Abstract
Functional recovery is typically poor after facial nerve transection and surgical repair. In rats, whisking amplitude remains greatly diminished after facial nerve regeneration, but can recover more completely if the whiskers are periodically mechanically stimulated during recovery. Here we present a robotic “whisk assist” system for mechanically driving whisker movement after facial nerve injury. Movement patterns were either pre-programmed to reflect natural amplitudes and frequencies, or movements of the contralateral (healthy) side of the face were detected and used to control real-time mirror-like motion on the denervated side. In a pilot study, twenty rats were divided into nine groups and administered one of eight different whisk assist driving patterns (or control) for 5–20 minutes, five days per week, across eight weeks of recovery after unilateral facial nerve cut and suture repair. All rats tolerated the mechanical stimulation well. Seven of the eight treatment groups recovered average whisking amplitudes that exceeded controls, although small group sizes precluded statistical confirmation of group differences. The potential to substantially improve facial nerve recovery through mechanical stimulation has important clinical implications, and we have developed a system to control the pattern and dose of stimulation in the rat facial nerve model.
Index Terms: facial nerve, facial paralysis, nerve regeneration, rehabilitation, reinnervation, robotic, vibrissae, whisking
I. Introduction
LOSS of facial nerve function arises from a wide range of clinical entities including developmental abnormalities, trauma, surgery, or infection. Despite advances in facial nerve surgical grafting and repair techniques over the past 60 years, functional outcomes are often disappointing [1], leaving patients with substantial impairment in facial muscle control. Facial paralysis can be functionally and emotionally devastating, leading to both depression and social isolation. In addition to considerable esthetic issues relating to asymmetry in static facial posture (Figure 1), facial nerve paralysis may also impair or eliminate dynamic facial expression, eye closure (causing corneal ulceration/discomfort), oral competence, nasal valve patency, and speech articulation [2].
Fig. 1.
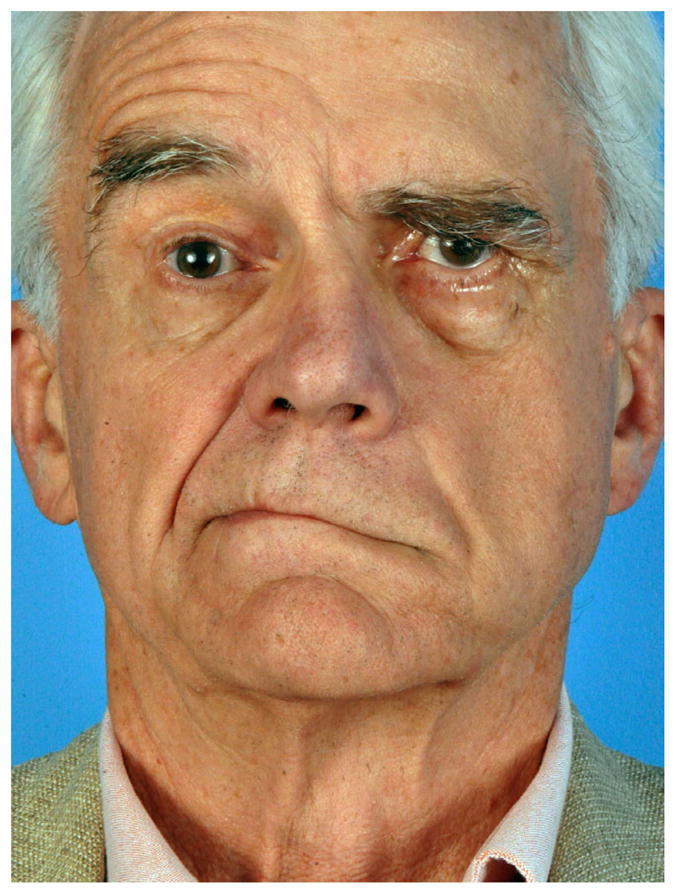
Left facial paralysis as evident by brow and eyelid ptosis (drooping), nasal valve collapse, effacement of the nasolabial fold, and oral commissure asymmetry at rest or during smile.
Several animal models have been used to test techniques for improving the speed and accuracy of facial nerve regeneration. Whisker motion in the rat (vibrissal whisking) is dynamic, readily quantified, and can be elicited when not produced spontaneously [3–7]. Modest improvements in whisking recovery after facial nerve injury have been observed in response to pharmacologic [8–10], electrical stimulatory [11, 12], and surgical interventions [4, 13]; however, simple mechanical stimulation of the whisker pad during the recovery period seems to provide the greatest benefit [14–17]. Angelov and colleagues have shown that repetitive protraction of the whiskers (by finger tip) for as little as 5 minutes a day during recovery can restore normal, symmetrical whisking after unilateral facial nerve cut and suture repair [14–17]. If stimulation is not given during daily handling, or if it is administered only to the contralateral (healthy) side of the face, then little whisking ability is regained even after months of recovery. Enhancement in functional outcome was associated with a reduction in polyinnervation (which typically occurs with facial nerve regeneration) [14, 16, 18], required intact sensory innervation of the whisker pad [19], and depended on insulin-like growth factor 1 [15], brain-derived neurotrophic factor, and its receptor tyrosine kinase B [17].
Our research group attempted to replicate the reported enhancement of facial nerve regeneration through mechanical stimulation of the paralyzed whiskers during recovery after transection and suture repair [12, 20], but has had limited success compared to previous studies [14–17]. Inconsistencies in manually applied stimulation rates, pressures, and ranges of motion may contribute to variability in outcome and discrepancies among research groups. To improve control over mechanical stimulation, we developed a system for driving horizontal whisker movements in pre-programmed patterns capable of recreating a majority of the largest and fastest whisks that rats can produce. Moreover, the system can track the healthy side using a laser micrometer and drive the recovering side to mirror its movements, effectively generating symmetrical (yoked) whisker movement despite unilateral denervation. Given that healthy whisking patterns are typically symmetrical [7, 21], the assisted whisking on the recovering side should approximate the animal’s intended movement, thereby delivering intention-driven movement akin to what several robot-assisted therapy devices provide [22–24]. This new methodology enables a comparison of passive (pre-programmed) movement versus robot-assisted movement on facial nerve recovery.
II. Robotic whisk assist (WA) system
A. System Overview
An initial description of the WA system has previously appeared as a conference report [25], and additional apparatus description and pilot testing of WA treatment effects on facial nerve regeneration is provided in the present manuscript. The robotic WA system can be used in conjunction with our previously published system for measuring whisking function in restrained rats [5]. Rats are restrained using a cloth bag covering the body, a PVC half-pipe that holds the body with Velcro straps across the back, and attachment of an implanted head restraint [26] to a set of four rigid posts to immobilize the head (see Figure 2). Rats receive extensive conditioning prior to undergoing such restraint (see below), and are able to maintain normal respiration while restrained.
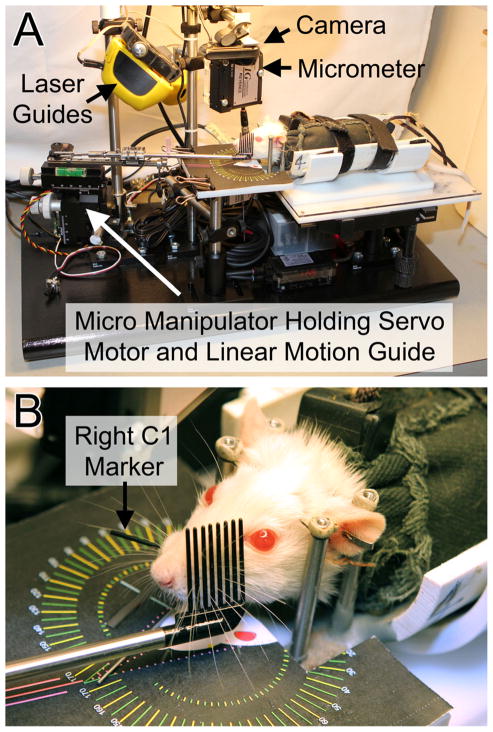
Fig. 2.
The Whisk Assist (WA) apparatus. A) Overview of the WA system (except for the associated computer). B) Rat in position to have the left whiskers mechanically driven and the right C1 tracked by the laser micrometer.
Rats are placed in the WA apparatus and aligned using a modified microscope stage for x-y-z axis, roll, and rotation adjustments. Two anatomical reference points on the rat head are used for alignment with the apparatus, including the mid-sagittal plane and the origin of the first whisker of row C on the right whisker pad (right C1 origin). Alignment is achieved by adjusting the position of each animal until the anatomical reference points are illuminated by lasers beams (Straight-Line Laser Lever 30) that are mounted on the WA framework. This ensures consistent head alignment among rats despite slight variations in head implant position.
Protration-retraction along a horizontal (anterior-posterior) plane is the dominant type of vibrissal motion in rats [7]. Moreover, horizontal movement of the C1 whisker has been shown to be a good indicator of overall whisking function [21] (even though some independent movement of whiskers is possible across different rows [27]). The WA system was thus designed to drive horizontal motion of all prominent whiskers on one side of the face while tracking horizontal C1 movement on the contralateral side (see Figure 2B). The WA system presented in this report is configured to drive the left whiskers and measure the right C1 position, but WA systems can be constructed to mechanically drive either the right or left whisker pad.
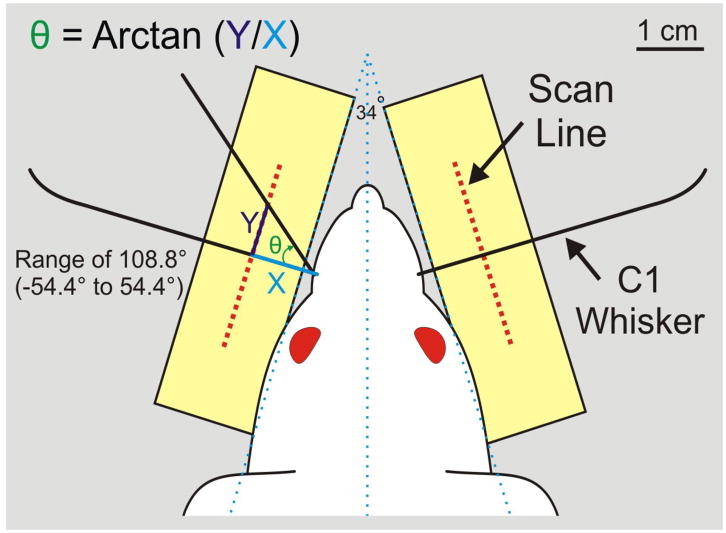
C1 whisker movement is tracked using non-contact laser micrometers (Keyence model IG-028 or MetraLight RX Series, respectively). Each micrometer pair has an emitter unit positioned above the whisker pad that emits an infrared laser light curtain onto the 28 mm scan line of a detector unit positioned below the whisker pad (see Figures 2 and 3). During testing, the C1 whisker is fitted with a polyimide tube (15mm long, 1.15mm diameter, 3–4mg) so that it casts a prominent shadow onto the detector while the other whiskers remain invisible to the detector. The detector scan line is positioned 1 cm from the lateral surface of the face (defined as 17° from the head midline [7]) and centered on the C1 origin such that it can detect 108.8° of whisking range (see Figure 3). This captures nearly all whisking motion (the maximum observed range is approximately 108° in restrained animals [28]).
Fig. 3.
Right and left C1 whiskers are drawn in relation to the micrometer scan lines, and the manner in which horizontal whisking amplitude is measured in degrees is demonstrated on the left.
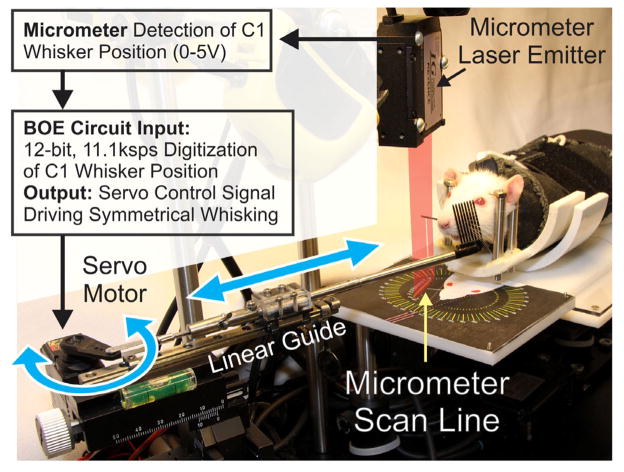
The WA system software, running on a microcontroller, allows the operator to define whisking frequency, amplitude, and session duration as either constant or randomly fluctuating variables. When operated in the yoked mode, motion is driven proportional to the contralateral side, and the software allows for adjustment in gain and baseline whisker position (Figure 4).
Fig. 4.
Flow diagram of healthy whisking detection on one side leading to robotic assistance of contralateral movement on the recovering side. The marked C1 whisker on the right is shown breaking a superimposed representation of the laser light curtain, causing a shadow that is detected by the micrometer scan line. The detected C1 position is digitized by the BOE circuit, and custom software generates a servo motor control signal that moves the rod holding the whisker comb and generates symmetrical, bilateral whisking. Double-headed arrows show direction of movement for the servo horn and linear guide holding the whisker comb rod.
Laser micrometer outputs are digitized and conditioned using a Board of Education (BOE) with a BS2px processor (Parallax Inc.) and 12-bit A/D sampling at 11.1kHz (Linear Technology, LTC1298). The processor controls a high-speed servo (Hitec HS-7940TH) attached to the whisker comb through a multi-jointed linkage and linear motion guide (THK RSR72M-130-1). The guide’s carriage holds a 14 cm long stainless steel tube (5 mm diameter) that ends with a segment of stainless steel pet comb (Four Paws, Model 11170) (Figure 2B). The comb consists of 8 tines, each 23 mm long, 1 mm in diameter, and centered 2 mm apart. All of the prominent whiskers are moved by motion of the comb. The servo motor, linear track, and tube with comb are mounted on a micro manipulator (World Precision Instrument model M3301L) that enables x-y-z positioning and rotation for placing the comb parallel to the whisker pad (guided by a printed template; see figure 2B). A video camera (Logitech C905), mounted above the animal’s head, captures whisker movements and apparatus position to a notebook computer.
B. System Calibration and Testing
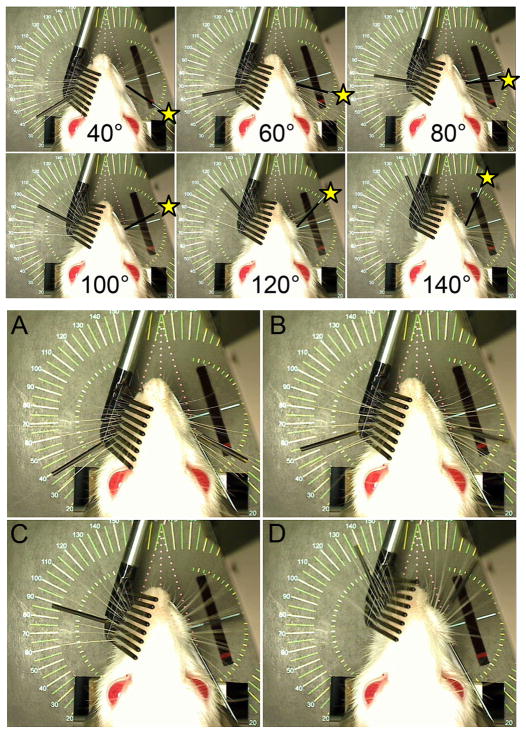
The positioning of the mechanically driven whiskers in the yoked mode is adjusted to mirror the contralateral whiskers by moving a mock whisker originating from the C1 origin and observing the resulting robotic whisker positioning. The servo controller’s software (Figure 4) has an input variable for determining the range of comb movement in relation to detected C1 position, and this variable can be adjusted until the detected and driven whisker positions match reasonably well across the 108.8° detection range. After this calibration step the correlation between measured (right) and positioned (left) whisker location was typically R2 > .98 (see Figure 5).
Fig. 5.
Top: Overhead camera view of WA system showing a testing rod on the right (marked with a yellow star) being moved from 40° to 140° and the left-side whiskers being moved by the servo motor mechanism (C1 whisker is marked with a thin black tube) as the system tracks the right-side marker. Bottom: Still video frames captured while the WA system tracked the C1 whisker on the right (intact) and simultaneously drove the paralyzed whiskers on the left in a yoked pattern across the full range of motion (e.g. panels A-D moving from 40° through approximately 140°). The rectangular hole in the platform below the rat (located 1 cm lateral to the right whisker pad) houses the laser micrometer detector which tracks the right C1 whisker position. Figured modified from IEEE Neural Engineering, 2011, pp. 629–633.
The dynamic response of the mechanically driven whiskers was tested using a function generator (Leader LFG-1300S) to deliver sine waves of 1–20 Hz in 1 Hz steps to the servo controller input, which would normally receive the laser micrometer signal tracking the C1 position on the healthy side. This enabled testing of the frequency, amplitude, and phase of WA-driven whisker movement in relation to a controlled driving signal spanning the range of potential whisking frequencies and amplitudes. Movement of the WA-driven whiskers was measured by tracking C1 movement with an additional laser micrometer emitter/detector pair temporarily positioned in the system for this test (i.e. not present in the system photographs). The WA-driven C1 whisker closely matched (R2 = .993) the input frequency across the tested range, but with a lag of approximately 60–70 ms that did not vary substantially with driving frequency. Motion amplitude was full range (108.8°) at low frequencies (< 3 Hz), and became progressively attenuated at higher frequencies. The unit achieved 65° of motion at 8Hz, which matches most vigorous whisking behavior [7, 21]. Driven whisking amplitude was < 20° for inputs above 14 Hz; however, whisking at such high frequencies is not high in amplitude and constitutes only a small fraction of the rat whisking repertoire [7, 21, 28, 29].
III. Pilot Study – WA treatment during facial nerve regeneration
A. Animals and Procedures
Twenty adult female Wistar rats weighing 250–300 grams were used in a pilot study of facial nerve regeneration. Each animal received one of 8 different pre-programmed or yoked WA treatment patterns, or was placed under restraint with no whisker stimulation as a control condition (n=2–3 per group; See Table 1). Experimental procedures are described in the following sections and include 1) head fixation implant surgery, 2) conditioning to the restraint apparatus, 3) facial nerve transection/repair surgery, 4) whisking assessment, and 5) daily WA treatment. At the conclusion of the study, animals were euthanized according to NIH guidelines, and all procedures were described in protocols approved by the Massachusetts Eye and Ear Infirmary institutional animal care committee.
TABLE 1.
Pilot Whisk Assist Daily Treatment Patterns
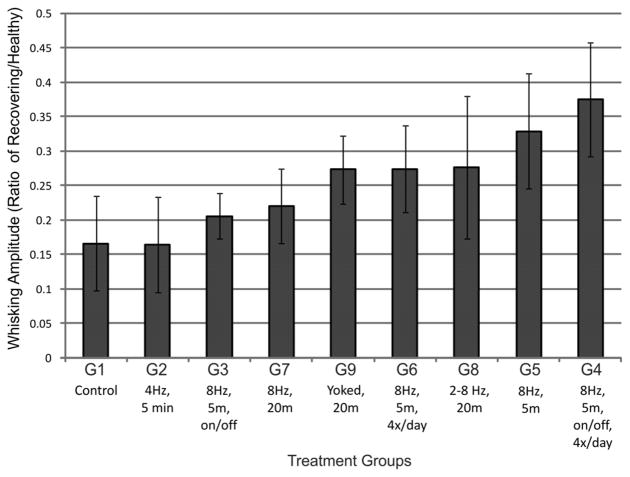
| Group | Daily Treatment |
|---|---|
| 1 | Control group - placed in restraint 5 min (N=2) |
| 2 | 4 Hz at 60–70 degrees for 5 minutes (N=2) |
| 3 | 8 Hz at 60–70 degrees for 5 seconds, twice per minute, for 5 minutes (N=2) |
| 4 | Same as group 3, repeated 4 times per day (N=2) |
| 5 | 8 Hz at 60–70 degrees for 5 minutes (N=2) |
| 6 | Same as group 5, repeated 4 times per day (N=2) |
| 7 | 8 Hz at 60–70 degrees for 20 minutes (N=2) |
| 8 | 2–8 Hz at 60–70 degrees, frequency randomly changing every 2 seconds, for 20 minutes (N=3) |
| 9 | Whisking yoked to match contralateral (healthy) side for 20 minutes (N=3) |
B. Head Restraint Device Implantation and Restraint Conditioning
Rats received surgical implantation of a titanium head fixation device 5–6 weeks before testing in order to provide attachment points for head immobilization during WA treatment and whisking measurement. Details of the implant design and implantation surgical procedure are available in Hadlock et al. [26].
Rats were conditioned to the testing apparatus according to previously described methods [5, 30]. Briefly, rats were handled daily for 2–3 days prior to the head restraint device implantation and not handled for 2 weeks after implantation surgery (to allow incision healing). Restraint conditioning then continued for 2 weeks prior to the initiation of WA treatment. Handling began with 5-minute daily sessions, progressing to 10 minutes per day. Once accustomed to being securely held for 10 minutes, rats were “sack trained” by being placed each day into a fitted cloth sack that restricted limb movement (see reference [5] for complete description). Head restraint conditioning involved placing rats in the head/body restraint apparatus for progressively longer periods, beginning for 30 seconds, and progressing to 20 minutes across a 5–7 day period. Bilateral whisking function was then tested for baseline performance [5] before undergoing the nerve transection and repair surgery.
C. Facial Nerve Transection and Repair Surgery
All rats underwent left facial nerve transection and repair surgery using procedures described previously [4]. Briefly, the facial nerve main trunk was exposed through an incision near the stylomastoid foramen after removal of the ipsilateral parotid gland. The main trunk was then cut transversely with microsurgical scissors under stereoscopic magnification (Leica Wild M65m, Wetzlar, Germany) and repaired with two or three 10-0 nylon epineurial sutures. The incision was then closed in a single layer. Rats recovered for one week before post-surgical bilateral whisking function was assessed, and WA treatment (or daily restraint for control rats) began on post-surgical day 8.
D. Whisking Assessment and WA Treatment
Twenty rats were divided into nine groups of 2–3 rats per group as described in Table 1. Whisking assessment was conducted weekly for each rat starting the week before nerve transection and repair surgery, and continued 1 week after surgery for 8 weeks. WA treatments were administered each weekday starting 8 days after facial nerve surgery and continued throughout 8 weeks of recovery. Weekly whisking assessment was performed using methods and custom apparatus previously described in detail [5]. Spontaneous whisking was recorded bilaterally for each rat under head and body restraint for 5 minutes per session (see apparatus description above). During each recording session, two 10-second flows of air scented with peanut butter or dilute isopropyl alcohol were directed towards the snout at random time intervals to elicit vigorous whisking.
WA treatments or control restraint were administered once (groups 1–3, 5, 7–9) or four times (groups 4, 6) per weekday across weeks 2–8 of recovery after facial nerve cut/repair or sham surgery. Individual WA treatments lasted 5 minutes (groups 2–6) or 20 minutes (7–9). WA treatments involved pre-programmed whisking patterns with fixed frequency (groups 2–7) or variable frequency which changed randomly in a range between 2–8 Hz every two seconds. All pre-programmed whisking patterns drove whiskers 60–70° per whisk. In the yoked treatment group, whiskers on the recovering side were moved in concert with detected whisker movement on the healthy side. The total driven distance was recorded for each 20 minute yoked treatment session.
IV. Results
Rats did not show signs of heightened stress (e.g. vocalizing, struggling, etc.) during the whisk assist (WA) treatment aside from occasional teeth chattering. This was true even for the fastest WA rate (8 Hz; groups 3–7) and longest testing period (20 minutes; groups 7–9). Not only did the rats appear to tolerate the WA-treatment well, they were also observed to “copy” the pre-programmed WA pattern delivered to the left (paretic) side of the face with their healthy contralateral whiskers (Figure 6).
Fig. 6.
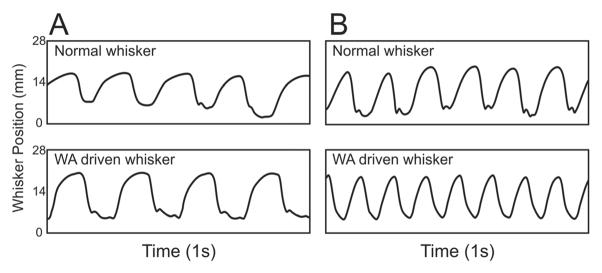
Examples of horizontal whisker movement during A) 4 Hz driven whisking and B) 8 Hz driven whisking. The tracked C1 whisker from the normal side of the face (top traces) was often found to approximate the rate at which the paralyzed/recovering side of the face was being driven (bottom traces)
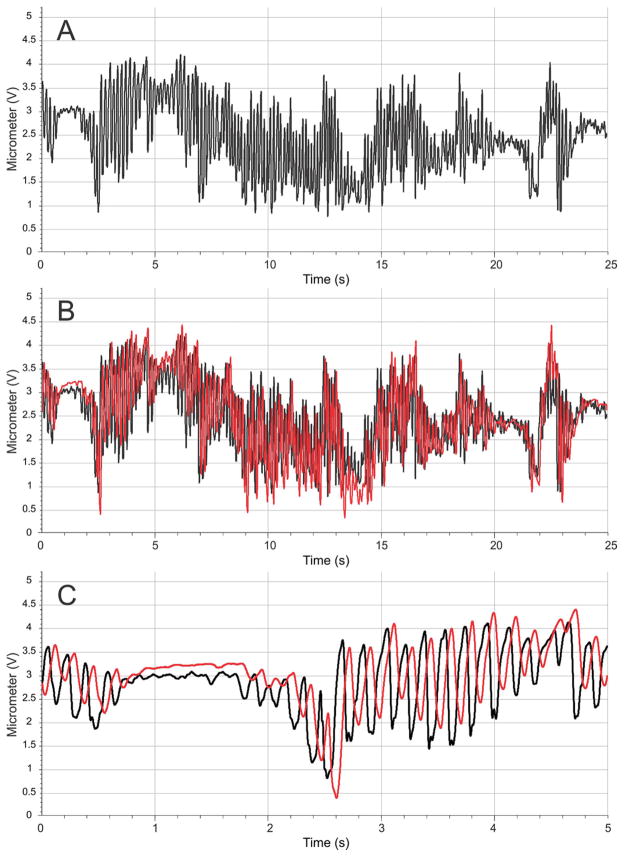
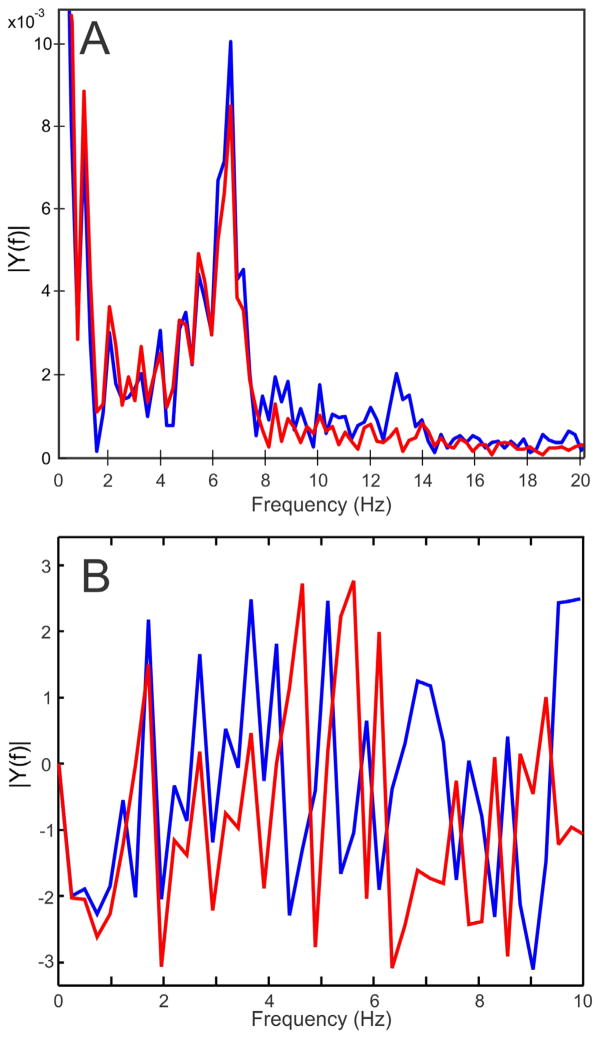
Three rats received daily yoked treatment (group 9 in Table 1). All three rats whisked without conspicuous pauses throughout the WA treatment sessions, with an average whisking distance of 4970 degrees/min across the 8 weeks of recovery. This shows that WA treatment did not inhibit whisking behavior. As a point of comparison, their average whisking distances were 5830 degrees/min during the weekly whisking assessment recording sessions, where head constraint was similar, but WA treatment was not delivered and whisk-eliciting scented air flows were used (see Methods). An example of whisking during WA treatment is provided in figure 7. Spectral analysis of healthy versus driven whisking from this example showed strong similarities in whisking amplitude across the frequency range representing a majority of rat whisking (i.e. > 8 Hz; see Fig 8). Yoked motion was delayed by approximately 60–70 ms. This delay caused a frequency-dependent phase difference between the tracked volitional movement and WA-driven movement which was approximately 160° out of phase for tracked whisks at the dominant frequency of 7 Hz.
Figure 7.
Micrometer recording from the C1 whisker position for the healthy whisker pad (A) and the healthy pad with the yoked contralateral paralyzed whisker pad C1 movement overlaid in red (B). The first 5 seconds in plot A/B are expanded in the bottom panel (C), demonstrating the time lag and phase difference for the driven movement (in red) relative to the healthy, volitional movement (in black).
Figure 8.
Amplitude (A) and phase (B) spectra of whisking from a 5 minute recording session comparing movement from the healthy whisker pad (in blue) versus the contralateral C1 whisker movement driven by the WA system (in red). The WA system was able to reproduce most dominant whisking frequencies (i.e. > 8Hz), but did not accurately reproduce some high-frequency whisking. Whisking was approximately 2.8 radians (160°) different in phase at the dominant frequency (≈ 7 Hz).
Average amplitudes of the largest three whisks per 5-minute recording session were tracked across the recovery period from the initiation of recovered whisker movement (week 3) to the termination of the study (week 8), and were used to compute average percent recovery values for each group. WA-treated groups recovered greater whisking amplitude compared to controls, but without an obvious pattern of recovery relating to WA treatment parameters (e.g. treatment times per day, WA frequency, continuous vs. intermittent movement, etc.). No group recovered more than 41% of healthy whisking amplitude. The small group sizes precluded meaningful statistical comparisons among treatment conditions. Nevertheless, it was apparent that none of the WA treatments restored normal, symmetrical whisking as has been previously reported for hand-delivered mechanical stimulation [14–17].
V. Discussion
The human facial nerve provides motor supply to muscles responsible for facial expression, eyelid closure, nasal valve patency, and configuration of the lips. In rats, an important additional facial nerve motor function is innervation of vibrissal muscles. Whisker movements are highly quantifiable and provide an objective means of tracking facial nerve function. Rats ‘whisk’ the approximately 25 whiskers embedded in each whisker pad through contraction of intrinsic and extrinsic pad musculature [28] innervated by the zygomatic, buccal and marginal mandibular branches of the facial nerve [31, 32]. Whisker follicles receive dense trigeminal sensory innervation, enabling rats to scan their immediate environment through tactile sensation, and obtain precise information about the location and surface characteristics of objects that they touch while whisking [33, 34]. Exploratory movements of the rat whisker array are predominantly in the anterior-posterior plane[7], with frequencies typically ranging 4–12 Hz (for review see [28]), and average about 7–9 Hz for freely exploring rats [35–37], or slightly slower (5–7 Hz) for head-fixed, constrained rats [28, 29]. Whisking amplitudes in the anterior-posterior plane typically range 10–100° (~35° of retraction to ~65° of protraction;[28, 29]), with average whisking excursion being approximately 30–50° [5, 21, 38]. Individual vibrissae can move independently from one another [27], but they usually move in concert within a whisker pad, and show a high degree of synchrony for paired whiskers across the two sides of the face [29].
In both rats and humans, functional recovery of facial motor control is generally poor if the facial nerve is injured beyond compression or crush, particularly if the nerve continuity has been disrupted. In humans, the face is rendered flaccid at rest and lacks dynamic expression if axons fail to reach denervated muscles after neurotmesis, or is often hypertonic and synkinetic when reinnervation does occur [39], presumably due to supernumerary axonal sprouting and misrouting [40]. In rats, if the facial nerve is transected and immediately repaired on one side of the face, then some whisking function begins to return by approximately 3 weeks on the affected side. Thereafter, only approximately 25% of whisking amplitude recovers compared to the largest contralateral whisks across the subsequent 2–4 months [10, 12, 16, 20, 30, 38].
Poor functional outcome after facial nerve regeneration can stem from a combination of potential factors, including (1) muscle flaccidity and atrophy if axons fail to reinnervate the affected facial muscles, (2) hyper-reinnervation and hypertonicity if too many regenerating axonal branches reach the affected muscles and/or sprout within the target muscle, (3) alteration of motor neuron drive associated with retraction of synaptic inputs, and (4) poor execution of intended movement if axons misroute during regeneration and reach one or more inappropriate muscle targets (e.g. if axons formerly controlling the lips reinnervate the eyelids and vice versa). All of these potential factors have been studied in the rat whisker pad model. For example, if the facial nerve main trunk is transected and not repaired, then ipsilateral facial function is lost and the facial muscles demonstrate substantial atrophy within 4 months [41]. If the cut ends of a transected facial nerve are successfully coapted, then sprouts from the cut proximal axons traverse the lesion site and send supernumerary axons to the facial muscles [16, 42]. Retrograde tracing of these regenerated neurons reveals a substantial loss of the normal myotopic organization of the facial motor nuclei after main trunk transection and repair [16, 43], indicating profound misrouting of axons en route to the whisker pad and creating the potential for synkinetic movement [30]. Moreover, regenerated axons poly-innervate whisker pad muscle fibers; many neuromuscular junctions receive input from multiple axon terminal branches instead of the typical single input [18, 44]. Therefore, even when the facial nerve successfully reinnervates the whisker pad in time to prevent appreciable muscle atrophy, both polyinnervation and profound misrouting may still prevent the return of normal whisking.
A wide range of interventions have been investigated in an attempt to improve whisking function after unilateral facial nerve transection and repair in rats. These have included surgical techniques and grafting strategies [4, 13], electrical stimulation of the nerve [12, 16] or whisker pad [45], systemic or local application of drugs [8–10, 46], and treatment of the nerve repair site with olfactory mucosa [38], stem cells [47], extracellular matrix proteins [48], or antibodies against neurotrophic factors [44, 49]. Although some of these interventions have hastened initial recovery of function [10, 12, 20], reduced aberrant axonal sprouting [49], and substantially increased the precision with which axons reach their targets [44], they have often surprisingly not lead to improved function (whisking).
The most successful manipulations to date have been those which prompt rats to make greater use of the paralyzed whisker pad and/or provided external stimulation to the whisker pad during the recovery period, akin to human forced-use and physical therapies, respectfully. For example, sightless rats (which rely heavily on vibrissal tactile information), or rats with trimmed or deafferented contralateral whisker pads (which rely more on the recovering pad to explore), recover substantially better whisking than controls [18, 43, 50]. Moreover, mechanical stimulation applied to the whisker pad for brief daily periods during the initial weeks of recovery have been reported by some groups to bring about full recovery of whisking function [14–17], provided that sensation of the recovering pad remains intact [19]. These forms of self and external stimulation do not prevent axonal misrouting or reduce supernumerary axons within the regenerating distal nerve [16], but have been shown to decrease the occurrence of polyinnervation of whisker pad muscle fibers [14, 16, 18]. Similar repetitive stroking of the submental region (under the chin) during hypoglossal nerve regeneration has likewise been reported to enhance recovery and reduce tongue polyinnervation [51, 52], suggesting a similar mechanism of action for physical therapy in these adjacent cranial motor systems. In both cases, mechanical stimulation has been delivered by repetitively stroking the denervated region with the investigator’s finger-tip. Although apparently effective, this delivery method presents several potential disadvantages and sources of experimental variation, including animal response to particular individuals holding the rats during the whisker-pad massage therapy, variation in stroking pressure and rate within and across administrators, limitations regarding the speed and duration for which fingertip stimulation can be delivered, and the inability to deliver random stimulation patterns or patterns which match intended (i.e. contralateral healthy) movements.
We developed the whisk assist (WA) system to explore what aspects of whisker pad mechanical stimulation have the greatest impact on functional recovery, so that we may better understand the underlying mechanisms, and ultimately apply that knowledge to improve human facial nerve regeneration. This system reduces potential sources of variation by delivering computer-controlled, consistent, and quantifiable doses of mechanical stimulation.
Many studies have shown enhanced functional recovery of peripheral nerve lesions through the use of either passive or active exercise (for review see [53]). However, the effects of exercise on nerve regeneration are not always positive, and depend on multiple factors including the type and intensity of exercise, how often and for how long it is delivered, the typical activity patterns of the affected motor system, and even the gender of the exercise recipient (for review see [54]). Some observations may help explain beneficial effects of exercise at a neuronal level, including the proliferation of Schwann cells in the regenerating nerve [55], increase in the growth rate of axons [56], improved precision in axon regrowth to functionally appropriate muscle groups [57], maintenance of muscle fiber end-plate structure [58], modulation of central and peripheral neurotrophic factors [59–61], and the reduction or reversal of synaptic stripping that typically occurs for motoneurons after nerve lesion [62, 63].
Animal studies have begun to relate the type, intensity and timing of exercise/physical therapy to nerve regeneration outcome. Functional recovery can be hindered by overexertion or overstimulation [64–67], highlighting the importance of determining the most effective and efficient therapy parameters for enhancing regeneration. For example, mechanical stroking treatment of the whisker pad for 5 minutes/day is just as effective for rat facial nerve regeneration as stimulation for 10 minutes/day , but daily treatments lasting only 1 or 2 minutes do not enhance whisking outcome [14]. Similarly in mice, high-intensity treadmill training for two, 2-minute sessions is just as effective in enhancing mouse sciatic nerve regeneration as 60 minutes of low-intensity training, yet a single 2-minute session each day is not effective [56]. Thus, it is important to test the effects of different stimulation characteristics on nerve regeneration, because effective therapy regimens are not necessarily predictable and relatively short treatment durations are often as effective as longer, potentially stressful or damaging durations.
Although mechanical stimulation of the whisker pad has been reported to restore normal, symmetrical whisking function within two months [14–17], none of the eight daily WA treatment patterns in the present study restored symmetrical whisking (N=18) across their two months of recovery despite receiving of a wide range of WA treatment frequencies and treatment durations. This difference in outcome compared to the findings of others might stem from differences in whisking assessment techniques, with our group using laser micrometer detection of the largest whisks in head-fixed rats versus the other groups using video-based detection of the largest whisks in unrestrained rats [14–17]. Head-restrained rats are known to produce larger whisks, on average, than when unrestrained [29], but probably not to the degree that would explain the discrepancy in functional recovery after WA treatment versus hand-delivered whisker pad stroking. Mechanical stimulation of the whiskers threaded through a comb oscillating adjacent to the pad may not produce as much pad tissue massage as repetitive finger stroking of the pad itself. Additional whisker/pad interface attachments could be developed to test this factor.
It was hypothesized that the intention-driven (yoked) WA treatment condition would prove particularly effective in enhancing whisking recovery because of the natural/physiologic whisking patterns being delivered, and the advantages demonstrated in human neural recovery by the use of intention-driven movement via robot-assisted therapy devices [22–24] when compared to passive movement of affected limbs. However, the intention-driven treatment group did not recover conspicuously better than the other WA treatment conditions. One initial concern was that the yoked treatment animals would not volitionally whisk enough to provide an effective WA treatment dose. Rats whisked an average of 83°/s during the 20 minute yoked treatments. This amount of self-delivered stimulation resembled the whisker movement rate delivered by hand-stroking the whisker pad in prior reports [12, 14–17], yet lasted four times longer (20 vs. 5 min) each treatment day than what has been shown to completely restore whisking function [14–17]. Therefore, under-stimulation in this group was likely not the cause of incomplete functional recovery. It is possible that the phase difference between the healthy whisker movements and contralateral WA-driven movements negatively affected the relationship between whisking motor drive and sensory feedback from the recovering whisker pad. Moreover, the completely assisted movement delivered by the WA system may be less effective than the assist-as-needed therapeutic movements provided by current robot-assisted therapy devices for humans [22–24]. Future iterations of the WA hardware could detect and assist volitional whisker movements (as needed) on the affected side throughout the recovery process instead of inferring movement efforts based on the healthy side whisking. To accomplish this, the system’s delay between detected and assisted/driven movements must be substantially reduced, perhaps by using servo hardware with a faster position update rate or a linear actuator.
VI. Conclusion
The Whisk Assist (WA) system is capable of delivering mechanical stimulation to the rat whisker pad in either preprogrammed patterns or yoked to contralateral volitional whisking. This report establishes the feasibility of delivering a wide range of physiologically relevant whisking frequencies and amplitudes, and pilot data for 8 different treatment patterns during facial nerve recovery indicate that rats tolerate the WA treatment well.
Future work is needed to 1) systematically test the effect of multiple pre-programmed patterns on facial nerve regeneration in larger groups of rats, 2) compare preprogrammed WA treatments with yoked whisking, and 3) relate enhancements in whisking recovery to anatomical and physiological features of the regenerated motor pathway, including the integrity of synaptic contacts within the brainstem, speed and accuracy of axonal regrowth, and muscle cell polyinnervation.
Figure 9.
Average whisking amplitude across weeks 3–8 of recovery as a ratio of whisking amplitude for the nerve-repaired side divided by the healthy side (i.e. score of 1 would be symmetrical movement). Groups 1–9 are described in Table 1. Error bars are ±SD.
Acknowledgments
This work was supported by R01NS071067 to T. A. Hadlock and J. T. Heaton.
Biographies
 James T. Heaton received the B.A. degree in psychology from Luther College in Decorah, IA, in 1990, and the M.S. and Ph.D. degrees in biopsychology from the University of Maryland, College Park, MD, in 1993 and 1997, respectively.
James T. Heaton received the B.A. degree in psychology from Luther College in Decorah, IA, in 1990, and the M.S. and Ph.D. degrees in biopsychology from the University of Maryland, College Park, MD, in 1993 and 1997, respectively.
He joined the faculty of Harvard Medical School, Boston, MA, in 1997 where he is currently an Assistant Professor in Surgery, and a faculty affiliate of the Harvard-MIT doctoral program in Speech and Hearing Bioscience and Technology (SHBT). He is also Professor in the Department of Communication Sciences and Disorders at the MGH Institute of Health Professions. His research interests include the neural control of head and neck structures involved in voice/speech production, creating an EMG-based interface for voice prosthesis control and speech recognition technology under funding from the NIH and DARPA.
 Christopher J. Knox was born in Stoneham, Massachusetts. He received a B.S. degree in biology from the University of New Hampshire, in 2008. His strong interest in translational research led him to join the Facial Nerve Regeneration Laboratory within the Harvard Medical School Department of Otology and Laryngology at the the Massachusetts Eye and Ear Infirmary, Boston, MA in 2009. Since then he has helped develop new quantitative measures of facial muscle control (movement rate, force, range and right/left symmetry) used to study nerve regeneration enhancing interventions in both animal models and human patients with acute and chronic facial nerve palsy.
Christopher J. Knox was born in Stoneham, Massachusetts. He received a B.S. degree in biology from the University of New Hampshire, in 2008. His strong interest in translational research led him to join the Facial Nerve Regeneration Laboratory within the Harvard Medical School Department of Otology and Laryngology at the the Massachusetts Eye and Ear Infirmary, Boston, MA in 2009. Since then he has helped develop new quantitative measures of facial muscle control (movement rate, force, range and right/left symmetry) used to study nerve regeneration enhancing interventions in both animal models and human patients with acute and chronic facial nerve palsy.
 Juan S. Malo was born and raised in Cuenca, Ecuador, where he received his medical degree from the University of Cuenca. Dr. Malo conducted research at Harvard Medical School from 2010–2011, focusing on pharmacologic, surgical, and stimulatory (mechanical and electrical) interventions for enhancing facial nerve regeneration. In 2011 he continued his research education as a postdoc at the CeTIR Department of Surgery at the University of Texas in the lab of Dr Charles Wade, focusing on the characterization and application of a large animal model for Penetrating Ballistic Brain Injury (PBBI). In 2012 Dr. Malo will be entering the medical residency program at the University of Illinois, Chicago.
Juan S. Malo was born and raised in Cuenca, Ecuador, where he received his medical degree from the University of Cuenca. Dr. Malo conducted research at Harvard Medical School from 2010–2011, focusing on pharmacologic, surgical, and stimulatory (mechanical and electrical) interventions for enhancing facial nerve regeneration. In 2011 he continued his research education as a postdoc at the CeTIR Department of Surgery at the University of Texas in the lab of Dr Charles Wade, focusing on the characterization and application of a large animal model for Penetrating Ballistic Brain Injury (PBBI). In 2012 Dr. Malo will be entering the medical residency program at the University of Illinois, Chicago.
 James B. Kobler graduated from Vassar College, Poughkeepsie, NY in 1975 and received a Ph.D. in neurobiology from the University of North Carolina, Chapel Hill, NC in 1983. He did a post-doctoral fellowship through the Research Laboratory of Electronics, MIT, at the Eaton-Peabody Laboratory at Massachusetts Eye and Ear Infirmary, Boston, MA. He is currently an Assistant Professor in Surgery, a faculty affiliate of the Harvard-MIT doctoral program in Speech and Hearing Bioscience and Technology (SHBT), and a member of the Center for Laryngeal Surgery and Voice Rehabilitation at the Massachusetts General Hospital, Boston, MA. In 2009 he received the Irving M. London teaching award from MIT. His research interests relate mainly to improving treatment of voice disorders.
James B. Kobler graduated from Vassar College, Poughkeepsie, NY in 1975 and received a Ph.D. in neurobiology from the University of North Carolina, Chapel Hill, NC in 1983. He did a post-doctoral fellowship through the Research Laboratory of Electronics, MIT, at the Eaton-Peabody Laboratory at Massachusetts Eye and Ear Infirmary, Boston, MA. He is currently an Assistant Professor in Surgery, a faculty affiliate of the Harvard-MIT doctoral program in Speech and Hearing Bioscience and Technology (SHBT), and a member of the Center for Laryngeal Surgery and Voice Rehabilitation at the Massachusetts General Hospital, Boston, MA. In 2009 he received the Irving M. London teaching award from MIT. His research interests relate mainly to improving treatment of voice disorders.
 Tessa A. Hadlock graduated from Bowdoin College in Brunswick Maine in 1990, then completed the combined Harvard M.I.T. MD program in 1994. This was followed by an internship in general surgery at University of Chicago and otolaryngology training at Massachusetts Eye and Ear InfirmaryShe has directed the Facial Nerve Center since 2002, and recently also became the Division Chief of Facial Plastic and Reconstructive Surgery at MEEI. She is an Associate Professor of Otology and Laryngology at Harvard Medical School, and spends most of her clinical effort on patients with facial nerve disorders. She is a principal investigator, together with Dr. Heaton, for an R-01 grant from the NINDS entitled “Surgical and Rehabilitative Management of Facial Nerve Injury”.
Tessa A. Hadlock graduated from Bowdoin College in Brunswick Maine in 1990, then completed the combined Harvard M.I.T. MD program in 1994. This was followed by an internship in general surgery at University of Chicago and otolaryngology training at Massachusetts Eye and Ear InfirmaryShe has directed the Facial Nerve Center since 2002, and recently also became the Division Chief of Facial Plastic and Reconstructive Surgery at MEEI. She is an Associate Professor of Otology and Laryngology at Harvard Medical School, and spends most of her clinical effort on patients with facial nerve disorders. She is a principal investigator, together with Dr. Heaton, for an R-01 grant from the NINDS entitled “Surgical and Rehabilitative Management of Facial Nerve Injury”.
Contributor Information
James T. Heaton, Email: james.heaton@mgh.harvard.edu, Massachusetts General Hospital Department of Surgery and Harvard Medical School, One Bowdoin Square Floor 11, Boston, MA 02114 USA. phone: 617-726-0211; fax: 617-643-0681;
Christopher Knox, Department of Otolaryngology/Head and Neck Surgery, Massachusetts Eye and Ear Infirmary and Harvard medical School, Boston, MA, 02114 USA.
Juan Malo, Department of Otolaryngology/Head and Neck Surgery, Massachusetts Eye and Ear Infirmary and Harvard medical School, Boston, MA, 02114 USA.
Tessa A. Hadlock, Email: tessa_hadlock@meei.harvard.edu, Department of Otolaryngology/Head and Neck Surgery, Massachusetts Eye and Ear Infirmary and Harvard medical School, Boston, MA, 02114 USA
References
- 1.Scaramella LF. On the repair of the injured facial nerve. Ear Nose Throat J. 1979 Mar;58:127–33. [PubMed] [Google Scholar]
- 2.May M, Schaitkin BM. History of facial nerve surgery. Facial Plast Surg. 2000;16:301–7. doi: 10.1055/s-2000-15543. [DOI] [PubMed] [Google Scholar]
- 3.Bermejo R, Harvey M, Gao P, Zeigler HP. Conditioned whisking in the rat. Somatosens Mot Res. 1996;13:225–33. doi: 10.3109/08990229609052578. [DOI] [PubMed] [Google Scholar]
- 4.Hadlock TA, Kowaleski J, Lo D, Mackinnon SE, Heaton JT. Rodent facial nerve recovery after selected lesions and repair techniques. Plast Reconstr Surg. 2010 Jan;125:99–109. doi: 10.1097/PRS.0b013e3181c2a5ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heaton JT, Kowaleski JM, Bermejo R, Zeigler HP, Ahlgren DJ, Hadlock TA. A system for studying facial nerve function in rats through simultaneous bilateral monitoring of eyelid and whisker movements. J Neurosci Methods. 2008 Jun 30;171:197–206. doi: 10.1016/j.jneumeth.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bermejo R, Friedman W, Zeigler HP. Topography of whisking II: interaction of whisker and pad. Somatosens Mot Res. 2005 Sep;22:213–20. doi: 10.1080/08990220500262505. [DOI] [PubMed] [Google Scholar]
- 7.Bermejo R, Vyas A, Zeigler HP. Topography of rodent whisking--I. Two-dimensional monitoring of whisker movements. Somatosens Mot Res. 2002;19:341–6. doi: 10.1080/0899022021000037809. [DOI] [PubMed] [Google Scholar]
- 8.Angelov DN, Neiss WF, Streppel M, Andermahr J, Mader K, Stennert E. Nimodipine accelerates axonal sprouting after surgical repair of rat facial nerve. J Neurosci. 1996 Feb 1;16:1041–8. doi: 10.1523/JNEUROSCI.16-03-01041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindsay RW, Heaton JT, Edwards C, Smitson C, Hadlock TA. Nimodipine and acceleration of functional recovery of the facial nerve after crush injury. Arch Facial Plast Surg. 2010 Jan-Feb;12:49–52. doi: 10.1001/archfacial.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vakharia KT, Lindsay RW, Knox C, Edwards C, Henstrom D, Weinberg J, Hadlock TA, Heaton JT. The effects of potential neuroprotective agents on rat facial function recovery following facial nerve injury. Otolaryngol Head Neck Surg. 2011 Jan;144:53–9. doi: 10.1177/0194599810390892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byers JM, Clark KF, Thompson GC. Effect of pulsed electromagnetic stimulation on facial nerve regeneration. Arch Otolaryngol Head Neck Surg. 1998 Apr;124:383–9. doi: 10.1001/archotol.124.4.383. [DOI] [PubMed] [Google Scholar]
- 12.Hadlock T, Lindsay R, Edwards C, Smitson C, Weinberg J, Knox C, Heaton JT. The effect of electrical and mechanical stimulation on the regenerating rodent facial nerve. Laryngoscope. 2010 Jun;120:1094–102. doi: 10.1002/lary.20903. [DOI] [PubMed] [Google Scholar]
- 13.Hadlock T, Sheahan T, Heaton J, Sundback C, Mackinnon S, Cheney M. Baiting the cross-face nerve graft with temporary hypoglossal hookup. Arch Facial Plast Surg. 2004 Jul-Aug;6:228–33. doi: 10.1001/archfaci.6.4.228. [DOI] [PubMed] [Google Scholar]
- 14.Angelov DN, Ceynowa M, Guntinas-Lichius O, Streppel M, Grosheva M, Kiryakova SI, Skouras E, Maegele M, Irintchev A, Neiss WF, Sinis N, Alvanou A, Dunlop SA. Mechanical stimulation of paralyzed vibrissal muscles following facial nerve injury in adult rat promotes full recovery of whisking. Neurobiol Dis. 2007 Apr;26:229–42. doi: 10.1016/j.nbd.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Kiryakova S, Sohnchen J, Grosheva M, Schuetz U, Marinova T, Dzhupanova R, Sinis N, Hubbers CU, Skouras E, Ankerne J, Fries JW, Irintchev A, Dunlop SA, Angelov DN. Recovery of whisking function promoted by manual stimulation of the vibrissal muscles after facial nerve injury requires insulin-like growth factor 1 (IGF-1) Exp Neurol. 2010 Apr;222:226–34. doi: 10.1016/j.expneurol.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 16.Skouras E, Merkel D, Grosheva M, Angelova SK, Schiffer G, Thelen U, Kaidoglou K, Sinis N, Igelmund P, Dunlop SA, Pavlov S, Irintchev A, Angelov DN. Manual stimulation, but not acute electrical stimulation prior to reconstructive surgery, improves functional recovery after facial nerve injury in rats. Restor Neurol Neurosci. 2009;27:237–51. doi: 10.3233/RNN-2009-0474. [DOI] [PubMed] [Google Scholar]
- 17.Sohnchen J, Grosheva M, Kiryakova S, Hubbers CU, Sinis N, Skouras E, Ankerne J, Kaidoglou K, Fries JW, Irintchev A, Dunlop SA, Angelov DN. Recovery of whisking function after manual stimulation of denervated vibrissal muscles requires brain-derived neurotrophic factor and its receptor tyrosine kinase B. Neuroscience. 2010 Sep 29;170:372–80. doi: 10.1016/j.neuroscience.2010.06.053. [DOI] [PubMed] [Google Scholar]
- 18.Angelov DN, Skouras E, Guntinas-Lichius O, Streppel M, Popratiloff A, Walther M, Klein J, Stennert E, Neiss WF. Contralateral trigeminal nerve lesion reduces polyneuronal muscle innervation after facial nerve repair in rats. Eur J Neurosci. 1999;11:1369–78. doi: 10.1046/j.1460-9568.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- 19.Pavlov SP, Grosheva M, Streppel M, Guntinas-Lichius O, Irintchev A, Skouras E, Angelova SK, Kuerten S, Sinis N, Dunlop SA, Angelov DN. Manually-stimulated recovery of motor function after facial nerve injury requires intact sensory input. Exp Neurol. 2008 May;211:292–300. doi: 10.1016/j.expneurol.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Lindsay RW, Heaton JT, Edwards C, Smitson C, Vakharia K, Hadlock TA. Daily facial stimulation to improve recovery after facial nerve repair in rats. Arch Facial Plast Surg. 2010 May-Jun;12:180–5. doi: 10.1001/archfacial.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bermejo R, Houben D, Zeigler HP. Optoelectronic monitoring of individual whisker movements in rats. J Neurosci Methods. 1998 Sep 1;83:89–96. doi: 10.1016/s0165-0270(98)00050-8. [DOI] [PubMed] [Google Scholar]
- 22.Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair. 2008 Mar-Apr;22:111–21. doi: 10.1177/1545968307305457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogan N, Krebs HI. Interactive robots for neuro-rehabilitation. Restor Neurol Neurosci. 2004;22:349–58. [PubMed] [Google Scholar]
- 24.Hogan N, Krebs HI, Rohrer B, Palazzolo JJ, Dipietro L, Fasoli SE, Stein J, Hughes R, Frontera WR, Lynch D, Volpe BT. Motions or muscles? Some behavioral factors underlying robotic assistance of motor recovery. J Rehabil Res Dev. 2006 Aug-Sep;43:605–18. doi: 10.1682/jrrd.2005.06.0103. [DOI] [PubMed] [Google Scholar]
- 25.Heaton JT, Knox C, Malo J, Hadlock TA. A system for delivering mechanical stimulation to the rat whisker pad during facial nerve regeneration. 5th International IEEE/EMBS Conference on Neural Engineering; Cancun, Mexico: IEEE Neural Engineering; 2011. pp. 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadlock T, Kowaleski J, Mackinnon S, Heaton JT. A novel method of head fixation for the study of rodent facial function. Exp Neurol. 2007 May;205:279–82. doi: 10.1016/j.expneurol.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachdev RN, Sato T, Ebner FF. Divergent movement of adjacent whiskers. J Neurophysiol. 2002 Mar;87:1440–8. doi: 10.1152/jn.00539.2001. [DOI] [PubMed] [Google Scholar]
- 28.Hill DN, Bermejo R, Zeigler HP, Kleinfeld D. Biomechanics of the vibrissa motor plant in rat: rhythmic whisking consists of triphasic neuromuscular activity. J Neurosci. 2008 Mar 26;28:3438–55. doi: 10.1523/JNEUROSCI.5008-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao P, Bermejo R, Zeigler HP. Whisker deafferentation and rodent whisking patterns: behavioral evidence for a central pattern generator. J Neurosci. 2001 Jul 15;21:5374–80. doi: 10.1523/JNEUROSCI.21-14-05374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hadlock T, Kowaleski J, Lo D, Bermejo R, Zeigler HP, Mackinnon S, Heaton JT. Functional assessments of the rodent facial nerve: a synkinesis model. Laryngoscope. 2008 Oct;118:1744–9. doi: 10.1097/MLG.0b013e31817f5255. [DOI] [PubMed] [Google Scholar]
- 31.Henstrom D, Hadlock TA, Lindsay R, Knox C, Malo J, Vakharia KT, Heaton JT. The convergence of facial nerve branches providing whisker pad motor supply in rats: Implications for facial reanimation study. Muscle & Nerve. doi: 10.1002/mus.23232. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semba K, Egger MD. The facial “motor” nerve of the rat: control of vibrissal movement and examination of motor and sensory components. J Comp Neurol. 1986 May 8;247:144–58. doi: 10.1002/cne.902470203. [DOI] [PubMed] [Google Scholar]
- 33.Carvell GE, Simons DJ. Biometric analyses of vibrissal tactile discrimination in the rat. J Neurosci. 1990 Aug;10:2638–48. doi: 10.1523/JNEUROSCI.10-08-02638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harvey MA, Bermejo R, Zeigler HP. Discriminative whisking in the head-fixed rat: optoelectronic monitoring during tactile detection and discrimination tasks. Somatosens Mot Res. 2001;18:211–22. doi: 10.1080/01421590120072204. [DOI] [PubMed] [Google Scholar]
- 35.Jin TE, Witzemann V, Brecht M. Fiber types of the intrinsic whisker muscle and whisking behavior. J Neurosci. 2004 Mar 31;24:3386–93. doi: 10.1523/JNEUROSCI.5151-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchinson B, Grant RA, Arkley K, Rankov V, Perkon I, Prescott TJ. Active vibrissal sensing in rodents and marsupials. Philos Trans R Soc Lond B Biol Sci. 2011 Nov 12;366:3037–48. doi: 10.1098/rstb.2011.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berg RW, Kleinfeld D. Vibrissa movement elicited by rhythmic electrical microstimulation to motor cortex in the aroused rat mimics exploratory whisking. J Neurophysiol. 2003 Nov;90:2950–63. doi: 10.1152/jn.00511.2003. [DOI] [PubMed] [Google Scholar]
- 38.Guntinas-Lichius O, Wewetzer K, Tomov TL, Azzolin N, Kazemi S, Streppel M, Neiss WF, Angelov DN. Transplantation of olfactory mucosa minimizes axonal branching and promotes the recovery of vibrissae motor performance after facial nerve repair in rats. J Neurosci. 2002 Aug 15;22:7121–31. doi: 10.1523/JNEUROSCI.22-16-07121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerrebijn JD, Freeman JL. Facial nerve reconstruction: outcome and failures. J Otolaryngol. 1998 Aug;27:183–6. [PubMed] [Google Scholar]
- 40.Sumner AJ. Aberrant reinnervation. Muscle Nerve. 1990 Sep;13:801–3. doi: 10.1002/mus.880130905. [DOI] [PubMed] [Google Scholar]
- 41.Jergovic D, Stal P, Lidman D, Lindvall B, Hildebrand C. Changes in a rat facial muscle after facial nerve injury and repair. Muscle Nerve. 2001 Sep;24:1202–12. doi: 10.1002/mus.1133. [DOI] [PubMed] [Google Scholar]
- 42.Borin A, Toledo RN, Faria SD, Testa JR, Cruz OL. Behavioral and histologic experimental model of facial nerve regeneration in rats. Braz J Otorhinolaryngol. 2006 Nov-Dec;72:775–84. [PubMed] [Google Scholar]
- 43.Tomov TL, Guntinas-Lichius O, Grosheva M, Streppel M, Schraermeyer U, Neiss WF, Angelov DN. An example of neural plasticity evoked by putative behavioral demand and early use of vibrissal hairs after facial nerve transection. Exp Neurol. 2002 Dec;178:207–18. doi: 10.1006/exnr.2002.8040. [DOI] [PubMed] [Google Scholar]
- 44.Guntinas-Lichius O, Irintchev A, Streppel M, Lenzen M, Grosheva M, Wewetzer K, Neiss WF, Angelov DN. Factors limiting motor recovery after facial nerve transection in the rat: combined structural and functional analyses. Eur J Neurosci. 2005 Jan;21:391–402. doi: 10.1111/j.1460-9568.2005.03877.x. [DOI] [PubMed] [Google Scholar]
- 45.Sinis N, Horn F, Genchev B, Skouras E, Merkel D, Angelova SK, Kaidoglou K, Michael J, Pavlov S, Igelmund P, Schaller HE, Irintchev A, Dunlop SA, Angelov DN. Electrical stimulation of paralyzed vibrissal muscles reduces endplate reinnervation and does not promote motor recovery after facial nerve repair in rats. Ann Anat. 2009 Oct;191:356–70. doi: 10.1016/j.aanat.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Yeh C, Bowers D, Hadlock TA. Effect of FK506 on functional recovery after facial nerve injury in the rat. Arch Facial Plast Surg. 2007 Sep-Oct;9:333–9. doi: 10.1001/archfaci.9.5.333. [DOI] [PubMed] [Google Scholar]
- 47.Sun F, Zhou K, Mi WJ, Qiu JH. Repair of facial nerve defects with decellularized artery allografts containing autologous adipose-derived stem cells in a rat model. Neurosci Lett. 2011 Jul 20;499:104–8. doi: 10.1016/j.neulet.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 48.Dohm S, Streppel M, Guntinas-Lichius O, Pesheva P, Probstmeier R, Walther M, Neiss WF, Stennert E, Angelov DN. Local application of extracellular matrix proteins fails to reduce the number of axonal branches after varying reconstructive surgery on rat facial nerve. Restor Neurol Neurosci. 2000;16:117–126. [PubMed] [Google Scholar]
- 49.Streppel M, Azzolin N, Dohm S, Guntinas-Lichius O, Haas C, Grothe C, Wevers A, Neiss WF, Angelov DN. Focal application of neutralizing antibodies to soluble neurotrophic factors reduces collateral axonal branching after peripheral nerve lesion. Eur J Neurosci. 2002 Apr;15:1327–42. doi: 10.1046/j.1460-9568.2002.01971.x. [DOI] [PubMed] [Google Scholar]
- 50.Skouras E, Popratiloff A, Guntinas-Lichius O, Streppel M, Rehm KE, Neiss WF, Angelov DN. Altered sensory input improves the accuracy of muscle reinnervation. Restor Neurol Neurosci. 2002;20:1–14. [PubMed] [Google Scholar]
- 51.Evgenieva E, Schweigert P, Guntinas-Lichius O, Pavlov S, Grosheva M, Angelova S, Streppel M, Irintchev A, Skouras E, Kuerten S, Sinis N, Dunlop S, Radeva V, Angelov DN. Manual stimulation of the suprahyoid-sublingual region diminishes polynnervation of the motor endplates and improves recovery of function after hypoglossal nerve injury in rats. Neurorehabil Neural Repair. 2008 Nov-Dec;22:754–68. doi: 10.1177/1545968308316387. [DOI] [PubMed] [Google Scholar]
- 52.Guntinas-Lichius O, Hundeshagen G, Paling T, Streppel M, Grosheva M, Irintchev A, Skouras E, Alvanou A, Angelova SK, Kuerten S, Sinis N, Dunlop SA, Angelov DN. Manual stimulation of facial muscles improves functional recovery after hypoglossal-facial anastomosis and interpositional nerve grafting of the facial nerve in adult rats. Neurobiol Dis. 2007 Oct;28:101–12. doi: 10.1016/j.nbd.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Udina E, Cobianchi S, Allodi I, Navarro X. Effects of activity-dependent strategies on regeneration and plasticity after peripheral nerve injuries. Ann Anat. 2011 Jul;193:347–53. doi: 10.1016/j.aanat.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 54.English AW, Wilhelm JC, Sabatier MJ. Enhancing recovery from peripheral nerve injury using treadmill training. Ann Anat. 2011 Jul;193:354–61. doi: 10.1016/j.aanat.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seo TB, Oh MJ, You BG, Kwon KB, Chang IA, Yoon JH, Lee CY, Namgung U. ERK1/2-mediated Schwann cell proliferation in the regenerating sciatic nerve by treadmill training. J Neurotrauma. 2009 Oct;26:1733–44. doi: 10.1089/neu.2008.0711. [DOI] [PubMed] [Google Scholar]
- 56.Sabatier MJ, Redmon N, Schwartz G, English AW. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol. 2008 Jun;211:489–93. doi: 10.1016/j.expneurol.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.English AW, Cucoranu D, Mulligan A, Sabatier M. Treadmill training enhances axon regeneration in injured mouse peripheral nerves without increased loss of topographic specificity. J Comp Neurol. 2009 Nov 10;517:245–55. doi: 10.1002/cne.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pachter BR, Eberstein A. Passive exercise and reinnervation of the rat denervated extensor digitorum longus muscle after nerve crush. Am J Phys Med Rehabil. 1989;68:179–82. doi: 10.1097/00002060-198908000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Wilhelm JC, Xu M, Cucoranu D, Chmielewski S, Holmes T, Lau KS, Bassell GJ, English AW. Cooperative roles of BDNF expression in neurons and schwann cells are modulated by exercise to facilitate nerve regeneration. J Neurosci. 2012 Apr 4;32:5002–9. doi: 10.1523/JNEUROSCI.1411-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002 Nov;88:2187–95. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 61.Funakoshi H, Belluardo N, Arenas E, Yamamoto Y, Casabona A, Persson H, Ibanez CF. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995;268:1495–9. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- 62.Lieberman AR. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- 63.English AW, Krakowiak JR, Wilhelm JC. Society for Neuroscience. San Diego, CA: 2010. Effect of treadmill training on synaptic stripping of axotomized mouse motoneurons. [Google Scholar]
- 64.Herbison GJ, Jaweed MM, Ditunno JF, Scott CM. Effect of overwork during reinnervation of rat muscle. Exp Neurol. 1973;41:1–14. doi: 10.1016/0014-4886(73)90176-3. [DOI] [PubMed] [Google Scholar]
- 65.Herbison GJ, Jaweed MM, Gordon EE, Ditunno JF., Jr Overwork of denervated skeletal muscle: effect on muscle proteins in rats. Arch Phys Med Rehabil. 1974;55:202–5. [PubMed] [Google Scholar]
- 66.Kinney CL, Jaweed MM, Herbison GJ, Ditunno JF. Overwork effect on partially denervated rat soleus muscle. Arch Phys Med Rehabil. 1986;67:286–9. [PubMed] [Google Scholar]
- 67.Kim WS, Lee SU. Harmful effect of land-based endurance exercise in rats with diabetic nerve. Med Sci Sports Exerc. 2010 Sep;42:1625–31. doi: 10.1249/MSS.0b013e3181d58e09. [DOI] [PubMed] [Google Scholar]