Abstract
Objectives
To evaluate IQ and academic skills in adults who experienced an episode of moderate to severe infantile malnutrition and a healthy control group, all followed since childhood in the Barbados Nutrition Study.
Methods
IQ and academic skills were assessed in 77 previously malnourished adults (mean age=38.4 years; 53% male) and 59 controls (mean age=38.1 years; 54% male). Group comparisons were carried out by multiple regression and logistic regression, adjusted for childhood socioeconomic factors.
Results
The previously malnourished group showed substantial deficits on all outcomes relative to healthy controls (p<0.0001). IQ scores in the Intellectual Disability range (< 70) were 9 times more prevalent in the previously malnourished group (OR=9.18; 95% CI=3.50-24.13). Group differences in IQ of approximately one standard deviation were stable from adolescence through mid-life.
Discussion
Moderate to severe malnutrition during infancy is associated with a significantly elevated incidence of impaired IQ in adulthood, even when physical growth is completely rehabilitated. An episode of malnutrition during the first year of life carries risk for significant lifelong functional morbidity.
Keywords: Academic achievement, IQ, Malnutrition, Socioeconomic
Introduction
Adequate nutrition in the early years of life is crucial for brain development. Laboratory studies have documented that pre- and post-natal nutritional deprivation can result in long-term alteration of structural and functional brain development,1,2 and, further, that these alterations are correlated with compromise of learning and memory.3,4 Moreover, there is ample documentation showing that previously malnourished and growth-stunted children display both cognitive and behavioral compromise in childhood and adolescence, presumed to reflect the impact of the malnutrition on the developing brain.5-8 The risk for impaired intellectual capacity across the lifespan, however, is less well appreciated.
The present study describes the IQ and academic skills of a group of Barbadian adults at mid-life who were of normal birth weight, but experienced an episode of moderate to severe malnutrition during the first year of life, requiring hospitalization. These individuals, along with a case-control comparison group recruited from the same community, have been followed through childhood, adolescence, and now into middle adulthood as participants in the Barbados Nutrition Study.
Evaluating the impact of infantile malnutrition on development is inevitably complicated by social context. Malnutrition typically occurs in the setting of significant poverty, which itself presents major risks for child development. Furthermore, even when children recover from an acute episode of malnutrition, many continue to experience chronic undernutrition, and the impacts of acute and chronic malnourishment can be difficult to discriminate. Disentangling the effects of the malnutrition itself from the very significant impact of such experiential adversities on brain and behavioral development thus presents a major research challenge.9
The Barbados Nutrition Study provides the opportunity to address some of these concerns. Although the children were reared in conditions of significant poverty that could have affected their development, the case-control design compared their performance to that of healthy children from the same neighborhoods and classrooms. Participants were enrolled in a nutritional intervention program following their hospitalization, which monitored and assured adequate nutrition and good health at least to age 12. Thus, for these individuals, the malnutrition episode can be assumed to have been limited to the first year of life. Importantly, detailed data on socioeconomic circumstances were collected at multiple time points during childhood and adolescence so that potentially confounding effects of standard of living on IQ and academic achievement could be estimated.
The primary finding from the Barbados Nutrition Study was that although the previously malnourished children achieved complete catch-up in their physical growth by adolescence, their cognitive and behavioral development remained adversely affected. Compared to a healthy control group from the same social environment, they had lower IQ, more attention problems, and lower grades in school.5,10,11 Their performance on the Common Entrance Examination, a standard academic competency test administered to all Barbadian children at age 11 to determine high school placement, was also poorer.12
In the present report, we document the intellectual functioning and academic skills of these previously malnourished individuals and the comparison group at mid-life. We specifically compare the prevalence of impaired IQ (in the range of Intellectual Disability) in the previously malnourished individuals to that of the control group.
Methods
Participants
Participants were born in Barbados, an English-speaking Caribbean country, between 1967 and 1972.5,11,13 The majority of the Barbadian population is of African origin (92%) and lower-middle class, with obligatory school attendance through age 16 and a 99% literacy rate. In 1970, during the period when the Barbados Nutrition Study sample was born, the infant mortality rate was 46 per 1000 live births, compared with 7.8 per 1000 today.
All index children had been admitted to Queen Elizabeth Hospital in their first year of life with grade II or III protein energy malnutrition (marasmus or kwashiorkor) based on the Gomez Scale, which classifies degree of malnutrition on the basis of expected weight for age.14 Children were eligible for inclusion if they met the following criteria: normal birth weight (>2500g), absence of pre- or postnatal complications, Apgar scores ≥8, no encephalopathic events during childhood, and no malnutrition after the first year of life. These children were subsequently enrolled in a government-supported intervention program that provided health care, growth monitoring, nutrition education, subsidized foods, and regular home visits, and were followed in the program until age 12. Healthy control children were recruited from the same classrooms and matched to the index children for gender, age (within three months), and handedness. They met the same inclusion criteria but had no histories of malnutrition.
Design
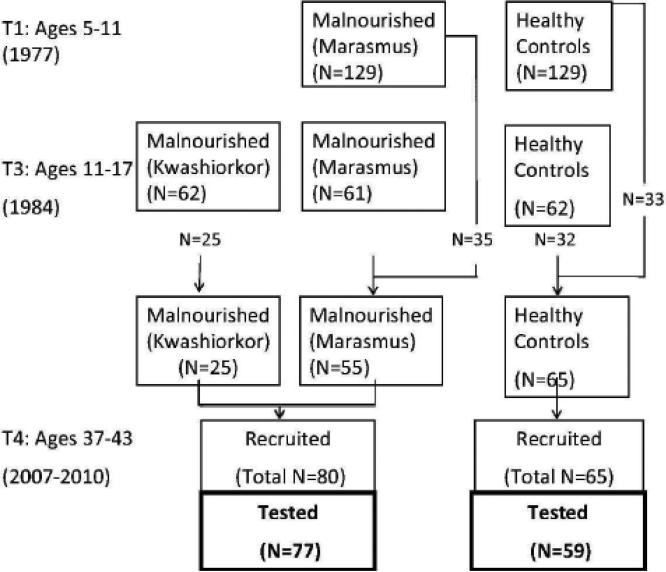
Figure 1 illustrates the design and specifies the source of the 136 individuals on whom the present study was based. Participants were evaluated comprehensively at three time points spanning childhood and adolescence and subsequently as adults. In 1977, we evaluated 129 children with histories of marasmus (growth retardation and wasting due to prolonged deficiency of protein and calories) and 129 healthy comparison children, all of whom were between the ages of 5 and 11 at that time. The same children were re-evaluated in 1982 (not shown in the Figure). In 1984, an additional group of children who had been hospitalized for kwashiorkor (N=62, insufficient protein in the diet) during the same period as the children with marasmus was recruited for comparison purposes. At that time, 123 (marasmus, N=61; healthy comparison, N=62) of the original 258 children were selected for evaluation because they were the best matches to the kwashiorkor group for age, sex, and grade in school. Note that the reduction in sample size at that point was not due to attrition, but to the specific focus of the study design at the third time point on potential differences in outcome between marasmus and kwashiorkor.
Figure 1.
Barbados Nutrition Study design, indicating the source of the 136 participants whose data were included in the present study.
Data collection was carried out between 2006 and 2010 when these individuals were in the latter part of their fourth decade of life. Since the kwashiorkor and marasmus groups did not differ on outcomes measured in adolescence, or in preliminary analyses of the adult outcomes, they were combined for the present study into a single, previously malnourished group. The sample included 80 previously malnourished participants and 63 control participants. Of these, 76 from the previously malnourished group and 59 from the healthy control group completed IQ and academic achievement testing. Median age of hospital admission (for the malnourished group) was 7.37 months (range 1-13 months).
Although we were able to account for 98% of the original participants through preliminary interviewing of community contacts, fewer were studied. A number of factors contributed to this reduction in numbers. Some individuals were deceased or incarcerated, and a number had moved off the island and were lost or inaccessible to follow-up. The primary source of attrition, however, was a limitation in funding resources to support the recruitment and data collection effort.
This study was approved by the Ministry of Health, Barbados. Written informed consent for the adult study was provided by all participants under the oversight of the Judge Baker Children's Center Human Research Review Committee (Assurance No. FWA 00001811).
Measures
Socioeconomic Status
Standard of living was measured at all 3 time points in childhood and adolescence using the Barbados Ecology Questionnaire,15 which assessed socioeconomic and microenvironmental conditions in the home, as well as educational level and employment history of the parents. The questionnaire contained 50 items assessing environmental conditions most relevant to the social context at the time. Factor analysis, based on data combined across all three time points, identified a first principal component (Armor ϴ = 0.86) that appeared to represent standard of living, including, for example, the number of rooms in the house or access to indoor plumbing.16 Scores based on this factor were standardized to have zero mean and unit variance.
IQ and Academic Achievement
Standardized screening tests of intelligence and academic achievement were applied. Because these tests are not normed in Barbados, US norms were applied.
Adult IQ
Wechsler Abbreviated Scale of Intelligence (WASI) – Vocabulary and Matrix Reasoning subtests. This 2-subtest version can be used to estimate Full Scale IQ. The correlation with the (4-subtest) WASI Full Scale IQ is reported to be 0.95, and the correlation with the Wechsler Adult Intelligence Scale-III Full Scale IQ is 0.92. Adolescent IQ (at 11-17 years) was measured using the WISC-R.10
Academic Achievement
Wide Range Achievement Test-III (WRAT-III) – Reading, Spelling, and Calculation subtests. These are brief measures of single word reading, single word spelling, and calculation.
Testing was carried out in a single session at the study laboratory, in the context of a more detailed neuropsychological assessment, by a trained local psychologist or psychometrician who was blind to group status.
Statistical Methods
Data were analyzed using SAS statistical software, version 9.2. Means, standard deviations, and ranges were calculated for all outcomes. Group differences in demographic characteristics were evaluated by t-tests and chi-square tests. Because of the known impact of socioeconomic circumstances on cognitive outcome, and the documented differences between the nutrition groups on this variable, we adjusted for its effects in all analyses. We adjusted for childhood standard of living but not adult socioeconomic status, since the latter was viewed as a potential outcome of longstanding functional compromise associated with the history of early malnutrition.
Group differences in the prevalence of IQ in the Intellectual Disability range (<70) and impaired academic functioning were evaluated by logistic regression, adjusting for the effects of childhood standard of living, using all available data points from childhood and adolescence. A similar set of analyses, with 70 as the cut-off, was applied to the academic achievement tests.
For descriptive purposes, group IQ means were calculated at adolescence (based on archival data) and adulthood for those individuals who participated in the Barbados Nutrition Study at both time points. Although these scores could not be compared directly because different test instruments had been used, the descriptive data provide an indication of potential continuity or change over time.
Results
Demographic Characteristics
Since the sample attrition raised concerns about bias, we compared the demographic characteristics, as documented at the time of their entry to the study as children or adolescents, of individuals who did and did not participate as adults. Participants and non-participants did not differ in terms of representation from the malnourished and control groups, childhood IQ, or childhood standard of living. Borderline range differences (p=0.10) were detected for age (participants older) and sex (proportionally fewer males among participants). Preliminary analyses indicated, however, that neither of these variables was related to the primary outcomes, IQ or academic achievement. Thus, the study participants could be assumed to be representative of the original sample.
Table 1 displays the demographic characteristics of the sample. The nutrition groups did not differ in terms of age or sex, but there were significant differences in childhood standard of living.
Table 1.
Demographic characteristics by group.
| Infant Malnutrition (N=77) | Control (N=59) | |||
|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | |
| Age (years) | 38.36 | 1.86 | 38.13 | 1.93 |
| Sex N(%) male | 41 (53.2) | 32 (54.2) | ||
| Childhood Standard of Living at T1*** | -0.91a | 0.93 | -0.27b | 0.80 |
| Childhood Standard of Living at T2* | 0.31c | 0.81 | 0.66d | 0.74 |
| Childhood Standard of Living at T3* | 0.16e | 0.73 | 0.53f | 0.62 |
N=53
N=59
N=47
N=52
N=58
N=28.
p<0.05
p<0.001
Intelligence and Academic Skills
Table 2 displays the unadjusted group means, as well as parameter estimates, for models including both malnutrition status and childhood standard of living as predictors. Malnutrition effects from the latter models can be interpreted as adjusted group mean differences controlling for childhood standard of living. After adjusting for childhood standard of living, there were statistically significant group differences on all outcome variables, generally on the order of one standard deviation, a very large effect size. The estimated group difference for IQ, for example, was 15 points.
Table 2.
Unadjusted Means for Previously Malnourished and Control Groups and Model Parameter Estimates (Adjusted for Childhood Standard of Living) for IQ and Academic Achievement.
| Unadjusted Mean |
|||||
|---|---|---|---|---|---|
| Infant Malnutrition (N=77) | Control (N=59) | Malnutrition Effect | |||
| Mean | S.D. | Mean | S.D. | Beta | |
| WASI | |||||
| Vocabulary (T-score) | 38.03 | 11.62 | 48.81 | 11.12 | -9.88**** |
| Matrix Reasoning (T-score) | 38.36 | 13.08 | 49.17 | 11.00 | -10.37**** |
| Full IQ (Standard Score) | 82.83 | 16.62 | 99.03 | 15.43 | -15.06**** |
| WRAT-III (Standard Score) | |||||
| Reading | 77.51 | 18.23 | 93.91 | 17.65 | -15.72**** |
| Spelling | 72.80 | 17.72 | 87.88 | 16.51 | -14.05**** |
| Calculation | 73.46 | 14.41 | 85.10 | 11.07 | -10.78**** |
*p<0.05
**p<0.01
***p<0.001
P<0.0001
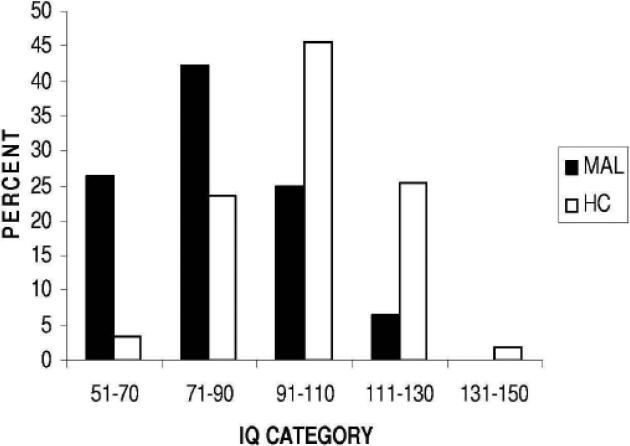
Participants from each group were classified according to whether their IQ fell within the range of Intellectual Disability (<70) or not. Twenty individuals (26.3%) from the previously malnourished group had impaired IQ compared with only 2 (3%) from the control group (Figure 2).
Figure 2.
Distribution of IQ scores for previously malnourished (MAL, N=76) and healthy control (HC, N=59) groups.
These data were then submitted to logistic regression to evaluate group differences in prevalence of impaired IQ, adjusted for childhood standard of living. Individuals in the previously malnourished group were estimated to be 9 times more likely to have impaired IQ than those in the control group (OR = 9.18, 95% CI=3.50-24.13).
Academic skills were also depressed in the malnourished group, similarly adjusted for childhood standard of living (Reading, OR=3.44, 95% CI=1.88-6.30; Spelling, OR=6.10, 95% CI=3.34-11.16; Calculation, OR=6.23, 95% CI=3.15-12.32, all p<0.0001).
Since infants who were younger when the malnutrition occurred could have been more vulnerable to impairment, an exploratory analysis was conducted to assess the potential association between age at hospital admission and adult IQ. This analysis did not confirm such a relationship (r=0.11, p=0.33).
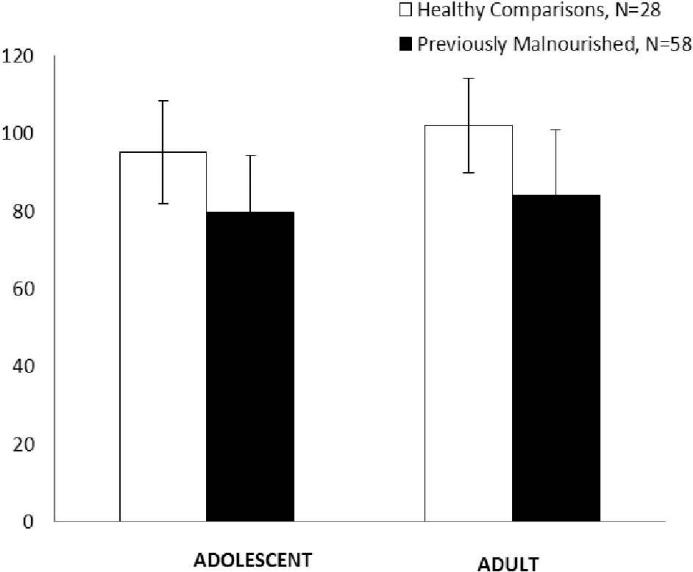
Finally, Figure 3 shows mean IQ scores of 86 individuals (58 previously malnourished; 28 control) who completed testing as both adolescents and adults. Group means differed at both time points, with differences approximating one standard deviation (Adolescent difference, 15.5; Adult difference, 18.1, both p<0.001). The number of individuals with IQ below 70 was also comparable (Adolescent: Previously malnourished, 22.4%, Control, 3.6%; Adult: Previously malnourished, 25.9%, Control, 0.0%).
Figure 3.
Mean IQ scores of previously malnourished (N=58) and healthy control (N=28) individuals who were tested in both adolescence (WISC-R) and adulthood (WASI).
Discussion
The present findings document the life-span cognitive burden of infantile malnutrition in a well-characterized sample that has been followed for over four decades from infancy to mid-life. Cognitive deficits documented in childhood persisted into adulthood. Likewise, of significant concern, the prevalence of IQ scores in the Intellectual Disability range was substantial, with an estimated 9-fold elevation in the previously malnourished group compared to the control group. These outcomes, moreover, appear to have been relatively stable over the more than two decades that have elapsed since the Barbados Nutrition Study cohort was evaluated as adolescents.
As discussed initially, the vast majority of cases of malnutrition occur in the setting of poverty, which greatly complicates interpretation of outcome data such as those reported here. Although the healthy control children in our study were selected from the same neighborhoods and classrooms as the probands, they nevertheless differed significantly in terms of their standard of living within that context. Including the standard of living indicator in our statistical models only minimally decreased the magnitude of the nutrition group differences, however.
A number of studies have pointed to an impact of early postnatal malnutrition on lifetime cognitive functioning. A recent compilation of data from five epidemiologic cohorts found that weight gain in the first two years of life predicted school attainment in adulthood.17 Intervention studies further corroborate the impact of early malnutrition. Grantham-McGregor and colleagues followed a sample of Jamaican children who displayed growth stunting at 9 to 24 months of age. Although their cognitive impairment persisted into young adulthood, early intervention consisting of cognitive stimulation and parent support mitigated these effects. The intervention was associated with a 7 point increase in IQ from 70 to 77 and a lower level of violent behavior at 22 years of age.18 Similarly, a Guatemalan study found benefits of a protein supplement (versus a calorie supplement) during infancy on cognitive outcomes and schooling at age 40.19 None of the studies, however, were designed with the primary intention of comparing long-term outcomes in individuals malnourished as infants to those of a healthy control group in order to ascertain the impact of early malnutrition. Thus, the findings from the present study, which includes participants from a healthy control group, and in which subsequent malnutrition did not occur, provides a more direct assessment of the potential developmental cost of a limited period of malnutrition during the first year of life.
The neural substrates involved in early malnutrition and its apparent lifelong functional consequences in humans are not well understood. Magnetic resonance imaging studies have documented structural changes in the brains of children during the acute phases of protein-energy malnutrition, the most consistent of which is cerebral atrophy.20 One longitudinal study reported, though, that by 90 days after admission for rehabilitation, the cerebral “shrinkage” was largely reversed, suggesting recovery.21 In rodent models, however, malnutrition can lead to microstructural and neurochemical changes that are not reversed by nutritional rehabilitation.22 Although such changes would not be detected by clinical MRI, neuropathological analysis of the brains of infants who died of malnutrition has in fact documented alterations in dendritic structure.23
From a developmental perspective, the observed outcomes cannot be interpreted strictly as sequelae of structural neurological impairment associated with the episode of malnutrition. They likely also reflect the altered interactions of the neurologically compromised child with the physical and social environment over the course of a lifetime. To some extent, therefore, the outcomes may reflect the continuing impact of the neurological impairment on these interactions, and the further impact of the interactions themselves on subsequent development.
Despite the high rate of intellectual impairment among the previously malnourished individuals, it is equally important to note that a number of them did exhibit favorable outcomes, suggesting some source of resilience. Protective factors could be experiential, having to do with the quality of the child's home life, or biological, such as a genetic variant that protected the brain against the adverse effects of the malnutrition or stress. More detailed exploration of risk and protective factors is thus highly relevant.
The broader social implications of the cognitive impairment associated with infantile malnutrition may be considerable. In a separate report, we documented substantial social and economic disparities in adulthood between the previously malnourished and healthy control groups.24 These differences were partially accounted for by childhood IQ. Thus, the intellectual impairment that ensues from early malnutrition apparently persists from childhood into adulthood, with likely impact on life circumstances, and with considerable social implications for populations where early malnutrition and undernutrition are widespread.
The study also had limitations. Although we did find significant group differences in childhood standard of living and adjusted for these effects in our models, our indicators could have failed to capture salient but unknown environmental influences that could account to some extent for the observed differences in outcomes. We did not, for example, have direct measures of potentially relevant aspects of the social environment, such as the quality of the mother's language and the mother-child interaction. Also, we obtained data from less than 50% of the original sample, raising the potential for ascertainment bias. Comparison of participants and non-participants on childhood characteristics, however, did not reveal meaningful differences.
In sum, this study provides strong evidence that an episode of moderate to severe malnutrition in infancy can lead to lifelong functional burdens, with a significantly elevated incidence of impaired IQ, in the range of Intellectual Disability, in adulthood. Given the millions of children who continue to be impacted by malnutrition world-wide, the findings have major social implications. Furthermore, since these adverse outcomes were seen in individuals who had the benefit of excellent nutritional rehabilitation and monitoring after the event, prevention of infantile malnutrition must remain the highest priority from a public health perspective, with potentially lifelong social and economic benefit.
Acknowledgements
This work was conducted with the cooperation of the Ministry of Health of Barbados and by grants R01 MH065877 and R01 HD060986 from the National Institutes of Health. No restrictions have been imposed by them on free access to or publication of the research data.
Footnotes
Institution and department addresses:
Barbados Nutrition Study, 17 Warrens Terrace, St. Thomas, Barbados
Boston Children's Hospital, 300 Longwood Ave, Boston, MA 02115
Harvard Medical School, 651 Huntington Avenue, Boston, MA 02115
Harvard School of Public Health, 677 Huntington Avenue, Boston, MA 02115
Judge Baker Children's Center, 53 Parker Hill Avenue, Boston, MA 02120
McLean Hospital, 115 Mill Street, Belmont, MA 02478
Conflicts of Interest: None
Contributor Information
Deborah P. Waber, Dept. of Psychiatry, Boston Children's Hospital and Harvard Medical School, Boston, MA, Postal: Boston Children's Hospital, 300 Longwood Ave, Pavilion 155, Boston, MA 02115
Cyralene P. Bryce, Barbados Nutrition Study, Bridgetown, Barbados, Postal: Barbados Nutrition Study, 17 Warrens Terrace, St. Thomas, Barbados
Jonathan M. Girard, Dept. of Psychiatry, Boston Children's Hospital, Boston, MA, Postal: Boston Children's Hospital, 300 Longwood Ave, Pavilion 152, Boston, MA 02115
Miriam Zichlin, Judge Baker Children's Center, Boston, MA, Postal: Judge Baker Children's Center, 53 Parker Hill Avenue, Boston, MA 02120
Garrett M. Fitzmaurice, Laboratory for Psychiatric Biostatistics, McLean Hospital and Department of Biostatistics, Harvard School of Public Health, Boston, MA, Postal: Laboratory for Psychiatric Biostatistics, McLean Hospital, 115 Mill Street, Belmont, MA 02478
Janina R. Galler, Judge Baker Children's Center and Harvard Medical School, Boston, MA, Postal: Judge Baker Children's Center, 53 Parker Hill Avenue, Boston, MA 02120
References
- 1.Bedi KS. Nutritional effects on neuron numbers. Nutr Neurosci. 2003;6(3):141–52. doi: 10.1080/1028415031000098549. [DOI] [PubMed] [Google Scholar]
- 2.Lister JP, Tonkiss J, Blatt GJ, Kemper TL, DeBassio WA, Galler JR, et al. Asymmetry of neuron numbers in the hippocampal formation of prenatally malnourished and normally nourished rats: A stereological investigation. Hippocampus. 2006;16(11):946–58. doi: 10.1002/hipo.20221. [DOI] [PubMed] [Google Scholar]
- 3.Durán P, Cintra L, Galler JR, Tonkiss J. Prenatal protein malnutrition induces a phase shift advance of the spontaneous locomotor rhythm and alters the rest/activity ratio in adult rats. Nutr Neurosci. 2005;8(3):167–72. doi: 10.1080/10284150400026117. [DOI] [PubMed] [Google Scholar]
- 4.Valadares CT, Fukuda MTH, Françolin-Silva AL, Hernandes AS, Almeida SS. Effects of postnatal protein malnutrition on learning and memory procedures. Nutr Neurosci. 2010;13(6):274–82. doi: 10.1179/147683010X12611460764769. [DOI] [PubMed] [Google Scholar]
- 5.Galler JR, Ramsey F, Solimano G, Lowell WE, Mason E. The influence of early malnutrition on subsequent behavioral development: I. Degree of impairment in intellectual performance. J Am Acad Child Adolesc Psychiatry. 1983;22(1):8–15. doi: 10.1097/00004583-198301000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Grantham-McGregor S. A review of studies of the effect of severe malnutrition on mental development. J Nutr. 1995;125(8 Suppl):2233S–8S. doi: 10.1093/jn/125.suppl_8.2233S. [DOI] [PubMed] [Google Scholar]
- 7.Ivanovic DM, Leiva BP, Perez HT, Inzunza NB, Almagià AF, Toro TD, et al. Long-term effects of severe undernutrition during the first year of life on brain development and learning in Chilean high-school graduates. Nutrition. 2000;16(11-12):1056–63. doi: 10.1016/s0899-9007(00)00431-7. [DOI] [PubMed] [Google Scholar]
- 8.Kar BR, Rao SL, Chandramouli BA. Cognitive development in children with chronic protein energy malnutrition. Behav Brain Funct. 2008:4. doi: 10.1186/1744-9081-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wachs TD. Multiple influences on children's nutritional deficiencies: A systems perspective. Physiol Behav. 2008;94(1):48–60. doi: 10.1016/j.physbeh.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Galler JR, Ramsey F, Forde V, Salt P, Archer E. Long-term effects of early kwashiorkor compared with marasmus. II. Intellectual performance. J Pediatr Gastroenterol Nutr. 1987;6(6):847–54. doi: 10.1097/00005176-198711000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Galler JR, Ramsey F, Solimano G, Lowell WE. The influence of early malnutrition on subsequent behavioral development: II. Classroom behavior. J Am Acad Child Adolesc Psychiatry. 1983;22:16–22. doi: 10.1097/00004583-198301000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Galler JR, Ramsey F, Morley DS, Archer E, Salt P. The long-term effects of early kwashiorkor compared with marasmus. IV. Performance on the national high school entrance examination. Pediatr Res. 1990;28(3):235–9. doi: 10.1203/00006450-199009000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Galler JR, Ramsey F, Salt P, Archer E. Long-term effects of early kwashiorkor compared with marasmus. I. Physical growth and sexual maturation. J Pediatr Gastroenterol Nutr. 1987;6(6):841–6. doi: 10.1097/00005176-198711000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Gomez F, Galvan R, Cravioto J, Frenk S. Malnutrition in infancy and childhood, with special reference to kwashiorkor. Adv Pediatr. 1955;7:131–69. [PubMed] [Google Scholar]
- 15.Galler JR, Ramsey F. The influence of early malnutrition on subsequent behavioral development: VI. The role of the microenvironment of the household. Nutrition & Behavior. 1985;2(3):161–73. [Google Scholar]
- 16.Galler JR, Bryce CP, Waber D, Hock RS, Exner N, Eaglesfield D, et al. Early childhood malnutrition predicts depressive symptoms at ages 11-17. J Child Psychol Psychiatry. 2010;51(7):789–98. doi: 10.1111/j.1469-7610.2010.02208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martorell R, Melgar P, Maluccio JA, Stein AD, Rivera JA. The nutrition intervention improved adult human capital and economic productivity. J Nutr. 2010;140(2):411–4. doi: 10.3945/jn.109.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker SP, Chang SM, Vera-Hernández M, Grantham-McGregor S. Early childhood stimulation benefits adult competence and reduces violent behavior. Pediatrics. 2011;127(5):849–57. doi: 10.1542/peds.2010-2231. [DOI] [PubMed] [Google Scholar]
- 19.Pollitt E, Gorman KS, Engle PL, Rivera JA, Martorell R. Nutrition in early life and the fulfillment of intellectual potential. J Nutr. 1995;125(4 Suppl):1111S–8S. doi: 10.1093/jn/125.suppl_4.1111S. [DOI] [PubMed] [Google Scholar]
- 20.Odabas D, Caksen H, Sar S, Unal O, Tuncer O, Atas B, et al. Cranial MRI findings in children with protein energy malnutrition. Int J Neurosci. 2005;115:829–37. doi: 10.1080/00207450590882082. [DOI] [PubMed] [Google Scholar]
- 21.Gunston G, Burkimsher D, Malan H, Sive A. Reversible cerebral shrinkage in kwashiorkor: An MRI study. Arch Dis Child. 1992;67:1030–2. doi: 10.1136/adc.67.8.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgane PJ, Austin-LaFrance R, Bronzino J, Tonkiss J, Díaz-Cintra S, Cintra L, et al. Prenatal malnutrition and development of the brain. Neurosci Biobehav Rev. Spring. 1993;17(1):91–128. doi: 10.1016/s0149-7634(05)80234-9. [DOI] [PubMed] [Google Scholar]
- 23.Benítez-Bribiesca L, De la Rosa-Alvarez I, Mansilla-Olivares A. Dendritic spine pathology in infants with severe protein-calorie malnutrition. Pediatrics. 1999;104(2):e21. doi: 10.1542/peds.104.2.e21. [DOI] [PubMed] [Google Scholar]
- 24.Galler JR, Bryce C, Waber D, Zichlin M, Fitzmaurice G, Eaglesfield G. Socioeconomic outcomes in adults malnourished in the first year of life: A 40-year study. Pediatrics. 2012;130(1):e1–7. doi: 10.1542/peds.2012-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]