Abstract
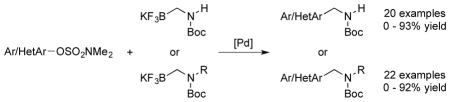
Sulfamates were studied as the electrophilic partners in the palladium-catalyzed Suzuki–Miyaura cross-coupling reaction with potassium Boc-protected primary and secondary aminomethyltrifluoroborates. A broad range of substrates was successfully coupled to provide the desired products. Complex molecules containing a new carbon-carbon bond and an aminomethyl moiety could be prepared through this developed method.
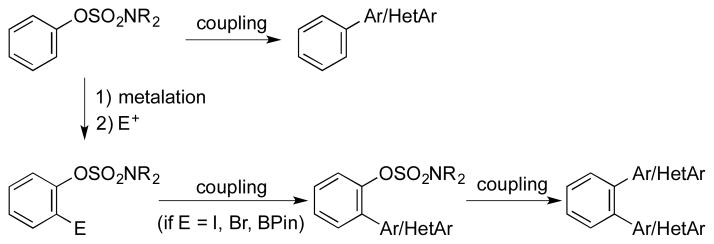
Transition metal catalyzed cross-coupling reactions of phenol derivatives, such as sulfonates1 and sulfamates,2–4 can complement procedures using more common aryl halide electrophiles. Such substrates are inexpensive and easy to prepare from the corresponding phenols. Moreover, phenolic moieties are readily found as intermediates in many target oriented syntheses, and can thus be utilized as coupling partners after activation when analogous halides would be difficult to access.
Recently, sulfamates have gained interest because of their facile preparation and ease of further functionalization.2,3a The installation of functional groups via metalation ortho or para to sulfamates have been reported, and the sulfamate group can also remain intact during transformations of other functional groups embedded within the molecules.
Subsequent to such transformations, sulfamates can serve as electrophiles in cross-coupling reactions. Although sulfamates have been utilized as partners in Kumada couplings,3 many functional groups, such as esters, ketones, and nitro groups are generally not compatible with the Grignard reagents used in these protocols. Therefore, Kumada couplings cannot be widely applied. More recently, Suzuki–Miyaura reactions with sulfamates have been reported by several research groups.2,3a,4 Boronic acids and neopentylglycolboronates have been successfully coupled with sulfamates in the presence of nickel catalysts. Only nickel-catalyzed reactions have been studied because palladium catalysts were thought to be inefficient for sulfamate coupling reactions.2a By a combination of these developed methods (functionalization and coupling reactions), the complexity of the starting materials used in these transformations can be rapidly increased (Scheme 1).
scheme 1.
As a continuation of our study on phenol coupling partners5,6a and aminomethylating reagents,6,7 we were interested in the use of sulfamates as the electrophilic coupling partners in the Suzuki–Miyaura reaction of potassium Boc-protected aminomethyltrifluoroborates. We envisioned that carbon(sp3)-carbon(sp2) bonds could be formed by the development of Suzuki–Miyaura coupling reactions with sulfamates and aminomethyltrifluoroborates, which were previously reported only with carbon(sp2)-carbon(sp2) bond formation. To the best of our knowledge, palladium-catalyzed Suzuki–Miyaura coupling reactions with potassium organotrifluoroborates and sulfamates have not been reported.
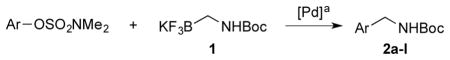
First, coupling reactions were investigated with the N,N-dimethylsulfamate derived from 1-naphthol and Boc-protected primary aminomethyltrifluoroborate 1. Various palladium catalysts, ligands, bases, solvents, concentrations, temperatures, and reaction times were screened extensively (see Supporting Information for details). After this process, the combination of 4 mol % of XPhos-Pd-G2 (Buchwald’s second generation preformed catalyst, Figure 1) and K2CO3 in t-BuOH/H2O (1:1, 0.5 M) at 85 °C for 3 h emerged as the best conditions. Inexplicably, the amount of base was not general to all substrates. Therefore, 3, 5, or 7 equiv of K2CO3 were required, depending on the nature of the sulfamates.
Figure 1.
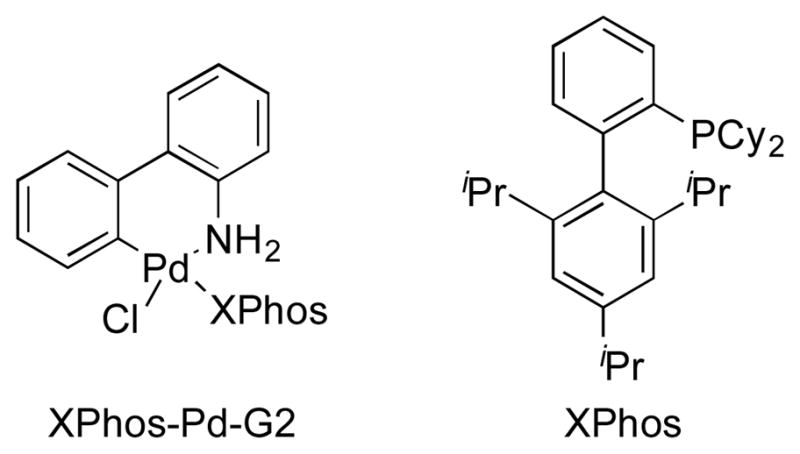
Buchwald’s 2nd generation preformed catalyst
With the optimized conditions in hand, we investigated the scope of the coupling reactions with aryl sulfamates (Table 1). 1-Naphthol and phenol sulfamates were successfully coupled to provide the desired products in 93% and 85% isolated yields, respectively (Table 1, entries 1 and 2). Unfortunately, the ortho or para-methyl substituted phenolic derivatives were observed in low conversions and low yields by 1H NMR with an internal standard (Table 1, entries 3 and 4). Moreover, electron-rich substrates in general proved to be inefficient coupling partners under the same set of reaction conditions (Table 1, entry 5). Interestingly, the aryl sulfamate with both electron-donating and electron-withdrawing groups on the aryl ring gave the desired product 2f in a high yield, 90%, with full conversion (Table 1, entry 6). In the case of fluoro substituted aryl sulfamates, a mixture of t-BuOH/H2O was not efficient because low conversion and yields were observed. After screening the solvents again, a mixture of n-PrOH/H2O emerged as a better solvent system for the fluoro substrate to obtain the desired product in a higher yield with a better conversion (Table 1, entry 9). Several functional groups, such as ketones, esters, nitriles, and nitro groups were compatible throughout the coupling reactions performed. Moreover, the reaction was scalable to 3 mmol of sulfamate with a lower catalyst loading, 2 mol % instead of 4 mol %, and the desired product was isolated in 93% yield (Table 1, entry 1).
Table 1.
Cross-Coupling of Various Aryl Sulfamates with Primary Aminomethyltrifluoroborate 1
| |
|---|---|
| entry | product yield(%)b/conversion(%)c |
| 1d |
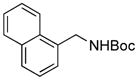 2a, 93(93)h/100 |
| 2e |
2b, 85/90 |
| 3d |
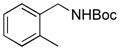 2c,13b/27 |
| 4d |
2d, 38b/38 |
| 5d |
2e, 23b/25 |
| 6d |
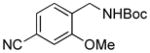 2f, 90/100 |
| 7d |
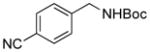 2g, 88/100 |
| 8d |
2h, 93/100 |
| 9f |
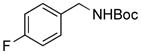 2i, 72/75 89/98g |
| 10d |
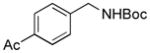 2j, 87/100 |
| 11e |
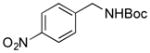 2k, 60/80 |
| 12e |
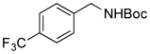 2l, 76/100 |
Reaction conditions: 1.0 equiv of aryl sulfamate, 1.05 equiv of trifluoroborate, 4 mol % XPhos-Pd-G2, K2CO3, t-BuOH/H2O (1:1, 0.5 M), 85 °C, 3 h.
isolated yield.
calculated by 1H NMR with 30 μL of CH2Cl2.
3 equiv K2CO3.
5 equiv K2CO3.
7 equiv K2CO3.
n-PrOH/H2O.
3 mmol of sulfamate, 2 mol % XPhos-Pd-G2, 3 equiv K2CO3, t-BuOH/H2O (1:1, 0.5 M), 85 °C, 18 h.
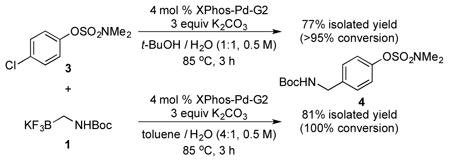
Subsequently, 4-chlorophenyl N,N-dimethylsulfamate 3 was examined as the electrophilic coupling partner using the same set of conditions (eq 1). Perhaps not unexpectedly, the product that was isolated resulted from coupling of the chloride site, and no trace of product resulting from coupling at the sulfamate could be detected by 1H NMR. Conditions that had been previously developed for coupling of aryl chlorides with Boc-protected primary aminomethyltrifluoroborate were also applied to this substrate, with essentially the same result. Therefore, oxidative addition to chlorides is evidently faster than that of sulfamates when the two substrates are on the same aryl ring. In fact, nickel-catalyzed Suzuki–Miyaura cross-coupling reactions provide the same result with competing sulfamate/halide electrophilic sites, albeit using an iodide as the halide as opposed to a chloride as in the present study.2b
 |
(1) |
To expand the array of electrophiles, hetaryl sulfamates were employed as the coupling partners using the same reaction conditions (Table 2). Various hetaryl sulfamates containing nitrogen and sulfur proved to be good electrophilic coupling counterparts. However, the desired product was not detected with indazole derivatives (Table 2, entry 7). In the case of indole, the reaction proceeded to give the corresponding product 5c without protection in 68% isolated yield (Table 2, entry 3). 2-Pyridyl sulfamate gave the product 5f in lower yield compare to 3-pyridyl sulfamate (Table 2, entries 5 and 6).
Table 2.
Cross-Coupling of Various Hetaryl Sulfamates with Primary Aminomethyltrifluoroborate 1
| |
|---|---|
| entry | product yield(%)b/conversion(%)c |
| 1d |
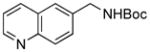 5a, 88/100 |
| 2f |
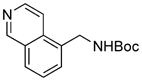 5b, 91/100 |
| 3f |
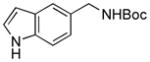 5c, 68/79 |
| 4d |
5d, 85/89 |
| 5e |
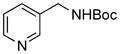 5e, 83/83 |
| 6e |
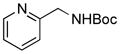 5f, 58/100 |
| 7d |
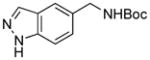 5g, 0/0 |
Reaction conditions: 1.0 equiv of hetaryl sulfamate, 1.05 equiv of trifluoroborate, 4 mol % XPhos-Pd-G2, K2CO3, t-BuOH/H2O (1:1, 0.5 M), 85 °C, 3 h.
isolated yield.
calculated by 1H NMR with 30 μL of CH2Cl2.
3 equiv K2CO3.
5 equiv K2CO3.
7 equiv K2CO3.
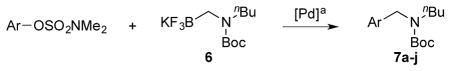
Based on the reaction conditions with Boc-protected primary aminomethyltrifluoroborate, we screened the coupling reactions of Boc-protected secondary aminomethyltrifluoroborate 6 with different bases, solvents and times. The optimal conditions were a combination of 4 mol % of XPhos-Pd-G2 and 7 equiv of K2CO3 in t-BuOH/H2O (1:1, 0.5 M) at 85 °C for 18 h. Although the amount of base was varied with primary aminomethyltrifluoroborate 1, 7 equiv of base was more efficient for secondary aminomethyltrifluoroborate 6. Moreover, a longer reaction time, 18 h rather than 3 h, was required for the reactions to go to completion.
We applied these optimized conditions to coupling reactions with various aryl sulfamates (Table 3). Unfortunately, many of the substrates did not give as high yields as the coupling reactions with the primary aminomethyltrifluoroborate 1. 1-Naphthol sulfamate was successfully coupled to provide the desired products in 92% isolated yield, but the substrate derived from phenol gave the corresponding product in only 33% yield (Table 1, entries 1 and 2). A sulfamate with both electron-rich and electron-poor functional groups on the aryl ring provided the desired product in 63% isolated yield, while lower yields and lower conversions were observed by 1H NMR in the case of an electron-rich sulfamate (Table 3, entries 3 and 4). Several functional groups, such as nitrile, ketones, and esters, were tolerated. In the case of the nitrile-containing substrate, a lower amount of base, 5 equiv of K2CO3, was required (Table 3, entry 5). An acyl substituted sulfamate gave a lower yield compared to other substrates (Table 3, entry 6). Even though the expected products were obtained with fluoro and trifluoromethyl substituted aryl sulfamates, the yields, 47% and 42%, respectively, were lower (Table 3, entries 8 and 9). Unfortunately, the desired product was not observed with a nitro group-containing aryl sulfamate (Table 3, entry 10).
Table 3.
Cross-Coupling of Various Aryl Sulfamates with Secondary Aminomethyltrifluoroborates 6
| |
|---|---|
| entry | product yield(%)b/conversion(%)c |
| 1 |
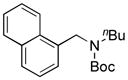 7a, 92/100 |
| 2 |
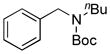 7b, 33/76 |
| 3 |
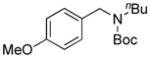 7c, 15b/16 |
| 4 |
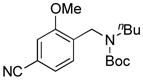 7d, 63/100 |
| 5d |
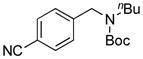 7e, 53/80 |
| 6 |
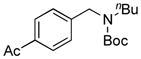 7f, 42/100 |
| 7 |
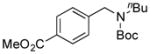 7g, 76/100 |
| 8 |
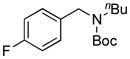 7h, 47/68 |
| 9 |
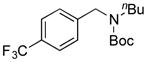 7i, 42/60 |
| 10 |
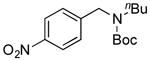 7j, 0/0 |
Reaction conditions: 1.0 equiv of aryl sulfamate, 1.05 equiv of trifluoroborate, 4 mol % XPhos-Pd-G2, 7 equiv of K2CO3, t-BuOH/H2O (1:1, 0.5 M), 85 °C, 18 h.
isolated yield.
calculated by 1H NMR with 30 μL of CH2Cl2.
5 equiv of K2CO3.
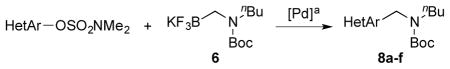
We also studied hetaryl sulfamates as the electrophilic coupling partners in the coupling with secondary aminomethyltrifluoroborate 6 (Table 4). Unfortunately, the reactions were limited to only a few hetaryl sulfamates. Quinoline, isoquinoline, and thiazole derivatives provided the desired products in good yields (Table 4, entries 1–3). However, pyridine derivatives were inefficient coupling partners under the same set of conditions (Table 4, entries 4 and 5). No conversion was observed in the case of the 2-pyridyl sulfamate, and with the 3-pyridyl sulfamate a complex mixture of products was formed. Moreover, the expected product was not detected when the indole derivative was applied (Table 4, entry 6), with again unreacted starting material being observed. Even though expanded studies were conducted with these substrates, all efforts were unsuccessful.
Table 4.
Cross-Coupling of Various Hetaryl Sulfamates with Secondary Aminomethyltrifluoroborates 6
| |
|---|---|
| entry | product yield(%)b/conversion(%)c |
| 1 |
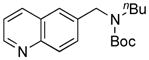 8a, 84/100 |
| 2 |
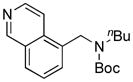 8b, 83/100 |
| 3 |
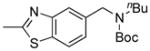 8c, 85/92 |
| 4 |
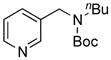 8d, 29b/100 |
| 5 |
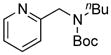 8e, 0/0 |
| 6 |
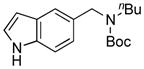 8f, 0/0 |
Reaction conditions: 1.0 equiv of hetaryl sulfamate, 1.05 equiv of trifluoroborate, 4 mol % XPhos-Pd-G2, 7 equiv of K2CO3, t-BuOH/H2O (1:1, 0.5 M), 85 °C, 18 h.
isolated yield.
calculated by 1H NMR with 30 μL of CH2Cl2.
Next, we employed various Boc-protected secondary aminomethyltrifluoroborates 9a–f as the nucleophilic coupling partners with N,N-dimethylsulfamated 1-naphthol using the same set of conditions (Table 5). An aliphatic alkyl group on the amine nitrogen was effectively coupled to give the desired product 10a in good yield (Table 5, entry 1). In the case of the substrates possessing cyclic alkyl groups on the nitrogen, only a cyclohexyl group provided the corresponding product 10b in 68% isolated yield (Table 5, entry 2). Unfortunately, the expected product was not observed with a cyclopropyl group (Table 5, entry 3). Benzyl groups on the nitrogen, even with electron-donating groups on an aryl ring, afforded the desired products in good yields (Table 5, entries 4 and 5). Moreover, the trifluoroborate with an embedded acetal also proved to be an effective coupling partner, providing the corresponding product 10f in 86% isolated yield with full conversion (Table 5, entry 6).
Table 5.
Cross-Coupling of 1-Naphthol Sulfamate with Various Secondary Aminomethyltrifluoroborates 9a–f
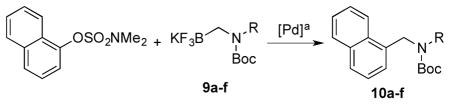
| |
|---|---|
| entry | product yield(%)b/conversion(%)c |
| 1 |
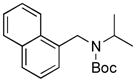 10a, 71/100 |
| 2 |
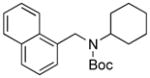 10b, 68/100 |
| 3 |
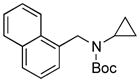 10c, 0/0 |
| 4 |
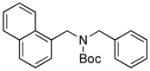 10d, 74/89 |
| 5 |
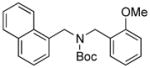 10e, 82/97 |
| 6 |
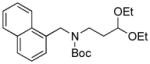 10f, 86/100 |
Reaction conditions: 1.0 equiv of hetaryl sulfamate, 1.05 equiv of trifluoroborate, 4 mol % XPhos-Pd-G2, 7 equiv of K2CO3, t-BuOH/H2O (1:1, 0.5 M), 85 °C, 18 h.
isolated yield.
calculated by 1H NMR with 30 μL of CH2Cl2.
In conclusion, a method has been developed to couple sulfamates as the electrophilic coupling partners with various potassium Boc-protected aminomethyltrifluoroborates, primary and secondary aminomethylating reagents, in the presence of palladium catalyst. By this method, new carbon(sp3)-carbon(sp2) bonds could be efficiently forged, and the complexity of the molecules could be readily increased. This study provides evidence that potassium alkyltrifluoroborates can be utilized to expand the scope of cross-coupling to diverse electrophilic partners, thus complementing similar methods that employ less advantageous nucleophilic partners.
Supplementary Material
Acknowledgments
We thank NIH (NIGMS R01-GM-081376) for their support of this research. We also acknowledge Dr. Rakesh Kohli (University of Pennsylvania) for obtaining HRMS data.
Footnotes
Supporting Information Available Experimental procedures and spectral data of all compounds synthesized. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For recent examples of Suzuki–Miyaura cross-coupling with sulfonate derivatives, see: Fan X-H, Yang L-M. Eur J Org Chem. 2011:1467.So CM, Lau CP, Chan ASC, Kwong FY. J Org Chem. 2008;73:7731. doi: 10.1021/jo8014819.Petersen MD, Boye SV, Nielsen EH, Willumsen J, Sinning S, Wiborg O, Bols M. Bioorg Med Chem. 2007;15:4159. doi: 10.1016/j.bmc.2007.03.069.Zhang LA, Meng TH, Wu J. J Org Chem. 2007;72:9346. doi: 10.1021/jo7019064.Lipshutz BH, Butler T, Swift E. Org Lett. 2008;10:697. doi: 10.1021/ol702453q.
- 2.(a) Quasdorf KW, Riener M, Petrova KV, Garg NK. J Am Chem Soc. 2009;131:17748. doi: 10.1021/ja906477r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Quasdorf KW, Antoft-Finch A, Liu P, Silberstein AL, Komaromi A, Blackburn T, Ramgren SD, Houk KN, Snieckus V, Garg NK. J Am Chem Soc. 2011;133:6352. doi: 10.1021/ja200398c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Macklin TK, Snieckus V. Org Lett. 2005;7:2519. doi: 10.1021/ol050393c. [DOI] [PubMed] [Google Scholar]; (b) When PM, Du Bois J. Org Lett. 2005;7:4685. doi: 10.1021/ol051896l. [DOI] [PubMed] [Google Scholar]; (c) Silberstein AL, Ramgren SD, Garg NK. Org Lett. 2012;14:3796. doi: 10.1021/ol301681z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Baghbanzadeh M, Pilger C, Kappe CO. J Org Chem. 2011;76:1507. doi: 10.1021/jo1024464. [DOI] [PubMed] [Google Scholar]; (b) Leowanawat P, Zhang N, Resmerita AM, Rosen BM, Percec V. J Org Chem. 2011;76:9946. doi: 10.1021/jo202037x. [DOI] [PubMed] [Google Scholar]
- 5.(a) Molander GA, Beaumard F. Org Lett. 2011;13:3948. doi: 10.1021/ol201469r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Molander GA, Beaumard F, Niethamer TK. J Org Chem. 2011;76:8126. doi: 10.1021/jo2015246. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Molander GA, Beaumard F. Org Lett. 2011;13:1242. doi: 10.1021/ol200128y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Molander GA, Beaumard F. Org Lett. 2010;12:4022. doi: 10.1021/ol101592r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Molander GA, Shin I. Org Lett. 2012;14:3138. doi: 10.1021/ol301221p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Molander GA, Shin I. Org Lett. 2011;13:3956. doi: 10.1021/ol2014768. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Molander GA, Shin I. Org Lett. 2012;14:4458. doi: 10.1021/ol301955s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Molander GA, Hiebel MA. Org Lett. 2010;12:4876. doi: 10.1021/ol102039c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Molander GA, Fleury-Brégeot N, Hiebel MA. Org Lett. 2011;13:16937. doi: 10.1021/ol200202g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Fleury-Brégeot N, Raushel J, Sandrock DL, Dreher SD, Molander GA. Chem–Eur J. 2012;18:9564. doi: 10.1002/chem.201200831. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Molander GA, Sandrock DL. Org Lett. 2007;9:1597. doi: 10.1021/ol070543e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


