via TGR5 activation. Thus, it is plausible that bile acids activate TGR5 on sensory nerves to stimulate the release of neuropeptides (e.g., GRP and opioid peptide) in the spinal cord for eliciting itch transmission. Although bile acids levels are elevated in circulation and skin in cholestatic patients, these levels do not necessarily correlate with itch intensity levels [21], suggesting that other itch mediators may also contribute to cholestatic itch.
Lysophosphatidic acid (LPA)
Recent study also identified LPA as a possible mediator for cholestasis-related itch [79]. The serum levels of LPA and autotaxin (ATX), the enzyme that converts lysophosphatidylcholine into LPA, are elevated in cholestatic patients with chronic itch. Notably, ATX levels are correlated with itch severity. Intradermal injection of LPA results in itching in mice [54;79]. LPA is a well known lipid mediator that can activate various cell types through the GPCRs LPA receptors (LPA1-6) [27]. It is of great interest to test whether LPA can activate peripheral itch fibers via LPA receptors to produce itch.
PARs
Spicules of cowhage, when inserted into skin, produce a typical form of nonhistaminergic itch, via activation of cutaneous polymodal C-fibers in human [68;122]. Subsequent biochemical studies revealed that the active itch-inducing constituent is a cystein protease named mucunain, which was shown to activate the protease-activated receptors (PARs), including PAR2 and PAR4 [122;135], by cleaving the extracellular N-terminus from PARs, allowing the N-terminus for being a ligand of its own receptor [135]. The signaling pathways downstream of PAR2 activation in sensory neurons and their role in itch are still incompletely understood. PAR2 couples to PLCβ to sensitize TRPV1 and TRPV4 via activation of protein kinase C and A [9;10;31;48]. PAR2 activation also sensitizes TRPA1 [32] (Figure 1). In addition to DRG neurons, PARs are also expressed in non-neuronal cells in the skin [125;152]. Endogenous proteases such as trypsin, mast cell tryptase, kallikreins, and cathepsin S have been demonstrated to generate itch via activation of PARs [28;29]. In atopic dermatitis patients, the endogenous agonists and PAR2 per se increased in skin and PAR-2 agonists provoked enhanced and prolonged itch [143]. Consistently, over-expression of cathepsin S or PAR2 in the mouse skin causes dermatitis and itching [41;73]. Thus, PARs especially PAR2 are key receptors to mediate acute and chronic itch via neuronal and non-neuronal mechanisms.
Mrgprs
Recent studies have revealed that several members of the Mrgpr family GPCRs, which are specifically expressed in subsets of small-sized DRG neurons, can serve as itch receptors [88;89;103;138] (Figure 1). Mrgprs family has more than 50 members in mouse and can be divided into several subfamilies: MrgprAs, MrgprBs, MrgprCs and MrgprD-G. However, there are only 10 members in human, and MrgprX1 is the mouse orthology of MrgprC11. Direct activation of MrgprA3 in a subset of primary sensory neurons by chloroquine, an antimalarial drug that can induce severe itch in human, induces itch in mice [88]. BAM8-22, an endogenous bovine adrenal medulla peptide induces itch through direct activation of MrgprC11 [88;138]. Activation of TRPA1 in DRG neurons is required for both MrgprA3 and MrgprC11-mediated and histamine-independent itch in mice [112;154]. In addition, β-alanine elicited histamine-independent itch through activation of a subset of MrgprD-expressing DRG neurons, which exclusively innervate the upper epidermis of the skin and are not-overlapped with the MrgprA3+ population [87]. The precise roles of Mrgprs and their endogenous agonists in chronic itch conditions are areas of interest.
TRP channels
Increasing evidence indicates that TRPV1 and TRPA1 ion channels are involved in both pain and itch. For example, capsaicin, the best known TRPV1 agonist, can elicit itching when applied, in a punctuate manner, to the epidermis [139]. Also, topically administrated capsaicin to normal human skin produces itch prior to burning sensation [22], suggesting that the superficial itch-mediating fibers express functional TRPV1. TRPV1 is also essential for mediating histamine-induced itch [136]. Furthermore, specific ablation of TRPV1-expressing neurons abolished both thermal pain and itch [62]. A regulatory subunit of TRPV1, Pirt (phosphoinositide binding protein), is required for both normal pain and itch [118]. TRPA1 agonists, such as AITC, allicin, and cinnamaldehyde, can elicit irritation in the skin, suggesting the role of TRPA1 in pain sensation [19]. TRPA1 is also required for Mrgprs-mediated and histamine-independent itch [112;148;154]. Our recent finding revealed that oxidative stress can also induce histamine-independent itch via activation of TRPA1, but not TRPV1 [92]. As oxidative stress is involved in various diseases associated with chronic itch, antioxidants and/or TRPA1 blockers may be used to treat chronic itch [92;112]. Although there is evidence suggesting that itch may be induced by activation of TRPV1 alone [139], TRP channels generally act as downstream effectors in pruriceptors for itch signaling transduction. Other TRP channels such as TRPC3 may also play a role for itch [148]. Thus, TRP channels represent molecular integrators for both pain and itch sensations.
Toll-like receptors (TLRs)
TLRs are typically expressed in immune cells to mediate innate immunity by recognition of pathogen-associated molecular patterns (PAMPs) [2]. Recently, several TLRs, such as TLR3, TLR4, and TLR7 have also shown to be functionally expressed in primary sensory neurons and play a role in detecting exogenous pathogens or endogenous danger signals [26;37;90;93;121]. We have demonstrated a novel role of TLRs in itch control [90;91;93]. Particularly, we identified TLR7 in a subset of primary sensory neurons that co-express TRPV1 and GRP [93]. TLR7 can recognize synthetic ligands such as imidazoquinoline derivatives (e.g., imiquimod and resiquimod) and guanine analogs (e.g., loxoribine) [55]. Intradermal application of TLR7 ligands induced dose-dependent scratching behavior in mice. Of note, imiquimod and loxoribine also elicit rapid inward currents and action potentials in capsaicin-sensitive DRG neurons, in a TLR7-dependent manner. This result suggests that the TLR7 ligands can directly activate and excitate itch-mediating fibers. Furthermore, we found that TRPV1-expressing C fibers, but not TRPV1 per se, are required for imiquimod-elicited itch [93]. These findings suggest a possible coupling of TLR7 with ion channels in primary sensory neurons that can trigger itch sensation. Notably, imiquimod also elicits TLR7-independent itch, which may be attributed to its off-target effects on adenosine receptors [133], inositol 1, 4, 5-trisphosphate receptors [74], and potassium channels [84]. Compared with wild-type (WT) mice, Tlr7−/− mice exhibit normal thermal and mechanical pain. In contrast, Tlr7−/− mice show a significant reduction in scratching behaviors in response to nonhistaminergic pruritogens, including chloroquine, endothelin-1, and SLIGRL peptide [93]. Thus, TLR7 may be involved in various forms of non-histaminergic itch.
We further observed TLR3 expression in small-sized primary sensory neurons that co-express TRPV1 and GRP [90]. The TLR3 agonist polyinosine-polycytidylic acid (poly(I:C)), a synthetic analog of double-strands (ds) RNA, also induces inward currents and action potentials in DRG neurons and elicits scratching in WT mice but not Tlr3−/− mice [90]. Thus, TLR3 ligand may directly activate and excite itch-fibers to elicit itch. Unlike Tlr7 deletion which only affects nonhistaminergic itch, deletion of Tlr3 results in a significant reduction in scratching behaviors induced by both histamine-dependent and -independent pruritogens, in part due to additional actions of TLR3 in modulating spinal cord synaptic transmission and central sensitization (see below).
Are there itch-specific intracellular signaling pathways in nociceptors?
It was postulated that primary sensory neurons may be able to separate multiple sensations through distinct intracellular signaling [98]. Mounting evidence shows that primary sensory neurons that respond to pruritogens are also sensitive to other noxious stimuli, such as heat and pain [77;88;117]. For example, histamine-responding sensory neurons are also sensitive to capsaicin [136] and chloroquine-sensitive neurons also respond to capsaicin and mustard oil [154]. In addition, TRPV1-expressing primary sensory neurons are required for both thermal pain and itch [62]. It is possible that irritative chemicals may selectively activate distinct receptor and intracellular signaling pathways in the same neurons for transducing distinct itch or pain signaling. This hypothesis was supported by recent finding that SLIGRL peptide, which was regarded as a PAR2 agonist, can activate both MrgprC11 and PAR2 in DRG neurons: MrgprC11 mediates SLIGRL-induced itch, but PAR2 mediates SLIGRL-induced pain [53;89] (also see a prominent role of PAR2 for itch in the previous session). In addition, pruritogens may also activate different intracellular signaling pathways in sensory neurons. For example, Mrgprs are coupled to TRPA1 via distinct signaling: MrgprA3 is coupled to TRPA1 via Gβγ signaling while MrgprC11 can be coupled to TRPA1 via PLC signaling [154].
Are there itch-specific cutaneous sensory fibers?
It is widely accepted that primary sensory neurons that respond to pruritogens are also sensitive to pain stimuli. Thus, it was doubted whether there are itch-specific neurons. However, continuous attempts have been made to identify itch-specific neurons. Of note, some mediators important for pain sensation and development of nociceptors, such as galanin, are not required for itch sensation [56]. Also, various pruritogens, such as histamine, chloroquine, and BAM8-22, were shown to activate a subpopulation of nociceptors [103]. Furthermore, Schmelz and collaborators identified a population of mechanical-insensitive C-fibers that are responsive to histamine, and the firing of these C-fibers matched the itch sensation in human [132], suggesting that these C-fibers might be specific for histamine-induced itch. Capsaicin, a highly potent algesic compound, is able to induce itch in human when specifically delivered to the epidermis [139], suggesting that a subpopulation of TRPV1-expressing fibers that exclusively innervate the superficial layers of the epidermis could be itch-specific.
Recently, Dong’s group, in collaboration with LaMotte’s group, has identified MrgprA3-expressing neurons as itch-specific neurons in mice [51]. MrgprA3 defines a unique population of DRG neurons that co-express calcitonin gene–related peptide (CGRP) but not substance P. In addition, MrgprA3 is also co-localized with IB4 (nonpeptidergic marker) and TRPV1. Interestingly, MrgprA3+ nerve fibers exclusively innervate the epidermis of the skin, providing an anatomical basis for skin-elicited itch. Ablation of MrgprA3-expressing neurons in mice attenuated itch, induced by various pruritogens such as histamine, chloroquine, SLIGRL, BAM8-22, 5-HT, and ET-1, except β-alanine. Moreover, chronic itch induced by dry skin and allergic responses was also decreased in these mice lacking MrgprA3-expressing neurons. Consistently, ablation of CGRP+ population abolished histamine and chloroquine-induced itch [102]. To determine whether selective activation of MrgprA3-expressing fibers by capsaicin would induce itch, Dong’s group employed an elegant strategy to re-express TRPV1 exclusively in MrgprA3-expressing neurons in TRPV1 knockout mice. Strikingly, intradermal injection of capsaicin into the cheek of these mice only induced itch-indicative hindlimb scratching behavior but not pain-indicative forelimb wiping behavior. Thus, selective activation of itch-specific neurons (MrgprA3) only induces itch, regardless the stimulus modality.
Central mechanisms of itch
Spinal cord mechanisms of itch
The itch-specific neuronal circuitry in the spinal cord has also begun to be revealed. Recent studies from Zhen’s group highlighted an essential role of the GRP/GRP receptor (GRPR)-expressing neurons in the spinal cord for itch transmission [94;144;145]. GRPR is expressed in superficial dorsal horn neurons in the laminae I and II [144]. GRPR mutant mice display reduced scratching induced by multiple pruritic agents, especially nonhistaminergic pruritogens, whereas pain sensation is intact in the mutants [144]. Importantly, ablation of GRPR+ neurons in the dorsal horn by intrathecal injection of the neurotoxin bombesin-saporin markedly reduced scratching, evoked by both histaminergic and nonhistaminergic pruritogens [145]. In contrast, elimination of GRPR+ neurons had no effects on pain behaviors. Thus, GRPR+ neurons define a specific population for mediating itch [145]. A follow-up study shows that intrathecal morphine can also activate GRPR to elicit itch by binding to the μ-opioid receptor (MOR) isoform MOR1D that is cross-linked to GRPR [94]. Intriguingly, MrgprA3+ primary afferents may form direct synaptic connections with GRPR+ spinal cord neurons [51]. Together, peripheral itch-specific fibers may directly project to the itch-mediating neurons in spinal cord.
Although GRP peptide was detected in a subset of DRG neurons [90;144], the role of DRG neurons-derived GRP in itch transmission is still unclear. It was found that GRP is more prominently expressed in spinal cord neurons within laminae I and II [40]. It has also recently been demonstrated: 1) capsaicin induced CGRP but not GRP release; and 2) preincubation of capsaicin to deplete neuropeptides from primary afferents prevented bile acids-evoked CGRP release but not GRP release in spinal cord slices [8]. This is consistent with an early observation that capsaicin induced SP but not GRP release in spinal cord slices, while depolarization with KCl evoked Ca2+-dependent GRP release [108]. Thus, activation of peripheral TGR5-expressing itch receptors by bile acids might release GRP from spinal neurons but not from primary afferents. Although glutamate was proposed as the principal excitatory neurotransmitter between C fibers and GRPR+ spinal neurons [78], SP and CGRP may also play a role in itch transmission in the spinal cord. In addition to a central role of GRP in itch control, serum levels of GRP were found to be correlated with itch severity in atopic dermatitis patients [69], and intradermal injection of GRP induced scratching behavior in mice through activation of mast cells [13]. Therefore, GRP also plays a role in peripheral itch signaling.
The population of the SP receptor NK1-expressing neurons, most of which are spinothalamic tract (STT) neurons, has been implicated in both pain and itch sensation [61]. In rats, elimination of NK1R+ neurons with intrathecal substance P-saporin in the spinal cord leads to deficits in both pain and itch, suggesting that this cell population of neurons is critical for both itch and pain [25;101;111]. However, the precise role of NK1+ neurons in mediating itch in mice still need further investigation. It will be of particular interest to examine whether NK1+ neurons and GRPR+ neurons are overlapping (Box-2).
A subset of spinal inhibitory interneurons, whose development requires the expression of the transcription factor Bhlhb5, was recently identified to be required for itch suppression [127;128]. Ablation of Bhlhb5 from an inhibitory interneuron population, which can also be labeled by the transcription factor Pax2, leads to skin lesions and enhanced scratching in mice [127]. Future studies are needed to determine the relationship between Bhlhb5+ neurons and GRPR+ neurons and the neurotransmitters that are used by Bhlhb5+ itch-suppressing neurons (Figure 2 and Box-2).
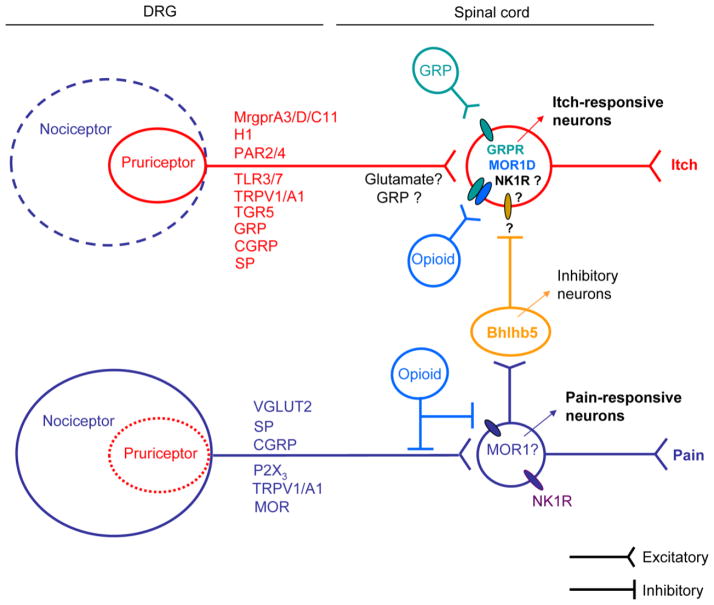
Figure 2. Crosstalk of the itch and pain pathways in physiological conditions.
Itch and pain are encoded by the unique labeled lines in the physiological conditions. In dorsal root ganglia or trigeminal ganglia, the large population of pain-responsive neurons (e.g., TRPV1-expressing neurons) consists of the small population of itch-responsive neurons (e.g. MrgprA3-expressing neurons) which are polymodal and also respond to painful stimuli. These pruriceptors may also express H1, PAR2, TLR3/7, TRPA1/V1, and neuropeptides (GRP, CGRP, SP). Activation of itch primary afferents stimulates neurotransmitter release from the central terminals of the pruriceptors in the spinal cord dorsa horn, leading to GRP and opioids release to activate GRPR and MOR1D-expressing neurons for itch transmission. Activation of nociceptors results in the activation of secondary nociceptive neurons (e.g., NK1R+ neurons) in the spinal cord for nociceptive transmission. Activation of nociceptors also causes subsequent activation of Bhlhb5+ inhibitory neurons to suppress itch transmission in GRPR+ neurons. In addition, spinal cord opioids can activate MOR1 to suppress pain and also activate MOR1D to elicit itch via its coupling to GRPR.
We recently identified TLR3 as a critical regulator of synaptic transmission and central sensitization underlying itch induction and sensitization in mice [90]. TLR3 is not only expressed in cell bodies of DRG neurons but is also transported to central axonal terminals in the spinal cord to modulate synaptic transmission in capsaicin-sensitive C-fibers [90]. Deletion of Tlr3 results in a reduction in excitatory synaptic transmission (i.e. decrease in the frequency of spontaneous excitatory postsynaptic currents, sEPSCs). Induction of long-term potentiation (LTP) in the intact spinal cord is also abolished in Tlr3−/− mice but not Tlr7−/− mice [90]. Thus, TLR3 may regulate itch via both peripheral and central mechanisms [90].
Brain Mechanisms of itch
Brain imaging techniques, such as positron emission tomography (PET) and functional magnetic resonance imaging or functional MRI (fMRI), have begun to unravel the brain circuitries for itch perception [61;153]. Itch is a multidimensional perception encompassing sensory discriminative, affective, and emotional components. It is extensively processed by the brain network, including the primary and secondary sensory cortex, supramarginal gyrus, inferior parietal lobe, anterior and posterior cingulate cortex, precuneus and insula-claustrum complex [72]. Although itch and pain are distinct sensations, the same brain regions, such as the amygdala, hippocampus, and hypothalamus, can be activated by both sensations. In healthy volunteers, histamine-induced itch activates a diffuse brain network, while cowhage-induced histamine-independent itch induces greater activation of the insula, thalamus, and putamen [120].
Mechanisms of chronic itch
Peripheral sensitization and neural-immune interactions
Skin injury and inflammation result in recruitment of immune cells (e.g., diverse innate immune cells, T lymphocytes). Activated immune cells release proinflammatory mediators to sensitize pruriceptors. This peripheral sensitization contributes importantly not only to chronic pain and but also to chronic itch [18;61]. In pathological conditions, the population of pruriceptors could be enlarged and also exhibits enhanced responses to pruritogens [3;5]. For example, in dry skin-induced chronic itch, the populations of primary sensory neurons responding to PAR2 agonist and 5-HT are enlarged, which is associated with enhanced scratching responses to these pruritogens [3]. In the skin, neural-mast cell and neural-keratinocyte interactions play an important role in peripheral sensitization [61;98]. Degranulation of mast cells leads to the release of histamine [30;38;136], serotonin [71;156], tryptase[143], TNF-α [157], prostaglandin E1/2 (PGE1/2) [97] and leukotriene B4 (LTB4) [11;12;14]. Activated keratinocytes also release ET-1, proteases (e.g. cathepsin S) [123] and β-endorphine [82;83;158]. These inflammatory mediators sensitize pruriceptors or/and disrupt barrier function in the epidermis and result in dry skin injury [61]. T cells can release cytokines such as IL-2 and IL-31, which have been shown to activate and/or sensitize pruriceptors. In addition, neurotrophins such as nerve growth factor (NGF) from keratinocytes or mast cells [129] and artemin from fibroblasts [109] may cause long-term structural reorganization (sprouting) of free nerve endings including itch fibers (Figure 3). In atopic dermatitis, contact dermatitis and prurigo nodularis, serum and local NGF is remarkably increased [59]. Interestingly, NGF up-regulation in dry skin is reduced in Tlr3−/− mice, which is associated with a substantial reduction of chronic itch in these knockout mice [90]. Anti-inflammation and anti-NGF strategies may be beneficial for treating both chronic itch and chronic pain.
Figure 3. Crosstalk of the itch and pain pathways in pathological conditions.
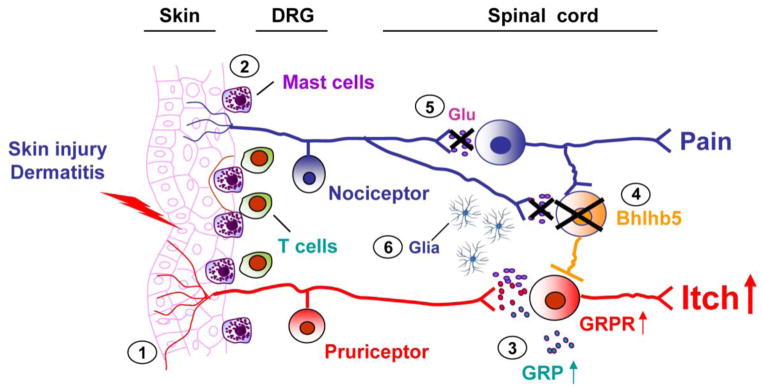
In pathological conditions, multiple mechanisms contribute to the development of chronic itch. (1) Activation of mast cells and T cells in injured skins causes the release of itch mediators such as histamine and IL-31, leading to the activation of pruriteptors and induction of peripheral sensitization. (2) Growth factors (e.g., NGF or artemin), released from skin cells may cause sprouting of C-fibers (including pruriceptive fibers), leading to itch hypersensitivity. Furthermore, tissue injury and damage or dysfunction of the nervous system causes central sensitization in spinal cord neurons which is associated with (3) up-regulation of GRP and GRPR, (4) decrease or loss of inhibitory control from Bhlhb5+ neurons, (5) decrease of VGLUT2-dependent glutamate release from nociceptors, and (6) activation of glial cells (astrocytes or microglia) in the spinal cord. Thus, the crosstalk of the pain and itch labeled lines are disrupted in pathological conditions. Central sensitization not only drives chronic pain but also potentiates and maintains chronic itch. In addition, descending facilitation from the brainstem may further potentiate chronic itch. These mechanisms of peripheral and central sensitization are also known to drive chronic pain.
Cytokines, including IL-2, IL-6 and IL-31, are recently highlighted as key mediators for itch sensation [142] (Figures 1 and 3). Among them, IL-31 is getting more attention. Transgenic mice over-expressing IL-31 developed chronic itch, alopecia, and skin lesions [36;141]. The activated T lymphocytes, particularly T helper type 2 cells, are one of the main sources for IL-31. IL-31 binds to a heterodimeric receptor composed of IL-31 receptor (IL-31RA) and oncostatin M receptor (OSMR) to activate several signaling pathways, such as the Janus tyrosine kinase (JAK), signal transducers and activators of transcription (STAT), phosphatidylinositol 3-kinase/AKT (PI3K/AKT) and p38 MAPK pathway in keratinocytes and monocytes [36;141]. Fluorescence activated cell sorting (FACS) analysis also showed that the skin of chronic atopic dermatitis patients contains increased levels of IL-31-producing T cells. IL-31 mRNA is significantly up-regulated in serum and skin of patients with pruritic atopic dermatitis [39;141;147]. Canine IL-31 was also detected in the majority of dogs with naturally occurring atopic dermatitis and intradermal injection of it induced pruritic behaviors in dogs [46]. Higher levels of blood β-endorphin and IL-31 significantly correlated itch severity in atopic dermatitis patients [83]. Moreover, immunohistochemistry data showed that IL-31RA and β-endorphin were increased and colocalized in the skin of atopic dermatitis patients [83]. IL-31 receptor activation in keratinocytes induces calcium influx and STAT3-dependent production of β-endorphin, leading to itch this is associated with atopic dermatitis [83]. Application of IL-31 antibody reduces scratching behavior in NC/Nga mice with atopic dermatitis [50]. IL-31RA and OSMR are co-expressed in a subset of DRG neurons [16], suggesting that T cell-derived IL-31 may directly excite primary sensory neurons to elicit itch sensation. However, the antibody used for the localization of IL-31RA in this paper also showed positive stains in IL-31RA KO mice [16], raising the concern of non-specific staining. Thus, the direct evidence for the expression of IL-31RA in DRG neurons and activation of DRG neurons by IL-31 is still lacking. Nevertheless, IL-31 may serve as an ideal mediator for neural-immune interaction in chronic itch.
Central sensitization and neural-glial interactions
In chronic pain, tissue injury and persistent noxious input to the spinal cord result in central sensitization [64], i.e. hyperactivity of spinal cord projection neurons and excitatory interneurons, which is responsible for allodynia (pain induced by normally innocuous stimuli, e.g., light touch), and hyperalgesia (enhanced responses to noxious stimuli, e.g. pinch) [18]. In chronic itch, ongoing activity of pruriceptors after skin injury also results in central sensitization to drive itch. Enhanced responses of spinal cord neurons to PAR-2 agonists were demonstrated in a dry skin model [5]. Peripheral GRP expression was up-regulated in a mouse model of pruritic atopic dermatitis [149]. GRP/GRPR signaling in the spinal cord may also be up-regulated in chronic itch to enhance itch-related central sensitization.
It is well established that activation of glial cells in the spinal cord plays an important role in the pathogenesis of chronic pain [23;42;124]. Microglia and astrocytes are activated following tissue or nerve injury [66;67]. Activation of p38 MAPK in microglia results in the expression and release of the cytokines TNF-α and IL-1β and the brain-derived neurotrophic factor (BDNF) [65]. On the other hand, activation of c-Jun N-terminal kinase (JNK) signaling in astrocytes results in the release of chemokine (C-C motif) ligand 2 (CCL2) [44]. These glial mediators (TNF-α, IL-1β, BDNF, and CCL2) can cause central sensitization and persistent pain by enhancing excitatory synaptic transmission and suppressing inhibitory synaptic transmission in spinal cord neurons [43;44]. Whether these glial mediators can cause central sensitization of GRPR-expressing pruriceptive neurons in the spinal cord warrants further investigation. Glial activation may also be involved in neuropathic itch [114;115]. Intramedullary cavernous hemangiomas was associated with chronic neuropathic itch in the corresponding dermatome and featured as gliosis and hemosiderin deposition after hemorrhage [24;35;81].
Central sensitization in the brain regions is also involved in chronic itch. Allergen-induced itch in patients provokes robust activation of the striato-thalamo-cortical circuit, which is involved in motivational processing and associated with the urge to scratch in chronic itch condition [85]. In addition, descending facilitation from the cortex/brain stem to the spinal cord, which has been strongly implicated in chronic pain sensitization, may also play a role in chronic itch [72].
Crosstalk between the itch and pain pathways
Crosstalk between the itch and pain pathways in physiological conditions
Itch and pain represent two distinct unpleasant sensations and evoke distinct behavioral responses: pain evokes withdrawal reflex while itch elicits scratching responses [61]. Itch and pain are also closely related: itch can be suppressed by scratching and painful stimuli, such as noxious heat, noxious chemicals, and electric shock in human [131]. In line of these observations, itch is also inhibited within the area that can generate capsaicin-induced secondary mechanical hypersensitivity in human [47]. Interestingly, scratching behaviors induced by chloroquine [49], imiquimod [74;93] and hydrogen peroxide [92] display inverted U-shaped dose-response curves in rodents, suggesting that these pruritogens at higher doses produce pain to suppress itch. It was also found that mouse strains that are sensitive to pain are less sensitive to itch and vice versa [49].
On the other hand, inhibition of pain may produce itch [61]. The best-known example is spinal administration of morphine, which inhibits pain but elicits itch [107]. It was found that spinal itch-responding neurons do not exhibit spontaneous activity, possibly due to the tonic inhibition of these neurons by pain-relay neurons that are spontaneously active [61;119]. It was speculated that morphine-induced itch may be attributable to disinhibition of itch, by relieving pain-induced suppression in the spinal cord [15]. However, this hypothesis was challenged by a recent study showing that intrathecal morphine produces itch via direct activation of the MOR isoform MOR1D that forms a heterodimerizer with GRPR, whereas morphine-induced spinal analgesia is mediated by a different isoform, MOR1 [94]. Thus, morphine may directly activate itch-selective neurons in spinal cord to produce itch, and therefore, morphine-induced analgesia is not a prerequisite for the generation of itch. However, it is still important to investigate whether inhibition of pain is sufficient for the induction of itch.
The population coding theory was proposed to explain the coding of pain versus itch and has gained experimental support [7;96;99;100;117]. As demonstrated in Figure 2, there are two populations of primary sensory neurons that respond to pain and itch, respectively. Itch-specific population (pruriceptors) such as MrgprA3+ neurons is a much smaller population within pain-specific population (nociceptors) expressing TRPV1. Notably, pruriceptors are polymodal and also respond to algogens such as capsaicin or mustard oil [88;89;144;145]. Activation of itch afferents stimulates release of certain neurotransmitters from the spinal cord central terminals of primary afferents, leading to subsequent release of GRP and opioid peptide(s) to activate itch-selective neurons that express GRPR and MOR1D. If a stimulus selectively activates the small itch-population, regardless of the stimulus modalities, it could generate itch. On the other hand, activation of a larger population of pain primary afferents will release glutamate and neuropeptides (SP and CGRP) in the spinal cord to activate pain-mediating neurons (e.g., NK1R+ neurons), and meanwhile, also activates Bhlhb5+ inhibitory neurons to suppress itch. If a strong stimulus activates both pain and itch afferents, pain would dominate by masking itch via an inhibitory circuit (Figure 2). Mixed pain and itch sensations may also be produced, if pain stimulus is not sufficient to mask itch [99].
Crosstalk between the itch and pain pathways in pathological conditions
Recent animal studies suggested that dysfunction of pain-itch crosstalk may be involved in the development of chronic itch [95;127] (Figure 3). Mice lacking a subset of inhibitory interneurons, labeled by the transcription factor Bhlhb5 in spinal cord dorsal horn, display enhanced chronic itch phenotype [127]. Another study showed that ablation of vesicular glutamate transporter 2 (VGLUT2)-dependent glutamate release from Nav1.8-expressing nociceptors leads to pain deficits and itch hypersensitivity in mice [95]. Notably, intradermal injection of capsaicin induces itch but not pain in these conditional knockout mice lacking vGlut2 in nociceptors [95]. It is suggested that glutamate released from the central branches of nociceptors not only triggers pain transmission but also activates inhibitory neurons for itch suppression.
Under the pathological conditions, painful stimuli may not be sufficient to suppress itch. Instead, pain may enhance itch in chronic itch patients. Itch stimuli can be perceived as pain in some chronic neuropathic pain condition [17]. For example, normally painful stimuli, such as electrical stimuli, bradykinin or acetylcholine evoked itching in patients with atopic dermatitis [57;113] and mice with inflamed skin [86]. It is noteworthy that in “itchy skin”, touch or brush evokes itch near the site of itch, termed “alloknesis” [6].
Concluding remarks
Although closely related to pain, itch is a distinct sensation that causes scratching. Recent progress has indicated the existence of an itch-specific neuronal circuitry, formed in part by the MrgprA3-expressing primary sensory neurons and GRPR-expressing neurons in the superficial spinal cord. There is distinct crosstalk of itch and pain in physiological and pathological conditions. Acute itch can be inhibited by scratching and painful stimuli. An antagonistic interaction between pain and itch is further revealed by morphine-elicited itch and analgesia. Itch and pain employ largely overlapping receptors, such as TRPV1, TRPA1, TLRs and PARs.
Itch and pain appear to have more similarities in pathological and chronic conditions (Box-2). Dysfunction of the pain-itch crosstalk in pathological conditions may trigger chronic itch. Central sensitization, which has been widely implicated in the genesis of chronic pain (e.g., touch-evoked pain, allodynia), also play an important role in chronic itch, as manifested by and touch-evoked itch (alloknesis). Chronic itch is often diagnosed and treated by dermatologists as an immune disease. However, chronic itch is also a disorder of the nervous system, triggered by immune dysfunction. The striking similarities between chronic itch and chronic pain have important clinical implications, because chronic pain and chronic itch are likely to coexist in patients, such as those with neuropathic postherpetic pain and itch [114;115]. Many treatments and prescribed drugs for chronic pain control are also effective for treating chronic itch. For example, treatments like vagus nerve stimulation [76] and acupuncture [110] and drugs like gabapentin and pregabalin [1;61;140] have been shown to reduce chronic itch after burn, renal failure and multiple sclerosis. Antidepressants, such as amtryptiline and mirtazapine, which are widely used for treating neuropathic pain, also reduced psychiatric itch and cholestasis itch [61].
There are many outstanding questions remaining in the itch field, as indicated in Box 2. The neurotransmitters for spinal cord itch transmission and the identities (projection neurons vs. interneurons) of GRPR-expressing neurons are still unclear. It is also of great interest to investigate whether and how glial cells control itch transmission. Do cytokines play distinct role in regulating pain and itch transmission? Notably, the currently used animal models focus on acute itch induced by individual pruritogens or chronic itch evoked by atopic dermatitis and dry skin injury. However, animal models are still unavailable to mimic chronic itch after systemic diseases, such as liver or kidney diseases. The development of clinically relevant animal models of chronic itch is urgently needed. Little is known about the brain modulation of itch. Brain-imaging techniques and psychophysical protocols have been developed to study itch in humans, which will help to clarify the brain mechanisms of chronic itch and further reveal the interactions of itch and pain in the brain. Despite the fact that drugs for chronic pain treatments have demonstrated some success in chronic itch relief, elucidation of unique mechanisms underlying chronic itch will eventually lead to more selective anti-itch treatments.
Acknowledgments
This study is supported in part by NIH R01 grants DE17794, DE22743, and NS67686.
Footnotes
The authors have no financial interest in this study.
Reference List
- 1.Ahuja RB, Gupta GK. A four arm, double blind, randomized and placebo controlled study of pregabalin in the management of post-burn pruritus. Burns. 2012 doi: 10.1016/j.burns.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010;151:378–383. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akiyama T, Carstens MI, Carstens E. Spontaneous itch in the absence of hyperalgesia in a mouse hindpaw dry skin model. Neurosci Lett. 2010;484:62–65. doi: 10.1016/j.neulet.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akiyama T, Carstens MI, Carstens E. Enhanced responses of lumbar superficial dorsal horn neurons to intradermal PAR-2 agonist but not histamine in a mouse hindpaw dry skin itch model. J Neurophysiol. 2011;105:2811–2817. doi: 10.1152/jn.01124.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, Carstens E. Mouse model of touch-evoked itch (alloknesis) J Invest Dermatol. 2012;132:1886–1891. doi: 10.1038/jid.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akiyama T, Merrill AW, Carstens MI, Carstens E. Activation of superficial dorsal horn neurons in the mouse by a PAR-2 agonist and 5-HT: potential role in itch. J Neurosci. 2009;29:6691–6699. doi: 10.1523/JNEUROSCI.6103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alemi F, Kwon E, Poole DP, Lieu T, Lyo V, Cattaruzza F, Cevikbas F, Steinhoff M, Nassini R, Materazzi S, Guerrero-Alba R, Valdez-Morales E, Cottrell GS, Schoonjans K, Geppetti P, Vanner SJ, Bunnett NW, Corvera CU. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest. 2013 doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, Bunnett NW. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–571. doi: 10.1113/jphysiol.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis JB, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24:4300–4312. doi: 10.1523/JNEUROSCI.5679-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andoh T, Katsube N, Maruyama M, Kuraishi Y. Involvement of leukotriene B(4) in substance P-induced itch-associated response in mice. J Invest Dermatol. 2001;117:1621–1626. doi: 10.1046/j.0022-202x.2001.01585.x. [DOI] [PubMed] [Google Scholar]
- 12.Andoh T, Kuraishi Y. Intradermal leukotriene B4, but not prostaglandin E2, induces itch-associated responses in mice. Eur J Pharmacol. 1998;353:93–96. doi: 10.1016/s0014-2999(98)00440-3. [DOI] [PubMed] [Google Scholar]
- 13.Andoh T, Kuwazono T, Lee JB, Kuraishi Y. Gastrin-releasing peptide induces itch-related responses through mast cell degranulation in mice. Peptides. 2011;32:2098–2103. doi: 10.1016/j.peptides.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Andoh T, Saito A, Kuraishi Y. Leukotriene B(4) mediates sphingosylphosphorylcholine-induced itch-associated responses in mouse skin. J Invest Dermatol. 2009;129:2854–2860. doi: 10.1038/jid.2009.155. [DOI] [PubMed] [Google Scholar]
- 15.Ballantyne JC, Loach AB, Carr DB. Itching after epidural and spinal opiates. Pain. 1988;33:149–160. doi: 10.1016/0304-3959(88)90085-1. [DOI] [PubMed] [Google Scholar]
- 16.Bando T, Morikawa Y, Komori T, Senba E. Complete overlap of interleukin-31 receptor A and oncostatin M receptor beta in the adult dorsal root ganglia with distinct developmental expression patterns. Neuroscience. 2006;142:1263–1271. doi: 10.1016/j.neuroscience.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Baron R, Schwarz K, Kleinert A, Schattschneider J, Wasner G. Histamine-induced itch converts into pain in neuropathic hyperalgesia. Neuroreport. 2001;12:3475–3478. doi: 10.1097/00001756-200111160-00020. [DOI] [PubMed] [Google Scholar]
- 18.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Bell JK, McQueen DS, Rees JL. Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in Balb C mice. Br J Pharmacol. 2004;142:374–380. doi: 10.1038/sj.bjp.0705754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergasa NV. The pruritus of cholestasis. J Hepatol. 2005;43:1078–1088. doi: 10.1016/j.jhep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Biro T, Toth BI, Marincsak R, Dobrosi N, Geczy T, Paus R. TRP channels as novel players in the pathogenesis and therapy of itch. Biochim Biophys Acta. 2007;1772:1004–1021. doi: 10.1016/j.bbadis.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Calvo M, Dawes JM, Bennett DL. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012;11:629–642. doi: 10.1016/S1474-4422(12)70134-5. [DOI] [PubMed] [Google Scholar]
- 24.Carstens E. Scratching the brain to understand neuropathic itch. J Pain. 2008;9:973–974. doi: 10.1016/j.jpain.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport. 2010;21:303–308. doi: 10.1097/WNR.0b013e328337310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15:1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi JW, Chun J. Lysophospholipids and their receptors in the central nervous system. Biochim Biophys Acta. 2013;1831:20–32. doi: 10.1016/j.bbalip.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa R, Manjavachi MN, Motta EM, Marotta DM, Juliano L, Torres HA, Pesquero JB, Calixto JB. The role of kinin B1 and B2 receptors in the scratching behaviour induced by proteinase-activated receptor-2 agonists in mice. Br J Pharmacol. 2010;159:888–897. doi: 10.1111/j.1476-5381.2009.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa R, Marotta DM, Manjavachi MN, Fernandes ES, Lima-Garcia JF, Paszcuk AF, Quintao NL, Juliano L, Brain SD, Calixto JB. Evidence for the role of neurogenic inflammation components in trypsin-elicited scratching behaviour in mice. Br J Pharmacol. 2008;154:1094–1103. doi: 10.1038/bjp.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cowden JM, Zhang M, Dunford PJ, Thurmond RL. The histamine H4 receptor mediates inflammation and pruritus in Th2-dependent dermal inflammation. J Invest Dermatol. 2010;130:1023–1033. doi: 10.1038/jid.2009.358. [DOI] [PubMed] [Google Scholar]
- 31.Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci. 2004;24:4293–4299. doi: 10.1523/JNEUROSCI.0454-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550–558. doi: 10.1016/j.tins.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat Neurosci. 2009;12:544–546. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dey DD, Landrum O, Oaklander AL. Central neuropathic itch from spinal-cord cavernous hemangioma: a human case, a possible animal model, and hypotheses about pathogenesis. Pain. 2005;113:233–237. doi: 10.1016/j.pain.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 36.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, Haugen HS, Maurer M, Harder B, Johnston J, Bort S, Mudri S, Kuijper JL, Bukowski T, Shea P, Dong DL, Dasovich M, Grant FJ, Lockwood L, Levin SD, LeCiel C, Waggie K, Day H, Topouzis S, Kramer J, Kuestner R, Chen Z, Foster D, Parrish-Novak J, Gross JA. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–760. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 37.Due MR, Piekarz AD, Wilson N, Feldman P, Ripsch MS, Chavez S, Yin H, Khanna R, White FA. Neuroexcitatory effects of morphine-3-glucuronide are dependent on Toll-like receptor 4 signaling. J Neuroinflammation. 2012;9:200. doi: 10.1186/1742-2094-9-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunford PJ, Williams KN, Desai PJ, Karlsson L, McQueen D, Thurmond RL. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J Allergy Clin Immunol. 2007;119:176–183. doi: 10.1016/j.jaci.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 39.Ezzat MH, Hasan ZE, Shaheen KY. Serum measurement of interleukin-31 (IL-31) in paediatric atopic dermatitis: elevated levels correlate with severity scoring. J Eur Acad Dermatol Venereol. 2011;25:334–339. doi: 10.1111/j.1468-3083.2010.03794.x. [DOI] [PubMed] [Google Scholar]
- 40.Fleming MS, Ramos D, Han SB, Zhao J, Son YJ, Luo W. The majority of dorsal spinal cord gastrin releasing peptide is synthesized locally whereas neuromedin B is highly expressed in pain- and itch-sensing somatosensory neurons. Mol Pain. 2012;8:52. doi: 10.1186/1744-8069-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frateschi S, Camerer E, Crisante G, Rieser S, Membrez M, Charles RP, Beermann F, Stehle JC, Breiden B, Sandhoff K, Rotman S, Haftek M, Wilson A, Ryser S, Steinhoff M, Coughlin SR, Hummler E. PAR2 absence completely rescues inflammation and ichthyosis caused by altered CAP1/Prss8 expression in mouse skin. Nat Commun. 2011;2:161. doi: 10.1038/ncomms1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao YJ, Xu ZZ, Liu YC, Wen YR, Decosterd I, Ji RR. The c-Jun N-terminal kinase 1 (JNK1) in spinal astrocytes is required for the maintenance of bilateral mechanical allodynia under a persistent inflammatory pain condition. Pain. 2010;148:309–319. doi: 10.1016/j.pain.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomes LO, Hara DB, Rae GA. Endothelin-1 induces itch and pain in the mouse cheek model. Life Sci. 2012;91:628–633. doi: 10.1016/j.lfs.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 46.Gonzales AJ, Humphrey WR, Messamore JE, Fleck TJ, Fici GJ, Shelly JA, Teel JF, Bammert GF, Dunham SA, Fuller TE, McCall RB. Interleukin-31: its role in canine pruritus and naturally occurring canine atopic dermatitis. Vet Dermatol. 2013;24:48–53. doi: 10.1111/j.1365-3164.2012.01098.x. [DOI] [PubMed] [Google Scholar]
- 47.GRAHAM DT, GOODELL H, WOLFF HG. Neural mechanisms involved in itch, itchy skin, and tickle sensations. J Clin Invest. 1951;30:37–49. doi: 10.1172/JCI102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, Altier C, Cenac N, Zamponi GW, Bautista-Cruz F, Lopez CB, Joseph EK, Levine JD, Liedtke W, Vanner S, Vergnolle N, Geppetti P, Bunnett NW. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green AD, Young KK, Lehto SG, Smith SB, Mogil JS. Influence of genotype, dose and sex on pruritogen-induced scratching behavior in the mouse. Pain. 2006;124:50–58. doi: 10.1016/j.pain.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 50.Grimstad O, Sawanobori Y, Vestergaard C, Bilsborough J, Olsen UB, Gronhoj-Larsen C, Matsushima K. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: a model of atopic dermatitis. Exp Dermatol. 2009;18:35–43. doi: 10.1111/j.1600-0625.2008.00766.x. [DOI] [PubMed] [Google Scholar]
- 51.Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, LaMotte RH, Dong X. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 53.Han SK, Simon MI. Intracellular signaling and the origins of the sensations of itch and pain. Sci Signal. 2011;4:e38. doi: 10.1126/scisignal.2002353. [DOI] [PubMed] [Google Scholar]
- 54.Hashimoto T, Ohata H, Momose K. Itch-scratch responses induced by lysophosphatidic acid in mice. Pharmacology. 2004;72:51–56. doi: 10.1159/000078632. [DOI] [PubMed] [Google Scholar]
- 55.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 56.Holmes FE, Vanderplank P, Wynick D. Galanin-expression and galanin- dependent sensory neurons are not required for itch. Mol Pain. 2012;8:87. doi: 10.1186/1744-8069-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain. 2006;126:16–23. doi: 10.1016/j.pain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Huang JF, Thurmond RL. The new biology of histamine receptors. Curr Allergy Asthma Rep. 2008;8:21–27. doi: 10.1007/s11882-008-0005-y. [DOI] [PubMed] [Google Scholar]
- 59.Ikoma A, Cevikbas F, Kempkes C, Steinhoff M. Anatomy and neurophysiology of pruritus. Semin Cutan Med Surg. 2011;30:64–70. doi: 10.1016/j.sder.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ikoma A, Rukwied R, Stander S, Steinhoff M, Miyachi Y, Schmelz M. Neurophysiology of pruritus: interaction of itch and pain. Arch Dermatol. 2003;139:1475–1478. doi: 10.1001/archderm.139.11.1475. [DOI] [PubMed] [Google Scholar]
- 61.Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 62.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeffry J, Kim S, Chen ZF. Itch signaling in the nervous system. Physiology (Bethesda ) 2011;26:286–292. doi: 10.1152/physiol.00007.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 65.Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang F, Liu T, Cheng M, Pang XY, Bai ZT, Zhou JJ, Ji YH. Spinal astrocyte and microglial activation contributes to rat pain-related behaviors induced by the venom of scorpion Buthus martensi Karch. Eur J Pharmacol. 2009;623:52–64. doi: 10.1016/j.ejphar.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 67.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kagami S, Sugaya M, Suga H, Morimura S, Kai H, Ohmatsu H, Fujita H, Tsunemi Y, Sato S. Serum Gastrin-Releasing Peptide Levels Correlate with Pruritus in Patients with Atopic Dermatitis. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.38. [DOI] [PubMed] [Google Scholar]
- 70.Kim BM, Lee SH, Shim WS, Oh U. Histamine-induced Ca(2+) influx via the PLA(2)/lipoxygenase/TRPV1 pathway in rat sensory neurons. Neurosci Lett. 2004;361:159–162. doi: 10.1016/j.neulet.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 71.Kim DK, Kim HJ, Kim H, Koh JY, Kim KM, Noh MS, Kim JJ, Lee CH. Involvement of serotonin receptors 5-HT1 and 5-HT2 in 12(S)-HPETE-induced scratching in mice. Eur J Pharmacol. 2008;579:390–394. doi: 10.1016/j.ejphar.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 72.Kim HS, Yosipovitch G. An aberrant parasympathetic response: a new perspective linking chronic stress and itch. Exp Dermatol. 2013;22:239–244. doi: 10.1111/exd.12070. [DOI] [PubMed] [Google Scholar]
- 73.Kim N, Bae KB, Kim MO, Yu DH, Kim HJ, Yuh HS, Ji YR, Park SJ, Kim S, Son KH, Park SJ, Yoon D, Lee DS, Lee S, Lee HS, Kim TY, Ryoo ZY. Overexpression of cathepsin S induces chronic atopic dermatitis in mice. J Invest Dermatol. 2012;132:1169–1176. doi: 10.1038/jid.2011.404. [DOI] [PubMed] [Google Scholar]
- 74.Kim SJ, Park GH, Kim D, Lee J, Min H, Wall E, Lee CJ, Simon MI, Lee SJ, Han SK. Analysis of cellular and behavioral responses to imiquimod reveals a unique itch pathway in transient receptor potential vanilloid 1 (TRPV1)-expressing neurons. Proc Natl Acad Sci U S A. 2011;108:3371–3376. doi: 10.1073/pnas.1019755108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kini SP, Delong LK, Veledar E, McKenzie-Brown AM, Schaufele M, Chen SC. The Impact of Pruritus on Quality of Life: The Skin Equivalent of Pain. Arch Dermatol. 2011;147:1153–1156. doi: 10.1001/archdermatol.2011.178. [DOI] [PubMed] [Google Scholar]
- 76.Kirchner A, Stefan H, Schmelz M, Haslbeck KM, Birklein F. Influence of vagus nerve stimulation on histamine-induced itching. Neurology. 2002;59:108–112. doi: 10.1212/wnl.59.1.108. [DOI] [PubMed] [Google Scholar]
- 77.Klein A, Carstens MI, Carstens E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J Neurophysiol. 2011;106:1078–1088. doi: 10.1152/jn.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koga K, Chen T, Li XY, Descalzi G, Ling J, Gu J, Zhuo M. Glutamate acts as a neurotransmitter for gastrin releasing peptide-sensitive and insensitive itch-related synaptic transmission in mammalian spinal cord. Mol Pain. 2011;7:47. doi: 10.1186/1744-8069-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kremer AE, Martens JJ, Kulik W, Rueff F, Kuiper EM, van Buuren HR, van Erpecum KJ, Kondrackiene J, Prieto J, Rust C, Geenes VL, Williamson C, Moolenaar WH, Beuers U, Oude Elferink RP. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139:1008–18. 1018. doi: 10.1053/j.gastro.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 80.LaMotte RH, Shimada SG, Sikand P. Mouse models of acute, chemical itch and pain in humans. Exp Dermatol. 2011;20:778–782. doi: 10.1111/j.1600-0625.2011.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lanotte M, Panciani PP, Magistrello M, Naldi A, Fontanella M, Ducati A, Giordana MT. Central neuropathic itch as the presenting symptom of an intramedullary cavernous hemangioma: Case report and review of literature. Clin Neurol Neurosurg. 2012 doi: 10.1016/j.clineuro.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 82.Lee CH, Chuang HY, Shih CC, Jong SB, Chang CH, Yu HS. Transepidermal water loss, serum IgE and beta-endorphin as important and independent biological markers for development of itch intensity in atopic dermatitis. Br J Dermatol. 2006;154:1100–1107. doi: 10.1111/j.1365-2133.2006.07191.x. [DOI] [PubMed] [Google Scholar]
- 83.Lee CH, Hong CH, Yu WT, Chuang HY, Huang SK, Chen GS, Yoshioka T, Sakata M, Liao WT, Ko YC, Yu HS. Mechanistic correlations between two itch biomarkers, cytokine interleukin-31 and neuropeptide beta-endorphin, via STAT3/calcium axis in atopic dermatitis. Br J Dermatol. 2012;167:794–803. doi: 10.1111/j.1365-2133.2012.11047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee J, Kim T, Hong J, Woo J, Min H, Hwang E, Lee SJ, Lee CJ. Imiquimod enhances excitability of dorsal root ganglion neurons by inhibiting background (K(2P)) and voltage-gated (K(v)1.1 and K(v)1. 2) potassium channels. Mol Pain. 2012;8:2. doi: 10.1186/1744-8069-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leknes SG, Bantick S, Willis CM, Wilkinson JD, Wise RG, Tracey I. Itch and motivation to scratch: an investigation of the central and peripheral correlates of allergen- and histamine-induced itch in humans. J Neurophysiol. 2007;97:415–422. doi: 10.1152/jn.00070.2006. [DOI] [PubMed] [Google Scholar]
- 86.Liang J, He Y, Ji W. Bradykinin-evoked scratching responses in complete Freund’s adjuvant-inflamed skin through activation of B1 receptor. Exp Biol Med (Maywood ) 2012;237:318–326. doi: 10.1258/ebm.2011.011308. [DOI] [PubMed] [Google Scholar]
- 87.Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte RH, Dong X. Mechanisms of Itch Evoked by beta-Alanine. J Neurosci. 2012;32:14532–14537. doi: 10.1523/JNEUROSCI.3509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Q, Weng HJ, Patel KN, Tang Z, Bai H, Steinhoff M, Dong X. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci Signal. 2011;4:ra45. doi: 10.1126/scisignal.2001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu T, Berta T, Xu ZZ, Park CK, Zhang L, Lu N, Liu Q, Liu Y, Gao YJ, Liu YC, Ma Q, Dong X, Ji RR. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J Clin Invest. 2012;122:2195–2207. doi: 10.1172/JCI45414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28:131–144. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu T, Ji RR. Oxidative stress induces itch via activation of transient receptor potential subtype ankyrin 1 in mice. Neurosci Bull. 2012;28:145–154. doi: 10.1007/s12264-012-1207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat Neurosci. 2010;13:1460–1462. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, Li QF, Jeffry J, Kim JY, Loh HH, Chen ZF. Unidirectional Cross-Activation of GRPR by MOR1D Uncouples Itch and Analgesia Induced by Opioids. Cell. 2011;147:447–458. doi: 10.1016/j.cell.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Y, Abdel SO, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Y, Ma Q. Generation of somatic sensory neuron diversity and implications on sensory coding. Curr Opin Neurobiol. 2011;21:52–60. doi: 10.1016/j.conb.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lovell CR, Burton PA, Duncan EH, Burton JL. Prostaglandins and pruritus. Br J Dermatol. 1976;94:273–275. doi: 10.1111/j.1365-2133.1976.tb04383.x. [DOI] [PubMed] [Google Scholar]
- 98.Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- 99.Ma Q. Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest. 2010;120:3773–3778. doi: 10.1172/JCI43426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ma Q. Population coding of somatic sensations. Neurosci Bull. 2012;28:91–99. doi: 10.1007/s12264-012-1201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- 102.McCoy ES, Taylor-Blake B, Street SE, Pribisko AL, Zheng J, Zylka MJ. Peptidergic CGRPalpha Primary Sensory Neurons Encode Heat and Itch and Tonically Suppress Sensitivity to Cold. Neuron. 2013 doi: 10.1016/j.neuron.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McNeil B, Dong X. Peripheral mechanisms of itch. Neurosci Bull. 2012;28:100–110. doi: 10.1007/s12264-012-1202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McQueen DS, Noble MA, Bond SM. Endothelin-1 activates ETA receptors to cause reflex scratching in BALB/c mice. Br J Pharmacol. 2007;151:278–284. doi: 10.1038/sj.bjp.0707216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Metz M, Grundmann S, Stander S. Pruritus: an overview of current concepts. Vet Dermatol. 2011;22:121–131. doi: 10.1111/j.1365-3164.2010.00945.x. [DOI] [PubMed] [Google Scholar]
- 106.Miyamoto T, Nojima H, Shinkado T, Nakahashi T, Kuraishi Y. Itch-associated response induced by experimental dry skin in mice. Jpn J Pharmacol. 2002;88:285–292. doi: 10.1254/jjp.88.285. [DOI] [PubMed] [Google Scholar]
- 107.Miyamoto T, Patapoutian A. Why does morphine make you itch? Cell. 2011;147:261–262. doi: 10.1016/j.cell.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 108.Moody TW, Thoa NB, O’Donohue TL, Jacobowitz DM. Bombesin-like peptides in rat spinal cord: biochemical characterization, localization and mechanism of release. Life Sci. 1981;29:2273–2279. doi: 10.1016/0024-3205(81)90560-9. [DOI] [PubMed] [Google Scholar]
- 109.Murota H, Izumi M, Abd El-Latif MI, Nishioka M, Terao M, Tani M, Matsui S, Sano S, Katayama I. Artemin causes hypersensitivity to warm sensation, mimicking warmth-provoked pruritus in atopic dermatitis. J Allergy Clin Immunol. 2012;130:671–682. doi: 10.1016/j.jaci.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 110.Napadow V, Li A, Loggia ML, Kim J, Schalock PC, Lerner E, Tran TN, Ring J, Rosen BR, Kaptchuk TJ, Pfab F. The Brain Circuitry Mediating Antipruritic Effects of Acupuncture. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- 112.Nilius B, Appendino G, Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch. 2012;464:425–458. doi: 10.1007/s00424-012-1158-z. [DOI] [PubMed] [Google Scholar]
- 113.Nilsson HJ, Schouenborg J. Differential inhibitory effect on human nociceptive skin senses induced by local stimulation of thin cutaneous fibers. Pain. 1999;80:103–112. doi: 10.1016/s0304-3959(98)00205-x. [DOI] [PubMed] [Google Scholar]
- 114.Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011;30:87–92. doi: 10.1016/j.sder.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oaklander AL. Common neuropathic itch syndromes. Acta Derm Venereol. 2012;92:118–125. doi: 10.2340/00015555-1318. [DOI] [PubMed] [Google Scholar]
- 116.Ohsawa Y, Hirasawa N. The antagonism of histamine H1 and H4 receptors ameliorates chronic allergic dermatitis via anti-pruritic and anti-inflammatory effects in NC/Nga mice. Allergy. 2012;67:1014–1022. doi: 10.1111/j.1398-9995.2012.02854.x. [DOI] [PubMed] [Google Scholar]
- 117.Patel KN, Dong X. An itch to be scratched. Neuron. 2010;68:334–339. doi: 10.1016/j.neuron.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Patel KN, Liu Q, Meeker S, Undem BJ, Dong X. Pirt, a TRPV1 Modulator, Is Required for Histamine-Dependent and -Independent Itch. PLoS One. 2011;6:e20559. doi: 10.1371/journal.pone.0020559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paus R, Schmelz M, Biro T, Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest. 2006;116:1174–1186. doi: 10.1172/JCI28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pfab F, Valet M, Napadow V, Tolle TR, Behrendt H, Ring J, Darsow U. Itch and the brain. Chem Immunol Allergy. 2012;98:253–265. doi: 10.1159/000336529. [DOI] [PubMed] [Google Scholar]
- 121.Qi J, Buzas K, Fan H, Cohen JI, Wang K, Mont E, Klinman D, Oppenheim JJ, Howard OM. Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J Immunol. 2011;186:6417–6426. doi: 10.4049/jimmunol.1001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28:4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reddy VB, Shimada SG, Sikand P, LaMotte RH, Lerner EA. Cathepsin S elicits itch and signals via protease-activated receptors. J Invest Dermatol. 2010;130:1468–1470. doi: 10.1038/jid.2009.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16:1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–1379. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 126.Ross SE. Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr Opin Neurobiol. 2011;21:880–887. doi: 10.1016/j.conb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 127.Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ross SE, McCord AE, Jung C, Atan D, Mok SI, Hemberg M, Kim TK, Salogiannis J, Hu L, Cohen S, Lin Y, Harrar D, McInnes RR, Greenberg ME. Bhlhb5 and Prdm8 form a repressor complex involved in neuronal circuit assembly. Neuron. 2012;73:292–303. doi: 10.1016/j.neuron.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rukwied RR, Main M, Weinkauf B, Schmelz M. NGF Sensitizes Nociceptors for Cowhage- but Not Histamine-Induced Itch in Human Skin. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.242. [DOI] [PubMed] [Google Scholar]
- 130.Saint-Mezard P, Krasteva M, Chavagnac C, Bosset S, Akiba H, Kehren J, Kanitakis J, Kaiserlian D, Nicolas JF, Berard F. Afferent and efferent phases of allergic contact dermatitis (ACD) can be induced after a single skin contact with haptens: evidence using a mouse model of primary ACD. J Invest Dermatol. 2003;120:641–647. doi: 10.1046/j.1523-1747.2003.12093.x. [DOI] [PubMed] [Google Scholar]
- 131.Schmelz M. Itch and pain. Neurosci Biobehav Rev. 2010;34:171–176. doi: 10.1016/j.neubiorev.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 132.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C- receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schon MP, Schon M, Klotz KN. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a. J Invest Dermatol. 2006;126:1338–1347. doi: 10.1038/sj.jid.5700286. [DOI] [PubMed] [Google Scholar]
- 134.Seike M, Ikeda M, Kodama H, Terui T, Ohtsu H. Inhibition of scratching behaviour caused by contact dermatitis in histidine decarboxylase gene knockout mice. Exp Dermatol. 2005;14:169–175. doi: 10.1111/j.0906-6705.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- 135.SHELLEY WB, ARTHUR RP. Mucunain, the active pruritogenic proteinase of cowhage. Science. 1955;122:469–470. doi: 10.1126/science.122.3167.469. [DOI] [PubMed] [Google Scholar]
- 136.Shim WS, Oh U. Histamine-induced itch and its relationship with pain. Mol Pain. 2008;4:29. doi: 10.1186/1744-8069-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sikand P, Dong X, LaMotte RH. BAM8-22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J Neurosci. 2011;31:7563–7567. doi: 10.1523/JNEUROSCI.1192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66–75. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Solak Y, Biyik Z, Atalay H, Gaipov A, Guney F, Turk S, Covic A, Goldsmith D, Kanbay M. Pregabalin versus gabapentin in the treatment of neuropathic pruritus in maintenance haemodialysis patients: A prospective, crossover study. Nephrology (Carlton ) 2012;17:710–717. doi: 10.1111/j.1440-1797.2012.01655.x. [DOI] [PubMed] [Google Scholar]
- 141.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, Alenius H, Dieu-Nosjean MC, Meller S, Rieker J, Steinhoff M, Hoffmann TK, Ruzicka T, Zlotnik A, Homey B. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411–417. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 142.Steinhoff M, Cevikbas F, Ikoma A, Berger TG. Pruritus: management algorithms and experimental therapies. Semin Cutan Med Surg. 2011;30:127–137. doi: 10.1016/j.sder.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, Luger TA, Schmelz M. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23:6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 145.Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Suto H, Matsuda H, Mitsuishi K, Hira K, Uchida T, Unno T, Ogawa H, Ra C. NC/Nga mice: a mouse model for atopic dermatitis. Int Arch Allergy Immunol. 1999;120 (Suppl 1):70–75. doi: 10.1159/000053599. [DOI] [PubMed] [Google Scholar]
- 147.Takaoka A, Arai I, Sugimoto M, Yamaguchi A, Tanaka M, Nakaike S. Expression of IL-31 gene transcripts in NC/Nga mice with atopic dermatitis. Eur J Pharmacol. 2005;516:180–181. doi: 10.1016/j.ejphar.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 148.Than JY, Li L, Hasan R, Zhang X. The excitation and modulation of TRPV1-, TRPA1-and TRPM8-expressing sensory neurons by the pruritogen chloroquine. J Biol Chem. 2013 doi: 10.1074/jbc.M113.450072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tominaga M, Ogawa H, Takamori K. Histological characterization of cutaneous nerve fibers containing gastrin-releasing peptide in NC/Nga mice: an atopic dermatitis model. J Invest Dermatol. 2009;129:2901–2905. doi: 10.1038/jid.2009.188. [DOI] [PubMed] [Google Scholar]
- 150.Tominaga M, Tominaga T. Structure and function of TRPV1. Pflugers Arch. 2005;451:143–150. doi: 10.1007/s00424-005-1457-8. [DOI] [PubMed] [Google Scholar]
- 151.Twycross R, Greaves MW, Handwerker H, Jones EA, Libretto SE, Szepietowski JC, Zylicz Z. Itch: scratching more than the surface. QJM. 2003;96:7–26. doi: 10.1093/qjmed/hcg002. [DOI] [PubMed] [Google Scholar]
- 152.Vergnolle N, Ferazzini M, D’Andrea MR, Buddenkotte J, Steinhoff M. Proteinase-activated receptors: novel signals for peripheral nerves. Trends Neurosci. 2003;26:496–500. doi: 10.1016/S0166-2236(03)00208-X. [DOI] [PubMed] [Google Scholar]
- 153.Vierow V, Fukuoka M, Ikoma A, Dorfler A, Handwerker HO, Forster C. Cerebral representation of the relief of itch by scratching. J Neurophysiol. 2009;102:3216–3224. doi: 10.1152/jn.00207.2009. [DOI] [PubMed] [Google Scholar]
- 154.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Woolf CJ. What is this thing called pain? J Clin Invest. 2010;120:3742–3744. doi: 10.1172/JCI45178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yamaguchi T, Nagasawa T, Satoh M, Kuraishi Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci Res. 1999;35:77–83. doi: 10.1016/s0168-0102(99)00070-x. [DOI] [PubMed] [Google Scholar]
- 157.Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361:690–694. doi: 10.1016/S0140-6736(03)12570-6. [DOI] [PubMed] [Google Scholar]
- 158.Zhu J, Cheng B, Liu H, Tang J, Xiang X, Peng Y. Expression of beta-endorphin in hypertrophic scar and its relationship with pruritus. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26:731–734. [PubMed] [Google Scholar]