Abstract
Crystallins are heterogeneous proteins classified into alpha, beta, and gamma families. Although crystallins were first identified as the major structural components of the ocular lens with a principal function to maintain lens transparency, further studies have demonstrated the expression of these proteins in a wide variety of tissues and cell types. Alpha crystallins (alpha A and alpha B) share significant homology with small heat shock proteins and have chaperone-like properties, including the ability to bind and prevent the precipitation of denatured proteins and to increase cellular resistance to stress-induced apoptosis. Stress-induced upregulation of crystallin expression is a commonly observed phenomenon and viewed as a cellular response mechanism against environmental and metabolic insults. However, several studies reported downregulation of crystallin gene expression in various models of glaucomatous nerodegeneration suggesting that that the decreased levels of crystallins may affect the survival properties of retinal ganglion cells and thus, be associated with their degeneration. This hypothesis was corroborated by increased survival of axotomized retinal ganglion cells (RGCs) in retinas overexpressing alpha A or alpha B crystallins. In addition to RGC protective functions of alpha crystallins, beta or gamma crystallins were implicated in RGC axonal regeneration. These findings demonstrate the importance of crystallin genes in RGC survival and regeneration and further in-depth studies are necessary to better understand the mechanisms underlying the functions of these proteins in healthy RGCs as well as during glaucomatous neurodegeneration, which in turn could help in designing new therapeutic strategies to preserve or regenerate these cells.
Keywords: crystallin, retina, ganglion cells, optic nerve, glaucoma, axotomy
INTRODUCTION
Mammalian crystallins represent a heterogeneous group of proteins classified into three major families: alpha, beta, and gamma crystallins. These proteins were first identified as the major structural components of the ocular lens fiber cells, with a principal function to maintain lens transparency, which is necessary for its refractive characteristics. The predominant type of lens crystallin is alpha crystallin, which consists of noncovalently associated A and B subunits that form high molecular weight aggregates. Further studies have demonstrated the expression of crystallins outside of the lens in a wide variety of tissues and cell types [1-4]. For instance, alpha crystallins (alpha A and alpha B) were found in the retina, cornea, optic nerve, astrocytes, Muller cells, as well as in non-ocular tissues such as the brain, kidneys, lungs, liver, spleen, skin, cardiac and skeletal muscles. Both alpha A and alpha B share significant homology with small heat shock proteins (HSP), and as molecular chaperones prevent protein aggregation [5-10]. In addition to their structural/refractive and chaperone functions, alpha A and alpha B have also been implicated in cell survival in response to stress by increasing cell resistance to stress-inducible apoptosis. Although the exact mechanism of the alpha A and alpha B crystallins’ cell protective effect is unknown, recent studies associate these proteins with the regulation of several anti-apoptotic pathways. Alpha B crystallin can inhibit apoptotic cell death induced by FAS, TNFalpha, TRAIL, or growth factor deprivation by interacting with and suppressing proteolytic activation of caspase-3 [11-13]. Alpha B crystallin may also inhibit stress-induced apoptosis through interaction with pro-apoptotic members of the Bcl-2 family, Bax and Bcl-Xs, which in turn prevents the translocation of Bax and Bcl-XS from cytosol to mitochondria and thus preserves the integrity of mitochondria, blocks the release of cytochrome c, and prevents the activation of caspase-3 [14]. The cell protective effect of alpha A crystallin was attributed to its ability to interact with cytochrome c, as well as to interact and block the activation of caspase-3 and caspase-6 [12,13,15-17].
STRESS-INDUCED MODULATION OF CRYSTALLIN EXPRESSION
Upregulation of crystallins in response to a multitude of stress stimuli or to tissue damage is a commonly observed phenomenon. For instance, in a streptozotocin-induced rat model for diabetes, the expression of alpha A crystallin was found to be elevated in the retina, whereas alpha B crystallin was upregulated in the heart, muscle, brain, lens, and retina [18]. Both alpha A and alpha B crystallins are upregulated in the retina in several other rat models for diabetes, including a genetic model of spontaneous obesity-induced type 2 diabetes, high fat diet- and alloxan-induced diabetes [18-21]. Increased level of alpha A crystallin expression was also observed in the human diabetic retinas [22]. The upregulation of alpha-crystallin in several different models of diabetes as well as in human diabetic eyes strongly suggests that alpha crystallins play a role in the pathophysiology of the disease. Selective upregulation of alpha A crystallin, but not of alpha B or other HSPs, in the photoreceptor inner segments was observed during the early phase of experimental autoimmune uveitis (EAU) and was suggested to suppress mitochondrial oxidative stress-mediated apoptosis [15]. Alpha B crystallin upregulation in the retina was reported during S. aureus-induced endophthalmitis and proposed to inhibit apoptosis during immune clearance of the bacteria [23]. Gene expression studies consistently report the upregulation of alpha and beta/gamma crystallins in the retina after ischemia-reperfusion injury, light injury and retinal tears [24-26]. Elevated levels of alpha B crystallin have been associated with several neurological disorders including Alzheimer’s disease (AD), Alexander’s disease, Creutzfeldt-Jakob disease, and Parkinson’s disease [27-31]. Based on their anti-apoptotic effect, the stress-induced upregulation of alpha crystallins in various tissues was suggested to be a cellular response mechanism against environmental and metabolic insults. Interestingly, however, Stege et al. [32], reported that when rat cerebral cortex or hippocampal neurons were incubated with amyloid-β (Aβ; Aβ (1–40) and Aβ (1–42) peptides are implicated in the pathogenesis of AD), and alpha B crystallin, instead of protecting cells against toxic effect of Aβ, actually increased its toxicity. In the brains of AD patients, immunoreactivity to alpha B-crystallin in astrocytes and microglia was restricted to areas with senile plaques and neurofibrillary tangles, suggesting the association of alpha B with amyloid deposition. Since crystallins as molecular chaperones can prevent protein aggregation, it was proposed that observed upregulation of alpha B as well as HSP27 in AD brains can be viewed as an attempt to prevent the formation of amyloid fibrils and consequently protect cells from amyloid-induced to toxicity [30]. Indeed, the presence of alpha B crystallin does prevent and delay the formation of fibrils by Aβ(1–40) and by Aβ(1–42), respectively, and leads to formation of Aβ/alpha B crystallin aggregates. But in contrast to observation by Stege et al. [32] that alpha B increases neurotoxic effect of Aβ, other studies found that co-incubation with alpha B crystallin completely abolishes the toxicity of aggregated Aβ(1–40) and Aβ(1–42) [33]. Alpha A crystallin has also been shown to completely inhibit fibril formation by Aβ(1–40) and suppress its toxicity [34].
GENE EXPRESSION PROFILES INDICATE DOWNREGULATION OF CRYSTALLIN mRNAs IN ANIMAL MODELS FOR GLAUCOMATOUS NEURODEGENERATION
We first came across crystallin genes during the analysis of retinal gene expression profiles of rat ocular hypertension-induced glaucoma model. Glaucoma is the most common form of optic neuropathy that affects more than 60 million people worldwide and is expected to reach 79.6 million in 2020 [35,36]. If left untreated, glaucoma can lead to severe visual impairment and blindness. It is the second leading cause of blindness in the world with an estimated 8.4 million people bilaterally blind in 2010, rising to 11.1 million by 2020 [36]. The vision loss in glaucoma is caused by degeneration of retinal ganglion cells (RGCs) and their axons in the optic nerve. The main treatment strategy to slow progression of the disease is reduction of intraocular pressure (IOP). Lowering IOP has been shown to benefit not only patients with elevated IOP but also patients with normal pressure glaucoma. Unfortunately, in many cases, glaucomatous neuropathy continues to progress even after IOP reduction. Since the mechanisms of RGC death in glaucoma are unknown, several studies, including one in our laboratory, were designed to analyze the gene profiles of retinas derived from an experimental glaucoma model, with the aim of identifying genes associated with RGC degeneration. The glaucoma model with chronic IOP elevation used in this study was generated in Wistar rats by trabecular laser photocoagulation. Glaucomatous changes in this model have been characterized by the evaluation of RGC degeneration with the first statistically significant ~12% loss of RGCs observed two weeks after IOP elevation, and reaching ~27% by five weeks, compared to the control eyes [37]. Expression of several members of the crystallin superfamily, including alpha A (Cryaa), alpha B (Cryab), beta A1/A3 (Cryba1/a3), beta A2 (Cryba2), beta A4 (Cryba4), beta B2 (Crybb2), beta B1 (Crybb1), gamma 4 (Cryg4), and beta B3 (Crybb3) were found to be downregulated in experimental glaucomatous retinas two weeks after IOP elevation [38]. The expression levels of Cryaa, Cryba1/a3, Cryba2, Cryba4, and Crybb2 were decreased by two-fold or more and the level of Cryab transcript was reduced 1.6 times in glaucomatous retinas, compared to control (Table 1). However, at the 5-week post IOP elevation, no significant change in mRNA levels of these genes in glaucomatous versus control retinas was observed. Downregulation of crystallins was also observed in gene profiles obtained from retinas of other animal models of glaucoma. In Brown Norway rats that received unilateral episcleral vein injection of hypertonic saline to elevate IOP, Cryaa, Cryab, and Crybb2 were reproducibly downregulated in the group exposed to elevated IOP for 8 days, but not for 5 weeks [39]. In a hereditary rat model of elevated IOP, retinal gene profiling revealed downregulation of only one member of the crystallin family, Cryba1 [40]. In a DBA/2J mouse, a model for secondary angle closure glaucoma due to iris atrophy and pigment dispersion, which ultimately lead to increased IOP, microarray analysis of retinal RNA showed downregulation of 9 crystallin genes including Crygb (gamma B), Crygd (gamma D), Crygn (gamma N), Crybb3, Cryba4, Crybb1, Cryba2, Cryaa, and Cryba1 at 8 months when IOP is elevated versus at 3 months before disease onset [41]. Contrary to these observations, analysis of retinal gene expression changes in Wistar rats after experimental IOP elevation induced by translimbal laser photocoagulation showed upregulation of several members of the crystallin family. However, the authors suspected that these findings were artefactual, most likely due to contamination of retinal samples with lens material [42].
Table 1. Crystallin Regulation in Glaucomatous Retina.
| Crystallin | Gene Acc. # | Control/Treated |
|
|---|---|---|---|
| 2 weeks | 5 weeks | ||
|
| |||
| Alpha A (Cryaa) | NM_012534 | 2.9 | 0.9 |
| Beta A1 (Crybal) | BG371697 | 3.0 | 1.0 |
| Beta A2 (Cryba2) | NM_173140 | 3.3 | 1.0 |
| Beta A4 (Cryba4) | NM_031689 | 4.2 | 0.9 |
| Beta B2 (Crybb2) | NM_012937 | 2.6 | 0.7 |
| Alpha B (Cryab) | NM_012935 | 1.6 | 0.9 |
| Alpha C (Cryac) | NM_053612 | 1.1 | 1.2 |
| Beta B1 (Crybbl) | NM_012936 | 1.5 | 1.3 |
| Beta B3 (Crybb3) | NM_031690 | 1.3 | 0.7 |
| Gamma 4 (Crygd) | NM_033095 | 1.5 | 1.0 |
| Mu (Crym) | NM_053955 | 1.1 | 1.0 |
| Zeta (Cryz) | AI232098 | 1.2 | 1.1 |
EXPRESSION OF CRYSTALLIN GENES IN THE RETINA
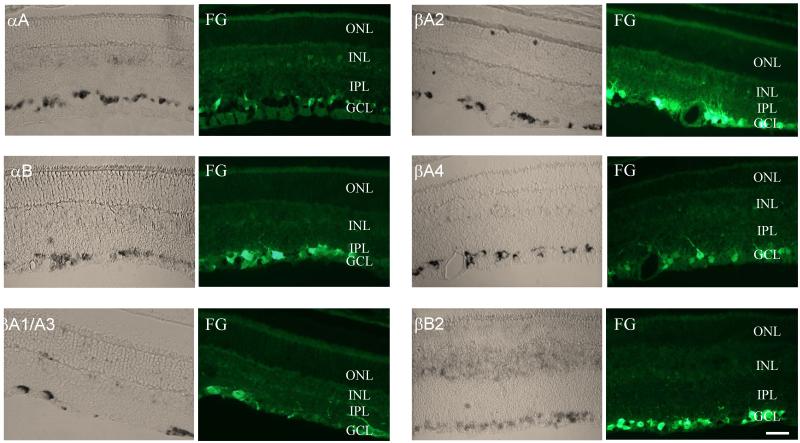
Since crystallins are associated with cell defense systems and are generally upregulated in response to stress, it can be hypothesized that the downregulation of these genes at early stages of the glaucomatous process observed on microarrays may affect the survival properties of RGCs, and thus contribute to their degeneration. In order to be able to speculate about the potential role of crystallin genes in RGC degeneration, in situ hybridization was performed to analyze their spatial distribution in the rat retina and first and foremost to determine whether or not these genes are expressed in RGCs. The expression pattern for all six crystallins that were downregulated two weeks after IOP elevation (Cryaa, Cryab, Cryba1/a3, Cryba2, Cryba4 and Crybb2) was very similar with the abundant staining observed in the ganglion cell layer (GCL), and to a lesser degree in the inner nuclear layer (INL, Fig. 1). In the mouse retina, Cryaa and Cryab crystallins also had similar distribution patterns and were localized to the GCL, INL, and outer nuclear layer (ONL) [9]. Cryab was also detected in the photoreceptor outer and inner segments. Since rodent GCL contains both RGCs and non-RGCs, such as displaced amacrine cells, in an approximately equal ratio, cells positive for Cryaa, Cryab, Cryba1/a3, Cryba2, Cryba4 and Crybb2 were co-localized with retrograde labeled RGCs to demonstrate that these crystalline-positive cells are RGCs. These results indicate that both alpha (Cryaa and Cryab) and beta (Cryba1/a3, Cryba2, Cryba4 and Crybb2) crystallin genes are expressed predominantly in the RGCs, suggesting a common mechanism regulating the expression of these genes in the retinas. Although there is virtually no information about the regulation of crystallin expression in the retina, regulation of crystallin transcription during lens differentiation has been extensively studied. Accumulated evidence suggests that temporal and spatial expression of all crystalline genes is regulated by different arrangements of developmentally regulated transcription factors, such as Pax-6, c-Maf, MafA/L-Maf, MafB, NRL, Sox2, Sox1, RARβ/RXRβ, RORα, Prox1, Six3, γFBP-B, HSF2, and HSF4, with ubiquitously expressed AP-1, CREB, pRb, TFIID, and USF factors [43-46]. Even though it is tempting to draw a parallel between lenticular and retinal regulation of crystallin transcription, the ensemble of transcription factors, and consequently, the mechanisms controlling expression of these genes could vary significantly from tissue to tissue. As evidence of this, evaluation of a 148 kb genomic fragment encompassing Cryaa showed that it contains all of the regulatory regions required for expression of this gene in the lens, but not in the retina, spleen or thymus, indicating the involvement of other genomic regions in modulation of alpha A extralenticular expression [47]. Nevertheless, it is noteworthy that among all non-ubiquitous factors that are involved in the regulation of crystallin transcription in the lens, only Pax-6 and RORα are known to be localized in the GCL of the differentiated retinas [44,48-51], suggesting that these factors play a role in the transcriptional regulation of crystallin genes in the RGCs. Pax-6 plays a crtical role in eye morphogenesis. Conditional knockout of the Pax6 in the developing retina leads to a failure in the specification of all cell types except amacrine cells [52]. Haploinsufficiency for Pax-6 causes aniridia, which is frequently accompanied by cataract, corneal opacification, and progressive glaucoma [53,54]. Mutations in the Pax6 have been associated with anophthalmia, nasal hypoplasia, and central nervous system defects [55]. The absence of RORα has no morphological effect on the retina, but causes dramatic changes in cerebellum development [49].
Figure 1.
In situ analysis of the crystallin expression in the retina. The expression of crystallins was primarily observed in the ganglion cell layer (GCL). Relatively weak staining can also be seen in the inner nuclear layer (INL) and, to a much lesser degree, in the outer nuclear layer (ONL). Crystallin-positive cells in the GCL were colocalized with RGCs retrogradely labeled with Fluorogold (FG). IPL, inner plexiform layer. Scale bars =20 μm.
CRYSTALLINS AND GLAUCOMATOUS NEURODEGENERATION
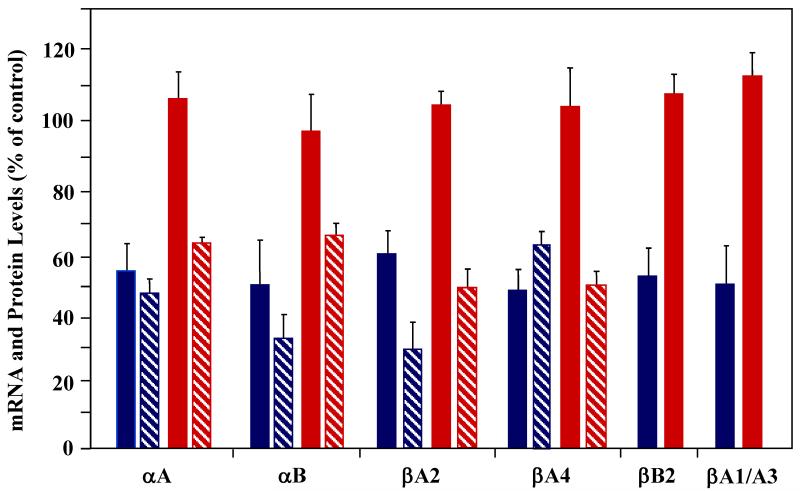
A notion about common mechanisms regulating expression of different crystallin genes in the retina based on their similar distribution pattern is supported by coordinated modulation of these genes during glaucomatous neurodegeneration. As stated above, gene profiles showed downregulation of six members of the crystallin superfamily, including Cryaa, Cryab, Cryba1/a3, Cryba2, Cryba4 and Crybb2 in glaucomatous retinas two weeks after IOP elevation, whereas the expression of these genes in retinas exposed to elevated IOP for five weeks was similar to that of control retinas. These dynamic changes in crystallin transcription in response to IOP elevation were evaluated by quantitative RNA and protein analysis [38]. In agreement with the microarray data, mRNA levels of alpha A, alpha B, beta A1/A3, beta A4, and beta B2 were approximately 50% and of beta A2 – 40% lower in experimental retinas than in controls (Fig. 2). By 5 weeks after IOP increase, the transcriptional levels of crystallin genes were elevated to the levels of control or even 5% to 10% higher. The estimated RGC loss was approximately 8% and 20% at two and five weeks post IOP elevation, respectively. Discordance between the 8% cell loss and ~ 50% reduction in crystallin expression at 2 weeks suggests at least two possible explanations: a) high IOP selectively kills RGCs that contribute ~ 50% of total expression of crystallins in the retina and b) high IOP at an early stage of the glaucomatous process by a yet unknown mechanism suppresses the transcription of crystallin genes in RGCs and other retinal cells. The first scenario is very unlikely to take place, since no apparent changes in the crystallin expression patterns were detected by in situ hybridization in 2-week experimental retinas and in GCL in particular compared to controls. Also, glaucomatous degeneration is not known to preferentially affect certain subgroups of RGCs. The second explanation suggests a deficiency at the level of expression or at the level of activity of one or more factors regulating transcriptional activity of crystallin genes. The mRNA levels of Pax-6 or RORα, that are known to control crystallin expression and to be expressed in the retina, were not significantly changed in the gene profiles of the glaucomatous retinas to associate these transcription factors with downregulation of crystallin expression. However, it is not known whether the expression of these factors is affected at the post-transcriptional level or at the level of activation in glaucomatous retinas. Pax-6, for instance, has been shown to be phosphorylated by the mitogen-activated protein kinases (MAPK), ERK (extracellular-signal regulated kinase) and p38 kinase, and phosphorylation by p38 kinase results in a strong increase of the transactivation potential of Pax-6 [56].
Figure 2.
Regulation of crystallin mRNA and protein expression in ocular hypertension rat model. mRNA levels of alpha and beta crystallin genes 2 (blue solid bars) and 5 (red solid bars) weeks after IOP elevation. mRNA levels of the six cristallin genes, expression of which was affected by IOP elevation as detected by gene profiling, was analyzed. Consistent with the gene profiling data, transcription of alpha A, alpha B, beta A1/A3, beta A2, beta A4 and beta B2 was transiently downregulated at 2 weeks but was similar to that of control at 5 weeks after IOP increase. Protein levels of alpha A, alpha B, beta A1/A3 and beta B2 were lower at 2 weeks (blue hatched bars) and at 5 weeks (red hatched bars) after IOP elevation compared to controls. Beta A2 and beta A4 crystallin proteins were undetected in the retinal extract, most likely due to their low expression level.
Unexpected downregulation of crystallins observed 2 weeks after IOP elevation, reversed its course with the progression of the glaucomatous process and followed a more generally accepted trend associated with regulation of crystallin expression in response to stress with the mRNA levels of the corresponding genes reaching and exceeding the levels of that in control retinas. We believe that this increase in crystallin levels is due to the activation of cell defense mechanisms in remaining RGCs and/or other retinal cells in response to progressive RGC degeneration. The notion of the upregulation of crystallin expression by non-RGC cells in the retina in response to a rapid and extensive RGC degeneration is supported by the analysis of crystallin profiles in retinas of the optic nerve axotomy model (see below). Nevertheless, the possibility that the crystallin upregulation in retinas with advanced neurodegeneration is irrelevant of the extent of RGC degeneration and is rather a cell response dynamic to IOP-induced stress cannot be excluded.
Interestingly, at the protein levels, although the upward trend was evident, alpha A, alpha B, beta A1/A3, and beta B2 crystallin expression in both 2 and 5-week post IOP elevation retinas was lower than in controls. Alpha A, alpha B, beta A1/A3 and beta B2 were approximately 2, 2.7, 3.3 and 1.6 fold lower in experimental retinas two weeks after IOP elevation, respectively, compared to controls. The levels of alpha A and alpha B proteins were decreased 1.6 fold and of beta A1/A3 and beta B2 almost twofold in retinas exposed to elevated IOP for five weeks. This deviation between mRNA and protein levels at five weeks could be explained by post-transcriptional regulation of crystallin expression or by an increased turnover rate of crystallins. Beta A2 and beta A4 crystallins were undetected in the retinal extract, suggesting the low expression level of these proteins. Miyara et al. reported a 2.7-fold downregulation of alpha A crystallin detected by quantitative proteomic analysis in retinas of a rat with a steroid-induced ocular hypertension [57].
In addition to chaperoning and death pathway regulatory properties of intracellular HSPs, extracellular and membrane-associated stress proteins have been reported to exhibit powerful effects on the immune response and were associated with autoimmune diseases, including neurodegenerative diseases [58-60]. For instance, van Noort et al. found that alpha B crystallin acts as an immunodominant myelin antigen to T cells when upregulated in oligodendrocytes and astrocytes at active multiple sclerosis (MS) lesion sites in the human brain. The authors suggest that although alpha B crystallin is unlikely to activate demyelinating autoimmunity in MS, it is a key myelin antigen that may contribute to the amplification of local inflammatory responses, disturbance of the blood-brain barrier, cytokine production, and expression of major histocompatibility antigens. With respect to glaucomatous neurodegeneration, antigens corresponding to alpha B-crystallin were identified in the aqueous humor of normal tension glaucoma patients [61]. Serum immunoreactivity against alpha A and alpha B crystallins and HSP27 was found in patients with primary open-angle and normal-pressure glaucoma [62]. Patients with normal-pressure glaucoma had a higher titer of autoantibodies to small heat shock proteins than age-matched primary open-angle glaucoma patients or control subjects. When applied in ex vivo and in vitro retina models, these antibodies have shown to trigger apoptotic cell death, suggesting that increased titers of circulating antibodies against small HSPs may have pathogenic significance in glaucoma patients.
CRYSTALLINS IN RGC PROTECTION AND REGENERATION
Since crystallins, particularly alpha A and alpha B, are known to play cell protective functions, the observed downregulation of these proteins in retinas of several glaucoma models could undermine cellular defense response to high IOP-induced stress and consequently be associated with or responsible for RGC death. We evaluated the cell protective effect of alpha A and alpha B crystallins in a rat optic nerve axotomy model for RGC degeneration [63]. This model is characterized by rapid, specific, and consistent degeneration of RGCs with approximately 90% of cell loss by 2 weeks after axotomy. Despite the loss of almost all RGCs, the overall level of alpha crystallin transcription was elevated in gene profiles of axotomized retinas. Quantitatively, the upregulation of Cryaa and Cryab expression 2 weeks after axotomy was 1.4 and 1.2 fold higher compared to control retinas, respectively. The loss of crystallin-expressing cells in the GCL (RGCs) on one hand, and the upregulation of alpha crystallin mRNAs on the other, suggest that crystallin transcription was induced in response to RGC loss in other retinal cells, including INL cells and photoreceptors. However, upregulation of alpha A and alpha B crystallins at the transcriptional level was not correlated with levels of the corresponding proteins which were 1.6 fold lower compared to control retinas. Remarkably, this discordance between the mRNA and protein levels of crystallin genes which observed in axotomized retinas with ~90% RGC loss was very comparable to that described above for glaucomatous retinas with 20% RGC loss. The difference between these models was the level of crystallin upregulation in experimental relative to control retinas: 40% for alpha A and 20% for alpha B in axotomized retinas versus 5% for alpha A and almost no change for alpha B in glaucomatous retinas. It appears that the difference in crystallin mRNA upregulation depends on the extent of the damage, i.e. more severe RGC loss caused by axotomy leads to higher crystallin mRNA levels. It is interesting to note, that at the protein level the expression of alpha crystallins in both axotomized and in glaucomatous retinas 5 weeks after IOP elevation was 1.6 fold lower than in control retinas. Since RGCs in untreated retinas show a very strong expression of these genes, similar level of these proteins in retinas with less than 10% (axotomy) and ~ 80% (ocular hypertension) remaining RGCs suggests that IOP elevation somehow suppresses the expression of crystallins. We believe that this downregulation of crystallins in RGCs may undermine the defense abilities of these cells to withstand the IOP-induced damage and thus make them more vulnerable to degeneration. RGC protective effect of alpha crystallins was clearly demonstrated in axotomized retinas [63]. Overexpression of alpha A and alpha B led to an increase in the number of survived RGCs by approximately 95% and 75%, respectively. Ying et al. [64] reported protection of RGC axons from optic nerve crush following a single intravitreal administration of alpha crystallin protein (unfortunately, it was not specified if it was alpha A or alpha B or both). Axonal survival was significantly greater in those crystallin treated than in control animals 2 weeks after injection: 16.0% vs 12%, 21% vs 11%, and 28% vs 17% at 0.5 mm, 2 mm, and 5 mm distal to the injury site, respectively. The protective effect of alpha crystallin declined by 4 weeks after injection but remained greater than in controls. Crystallins were also associated with the neuroprotective effect of wolfberry (fruit of Lycium barbarum Linn) in a rat ocular hypertension model. Wolfberry has been known in oriental countries for more than 2,500 years as a remedy that among other things improves visual acuity [65]. One of the active components in wolfberry, L. barbarum polysaccharide (LBP), has been shown to elicit anti-oxidative effects and inhibit JNK and double-stranded RNA-dependent protein kinase (PKR) pro-apoptotic signaling pathways [66-68]. Daily feeding of LBP starting 7 days prior to IOP elevation led to a decrease in RGC loss from ~ 18% to about 1% at 2 weeks after the laser photocoagulation [69]. Both alpha (A and B) and beta (A4 and B2) crystallins were found to be increased more than 10 times in LBP-treated rats compared to control 2 days after IOP elevation, suggesting their roles in protection of RGCs in experimental glaucoma [65].
Several studies suggest that lens injury (LI) increases RGC survival (up to eightfold) and transforms these cells into an active regenerative state, enabling axonal regrowth (100 fold increase in the number of axons regenerating beyond the crush site) after optic nerve crush or cut [70-72]. Mature RGCs, as typical neurons of the central nervous system (CNS) under normal circumstances fail to regenerate their axons partly because of the gradual decline in their intrinsic growth ability [73-76] as well as extrinsic inhibition by the glial environment of the adult CNS that includes myelin-associated inhibitors and proteoglycans involved in astroglial scarring [77-79]. Lentogenic axon growth-promoting effect was attributed to induction of intraocular inflammation. It was proposed that LI as well as peripheral nerve implants into the eye or intraocular injections of zymosan (a yeast cell wall preparation) lead to the activation and infiltration of macrophages into the eye, which secrete factors stimulating RGCs to regenerate their axons [70,80,81]. Yin et al. [82] identified oncomodulin (Ocm), a small Ca2+-binding protein, as the principal mediator of inflammation-induced axonal regeneration. Ocm is expressed and secreted by macrophages; it binds to RGCs with high affinity and stimulates axonal regeneration via a Ca2+/calmodulin (CaM) kinase—dependent pathway. However, since intravitreal injection of activated macrophages alone was not sufficient to induce axon regeneration [70] and oncomodulin’s in vivo axon-growth-promoting effect requires the presence of agents elevating intracellular cAMP level [82], it was suggested that in addition to the inflammation-induced axonal regeneration, there are macrophage-independent mechanisms by which LI-associated factors contribute to the regeneration-promoting effects [83-85]. Based on observations that an LI-induced switch of RGCs to a regenerative state is closely correlated with the activation of retinal astrocytes and Müller cells, the increased expression and release of ciliary neurotrophic factor (CNTF) from astrocytes after LI, activation of CNTF’s JAK/STAT3 (the Janus-kinase/signal transducers and activators of transcription 3) downstream pathway, it was proposed that both CNTF and JAK activation are required for the LI-induced axonal regeneration [84,86]. Furthermore, the LI-induced regeneration effect was observed following intravitreal injections of purified lens beta or gamma crystallins, but not of alpha crystallin. This is associated with an influx of circulating macrophages, an activation of retinal astrocytes, Müller cells, and resident microglia, elevated expression CNTF in astrocytes, and activation of the JAK/STAT3 signaling pathway [87]. Finally, crystallin beta B2 via an autocrine mechanism was implicated in neurite-promoting activity in retinal explants and dissociated neurons from the retina and the hippocampus [88].
CONCLUDING REMARKS
Although crystallins were first characterized as major structural proteins of the ocular lens with a principal function to maintain lens transparency, further studies identified extralenticular crystallin expression, including expression in the retinal cells and particularly in RGCs and demonstrated dynamic modulation of these genes, general upregulation, in response to stress or injury. As members of a small HSP family, crystallins demonstrate chaperone-like activities and were shown to suppress apoptotic cell death and consequently protect cells from insult. Therefore, the upregulation of crystallins observed in various cells and tissues in response to stress is commonly viewed as a component of cell defense mechanism activation. In contrast to these observations, a number of independent studies show downregulation of crystallin genes in retinas of various animal models for glaucoma, suggesting that the downregulation of crystallins could impair the survival properties of RGCs, and consequently be associated with RGC death in glaucoma. And as expected, a single intravitreal administration of alpha crystallin protein or overexpression of alpha A or alpha B crystallins led to an increase in RGC survival rate. Moreover, beta/gamma crystallins were implicated in RGC axonal regeneration via an autocrine, inflammation-induced or astrocyte-derived CNTF-mediated mechanism. Further in-depth studies on the roles of crystallins in RGC survival and regeneration will help us to better understand the pathophysiology of optic neuropathies such as glaucoma and to design new strategies to preserve/regenerate these cells and restore their synaptic connections with their target neurons.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health/National Eye Institute Grant EY018644 (NP) and the Research to Prevent Blindness (JC).
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Bhat SP, Nagineni CN. alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989;158:319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan AN, Nagineni CN, Bhat SP. alpha A-crystallin is expressed in non-ocular tissues. J Biol Chem. 1992;267:23337–23341. [PubMed] [Google Scholar]
- 3.Dubin RA, Wawrousek EF, Piatigorsky J. Expression of the murine alpha B-crystallin gene is not restricted to the lens. Mol Cell Biol. 1989;9:1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato K, Shinohara H, Kurobe N, Inaguma Y, Shimizu K, Ohshima K. Tissue distribution and developmental profiles of immunoreactive αB crystallin in the rat determined with a sensitive immunoassay system. Biochim Biophys Acta. 1991;1074:201–208. doi: 10.1016/0304-4165(91)90062-l. [DOI] [PubMed] [Google Scholar]
- 5.Piatigorsky J. Multifunctional lens crystallins and corneal enzymes: more than meets the eye. Ann N Y Acad Sci. 1998;842:7–15. doi: 10.1111/j.1749-6632.1998.tb09626.x. [DOI] [PubMed] [Google Scholar]
- 6.Andley UP, Song Z, Wawrousek EF, Fleming TP, Bassnett S. Differential protective activity of alpha A- and alphaB-crystallin in lens epithelial cells. J Biol Chem. 2000;275:36823–36831. doi: 10.1074/jbc.M004233200. [DOI] [PubMed] [Google Scholar]
- 7.Alge CS, Priglinger SG, Neubauer AS, Kampik A, Zillig M, Bloemendal H, Welge-Lussen U. Retinal pigment epithelium is protected against apoptosis by alphaB-crystallin. Invest Ophthalmol Vis Sci. 2002;43:3575–3582. [PubMed] [Google Scholar]
- 8.Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 9.Xi JH, Bai F, Andley UP. Reduced survival of lens epithelial cells in the alphaA-crystallin-knockout mouse. J Cell Sci. 2003;116:1073–1085. doi: 10.1242/jcs.00325. [DOI] [PubMed] [Google Scholar]
- 10.Liu JP, Schlosser R, Ma WY, Dong Z, Feng H, Lui L, Huang XQ, Liu Y, Li DW. Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res. 2004;79 [PubMed] [Google Scholar]
- 11.Mehlen P, Kretz-Remy C, Préville X, Arrigo AP. Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha-induced cell death. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- 12.Kamradt MC, Chen F, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem. 2001;276 doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- 13.Kamradt MC, Chen F, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J Biol Chem. 2002;277:38731–38736. doi: 10.1074/jbc.M201770200. [DOI] [PubMed] [Google Scholar]
- 14.Mao YW, Liu JP, Xiang H, Li DW. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- 15.Rao NA, Saraswathy S, Wu GS, Katselis GS, Wawrousek EF, Bhat S. Elevated retina-specific expression of the small heat shock protein, alphaA-crystallin, is associated with photoreceptor protection in experimental uveitis. Invest Ophthalmol Vis Sci. 2008;49:1161–1171. doi: 10.1167/iovs.07-1259. [DOI] [PubMed] [Google Scholar]
- 16.Kamradt MC, Lu M, Werner ME, Kwan T, Chen F, Strohecker A, Oshita S, Wilkinson JC, Yu C, Oliver PG, Duckett CS, Buchsbaum DJ, LoBuglio AF, Jordan VC, Cryns VL. The small heat shock protein alpha B-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem. 2005;280:11059–11066. doi: 10.1074/jbc.M413382200. [DOI] [PubMed] [Google Scholar]
- 17.Morozov V, Wawrousek EF. Caspase-dependent secondary lens fiber cell disintegration in {alpha}A-/{alpha}B-crystallin double-knockout mice. Development. 2006;133:813–821. doi: 10.1242/dev.02262. [DOI] [PubMed] [Google Scholar]
- 18.Kumar PA, Haseeb A, Suryanarayana P, Ehtesham NZ, Reddy GB. Elevated expression of alphaA- and alphaB-crystallins in streptozotocin-induced diabetic rat. Arch Biochem Biophys. 2005;444:77–83. doi: 10.1016/j.abb.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Kim YH, Choi MY, Kim YS, Han JM, Lee JH, Park CH, Kang SS, Choi WS, Cho GJ. Protein kinase C delta regulates anti-apoptotic alphaB-crystallin in the retina of type 2 diabetes. Neurobiol Dis. 2007;28:293–303. doi: 10.1016/j.nbd.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Wang YD, Wu JD, Jiang ZL, Wang YB, Wang XH, Liu C, Tong MQ. Comparative proteome analysis of neural retinas from type 2 diabetic rats by two-dimensional electrophoresis. Curr Eye Res. 2007;32:891–901. doi: 10.1080/02713680701593702. [DOI] [PubMed] [Google Scholar]
- 21.Fort PE, Freeman WM, Losiewicz MK, Singh RS, Gardner TW. The retinal proteome in experimental diabetic retinopathy: up-regulation of crystallins and reversal by systemic and periocular insulin. Mol Cell Proteomics. 2009;8:767–779. doi: 10.1074/mcp.M800326-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kase S, Ishida S, Rao NA. Increased expression of αA-crystallin in human diabetic eye. Int J Mol Med. 2011;28:505–511. doi: 10.3892/ijmm.2011.708. [DOI] [PubMed] [Google Scholar]
- 23.Whiston EA, Sugi N, Kamradt MC, Sack C, Heimer SR, Engelbert M, Wawrousek EF, Gilmore MS, Ksander BR, Gregory MS. alphaB-crystallin protects retinal tissue during Staphylococcus aureus-induced endophthalmitis. Infect Immun. 2008;76:1781–1790. doi: 10.1128/IAI.01285-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshimura N, Kikuchi T, Kuroiwa S, Gaun S. Differential temporal and spatial expression of immediate early genes in retinal neurons after ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2003;44:2211–2220. doi: 10.1167/iovs.02-0704. [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi H, Miyagi M, Darrow RM, Crabb JS, Hollyfield JG, Organisciak DT, Crabb JW. Intense light exposure changes the crystallin content in retina. Exp Eye Res. 2003;76:131–3. doi: 10.1016/s0014-4835(02)00249-x. [DOI] [PubMed] [Google Scholar]
- 26.Vazquez-Chona F, Song BK, Geisert EE. Temporal changes in gene expression after injury in the rat retina. Invest Ophthalmol Vis Sci. 2004;45:2737–2746. doi: 10.1167/iovs.03-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwaki T, Iwaki A, Tateishi J, Sakaki Y, Goldman JE. Alpha B-crystallin and 27-kd heat shock protein are regulated by stress conditions in the central nervous system and accumulate in Rosenthal fibers. Am J Pathol. 1993;143:487–495. [PMC free article] [PubMed] [Google Scholar]
- 28.Head MW, Corbin E, Goldman JE. Overexpression and abnormal modification of the stress proteins alpha B-crystallin and HSP27 in Alexander disease. Am J Pathol. 1993;143:1743–1753. [PMC free article] [PubMed] [Google Scholar]
- 29.Renkawek K, de Jong WW, Merck KB, Frenken CW, van Workum FP, Bosman GJ. Alpha B-crystallin is present in reactive glia in Creutzfeldt–Jakob disease. Acta Neuropathol. 1992;83:324–327. doi: 10.1007/BF00296796. [DOI] [PubMed] [Google Scholar]
- 30.Renkawek K, Voorter CE, Bosman GJ, van Workum FP, de Jong WW. Expression of alpha B-crystallin in Alzheimer’s disease. Acta Neuropathol. 1994;87:155–160. doi: 10.1007/BF00296185. [DOI] [PubMed] [Google Scholar]
- 31.Renkawek K, Stege GJ, Bosman GJ. Dementia, gliosis and expression of the small heat shock proteins hsp27 and alpha B-crystallin in Parkinson’s disease. Neuroreport. 1999;10:2273–2276. doi: 10.1097/00001756-199908020-00009. [DOI] [PubMed] [Google Scholar]
- 32.Stege GJ, Renkawek K, Overkamp PS, Verschuure P, van Rijk AF, Reijnen-Aalbers A, Boelens WC, Bosman GJ, de Jong WW. The molecular chaperone alphaB-crystallin enhances amyloid beta neurotoxicity. Biochem Biophys Res Commun. 1999;262:152–156. doi: 10.1006/bbrc.1999.1167. [DOI] [PubMed] [Google Scholar]
- 33.Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, de Waal RM, Verbeek MM. Small heat shock proteins inhibit amyloid-beta protein aggregation and cerebrovascular amyloid-beta protein toxicity. Brain Res. 2006;1089:67–78. doi: 10.1016/j.brainres.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 34.Santhoshkumar P, Sharma KK. Inhibition of amyloid fibrillogenesis and toxicity by a peptide chaperone. Mol Cell Biochem. 2004;267:147–155. doi: 10.1023/b:mcbi.0000049373.15558.b8. [DOI] [PubMed] [Google Scholar]
- 35.Leske MC. The epidemiology of open-angle glaucoma: a review. Am J Epidemiol. 1983;118:166–191. doi: 10.1093/oxfordjournals.aje.a113626. [DOI] [PubMed] [Google Scholar]
- 36.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishii Y, Kwong JM, Caprioli J. Retinal ganglion cell protection with geranylgeranylacetone, a heat shock protein inducer, in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003;44:1982–1992. [PubMed] [Google Scholar]
- 38.Piri N, Song M, Kwong JM, Caprioli J. Modulation of alpha and beta crystallin expression in rat retinas with ocular hypertension-induced ganglion cell degeneration. Brain Res. 2007;1141:1–9. doi: 10.1016/j.brainres.2006.11.095. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed F, Brown KM, Stephan DA, Morrison JC, Johnson EC, Tomarev SI. Microarray analysis of changes in mRNA levels in the rat retina after experimental elevation of intraocular pressure. Invest Ophthalmol Vis Sci. 2004;45:1247–1258. doi: 10.1167/iovs.03-1123. [DOI] [PubMed] [Google Scholar]
- 40.Naskar R, Thanos S. Retinal gene profiling in a hereditary rodent model of elevated intraocular pressure. Mol Vis. 2006;12:1199–1210. [PubMed] [Google Scholar]
- 41.Steele MR, Inman DM, Calkins DJ, Horner PJ, Vetter ML. Microarray analysis of retinal gene expression in the DBA/2J model of glaucoma. Invest Ophthalmol Vis Sci. 2006;47:977–985. doi: 10.1167/iovs.05-0865. [DOI] [PubMed] [Google Scholar]
- 42.Yang Z, Quigley HA, Pease ME, Yang Y, Qian J, Valenta D, Zack DJ. Changes in gene expression in experimental glaucoma and optic nerve transection: the equilibrium between protective and detrimental mechanisms. Invest Ophthalmol Vis Sci. 2007;48:5539–5548. doi: 10.1167/iovs.07-0542. [DOI] [PubMed] [Google Scholar]
- 43.Cvekl A, Piatigorsky J. Lens development and crystallin gene expression: many roles for Pax-6. Bioessays. 1996;18:621–630. doi: 10.1002/bies.950180805. [DOI] [PubMed] [Google Scholar]
- 44.Cvekl A, Yang Y, Chauhan BK, Cveklova K. Regulation of gene expression by Pax6 in ocular cells: a case of tissue-preferred expression of crystallins in lens. Int J Dev Biol. 2004;48:829–844. doi: 10.1387/ijdb.041866ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Somasundaram T, Bhat SP. Developmentally dictated expression of heat shock factors: exclusive expression of HSF4 in the postnatal lens and its specific interaction with alphaB-crystallin heat shock promoter. J Biol Chem. 2004;279:44497–44503. doi: 10.1074/jbc.M405813200. [DOI] [PubMed] [Google Scholar]
- 46.Cvekl A, Duncan MK. Genetic and epigenetic mechanisms of gene regulation during lens development. Prog Retin Eye Res. 2007;26:555–597. doi: 10.1016/j.preteyeres.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolf L, Yang Y, Wawrousek E, Cvekl A. Transcriptional regulation of mouse alpha A-crystallin gene in a 148kb Cryaa BAC and its derivates. BMC Dev Biol. 2008;8:88. doi: 10.1186/1471-213X-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones SE, Jomary C, Grist J, Thomas MR, Neal MJ. Expression of Pax-6 mRNA in the retinal degeneration (rd) mouse. Biochem Biophys Res Commun. 1998;252:236–240. doi: 10.1006/bbrc.1998.9631. [DOI] [PubMed] [Google Scholar]
- 49.Steinmayr M, Andre E, Conquet F, Rondi-Reig L, Delhaye-Bouchaud N, Auclair N, Daniel H, Crepel F, Mariani J, Sotelo C, Becker-Andre M. staggerer phenotype in retinoid-related orphan receptor alpha-deficient mice. Proc Natl Acad Sci USA. 1998;95:3960–3965. doi: 10.1073/pnas.95.7.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaneko Y, Matsumoto G, Hanyu Y. Pax-6 expression during retinal regeneration in the adult newt. Dev Growth Differ. 1999;41:723–729. doi: 10.1046/j.1440-169x.1999.00476.x. [DOI] [PubMed] [Google Scholar]
- 51.Bhat SP, Rayner SA, Chau SC, Ariyasu RG. Pax-6 expression in posthatch chick retina during and recovery from form-deprivation myopia. Dev Neurosci. 2004;26:328–335. doi: 10.1159/000082274. [DOI] [PubMed] [Google Scholar]
- 52.Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 53.Ton CC, Hirvonen H, Miwa H, Weil MM, Monaghan P, Jordan T, van Heyningen V, Hastie ND, Meijers-Heijboer H, Drechsler M, Royer-Pokora B, Collins F, Swaroop A, Strong LC, Saunders GF. Positional cloning and characterization of a paired box and homeobox containing gene from the aniridia region. Cell. 1991;67:1059–1074. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- 54.Glaser T, Walton DS, Maas RL. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nature Genet. 1992;2:232–238. doi: 10.1038/ng1192-232. [DOI] [PubMed] [Google Scholar]
- 55.Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL. Pax6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nature Genet. 1994;7:463–471. doi: 10.1038/ng0894-463. [DOI] [PubMed] [Google Scholar]
- 56.Mikkola I, Bruun JA, Bjorkoy G, Holm T, Johansen T. Phosphorylation of the transactivation domain of Pax6 by extracellular signal-regulated kinase and p38 mitogen-activated protein kinase. J Biol Chem. 1999;274:15115–15126. doi: 10.1074/jbc.274.21.15115. [DOI] [PubMed] [Google Scholar]
- 57.Miyara N, Shinzato M, Yamashiro Y, Iwamatsu A, Kariya K, Sawaguchi S. Proteomic analysis of rat retina in a steroid-induced ocular hypertension model: potential vulnerability to oxidative stress. Jpn J Ophthalmol. 2008;52:84–90. doi: 10.1007/s10384-007-0507-5. [DOI] [PubMed] [Google Scholar]
- 58.Young DB. Heat-shock proteins: immunity and autoimmunity. Curr Opin Immunol. 1992;4:396–400. doi: 10.1016/s0952-7915(06)80029-4. [DOI] [PubMed] [Google Scholar]
- 59.van Noort JM, van Sechel AC, Bajramovic JJ, el Ouagmiri M, Polman CH, Lassmann H, Ravid R. The small heat-shock protein alpha B-crystallin as candidate autoantigen in multiple sclerosis. Nature. 1995;375:798–801. doi: 10.1038/375798a0. [DOI] [PubMed] [Google Scholar]
- 60.Srivastava PK. Heat shock protein-based novel immunotherapies. Drug News Perspect. 2000;13:517–522. doi: 10.1358/dnp.2000.13.9.858479. [DOI] [PubMed] [Google Scholar]
- 61.Joachim SC, Bruns K, Lackner KJ, Pfeiffer N, Grus FH. Antibodies to alpha B-crystallin, vimentin, and heat shock protein 70 in aqueous humor of patients with normal tension glaucomaand IgG antibody patterns against retinal antigen in aqueous humor. Curr Eye Res. 2007;32:501–509. doi: 10.1080/02713680701375183. [DOI] [PubMed] [Google Scholar]
- 62.Tezel G, Seigel GM, Wax MB. Autoantibodies to small heat shock proteins in glaucoma. Invest Ophthalmol Vis Sci. 1998;39:2277–3287. [PubMed] [Google Scholar]
- 63.Munemasa Y, Kwong JM, Caprioli J, Piri N. The role of alphaA- and alphaB-crystallins in the survival of retinal ganglion cells after optic nerve axotomy. Invest Ophthalmol Vis Sci. 2009;50:3869–3875. doi: 10.1167/iovs.08-3138. [DOI] [PubMed] [Google Scholar]
- 64.Ying X, Zhang J, Wang Y, Wu N, Wang Y, Yew DT. Alpha-crystallin protected axons from optic nerve degeneration after crushing in rats. J Mol Neurosci. 2008;35:253–258. doi: 10.1007/s12031-007-9010-1. [DOI] [PubMed] [Google Scholar]
- 65.Chiu K, Zhou Y, Yeung SC, Lok CK, Chan OO, Chang RC, So KF, Chiu JF. Up-regulation of crystallins is involved in the neuroprotective effect of wolfberry on survival of retinal ganglion cells in rat ocular hypertension model. J Cell Biochem. 2010;110:311–320. doi: 10.1002/jcb.22539. [DOI] [PubMed] [Google Scholar]
- 66.Li XM. Protective effect of Lycium barbarum polysaccharides on streptozotocin-induced oxidative stress in rats. Int J Biol Macromol. 2007;40:461–465. doi: 10.1016/j.ijbiomac.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 67.Yu MS, Leung SKY, Lai SW, Che CM, Zee SY, So KF, Yuen WH, Chang RC. Neuroprotective effects of anti-aging oriental medicine Lycium barbarum against [beta]-amyloid peptide neurotoxicity. Exp Gerontol. 2005;40:716–727. doi: 10.1016/j.exger.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 68.Yu MS, Lai CS, Ho YS, Zee SY, So KF, Yuen WH, Chang RC. Characterization of the effects of anti-aging medicine Fructus lycii on beta-amyloid peptide neurotoxicity. Int J Mol Med. 2007;20:261–268. [PubMed] [Google Scholar]
- 69.Chan HC, Chang RCC, Ip AKC, Chiu K, Yuen WH, Zee SY, So KF. Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Exp Neurol. 2007;203:269–273. doi: 10.1016/j.expneurol.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 70.Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fischer D, Pavlidis M, Thanos S. Cataractogenic lens injury prevents traumatic ganglion cell death and promotes axonal regeneration both in vivo and in culture. Invest Ophthalmol Vis Sci. 2000;41:3943, 3954. [PubMed] [Google Scholar]
- 72.Fischer D, Heiduschka P, Thanos S. Lens-injury-stimulated axonal regeneration throughout the optic pathway of adult rats. Exp Neurol. 2001;172:257, 272. doi: 10.1006/exnr.2001.7822. [DOI] [PubMed] [Google Scholar]
- 73.Teng FY, Tang BL. Axonal regeneration in adult CNS neurons--signaling molecules and pathways. J Neurochem. 2006;96:1501–1508. doi: 10.1111/j.1471-4159.2006.03663.x. [DOI] [PubMed] [Google Scholar]
- 74.Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K, Martinez-Carrasco I, Connolly L, He Z. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64:617–623. doi: 10.1016/j.neuron.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong EV, David S, Jacob MH, Jay DG. Inactivation of myelin-associated glycoprotein enhances optic nerve regeneration. J Neurosci. 2003;23:3112–3117. doi: 10.1523/JNEUROSCI.23-08-03112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 79.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR, Benowitz LI. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23:2284–2293. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berry M, Carlile J, Hunter A. Peripheral nerve explants grafted into the vitreous body of the eye promote the regeneration of retinal ganglion cell axons severed in the optic nerve. J Neurocytol. 1996;25:147–170. doi: 10.1007/BF02284793. [DOI] [PubMed] [Google Scholar]
- 82.Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci. 2006;9:843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- 83.Lorber B, Berry M, Logan A. Lens injury stimulates adult mouse retinal ganglion cell axon regeneration via both macrophage- and lens-derived factors. Eur J Neurosci. 2005;21:2029–2034. doi: 10.1111/j.1460-9568.2005.04034.x. [DOI] [PubMed] [Google Scholar]
- 84.Müller A, Hauk TG, Fischer D. Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation. Brain. 2007;130:3308–3320. doi: 10.1093/brain/awm257. [DOI] [PubMed] [Google Scholar]
- 85.Hauk TG, Müller A, Lee J, Schwendener R, Fischer D. Neuroprotective and axon growth promoting effects of intraocular inflammation do not depend on oncomodulin or the presence of large numbers of activated macrophages. Exp Neurol. 2008;209:469–482. doi: 10.1016/j.expneurol.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 86.Leibinger M, Müller A, Andreadaki A, Hauk TG, Kirsch M, Fischer D. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J Neurosci. 2009;29:14334–14341. doi: 10.1523/JNEUROSCI.2770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fischer D, Hauk TG, Müller A, Thanos S. Crystallins of the beta/gamma-superfamily mimic the effects of lens injury and promote axon regeneration. Mol Cell Neurosci. 2008;37:471–479. doi: 10.1016/j.mcn.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 88.Liedtke T, Schwamborn JC, Schröer U, Thanos S. Elongation of axons during regeneration involves retinal crystallin beta b2 (crybb2) Mol Cell Proteomics. 2007;6:895–907. doi: 10.1074/mcp.M600245-MCP200. [DOI] [PubMed] [Google Scholar]