Abstract
Objectives/Introduction
To report the initial anatomic, radiographic, and genetic evaluations of a novel form of spontaneous pelvic organ prolapse (S-POP) in mice.
Methods
We observed S-POP in a colony of UPII-SV40T transgenic mice developed for studies on bladder cancer. We utilized magnetic resonance imaging and necropsy to characterize this finding. We have established a breeding colony to identify inheritance patterns and for future studies.
Results
Selective breeding isolated the S-POP phenotype from the transgene. In contrast to other animal models, the S-POP mouse does not require an obligatory antecedent event to manifest pelvic organ prolapse. Necropsy and imaging demonstrate significant displacement of the pelvic organs distal to the pelvic floor in both sexes. The appearance of the POP is similar to that seen in the human female phenotype. Preliminary breeding studies indicate an autosomal dominant inheritance pattern.
Conclusions
This mouse may be an effective animal model for the study of POP in humans.
Keywords: genetic, magnetic resonance imaging, mouse, pelvic organ prolapse
Introduction
Pelvic organ prolapse (POP) is a common condition, estimated to affect up to one third of women of all ages and up to one half of all women over age fifty [1,2]. The condition, which appears to manifest as defects in the supporting connective tissue of the pelvic organs, adversely affects quality of life and is a major health issue. A detailed mechanism explaining the development of the connective tissue defects remains elusive. However, genetic factors are increasingly viewed as a major determinant for the development of POP [3–8].
To date, a definitive genetic defect causing POP has not been identified. One of the greatest obstacles to studying this condition in humans is the gradual development of POP. Because POP may be initiated by events occurring decades earlier and then develop gradually over decades, cause and effect correlations are very difficult to examine.
Animal models may allow us to better understand the causes and natural history of the condition and to develop specific rather then empiric treatments. Primate models for POP have provided valuable anatomic, imaging, and histologic data in furthering our understanding of POP [9,10]. However, primate models can be both expensive and very time consuming to develop and manage. Because POP develops in rodents over weeks or months rather than years, a rodent model could be far more productive than current primate models.
We made the serendipitous discovery of inherited and spontaneous pelvic organ prolapse (S-POP) in mice. The goal of this paper is to describe our preliminary anatomic, radiographic, and genetic observations.
Materials and Methods
Mice were housed in the University of Rochester Medical Center Vivarium in micro-isolator cages. University Committee on Animal Resources (UCAR) protocols were followed. All work was performed in accordance with New York State Department of Health, Association for the Assessment and Accreditation of Laboratory Animal Care, and Public Health Service guidelines. For clarity, we use the abbreviation POP to refer to the condition of pelvic organ prolapse and S-POP for specific reference to the spontaneous pelvic organ prolapse phenotype and genotype in these mice.
Our laboratory has been studying bladder cancer in the UPII-SV40T transgenic mouse. The colony was established from two breeding pairs supplied by Dr. Tung-Tien Sun and Dr. Xue-Ru Wu of New York University. UPII-SV40T transgenic mice express the simian virus 40 T antigen protein specifically in urothelium, driven by the uroplakin II promoter [11]. We utilized a unique flat panel detector-based, cone beam computed tomography instrument (CT) to monitor bladder tumor growth in the UPII-SV40T mice [12].
A male mouse was observed to have a profoundly enlarged scrotum just prior to a scheduled CT imaging session. It was assumed that the mouse had developed a large and perhaps invasive bladder tumor. However, CT imaging showed that the bladder had prolapsed into the scrotum. This abnormal position of the bladder can be seen clearly in Figure 1. At necropsy, bladder, prostate, seminal vesicles, and bowel were found in the scrotum. Once alerted to the S-POP phenotype, it became obvious that it was occurring in both males and females in the UPII-SV40T colony and that the phenotype was not genetically linked with the UPII-SV40T transgene. Pelvic organ prolapse has not been observed in the NYU colony (Dr. Xue-Ru Wu, personal communication).
Figure 1.
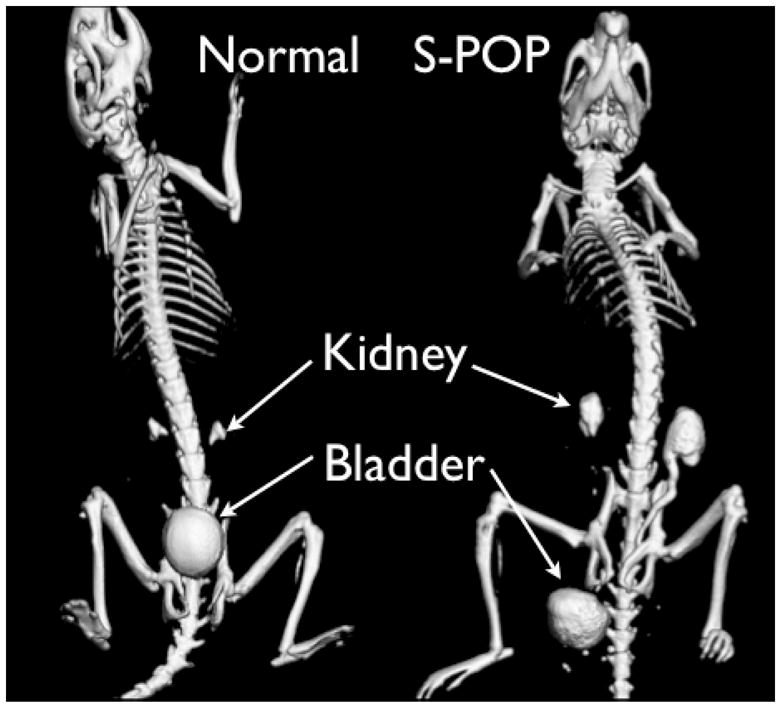
CT images of normal (left) and S-POP male mice (right). The mice were scanned following injection of contrast agent to aid in visualization of the urinary tract. In the normal mouse the smooth spherical object located above the pubic bones is the bladder. Above that, the contrast agent can be seen in the renal pelvi (pyramid shaped objects above the bladder). The bladder in the S-POP mouse is distal to the skeletal pelvis, and the prolapse has resulted in significant retention of contrast agent in the kidneys and ureter, consistent with hydroureter and hydronephrosis.
To assess the extent of POP in these mice without disrupting the in vivo relationships of anatomic structures, we explored the use of magnetic resonance imaging (MRI) microscopy. MRI permits the visualization of both hard and soft tissues in situ and organs can be identified and measured without the vagaries introduced by the manipulations inherent to gross dissection.
S-POP mice were identified by visible phenotype. Three representative animals (one wildtype and two S-POP mice) were euthanized using carbon dioxide. An incision was made to open the upper abdominal cavity to aid in fixation and, without disturbing the altered anatomy, the animals were immersed in 10% formalin containing the MRI gadolinium contrast agent (Omniscan, GE Healthcare). The specimen was suspended in normal buffered formalin with contrast agent added at a 400 fold dilution. The image was acquired at 9.4T using a gradient echo pulse sequence with TR/TE = 275/6 msec and flip angle of ~80°. The matrix size was 512 (readout) × 512 × 256 over a field of view of 60 X 30 X 30 mm, yielding voxel dimensions of 117 × 58 × 117 μm. Two signals were averaged for each phase-encode step. The data were pre-processed to select for regions of interest using ImageJ image processing and analysis software (NIH, Bethesda, MD; http://rsb.info.nih.gov/ij/), and then segmented using Amira software (Visage Imaging, Carlsbad, CA; www.visageimaging.com).
Male and female S-POP mice have proven to be fertile, successful breeders, although breeding female S-POP to male S-POP (n=3 successful matings) resulted in decreased litter size (1, 3, and 5 pups per litter). The reduced litter size may be a result of embryonic lethality in homozygous mice. To better characterize the inheritance of this defect, a male with S-POP and not carrying the UPII-SV40T transgene was selected to establish a S-POP breeding colony. This male was bred to two FVB female mice acquired from Jackson Laboratories. A total of 8 females and 9 males in two litters were born in mid October 2007.
Seven representative mice (three wildtype and four affected) were identified by visible phenotype and sacrificed for necropsy. These mice did not carry the UPII-SV40T transgene. Following euthanasia, the ventral skin and fat pads were removed. The pubic symphysis was disarticulated and in females, the uterus was freed at the level of the ovarian attachments and reflected forward to expose the uterosacral ligaments and pelvic floor musculature.
Results
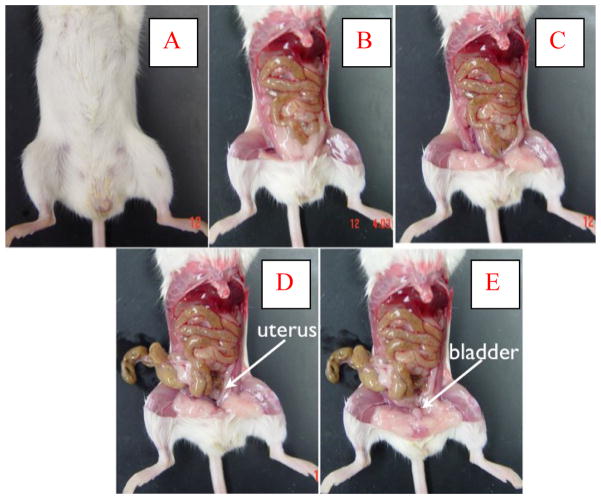
The S-POP phenotype was investigated using necropsy, computed tomography (Figure 1 described above) and magnetic resonance (MR) imaging. Each of these modalities demonstrated significant displacement of the pelvic organs distal to the pelvic floor. Figure 2 contains a series of photographs from necropsy of a male S-POP mouse. Box A shows the appearance of the external genitalia. The scrotum is distended. Testes were found in the abdominal cavity (Box C). The cecum was extracted from the scrotum, as were the urinary bladder and seminal vesicles (Boxes E and F). The anatomy of the female mouse is less complex than the male. Necropsy of a female S-POP mouse is presented in Figure 3. In this mouse, a bulge below the pelvis is appreciated, and bowel and bladder are prolapsed through the pelvic floor. The horned uterus appears to be displaced distally and is compressed by the bladder and bowel (Boxes D and E).
Figure 2.
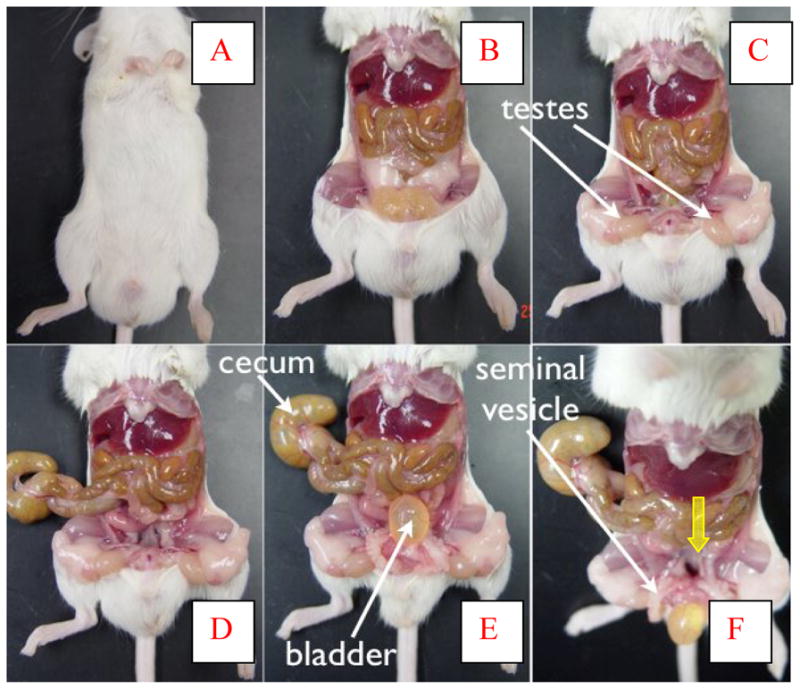
Necropsy of male S-POP mouse, proceeding Box A to Box E. The enlarged scrotum is clearly evident (A). After removal of the abdominal wall (B), the tstes were located in the abdomen (C). Extraction of the prolapsed cecum (D), and then bladder (E) empty the scrotum. The cavity through which the organs were withdrawn can be seen in Box F (yellow arrow).
Figure 3.
Necropsy of female S-POP mouse. The abdominal wall is removed (B) and fat pads are evident (C). In this mouse, a segment of bowel, uterus, and the bladder were withdrawn from the prolapse (D and E).
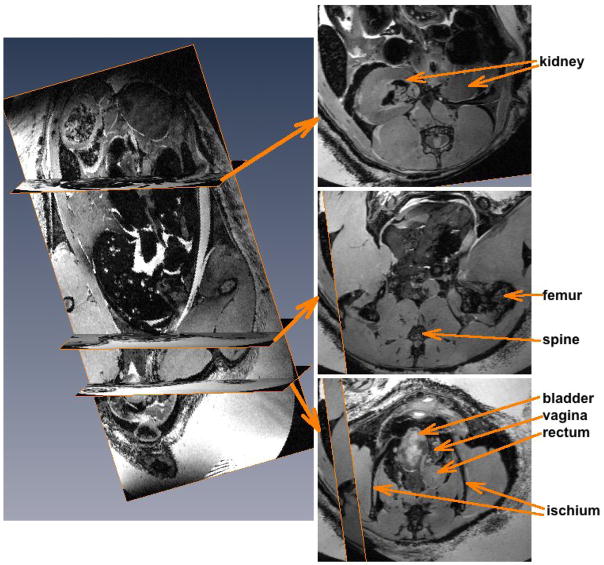
A representative set of MRI images for a female mouse is shown in Figure 4. It is clear that the bladder has prolapsed distal to the ischia. The vagina is compressed and displaced laterally. There is a loop of bowel at this level as well. The middle axial slice in the image set is presented to show the region of the body cavity normally occupied by the urinary bladder, but only bowel is seen at this level. In addition, there are several dark regions in the kidney suggestive of hydronephrosis and damage due to lower urinary tract obstruction. Both ureters appeared to be dilated (not shown).
Figure 4.
MRI images of female S-POP mouse. The image on the left is a coronal slice of the three dimensional MRI dataset. The locations of the three axial slices to the right are indicated. The top slice is at the level of the renal pelvis. The dark regions in the kidney are not normal and perhaps indicative of lower urinary tract obstruction. The middle slice is at the level at which the bladder is normally positioned. The bottom slice shows the severe organ prolapse at the level of the ischia with a full bladder, displaced and compressed vagina and rectum.
An analysis of UPII-SV40T breeding colony records suggested that S-POP was inherited independent of the UPII-SV40T transgene in both males and females and appeared to be a dominant trait (pedigree, Figure 5). At the time of this writing, S-POP was identified in more females than males in the pedigree outlined in Figure 5; however, S-POP is identified at an earlier age in females than in males. The appearance of S-POP in additional male progeny in the pedigree illustrated in Figure 5 may occur at a later time. Furthermore, UPII-SV40T mice have been backcrossed to FVB in an attempt to rid the transgenic line of S-POP. To date, S-POP has been noted in FVB backcrosses up to the F4 generation. However, these data were examined retrospectively and were not originally collected to characterize a S-POP mutation.
Figure 5.
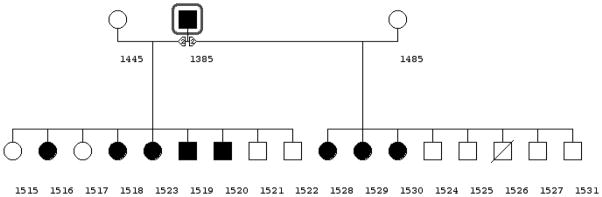
Pedigree showing autosomal dominant inheritance of S-POP phenotype. Mouse number 1385 was selected for breeding based on exhibition of the S-POP phenotype. Mouse 1385 may be homozygous for the S-POP mutation and if so we expect all of his progeny to develop prolapse. He was mated to two FVB female mice and two litters were produced. One male mouse was found moribund shortly after weaning. Of the eight females, 6 females are exhibiting S-POP at 4 months of age. Of the 8 surviving male mice, two have developed S-POP. The evaluation is based on external examination with comparison to wildtype animals.
Because pinworm infection in mice can cause anal prolapse, S-POP mice were screened for pinworm infection. The screening was conducted in addition to the routine monitoring for pinworm in the vivarium. No pinworm has been detected in S-POP mice or in rooms in which they are housed.
Comment
There is abundant epidemiological data implicating genetic susceptibility in the development of POP [13]. Researchers have demonstrated severe POP may occur in nulliparous women whose only risk factor is a familial link [3]. These data are consistent with the multitude of multiparous women who do not develop symptoms of POP, suggesting a clear genetic role for the development of POP. Jack and colleagues determined that the risk of POP among siblings of women younger than age 55 with advanced POP was five times higher than in the general population [4]. In addition, ethnic and racial associations with POP have been described, indicating that women of European and Hispanic heritage are at higher risk for development of POP than African, Asian, or Native American women [5,6]. In one prospective observational clinical study, Asian women, in particular, had significantly less pelvic organ mobility during pregnancy than their Caucasian counterparts [6]. The mouse model we have observed may have particular translational value, as parturition is not required in order for prolapse to occur, highlighting the role of genetics in pelvic organ prolapse.
While the evidence for a genetic influence is compelling, researchers have not yet demonstrated a single gene responsible for POP. The potential clinical implications of identifying specific genetic linkages for pelvic organ prolapse are many. In those women who have already developed POP, knowledge of one’s genetic propensities might influence management decisions. For instance, there are several surgical approaches to the repair of POP, with variable associated failure rates (up to 30% in some instances) [14]. Incorporating patient-specific genetic factors could allow physicians to better inform their patients preoperatively. This might include individualized counseling to offer more accurate predictions of surgical success of different techniques or the need for augmentation of the repair with synthetic mesh or a biologic graft. Identification of a gene that predisposes to POP may also allow for the development of strategies to prevent POP in susceptible women.
There has been some preliminary work examining specific genes in women with POP. Nikolova and colleagues identified and analyzed a family in which three generations of female relatives demonstrated POP at a very early age. The genetic analysis of this cohort suggested a dominant inheritance pattern, with a polymorphism in the promoter of the LAMC1 gene conveying increased susceptibility to POP [7]. The polymorphism reduces binding of the NFIL3 transcription factor. This gene encodes for laminin γ1, which is essential in modulating interactions between extracellular matrix molecules including collagen [7]. In addition, there is recent evidence of differential gene expression of structural proteins related to actin and myosin in the pubococcygeus muscle in women with POP [8].
Prior studies in mice found that failure of elastic fiber homeostasis contributed to poor functional integrity of the pelvic floor. Knockout mice lacking LOXL1 or Fibulin-5 expression have connective tissue defects and females develop postpartum prolapse [15–17]. Approximately one third of female LOXL1 mice develop severe POP after delivery of the first litter, and all of the remaining two thirds develop severe POP after delivery of the second litter [15]. Additionally, Connell and colleagues recently studied genetic influence on uterosacral ligament development in mice. Their research identified the HOXA11 gene as essential in the development of uterosacral ligaments [18].
The major weakness of our model is that the characterization is preliminary. The population we have studied is small, and the specific anatomic defect(s) is/are not yet well characterized. We have determined, however, that individual pelvic floor muscles such as the levator ani obturator internus muscles, are discretely identified by MRI (Figure 6). Our expectation is that dissecting and imaging a larger number of mice and correlating our findings to histologic analysis will yield a clear description of these defects.
Figure 6.
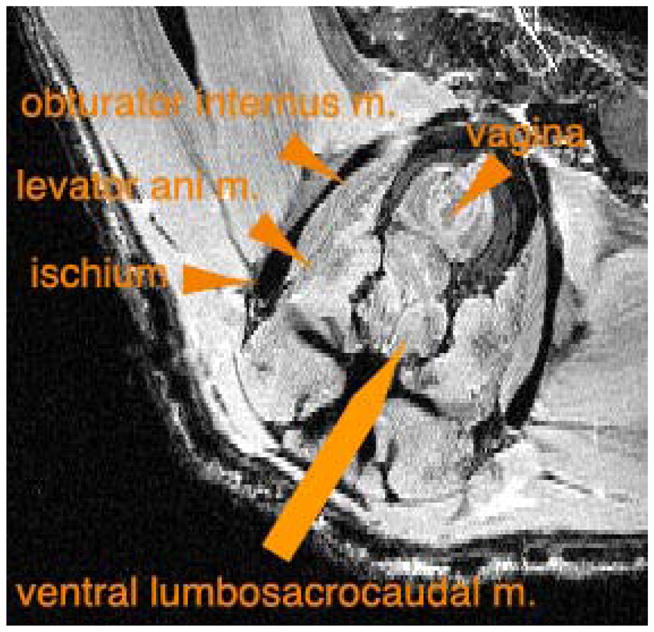
Identification of pelvic muscles in wildtype female mouse by MRI.
Our mouse model is distinct from previously described models, in that POP does not require parturition as a precipitating event, as has been reported in knockout mice. The mouse model we describe in this paper is unique in that no antecedent event is required in order for the prolapse to appear. This suggests a different, and perhaps more severe, form of S-POP than has been previously described. Identification of the gene responsible for this phenotype may offer greater insight into the pathophysiology of POP.
We are in the process of establishing a S-POP breeding colony, which will be used to produce adequate stock for future studies and for cryopreservation. Our goal is to map the S-POP locus and screen candidate genes. Identifying the gene responsible for the S-POP phenotype in the mouse will allow us to study the homologous gene in humans. We expect the results of this investigation to provide the basis for a wide variety of studies on the genetic etiology of POP in women.
Summary.
We describe the initial genetic, anatomic, and imaging observations of a novel form of inherited, spontaneous pelvic organ prolapse in mice.
Acknowledgments
RWW and SDK were supported by a grant from the National Institutes of Health (DK075036-01A2).
Footnotes
Reprints will not be available from the authors
No conflicts of interest.
References
- 1.Bump RC, Norton PA. Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin North Am ; 1998;25:723–46. doi: 10.1016/s0889-8545(05)70039-5. [DOI] [PubMed] [Google Scholar]
- 2.Subak LL, Waetjen LE, van den Eeden S. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol ; 2001;98:646–61. doi: 10.1016/s0029-7844(01)01472-7. [DOI] [PubMed] [Google Scholar]
- 3.Buchsbaum GM, Duecy EE, Kerr LA, et al. Urinary incontinence in nulliparous women and their parous sisters. Obstet Gynecol ; 2005;106:1253–58. doi: 10.1097/01.AOG.0000187309.46650.b2. [DOI] [PubMed] [Google Scholar]
- 4.Jack GS, Nikolova G, Vilain E. Familial transmission of genitovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct ; 2005;20:1–4. doi: 10.1007/s00192-005-0054-x. [DOI] [PubMed] [Google Scholar]
- 5.Kim S, Harvey MA, Johnston S. A review of the epidemiology and pathophysiology of pelvic floor dysfunction: do racial differences matter? J Obstet Gynecol. 2005;27:251–59. doi: 10.1016/s1701-2163(16)30518-7. [DOI] [PubMed] [Google Scholar]
- 6.Dietz HP. Do Asian women have less pelvic organ mobility than Caucasians? Int Urogynecol J Pelvic Floor Dysfunct ; 2003;14:250–53. doi: 10.1007/s00192-003-1073-0. [DOI] [PubMed] [Google Scholar]
- 7.Nikolova G, Lee H, Berkovitz S, et al. Sequence variant in the laminin γ1 (LAMC1) gene associated with familial pelvic organ prolapse. Hum Genet ; 2007;120:847–56. doi: 10.1007/s00439-006-0267-1. [DOI] [PubMed] [Google Scholar]
- 8.Visco AG, Yuan L. Differential gene expression in pubococcygeus muscle from patients with pelvic organ prolapse. Am J Obstet Gynecol ; 2003;189:102–12. doi: 10.1067/mob.2003.372. [DOI] [PubMed] [Google Scholar]
- 9.Kramer L, Gendron J, Pierce L, et al. Magnetic resonance imaging of the levator ani in the squirrel monkey: A comparison of muscle volume between a cohort with pelvic organ prolapse and matched normals. Am J Obstet Gynecol ; 2006;194:1467–71. doi: 10.1016/j.ajog.2006.01.062. [DOI] [PubMed] [Google Scholar]
- 10.Mattson J, Kuehl T, Yandell P, et al. Evaluation of the aged female baboon as a model of pelvic organ prolapse and pelvic reconstructive surgery. Am J Obstet Gynecol ; 2005;192:1395–98. doi: 10.1016/j.ajog.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 11.Zhang ZT, Pak J, Shapiro E, Sun TT, Wu XR. Urothelium-specific expression of an oncogene in transgenic mice induced carcinoma in situ and invasive transitional cell carcinoma. Cancer Research ; 1999;59:3512–17. [PubMed] [Google Scholar]
- 12.Johnson AM, Conover DL, Huang J, Messing EM, Ning R, O’Connell MJ, Rossi MA, Sun TT, Wood RW, Wu XR, Reeder JE. Early detection and measurement of urothelial tumors in mice. Urology ; 2006;67:1309–14. doi: 10.1016/j.urology.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Weber AM, Buchsbaum GM, Chen B. Basic science and translational research in female pelvic floor disorders: proceedings of an NIH-sponsored meeting. Neurourol Urodyn ; 2004;23:288–301. doi: 10.1002/nau.20048. [DOI] [PubMed] [Google Scholar]
- 14.Birch C, Fynes MM. The role of synthetic and biologic prostheses in reconstructive pelvic floor surgery. Curr Opin Obstet Gynecol ; 2002;14:527–35. doi: 10.1097/00001703-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Zhao Y, Gao J, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nature Genetics ; 2004;36:178–82. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 16.Drewes PG, Yanagisawa H, Starcher B, et al. Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. Am J Pathology ; 2007;170:578–89. doi: 10.2353/ajpath.2007.060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiaoqing L, Zhao Y, Pawlyk B. Failure of elastic fiber homeostasis leads to pelvic floor disorders. Am J Pathology. 2006;168:519–27. doi: 10.2353/ajpath.2006.050399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connell K, Guess M, Chen H, et al. HOXA11 is critical for development and maintenance of uterosacral ligaments and deficient in pelvic prolapse. J Clin Invest. 2008;118:1050–55. doi: 10.1172/JCI34193. [DOI] [PMC free article] [PubMed] [Google Scholar]