Abstract
Background
We evaluated time to resumption of sex in relation to wound healing following circumcision of adult males. The purpose was to assess factors associated with adherence to the WHO recommendation of 42-days postcircumcision sexual abstinence and with engaging in sex before complete healing.
Methods
Participants were circumcised then followed weekly for 7 weeks and at 3 months. At each follow-up, participants were asked if they had engaged in sex since circumcision and their postcircumcision wounds examined to determine if they were fully healed. Log binomial regression identified risk factors for early sex before 42 days and sex before complete healing.
Results
Overall, 37.7% (120/318) of men reported sex before 42 days and 18.8% (60/319) reported sex before complete healing. Only 7% of men had unprotected sex before complete healing. There were no differences between HIV-positive and HIV-negative men in either healing time or sex before healing. Risk factors for sex before healing were being married or having 2 or more sex partners in the last year. Among single men, age older than 24 years and consistent alcohol consumption were associated with sex before healing.
Conclusion
The risk of HIV transmission because of unprotected sex before wound healing is low and transient, because most men reporting early sex either used a condom or had wound already healed. Adherence to the 42-day abstinence period and condom use at every sexual intercourse within 3 months postcircumcision should minimize risk of HIV spread because of sex before complete healing.
Keywords: male circumcision, sexual behavior, wound healing, HIV prevention, Africa, Kenya
INTRODUCTION
Three randomized-controlled trials confirmed that voluntary medical male circumcision (VMMC) reduces the risk of HIV acquisition among heterosexual men by approximately 60%.1–3 Consequently, the World Health Organization (WHO) and the Joint United Nations Program on HIV/AIDS (UNAIDS) endorsed VMMC in 2007 as part of a comprehensive HIV prevention package in areas with high prevalence of heterosexually transmitted HIV and low rates of male circumcision.4 A key component of the WHO guidelines is the promotion of sexual abstinence for 42 days after circumcision. This guidance is intended to minimize the risk of resuming sex before the postcircumcision wound is fully healed. Sex before complete wound healing could increase the risk of HIV acquisition by providing a portal of entry for the virus or of HIV transmission through increased viral shedding. If frequent, sex without condoms before complete wound healing may transiently increase the risk of HIV spread and erode the HIV prevention benefits of VMMC.5–7
Recent studies assessing sexual behavior postcircumcision have shown that resumption of sex before the recommended 42 days of sexual abstinence is common among adults. Reported rates of early sex vary from 3.9% to 30.7%, with being married and risky sexual behaviors before circumcision being associated with increased likelihood of early postcircumcision sex.7–9 However, differences between studies in the definition and ascertainment of early sex and wound healing, in the age distributions of study populations and in reporting methodologies, make it difficult to gauge the magnitude of early sex and the factors contributing to it.
The 42-day abstinence recommended by WHO is based on the assumption that all or nearly all men will be healed within 6 weeks after circumcision. Most previous studies have defined “early sex” as resumption of sex before 42 days postsurgery.7–9 However, wound in some men may be fully healed before that time, whereas wound in others may not be fully healed within the 42-day interval. No studies to date have assessed the postcircumcision wound and the onset of sexual activity at intervals frequent enough to establish the temporality between complete wound healing and resumption of sex. For example, an often-cited study of serodiscordant couples in Uganda found that HIV-positive men who resumed sex before complete wound healing were at higher risk [relative risk (RR) = 2.92; 95% confidence interval (CI) = 1.01 to 8.46] of infecting their female partners than those who waited for complete healing. Participants were followed up between 24 and 48 hours, 5 and 9 days, 4 and 6 weeks, and 6, 12, and 24 months postsurgery. Such a follow-up schedule is not sufficiently frequent to assess the relative timing of complete healing and resumption of sex. Sex before healing was, therefore, prone to misclassification. In addition, HIV infection in the female partners was assessed at 6 months postsurgery, an interval during which many exposures to HIV may have occurred apart from sex with an unhealed HIV-positive partner.10
To overcome the shortcomings of previous studies, we assessed time to resumption of sex after circumcision in relation to both the WHO-recommended 42-day abstinence and in relation to observed wound healing through weekly postsurgical observations for better determination of temporality between resumption of sex and complete wound healing.
METHODS
Participants
From March to December 2011, men seeking circumcision at the UNIM clinic in Kisumu were given written and verbal information about the study. Those who expressed interest in participating were screened to confirm eligibility for circumcision and participation. Men were eligible if they were aged 18–35 years, had no contraindication to circumcision, and were residents and intended to stay within Kisumu for the next 3 months. The study was designed to include 108 HIV-positive men and 215 HIV-negative men. Because HIV status, sexual activity, marital status, and wound healing may vary with age, we ensured that the HIV-positive men were matched with HIV-negative men of similar age (within 2 years). All participants gave written informed consent before enrollment. The study was approved by the Institutional Review Board of the University of Illinois at Chicago and by the Ethics and Research Committee of Kenyatta National Hospital.
Study Procedures
A total of 215 HIV-negative and 108-HIV positive men aged 18–35 years were enrolled and followed up weekly for 7 weeks and at 3 months following circumcision by the forcepsguided method.11,12 At enrollment, a baseline questionnaire was administered face-to-face to collect data on demographic and behavioral characteristics. Following WHO and Kenya National guidelines,13,14 men were advised to abstain from sex for 42 days postcircumcision. Participants were asked at each follow-up if they had engaged in sex since surgery. Men who answered yes were prompted to provide the date of first postsurgical sexual intercourse and to provide details regarding type of partner, condom use, alcohol use, and personal perception of wound healing at the time of resuming sex. Counseling on behavioral risk reduction including postcircumcision abstinence was reinforced at each follow-up.
The wound was inspected at each study visit by a clinician who was not blinded to the purpose of the study. Participants were considered healed if they had apposition of wound edges, no gaps or disruptions along the incision line, no scab or exposed underlying tissue, and a mature scar. Reported sex before the day of confirmed healing was considered sex before wound healing. Reported sexual intercourse was recorded as the number of postoperative days that had elapsed postcircumcision. Any reported sex before 42 days was classified as early sex.
Statistical Analyses
Baseline demographic and behavioral characteristics were summarized using counts and percentages. A priori identified covariates including age, marital status, personal perception of healing, HIV status, education, number of sex partners in the last year, and alcohol use were examined for their relationship with sex before healing and early sex. We used log binomial regression to calculate RRs and 95% CIs to estimate the crude relationship between these covariates and the 2 outcome variables. Multivariate models for the 2 outcomes of interest were constructed using the covariates that displayed a relationship with the outcomes under univariate analysis at the P < 0.05 level. In addition, we used Kaplan–Meier plots and log rank tests to assess differences in time to resumption of sex by marital status and HIV status.
RESULTS
A total of 215 HIV-negative and 108 HIV-positive men aged 18–35 years with a median of 26 years [interquartile range (IQR), 23–30] were enrolled. Nearly, all men (319/323) reported ever having had sex, whereas 46% (149/323) were married or cohabiting and 15% (47/323) reported consistent alcohol use of 3 days or more per week (Table 1). Of those who reported ever having had sex, 84% (267/319) reported having ever used a condom and 44% (117/267) reported using a condom at last sex. About half of the married or cohabiting men (71/149) reported an ongoing extramarital sexual partnership. Each of the 323 participants was scheduled for weekly postcircumcision visits till week 7 and at week 12, giving a total of 2584 expected visits, of which 2510 (97.1%) were completed. Three hundred and one men (93.2%) completed 12 weeks of follow-up, and an additional 18 men who did not complete follow-up were certified fully healed during follow-up.
TABLE 1.
Demographic and Behavioral Characteristics of Study Participants at Baseline (n = 323)
| Baseline Characteristics | n (%) |
|---|---|
| Reported age at enrollment, years | |
| 18–24 | 111 (34.4) |
| 25–29 | 127 (39.3) |
| 30+ | 85 (26.3) |
| Marital status | |
| Single | 174 (53.9) |
| Married or cohabiting | 149 (46.1) |
| HIV status | |
| HIV-negative | 215 (66.6) |
| HIV-positive | 108 (33.4) |
| Education | |
| None | 3 (0.9) |
| Primary | 151 (46.8) |
| Secondary | 113 (35.0) |
| Postsecondary | 56 (17.3) |
| Ever had sex | |
| Yes | 319 (98.8) |
| No | 4 (1.2) |
| Ever used a condom | |
| Yes | 267 (83.7) |
| No | 52 (16.3) |
| Condom use at last sex | |
| Yes | 117/267 (43.8) |
| No | 150/267 (56.2) |
| Sexual partners in past year | |
| None | 10 (3.1) |
| One | 141 (43.6) |
| Two | 91 (28.2) |
| Three or more | 81 (25.1) |
| Current extramarital partnerships | |
| Yes | 71 (47.7) |
| No | 78 (52.3) |
| Alcohol use (days per week) | |
| None | 179 (55.4) |
| Less than one | 32 (9.9) |
| One to two | 65 (20.1) |
| Three or more | 47 (14.6) |
Sex Before Complete Wound Healing
Of the 319 men who completed 12 weeks follow-up or were certified healed during follow-up, 18.8% (60/319) resumed sex before complete wound healing. Mean time to resumption of sex following circumcision was 51 days (SE, 1.2) whereas the median was 46 days (IQR, 34–74). None of the 4 participants who had never had sex before circumcision initiated sex during follow-up. Of the 60 men who resumed sex before complete healing, 37 (61.7%) used a condom and 54 (90%) reported sex before 42 days postsurgery. Of the 60 men, 37 (62%) were married; among these 2 (5%) had first sex after circumcision with an extramarital partner, and 23 (62%) used a condom at first sex. Five of those classified as having sex before healing had delayed wound healing beyond 7 weeks but missed all visits between weeks 7 and 12 and resumed sex at some point during this large gap between visits. It is possible that some or all of them were already healed at the time of resuming sex; 4 of them reported using a condom at resumption of sex. One man with wound healing delayed past week 7 completed later weekly follow-ups and reported sex before healing but with a condom.
Risk Factors for Sex Before Complete Healing
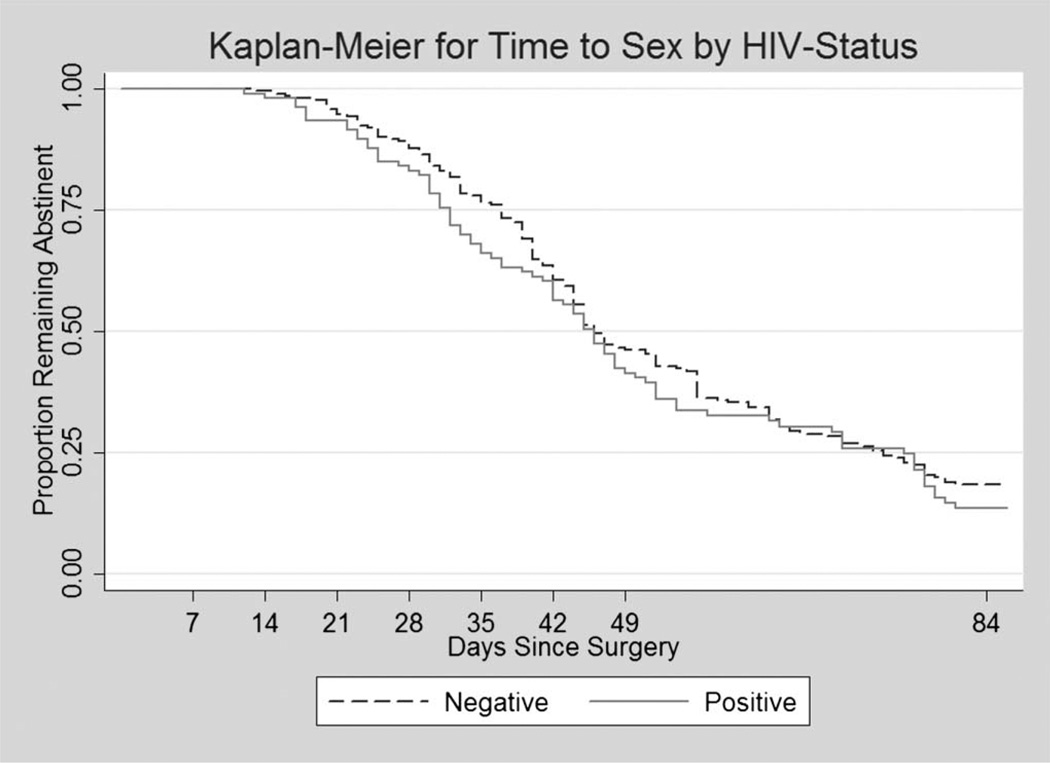
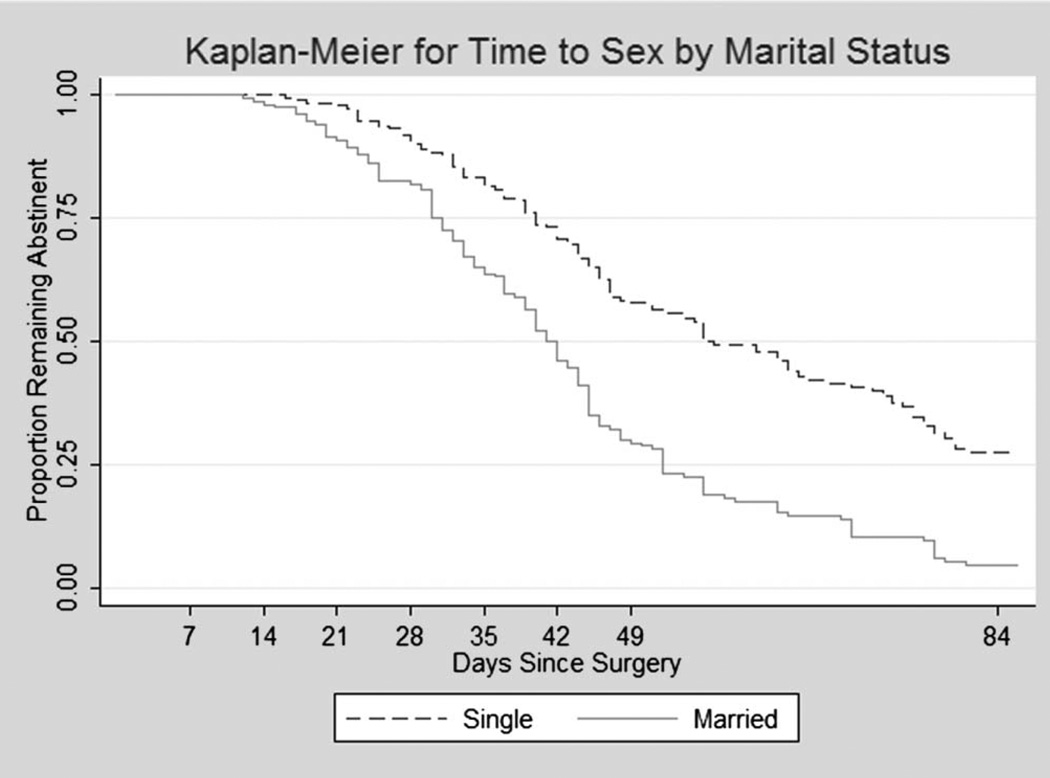
We generated Kaplan–Meier plots of time to first sex by HIV status (Fig. 1) and by marital status (Fig. 2). The log rank test showed no difference between HIV-positive and HIV-negative participants in the time to resumption of sex (P = 0.30). Married men, however, resumed sex significantly earlier than single men (log rank test, P = 0.0001), with mean time to sex in married men was being 43.5 days vs 58 days for single men.
FIGURE 1.
Kaplan–Meier plots of time to first sex following circumcision by HIV status (log rank test for equality =1.09; P = 0.30).
FIGURE 2.
Kaplan–Meier plots of time to first sex following circumcision by marital status (log rank test for equality =43.9; P < 0.0001).
Married men were more likely to engage in sex before complete wound healing (RR = 1.84; 95% CI = 1.15 to 2.94), as were older men (RR = 2.86; 95% CI = 1.46 to 5.59), HIV-positive men (RR = 1.64; 95% CI = 1.05 to 2.58), those who consumed alcohol 3 or more times per week (RR = 2.10; 95% CI = 1.30 to 3.41), and those who had 2 or more sex partners in the last year (RR = 2.25; 95% CI = 1.34 to 3.67). Level of education was not significantly associated with sex before healing (Table 2).
TABLE 2.
Univariate Analysis of Sex Before 42 Days and of Sex Before Complete Healing by Baseline Characteristics
| Baseline Characteristics | Sex Before 42 Days, % by Category (n = 318) |
Relative Risk (95% CI) | Sex Before Healed, % by category (n = 319) |
Relative Risk (95% CI) |
|---|---|---|---|---|
| Age, years | ||||
| 18–24 | 27/108 (25.0) | Ref. | 9/107 (8.4) | Ref. |
| 25+ | 93/210 (44.3) | 1.77 (1.24 to 2.54) | 51/212 (24.1) | 2.86 (1.46 to 5.59) |
| Marital status | ||||
| Single | 46/171 (26.9) | Ref. | 23/170 (13.5) | Ref. |
| Married or cohabiting | 74/147 (50.3) | 1.87 (1.39 to 2.51) | 37/149 (24.8) | 1.84 (1.15 to 2.94) |
| HIV status | ||||
| HIV-negative | 78/214 (36.5) | Ref. | 33/213 (15.5) | Ref. |
| HIV-positive | 42/104 (40.4) | 1.11 (0.83 to 1.49) | 27/106 (25.5) | 1.64 (1.05 to 2.58) |
| Sexual partners in last year | ||||
| None or one | 47/150 (31.3) | Ref. | 17/150 (11.3) | Ref. |
| Two or more | 73/168 (43.4) | 1.39 (1.03 to 1.86) | 43/169 (25.4) | 2.25 (1.34 to 3.67) |
| Alcohol use (d/wk) | ||||
| 2 or fewer | 95/271 (35.1) | Ref. | 44/272 (16.2) | Ref. |
| 3 or more | 25/47 (53.2) | 1.52 (1.11 to 2.08) | 16/47 (34.0) | 2.10 (1.30 to 3.41) |
| Education | ||||
| Primary or less | 68/150 (45.3) | 1.21 (0.83 to 1.77) | 34/150 (22.7) | 1.59 (0.73 to 3.22) |
| Secondary | 31/112 (27.7) | 0.74 (0.47 to 1.16) | 18/113 (15.9) | 1.12 (0.52 to 2.41) |
| Postsecondary | 21/56 (37.5) | Ref. | 8/56 (14.3) | Ref. |
| Ever used a condom | ||||
| Yes | 104/262 (39.7) | Ref. | 48/264 (18.2) | Ref. |
| No | 16/56 (28.6) | 0.72 (0.46 to 1.12) | 12/55 (21.8) | 1.20 (0.68 to 2.10) |
| Condom use at last sex | ||||
| Yes | 50/147 (34.0) | Ref. | 20/148 (13.5) | Ref. |
| No | 54/115 (47.0) | 1.38 (1.03 to 1.86) | 28/116 (24.1) | 1.79 (1.06 to 3.00) |
| Current extramarital partnership | ||||
| Yes | 38/70 (54.3) | 1.16 (0.84 to 1.60) | 22/71 (31.0) | 1.61 (0.91 to 2.86) |
| No | 36/77 (46.8) | Ref. | 15/78 (19.2) | Ref. |
Because the effect of marital status is modified by age, we generated separate multivariable models for single and married participants. Among single men, the largest risk factor for sex before complete healing was age; those aged 25 years and older were at 3 times greater risk of engaging in sex before healing (RR = 3.36; 95% CI = 1.31 to 8.62) than younger men; as expected, this factor was not significant in married men (RR = 0.96; 95% CI = 0.39 to 2.32). Drinking alcohol 3 or more days per week was also significantly associated with sex before healing in single men (RR = 2.32; 95% CI = 1.14 to 4.75) but not in married men (RR = 1.33; 95% CI = 0.68 to 2.53). Having sex with 2 or more partners in the last year was significant in both single (RR = 2.96; 95% CI = 1.06 to 8.29) and married men (RR = 1.93; 95% CI = 1.06 to 3.49).
Early Sex (Before 42 Days)
Of the 318 men who either completed 42 days of follow-up or initiated sex before 42 days, 37.7% (120/318) had early sex and 66.7% (80/120) of them used a condom at resumption of sex. Of the 120 men who resumed sex early, 84.1% (101/120) engaged in sex after 21 days, 60% (72/120) after 28 days, 15% (18/120) consumed alcohol at resumption of sex, and 39.2% (47/120) resumed sex with a nonregular partner. A comparison between men’s personal perception of their own healing and the clinician’s assessment showed only 65% agreement. Although 79.2% (95/120) of the men reporting early sex perceived themselves as already healed, only 63.2% of them were certified as healed by the study clinicians. Overall, 81.7% (98/120) of the men who resumed sex before 42 days were already healed (55.0%), reported condom use at first sex following surgery (66.7%), or both (40.0%).
Univariate analysis revealed that early sex was significantly associated with older age (RR = 1.77; 95% CI = 1.24 to 2.54), being married (RR = 1.87; 95% CI = 1.39 to 2.51), having 2 or more sex partners in the last year (RR = 1.39; 95% CI = 1.03 to 1.86), and using alcohol 3 days or more per week (RR = 1.52; 95% CI = 1.11 to 2.08). Level of education and HIV status were not associated with early sex. In multivariate analysis, being married (RR = 1.62; 95% CI = 1.16 to 2.24) and having 2 or more sex partners (RR = 1.34; 95% CI = 1.01 to 1.78) remained significant (Table 3). In summary, those who engaged in sex before the WHO recommended 42-day abstinence were more likely to be married and to have multiple sex partners in the last year.
TABLE 3.
Multivariate Analysis of Variables Associated With Sex Before 42 Days and With Sex Before Complete Healing*
| Sex Before Complete Healing |
|||
|---|---|---|---|
| Baseline Characteristic |
Sex Before 42 Days, Multivariate |
Multivariate Single Men Only |
Multivariate Married Men Only |
| Age, years | |||
| 18–24 | Ref. | Ref. | Ref. |
| 25+ | 1.33 (0.89–1.99) | 3.36 (1.31–8.62) | 0.96 (0.39–2.32) |
| Marital status | |||
| Single | Ref. | — | — |
| Married or cohabiting | 1.62 (1.16–2.24) | — | — |
| HIV status | |||
| HIV-negative | — | Ref. | Ref. |
| HIV-positive | — | 1.01 (0.50–2.07) | 1.61 (0.93–2.78) |
| Sexual partners in last year | |||
| None or one | Ref. | Ref. | Ref. |
| Two or more | 1.34 (1.01–1.78) | 2.96 (1.06–8.29) | 1.93 (1.06–3.49) |
| Alcohol use (d/wk) | |||
| 2 or fewer | Ref. | Ref. | Ref. |
| 3 or more | 1.26 (0.93–1.69) | 2.32 (1.14–4.75) | 1.33 (0.69–2.53) |
All variables significantly associated with resumption of sex at the P < 0.05 level under univariate analysis were included in the multivariate models with the exception of condom use at last sex because the question was asked only of individuals who reported ever using a condom (n = 262 for sex before 42 days; n = 264 for sex before healing).
DISCUSSION
WHO recommends 42 days of sexual abstinence after circumcision to allow for complete wound healing before engaging in sex11; yet, no published studies have examined resumption of sex in relation to time of healing observed at regular and frequent enough intervals. We found that 18.8% of men circumcised at our clinic in Kisumu, Kenya, resumed sex before clinicians judged them to be fully healed. This is in contrast to our finding that 37.7% resumed sex before the recommended 42 days of abstinence, a finding that is similar to the 30.7% rate found by Herman-Roloff et al.9 in western Kenya but higher than the 24% estimate reported by Hewett et al.7 from Zambia. The higher rate of noncompliance to the WHO recommendations in our sample may be because of the shorter (weekly) intervals between our assessments of both healing and resumption of sex, which likely reduced misclassification. In contrast, the earlier Kenyan study asked about resumption of sex between 28 and 42 days postsurgery, which would result in underestimation of the proportion resuming sex before 42 days. For the Zambian study, men were interviewed just once at 42 days postsurgery. The higher proportion of men engaging in early sex in our study may also be because of our inclusion of HIV-positive men and recruiting HIV-negative men matched for age. HIV-positive men are older and more likely to be married, and both these variables are associated with early resumption of sex. In contrast, the Zambian study included men of unknown HIV status aged 15–29 years, of whom only 7.6% were married.7
Only 23 of 319 men (7.2%) had unprotected sex before complete wound healing. All but 1 of these 23 men resumed sex before the WHO-recommended 42-day abstinence period. These results reflect poor compliance (62.3%) with the WHO recommendation for 42 days of postsurgical abstinence; however, they indicate that few men (6.9%) are at risk of HIV acquisition or transmission because of unprotected sex within the recommended abstinence period. Our results thus suggest that the current policy of counseling men undergoing circumcision to abstain from sex for at least 42 days postsurgery and to use a condom upon resumption of sex is likely to minimize transmissions during the postoperative period.
Those with the highest risk of resuming sex early, whether measured as sex before complete wound healing or as noncompliance with the WHO recommendations, are married men and men with 2 or more sex partners in the year before they are circumcised. Our results for married men are consistent with others from Kenya8,9 and Zambia7 and have led to calls for greater emphasis on counseling couples about male circumcision and to provide education targeted to women about the importance of abstaining from sex for a sufficient interval after their partner’s circumcision.9,15 Text messaging to circumcised men and their spouses has also been proposed, although trial of repeated text messages in 1 study had limited efficacy in delaying time to resumption of sex.16 Older single men should also be targeted for tailored counseling about the risks of early resumption of sex. We found that single men aged 25 years and older are more than 3 times more likely to resume sex before complete wound healing than their younger counterparts. Single men who drink more than 3 times per week are also at greater risk of resuming sex before their wounds are fully healed. Alcohol consumption is also associated with early first sex after circumcision. Thus, additional innovative means of counseling men to be aware of the influence of alcohol on their sexual risk behaviors would be prudent.
HIV-positive and HIV-negative men had similar levels of adherence to the WHO-recommended 42-day sexual abstinence. HIV-positive men were, however, one and half times more likely to have sex before complete healing, but this difference was not significant after adjusting for age and marital status. Although the mean HIV testing rate for men seeking circumcision in Kenya is approximately 92%,17 not all programs are this successful, and many HIV-positive men may be getting circumcised without their serostatus being known. Therefore, it is encouraging that we found that HIV-positive men heal at the same rate as HIV-negative men, and they are at the same risk of engaging in early sex. In Rakai, Uganda, HIV-positive men were found to take slightly longer to heal and were more likely to engage in sex before healing compared with HIV-negative men.18
A comparison between men’s personal perception of own healing and clinician’s assessment showed an agreement rate of only 65%. Unfortunately, we did not collect information from participants on how they made judgments about the healing status of their wound. Such information could be useful for designing more effective counseling to reduce early onset of sex after circumcision. However, because instances of early sex may be driven by false personal perception of healing, VMMC programs should aggressively promote abstinence for 42 days and condom use for the first 3 months after circumcision regardless of the status of healing or clients’ personal perception of risk.
This study has the advantage of weekly assessments of both the postsurgical wound and resumption of sex. It also affords a comparison between HIV-positive and HIV-negative men. A possible disadvantage is that older HIV-negative men were oversampled to have age-comparable groups by HIV status. Thus, the age distribution of our study population does not accurately reflect the ages of men seeking VMMC, who tend to be younger, more likely to be single, and thus likely have lower rates of sex before wound healing. Another disadvantage is that the clinicians assessing the wounds were not blinded to the purposes of the study. This could have resulted in a reporting bias, despite extensive training to the contrary. A further limitation of our study is the reinforcement of counseling on postcircumcision abstinence at weekly follow-ups, whereas men served in the national VMMC program receive similar counseling only once at surgery and possibly at 1 week follow-up. Such frequent follow-ups with counseling could result in reporting bias by participants, who might want to please the interviewer with a “correct” answer. It is possible that, under the Kenya National VMMC program, the frequency of early onset of sex is greater than we found in this study, because men are not so frequently counseled about postcircumcision sexual abstinence and condom use. This reinforces the need for well-designed, targeted individual counseling and widespread communication strategies to minimize unprotected sex after circumcision for HIV prevention.
In summary, although nearly 38% of men did not adhere to the WHO recommended 42 days of postcircumcision abstinence, only half that proportion (19%) resumed sex before complete healing, and only 7% had unprotected sex before healing. There were no differences between HIV-positive and HIV-negative men in their rate of healing and in the time to resumption of sex. To reduce the proportion of men who resume sex before they are healed, programs should develop innovative ways to reach and educate married men and men who have more than 1 sex partner. Older single men are at especially high risk of resuming sex early, particularly those who drink alcohol consistently. The period of healing after circumcision is brief and the levels of unprotected sex before complete wound healing are not high enough to offset the long-term protective effects of VMMC.7 However, we now have sufficient information to design interventions to address this issue directly, and more attention should be devoted to reducing early sex and promoting condom use in VMMC programs for HIV prevention.
Acknowledgments
Supported by University Illinois at Chicago through a grant from the Bill and Melinda Gates Foundation to FHI360.
Footnotes
Parts of the results were presented at the 16th International Conference on AIDS & STIs in Africa (ICASA), December 4–8, 2011, Addis Ababa-Ethiopia (A-403-0102-04,312) and the XIX International AIDS Conference (AIDS 2012) Washington, DC, 22–27 July 2012 (6542-MOPE170).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Auvert B, Taljaard D, Lagarde E, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. Available at: http://www.plosmedicine.org/article/info%3Adoi%2F10.1371%2Fjournal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomized controlled trial. Lancet. 2007;396:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 3.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Joint United Nations Programme on HIV/AIDS. New data on male circumcision and HIV prevention: policy and programme implications. [Accessed February 29, 2012];2007 Available at: http://libdoc.who.int/publications/2007/9789241595988_eng.pdf.
- 5.Hallett TB, Singh K, Smith JA, et al. Understanding the impact of male circumcision interventions on the spread of HIV in southern Africa. PLoS One. 2008;3:e2212. doi: 10.1371/journal.pone.0002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallett TB, Alsallaq RA, Baeten JM, et al. Will circumcision provide even more protection from HIV to women and men? New estimates of the population impact of circumcision interventions. Sex Transm Infect. 2011;87:88–93. doi: 10.1136/sti.2010.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hewett PC, Hallett TB, Mensch BS, et al. Sex with stitches: assessing the resumption of sexual activity during the postcircumcision wound-healing period. AIDS. 2012;26:749–756. doi: 10.1097/QAD.0b013e32835097ff. [DOI] [PubMed] [Google Scholar]
- 8.Mehta SD, Gray RH, Auvert B, et al. Does sex in the early period after circumcision increase HIV-seroconversion risk? Pooled analysis of adult voluntary medical male circumcision clinical trials. AIDS. 2009;23:1557–1564. doi: 10.1097/QAD.0b013e32832afe95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman-Roloff A, Bailey RC, Agot K. Factors associated with the early resumption of sexual activity following medical male circumcision in Nyanza province, Kenya. AIDS Behav. 2012;16:1173–1181. doi: 10.1007/s10461-011-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wawer MJ, Makumbi F, Kigozi G, et al. Circumcision in HIV-infected men and its effect on HIV transmission to female partners in Rakai, Uganda: a randomised controlled trial. Lancet. 2009;374:229–237. doi: 10.1016/S0140-6736(09)60998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Manual for male circumcision under local anaesthesia. [Accessed September 19, 2011];WHO/UNAIDS/JHPIEGO, Version 2.5C. 2008 Available at: http://www.who.int/hiv/pub/malecircumcision/who_mc_local_anaesthesia.pdf.
- 12.Clinical Manual for Male Circumcision Under Local Anaethesia. Republic of Kenya: Ministry of Public Health and Sanitation; 2009. [Google Scholar]
- 13.World Health Organization, Joint United Nations Programme on HIV/AIDS. Operational Guidance for Scaling Up Male Circumcision Services for HIV Prevention. Geneva: WHO and UNAIDS; 2008. [Google Scholar]
- 14.National Guidance for Voluntary Male Circumcision in Kenya. Republic of Kenya: Ministry of Health; 2008. [Google Scholar]
- 15.Marya P, Hawa M, Jan K, et al. The Unpeeled Mango: A Qualitative Assessment of Views and Preferences Concerning Voluntary Medical Male Circumcision in Iringa Region, Tanzania. 2011 [Google Scholar]
- 16.Odeny TA, Bailey RC, Bukusi EA, et al. Text messaging to improve attendance at post-operative clinic visits after adult male circumcision for HIV prevention: a randomized controlled trial. PLoS One. 2012;7:e43832. doi: 10.1371/journal.pone.0043832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mwandi Z, Murphy A, Reed J, et al. Voluntary medical male circumcision: translating research into the rapid expansion of services in Kenya, 2008–2011. PLoS Med. 2011;8:e1001130. doi: 10.1371/journal.pmed.1001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kigozi G, Gray RH, Wawer MJ, et al. The safety of adult male circumcision in HIV-infected and uninfected men in Rakai, Uganda. PLoS Med. 2008;5:e116. doi: 10.1371/journal.pmed.0050116. [DOI] [PMC free article] [PubMed] [Google Scholar]