Abstract
Children and adolescents with traumatic brain injury (TBI) often experience behavior difficulties that may arise from problem-solving deficits and impaired self-regulation. However, little is known about the relationship of neurocognitive ability to post-TBI behavioral recovery. To address this question, we examined whether verbal intelligence, as estimated by Vocabulary scores from the Wechsler Abbreviated Scale of Intelligence, predicted improvements in behavior and executive functioning following a problem-solving intervention for adolescents with TBI. 132 adolescents with complicated mild to serve TBI were randomly assigned to a 6 month web-based problem-solving intervention (CAPS; n = 65) or to an internet resource comparison (IRC; n = 67) group. Vocabulary moderated the association between treatment group and improvements in meta-cognitive abilities. Examination of the mean estimates indicated that for those with lower Vocabulary scores, pre-intervention Metacognition Index scores from the Behavior Rating Inventory of Executive Function (BRIEF) did not differ between the groups, but post-intervention scores were significantly lower (more improved) for those in the CAPS group. These findings suggest that low verbal intelligence was associated with greater improvements in executive functioning following the CAPS intervention and that verbal intelligence may have an important role in response to intervention for TBI. Understanding predictors of responsiveness to interventions allows clinicians to tailor treatments to individuals, thus improving efficacy.
Introduction
Children and adolescents with traumatic brain injury (TBI) commonly experience behavior difficulties (Taylor et al. 2002; Chapman et al., 2010), stemming in part from deficits in problem-solving skills and self-regulation (Chevignard, 2009; Ganesalingam, Sanson, Anderson, & Yeates, 2006; Ganesalingam, Sanson, Anderson, & Yeates, 2007). Although post-TBI behavior problems in children have been widely researched, relatively little is known about the efficacy of interventions to reduce behavior and problem-solving deficits and potential predictors of responsiveness to interventions. The current study sought to investigate the relationship between verbal intelligence and response to a problem-solving intervention for adolescents with TBI; in doing so, we aimed to identify adolescents who are most likely to benefit from intervention with the larger goal of tailoring treatments to individual needs.
Post-TBI neurocognitive deficits often occur across cognitive domains, including general intelligence (Catroppa et al., 2008), working memory (Schwartz et al., 2003), attention (DeJong & Donders, 2008; Mottram & Donders, 2005; Jacobs & Donders, 2008), executive functions (Yeates et al., 2002; Ganesalingam, Sanson, Anderson, & Yeates, 2006; Ganesalingam, Sanson, Anderson, & Yeates, 2007), and processing speed (Donders & Janke, 2008; Donders, 1997; Tremont, Mittenberg, & Miller, 1999; van der Heijden & Donders, 2003). Although deficits are greater in moderate to severe TBI (Schwartz et al., 2003; Catroppa et al., 2008; Yeates et al., 2002), existing research suggests that, relative to uncomplicated mild TBI and typically developing controls, children with complicated mild TBI often evidence impaired episodic memory and cognitive processing along with a diminished ability to manage cognitive interference (Levin et al., 2008). Neurocognitive deficits have been associated with persistent behavior problems and may be related to an impaired ability to regulate attention and emotional reactions (Barkley, 1997; Baum et al., 2010; Catroppa et al., 2008; Morgan & Lilenfeld, 2000 Schwartz et al., 2003). While some investigators have reported that verbal intelligence is negatively affected by neurological insult, such as TBI (Ewing-Cobbs et al., 1997; Verger et al., 2001), others have reported that verbal intelligence can be relatively unaffected when injury occurs later in development (Schmand, Smit, Geerlings, & Lindeboom, 1997; Anderson, Catroppa, Morse, Haritou, & Rosenfeld, 2000), suggesting that measures of verbal intelligence may provide reliable estimates of pre-morbid neurocognitive functioning after injury in adolescence (Lezak, Howieson, & Loring, 2004).
Cerebral reserve refers to the ability of the brain to often remain functional despite insult or pathology (Staff, Murray, Deary, & Whalley, 2004). Cognitive Reserve Hypothesis states that higher pre-morbid neurocognitive functioning may help to preserve functional capacity following neurologic insult and allow for more efficient utilization of existing brain networks (Kesler, Adams, Blasey, & Bigler, 2003; Stern, 2003). Cognitive reserve has been studied in relation to a variety of clinical disorders, including dementia and HIV-related cognitive decline (Roe, Xiong, Miller, Morris; 2007; Stern, 2006; Stern, Silva, Chaisson, & Evans, 1996). Within the dementia literature, for example, higher pre-morbid neurocognitive functioning has been associated with reduced dementia-related pathology and a less precipitous decline in cognitive ability (Roe et al., 2007; Stern, 2006). Examining cognitive reserve in TBI, Kesler and colleagues (2003) reported that pre-injury education level in adults partially predicted post-TBI outcomes, supporting the notion that high pre-morbid neurocognitive functioning may blunt the negative effects of TBI.
In one of the few investigations of cognitive reserve as a moderator of outcomes following pediatric TBI, Fay and colleagues (2010) examined postconcussive symptoms in children with mild TBI across the first year post injury. Their findings revealed that symptoms were related to a composite measure of cognitive ability for the subset of children with complicated mild TBI (i.e., injuries associated with intracranial findings on neuroimaging). Specifically, the children in this subset with low cognitive ability self reported more postconcussive symptoms at the initial post-acute assessment than did those with high cognitive ability. The subset of children with mild TBI who had low cognitive ability also displayed the most pronounced increases in cognitive symptoms at 3 months post injury relative to children in an orthopedic injury comparison group. In view of evidence that cognitive abilities were not affected by mild TBI in this study and that baseline cognitive skill was thus a reasonable measure of premorbid status, the findings were interpreted as evidence for a protective effect of cognitive reserve on the emergence of postconcussive symptoms. In a separate study, Farmer and colleagues (2002) reported that children with TBI and premorbid learning difficulties evidenced significantly poorer memory abilities than both healthy controls without a history of head injury and children with TBI without premorbid learning difficulties, providing additional evidence for moderating effects of premorbid cognitive abilities on post-TBI outcomes.
Limited evidence suggests that pre-intervention neurocognitive functioning is predictive of intervention response. Bierman and colleagues (2008) reported that better parent-reported pre-intervention executive functions, including self-regulation, was associated with greater response to an intervention that promoted development of social- emotional functioning and literacy in pre-school aged children. In examining moderators of treatment response for the multimodal treatment of ADHD, children with lower IQ scores were reportedly less likely to evidence a response to the intervention (Hinshaw, 2007). However, to our knowledge, no studies have examined indices of pre-injury cognitive functioning as moderators of the effects of interventions for children with TBI. Wade and colleagues established the efficacy of online, problem-solving approach in improving child and family outcomes in children with TBI ages 5–17 years (Wade, Carey, & Wolfe, 2006; Wade et al., 2010). The intervention consisted of self-guided web content along with live videoconferencing with a therapist. Relative to a demographically matched cohort that received only internet-based resources about TBI, the children who received the intervention had better parent-reported self-management, compliance with parental requests, social competence, and executive function (Wade, Carey, & Wolfe, 2006; Wade et al., 2010). Parents of children in the intervention group also showed more improvement in measures of parental injury-related burden, psychiatric symptoms, depression, and parenting stress than the parents of children in the internet resources only group (Wade, Wolfe, Brown, & Pestian, 2005; Wade, Carey, & Wolfe 2006b). As evidence for individual differences in treatment effects, improvements in the intervention group were more pronounced in children with more severe TBI and in those at greater socioeconomic disadvantage (Wade, Carey, & Wolfe, 2006; Wade et al., 2010). In view of research indicating that behavior problems are more common in these subgroups (Taylor et al., 2002), the findings suggest that the intervention was most effective for the children at greatest risk for these problems.
Neither the study by Wade et al. (2006) nor any subsequent identified studies have investigated the role of children’s pre-injury cognitive functioning as a potential moderator of intervention effects. Our aim in this study was to test the Cognitive Reserve Hypothesis by determining if cognitive reserve, as estimated by Vocabulary scores from the Wechsler Abbreviated Scale of Intelligence (WASI; Psychological Corporation, 1999), predicted response to a revised version of the web-based, problem-solving intervention for adolescents with TBI originally developed by the Wade team. Based on previous findings, we would anticipate that children at higher risk for the development of behavior problems, in this case because of lower cognitive reserve, would have greater potential to benefit from a post-TBI intervention than children at lower risk. We thus hypothesize that lower Vocabulary scores would predict greater short-term (6 month) improvement in parent ratings of executive dysfunction and other behavior problems.
Method
Participants
The current study was conducted at four major trauma centers located in the Midwestern and Western United States. Participants included 132 children between the ages of 12 and 17 years who were hospitalized overnight for a complicated mild to severe TBI within the previous 1–6 months. Eligibility requirements included alteration of neurological functioning as measured by a Glasgow Coma Scale (Teasdale & Jennett, 1974) score < 13 or evidence of neurological insult on magnetic resonance imaging (MRI) or computerized tomography (CT) scans, as well as English as the primary language spoken in the home, availability of the adolescent to participate in the intervention, and the family residence being within a 3-hour drive of the hospital (the latter implemented to increase feasibility of conducting study visits at the families’ homes). Seventeen percent (52/308) of those screened were ineligible for one or more of these reasons, with a primary language other than English constituting the most common reason for exclusion. More detail about recruitment, including the CONSORT flow chart, can be found in Wade et al., in press.
Of the 337 families initially identified as potentially eligible, 49 were found to be ineligible, 70 refused participation, 9 were unable to be contacted, and 77 were unable to be recruited during the initial six months post-injury, causing them to become ineligible. 132 families agreed to participate, 65 of which were randomized to Counselor Assisted Problem Solving (CAPS) group and 67 to the Internet Resources Comparison (IRC) group. For participating families, 86% of primary parent respondents (104/121) were mothers, 10% were fathers (12/121), and 4% (5/121) were grandparents or other relations. This did not differ by treatment group. Among participating adolescents, 50 had severe TBI and 82 had complicated mild to moderate TBI. Of those with complicated mild to moderate TBI, 54 (26 in IRC and 28 in CAPS) had documented GCS scores about 13.
Age at injury, injury severity, family census track income, and race were compared between participating and non-participating families. Compared to participants, non-participants were more often non-white (24.4 % to 18.2%; p < .001) and had less severe TBI [mean (SD) GCS score = 10.00 (4.64) for participants and 11.94 (3.89) for non-participants]. Sample characteristics are summarized in Table 1 and means and standard deviations for parent report measures at baseline and 6 months are included in Table 2.
Table 1.
Means and standard deviations for baseline data.
| CAPS | IRC | P | |
|---|---|---|---|
| N | 65 | 67 | – |
| Injury severity (N/% severe) |
24/39.3 | 26/40.6 | .88 |
| Gender (N/% male) | 44/67.7 | 42/62.7 | .54 |
| Age at injury | 14.40 (1.68) | 14.67 (1.77) | .36 |
| Time since injury to baseline in months | .30 (.15) | .29 (.13) | .55 |
| Median family income | $71,305.02 ($21,186.99) |
$65,911.61 ($22,838.64) |
.27 |
| Ethnicity (N/% white) | 52/80.0 | 54/80.6 | .42 |
| Vocabulary T-score | 48.78 (9.73) | 47.55 (10.89) | .49 |
| Estimated Full-Scale IQ | 99.15 (13.69) | 99.37 (12.45) | .33 |
Note: Estimated Full-Scale IQ is based on the 2-subtest version of the WASI.
Table 2.
Means and standard deviations for parent report measures at baseline and 6 months.
| CAPS | IRC | |||
|---|---|---|---|---|
|
| ||||
| Baseline | 6-months | Baseline | 6-months | |
| BRIEF MI | 58.56 (9.24) | 56.92 (9.73) | 61.56 (9.47) | 60.91 (11.20) |
| Externalizing | 52.76 (10.29) | 50.37 (11.32) | 54.07 (10.50) | 54.09 (11.64) |
| Internalizing* | 51.90 (10.67) | 49.87 (12.14) | 55.61 (11.22) | 53.18 (11.47) |
| CAFAS Total Score | 45.69 (35.08) | 43.00 (39.41) | 51.81 (37.61) | 48.38 (41.01) |
| CAFAS Total Score Log | 1.59 (.32) | 1.57 (.34) | 1.64 (.32) | 1.59 (.33) |
Significant group difference (CAPS versus IRC) for Internalizing baseline (p < .05)
Note: CAFAS Total Score log used for regression analyses.
CAPS intervention
As part of the ongoing parent study, an initial baseline assessment at the family’s home was completed by study personnel. The primary caregiver completed standardized measures that assessed the functioning and behavior of the teen and relevant demographic information and the adolescent was administered the WASI by a trained research assistant. All families were provided with a new computer and high speed internet access and randomly assigned to one of two internet-based interventions: CAPS, a 6 month online, family-centered intervention that focused on problem solving, communication, and self-regulation, or IRC, a program that provided families with links to online education and resources regarding TBI. A follow-up assessment was conducted 6 months later when parents repeated the measures of behavioral functioning that were completed at the baseline assessment. A sealed envelope containing group assignment was handed to the participants at the end of the baseline visit allowing interviewers to remain naïve to group assignment. Thus, interviewers/research assistants were unaware of group assignment at both assessments, whereas parents were naïve to group assignment only at the baseline visit.
Treatment Conditions
For those families that were randomly assigned to the CAPS group, study personnel offered instructions and demonstrations of how to access the web-based modules and individual online videoconferencing sessions with the therapist. Each CAPS session consisted of a self-guided online module that provided didactic content of problem-solving, communication, and self-regulation skills. Modules included video clips that modeled the skills and exercises to practice the skills. After the completion of each self-guided module, a videoconferencing session was conducted with the therapist to discuss the material presented and practice the problem-solving process using a problem identified by the family. The next module that contained new material was released upon completion of each online session with the therapist. Online videoconference sessions with the therapist were scheduled biweekly for the first 3 months of the intervention, for a total of six sessions. After completion of the six core sessions, an individualized portion that included up to four additional sessions was administered to address unresolved issues. All families were then scheduled for a final session with the therapist to review progress and plan for future transitions.
Families in the IRC group received access to a home page with links to online resources such as local, state, and national brain-injury associations and sites focusing on pediatric brain injury, such as the Center on Brain Injury Research and Training, Brain Injury Partners and the National Database of Educational Resources on Traumatic Brain Injury. In addition to providing information, these sites also provided training in working with schools and effective self-advocacy (McLaughlin, Glang, Beaver, Gau, & Keen, in press), handling stress, and problem-solving around common issues. Families were instructed to spend one or more hours per week accessing information on these sites throughout the intervention period and to track the sites that they visited.
Measures
Relevant medical information was obtained from hospital records and demographic information was collected from the parent at the baseline study visit. The WASI, a brief screening measure of intelligence, was administered to the adolescent at the baseline visit. The two-subtest WASI was administered, consisting of Vocabulary and Matrix Reasoning. This two subtest version allows for a reliable estimation of Full Scale IQ. Validation studies of the WASI have reported that the Vocabulary subtest corresponds closely to and provides a reliable estimate of the Verbal Intelligence Quotient (VIQ) obtained from the Wechsler Intelligence Scale for Children-III and the Wechsler Adult Intelligence Scale-III (Psychological Corporation, 1999).
Parent ratings of adolescent problems in executive function and symptoms of internalizing and externalizing were collected at the baseline and 6-month visits. The Behavior Rating Inventory of Executive Function (BRIEF; Gioia, Isquith, Guy, & Kenworthy, 2000) was completed to obtain information about behaviors reflecting executive functioning in activities of daily living. The BRIEF has high internal consistency and stability and acceptable inter-rater and test-retest reliability and was recommended as a supplemental measure of executive functions by the pediatric Common Data Elements workgroup (McCauley et al., 2011). Internalizing and externalizing behavior problems were assessed using the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2000; 2001), a recommended core common data element for pediatric behavior problems post- TBI (McCauley et al., 2011), was also completed by parents. The CBCL has high test-retest reliability and criterion validity (Achenbach & Rescorla, 2000; 2001) and is sensitive to behavior problems following TBI (Taylor et al., 2002; Schwartz et al., 2003). The Metacognition Index (MI) of the BRIEF was used to obtain information about the adolescent’s ability to initiate, plan, organize, and sustain future-oriented problem-solving behaviors, self-manage one’s behavior while completing a task, and monitor one’s own performance (Gioia, Isquith, Guy, & Kenworthy, 2000). T-scores for CBCL Internalizing and Externalizing were used to assess these behavior domains. The BRIEF Behavior Regulation Index (BRI) was excluded from analysis due to its high correlation with CBCL Externalizing (r = .80).
The Child Adolescent Functional Assessment Scale (CAFAS; Hodges, 1999), a clinical rating conducted with the parent by an interviewer, unaware of group assignment, was used to assess the adolescent’s functioning across domains including school, home, community, behavior toward others, mood/emotions, self-harmful behaviors, substance use, and thinking. The CAFAS is reliable at detecting impaired behavior functioning in adolescents across a variety of contexts and domains (Hodges, 1999). The Total Score provided a composite of impaired functioning across domains for analysis. For the current study, every tenth parent interview was videotaped and ratings were completed by an independent rater to assess inter-rater reliability. Overall inter-rater reliability across study sites was 93.9%.
Statistical Approach
Our primary hypothesis stated that lower Vocabulary scores would be associated with greater improvements in behavior following the CAPS intervention, relative to the IRC control condition, at the 6 month study visit, controlling for baseline behavioral ratings and injury severity. To address this hypothesis, linear regression was used to examine the interaction of treatment group with age-normed Vocabulary scaled scores in relation to parent ratings of the adolescent’s behavior at the 6 month visit, after controlling for baseline behavior ratings and injury severity. Dependent variables were the BRIEF MI, CBCL Externalizing and Internalizing Behaviors subscales, and CAFAS Total Score. R2 and standardized beta values provided measures of effect size. To address the issue of multiple comparisons, we categorized the dependent measures into three domains: executive functions (BRIEF MI), behavioral functioning (CBCL Externalizing and Internalizing subscales), and global functioning (CAFAS Total). We then made domain-wise corrections in alpha level by dividing the significance value by the number of measures in each domain (i.e., p < .025 for the CBCL scales and p < .05 for the BRIEF MI and CAFAS Total score).
Results
Preliminary analyses
The distributions of the CAFAS Total Score were skewed at baseline (skewness = .79; kurtosis = .25) and 6-months (skewness = 1.29; kurtosis = 1.47) with the majority of the participants reporting few problems. The logarithm of the CAFAS Total Score resulted in a more normal distribution at baseline (skewness = −.40; kurtosis = −.69) and 6-months (skewness = −.12, kurtosis = .25). Examination of the BRIEF validity scales revealed no primary caregivers whose response patterns were thought to represent overly negative evaluations or inconsistent responding. On the BRIEF MI, one participant scored approximately three standard deviations above the sample mean at baseline; as supported by prior research (Tabachnick & Fidell, 2007), we reduced the effect that this extreme value might have on statistical analyses by replacing it with a T-score of 80.
Examining group differences (CAPS versus IRC) in exposure to and use of web-based resources, time spent on the web viewing information regarding TBI did not differ by group. Forty-three percent of parents in the CAPS group and 48% in the IRC group reported spending fewer than 30 minutes per week viewing information about TBI (either the CAPS website or other links) and 50% of the CAPS group and 47% of the IRC group reported spending between 30 minutes and 2 hours per week. The distribution of time that adolescents reported spending at TBI websites was similar to that of their parents, with 43% of CAPS teens and 48% of IRC teens spending less than 30 minutes per week at TBI websites. As reported in Tables 1 and 2, group comparisons at baseline failed to reveal significant differences in injury severity, Vocabulary, Full-Scale IQ, BRIEF MI, Externalizing Behaviors, and CAFAS (all p’s > 0.05). A significant group difference was found on baseline Internalizing Behaviors [t (127) = 1.92, p = 0.05]. In Table 1, Full-Scale IQ was included for descriptive purposes, although it was not used in further analyses. Correlations between Vocabulary and the baseline behavior measures ranged from .19 for the BRIEF MI to .28 for the CBCL Externalizing total. Correlations between the dependent measures ranged from .39–.59. Using the criteria of Variance Inflation Factor (VIF) greater than 2.5 and tolerance less than .40 (Allison, 1999), no cases of multicollinearity were identified.
Vocabulary scores and post-intervention behavior ratings
Analysis of the BRIEF MI revealed a group × Vocabulary interaction, unstandardized β = .29, t = 2.13, p < .03 (Table 3). Follow-up testing of the interaction revealed that adolescents with lower Vocabulary scores profited from the CAPS intervention, but not the IRC condition (Figure 1a). In contrast, pre- to post- treatment changes did not differ significantly for adolescents with higher Vocabulary (Figure 1b). Among participants with lower Vocabulary, the difference in mean estimates of 6 month BRIEF MI scores between CAPS and IRC was significant (p = .003) and corresponded to a large effect size (Cohen’s d = .79). Group differences were not significant for adolescents with higher Vocabulary (p > .05). The change in BRIEF MI scores from baseline to 6 months among those with higher Vocabulary scores was non-significant for CAPS [t (127) = .84, p = .40] and IRC [t (126) = 1.48, p = .14], suggesting that higher Vocabulary participants did not evidence significant changes in BRIEF MI scores over the course of the intervention period in either treatment condition. In post-hoc analyses that were conducted to determine if injury severity moderated response to the CAPS intervention, we examined the interaction of injury severity (dichotomizing participants into those with complicated mild or moderate to severe injury, thereby examining the differential effects in those with moderate to severe injuries relative to complicated mild), treatment group (CAPS or IRC), and Vocabulary. The triple interaction of injury severity, treatment group, and Vocabulary and the two-way interactions of injury severity with Vocabulary and treatment group with injury severity were non-significant (p > .05). The interaction of treatment group with Vocabulary remained significant (p < .05), offering evidence to suggest that injury severity did not moderate response to the CAPS intervention.
Table 3.
Linear regression examining relationship of Vocabulary scores to post-intervention behavioral outcomes
| MI | Externalizing | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Variable | β | SE | Std β | t | p | Variable | β | SE | Std β | t | p |
| BL behavior | .79 | .07 | .70 | 10.75 | < .001 | BL behavior | .74 | .07 | .67 | 9.44 | < .001 |
| Injury severity | 2.28 | 1.39 | .10 | 1.64 | .10 | Injury severity | 1.13 | 1.58 | .04 | .71 | .47 |
| Group | –15.58 | 6.72 | –.72 | –2.31 | .02 | Group | –3.67 | 7.88 | –.15 | –.46 | .64 |
| Vocabulary | –.24 | .10 | –.23 | –2.36 | .02 | Vocabulary | –.15 | .11 | –.13 | –1.30 | .19 |
| Group × Vocabulary | .29 | .13 | .66 | 2.13 | .03 | Group × Vocabulary | .02 | .15 | .05 | .14 | .88 |
| R2 | .57 | R2 | .54 | ||||||||
| Internalizing | CAFAS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Variable | β | SE | Std β | t | p | Variable | β | SE | Std β | t | p |
| BL behavior | .71 | .07 | .69 | 9.84 | < .001 | BL behavior | .51 | .08 | .51 | 5.93 | < .001 |
| Injury severity | 1.20 | 1.62 | .05 | .73 | .46 | Injury severity | –.05 | .05 | –.07 | –.92 | .35 |
| Group | –5.30 | 7.92 | –.22 | –.66 | .50 | Group | –.01 | .25 | –.01 | –.04 | .96 |
| Vocabulary | –.13 | .11 | –.12 | –1.18 | .23 | Vocabulary | –.007 | .004 | –.22 | –1.73 | .08 |
| Group × Vocabulary | .09 | .16 | .19 | .58 | .55 | Group × Vocabulary | < .001 | .005 | .002 | .004 | .99 |
| R2 | .52 | R2 | .36 | ||||||||
Figure 1a.
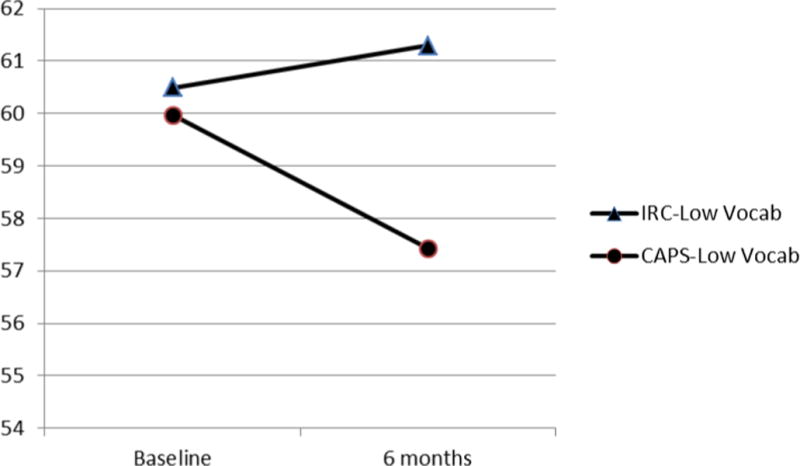
Estimated values of BRIEF MI scores for a Vocabulary score 1 SD below the sample mean.
Note: low Vocab = 1 SD below the sample mean Vocabulary score.
Difference in six-month mean estimates significant (p = .003).
Figure 1b.

Estimated values of BRIEF MI scores for a Vocabulary score 1 SD above the sample mean.
Note: High Vocab = 1 SD above the sample mean Vocabulary score.
Difference in six-month mean estimates non-significant (p = .12).
No significant interactions or main effects were identified for CBCL Externalizing or Internalizing subscales and the CAFAS Total Score logarithm. See Table 3.
Discussion
The current study sought to examine whether cognitive reserve, as estimated by WASI Vocabulary scores, predicted response to an online problem-solving intervention. Our larger goal was to identify the adolescents most likely to benefit from the intervention, affording clinicians greater ability to tailor treatments to meet the individual needs of adolescents and their families. We hypothesized that lower Vocabulary scores would be associated with the greatest reduction in parent-reported behavior problems in the CAPS intervention condition, reflecting the greater vulnerability of teens with lower Vocabulary scores to post-TBI deficits. Consistent with Cognitive Reserve Hypothesis, we also expected that higher Vocabulary scores would be associated with fewer post-intervention behavior problems due to the protective effect of higher cognitive reserve against post-TBI behavior problems.
In partial support of these hypotheses, results revealed a significant group × Vocabulary interaction effect for the BRIEF MI subscale. Follow-up tests indicated an association of lower Vocabulary scores with greater reduction in BRIEF MI scores in the CAPS condition. While these results are in contrast to limited evidence in other clinical populations suggesting that lower neurocognitive ability is related to less response to intervention (Hinshaw, 2007; Bierman et al., 2008), these findings are consistent with prior evidence that TBI interventions may be most effective in children who are most vulnerable to the development of post-TBI behavior problems (Wade, Carey, & Wolfe, 2006; Wade et al., 2010,Taylor et al., 2002). These findings suggest that adolescents with lower cognitive reserve have more potential to benefit from the CAPS intervention than those with higher reserve. For the current study, the MI summary score, which assesses initiation, planning, organization, future-oriented problem-solving behaviors, self-management, and monitoring one’s own performance (Gioia, Isquith, Guy, & Kenworthy, 2000), may be especially sensitive to the effects of CAPS, as it specifically targets deficits in problem-solving. Assuming lesser effects of TBI on Vocabulary than on other skills, the findings suggest that teens with lower pre-injury verbal skills may be limited in their ability to adapt to injury consequences. To the extent that lower Vocabulary scores are a reflection of the effects of TBI on cognition rather than premorbid abilities, an alternative interpretation is that the CAPS intervention is more effective in teens who sustain greater cognitive consequences of injury. However, regardless of whether behavior problems stemmed from TBI or were present prior to injury, these findings document the utility of cognitive-behavioral problem-solving interventions for adolescents with poorer cognitive functioning, a group that may not typically be considered for this type of intervention.
Evidence for moderating effects of Vocabulary on response to the CAPS intervention was found only for the BRIEF MI and not for the three other measures of treatment outcome. Internalizing and externalizing behaviors have been reported as a common consequence of TBI (Bloom et al., 2001; Grados et al., 2008; Levi, Drotar, Yeates, & Taylor, 1999; Max et al., 1998; Max et al., 1998d; Schwartz et al., 2003; Wade, Michaud, & Brown, 2006). Hence, one might have anticipated that these outcomes would also be subject to improvement with the CAPS intervention. The fact that CBCL Internalizing and Externalizing scores were not clinically elevated at baseline in the current study, thereby limiting our ability to predict improvements on these measures, may help to account for the lack of effects on these measures. The limited numbers of teens with severe impairments in behavior functioning on the CAFAS may also help to explain the absence of effects on this measure. The structure of the CAFAS and its focus on more extreme impairments may also have contributed to its lack of sensitivity to treatment effects. The CAFAS score is based on the occurrence of critical behaviors during the previous 3 months, regardless of their frequency. These issues may limit the utility of the CAFAS in assessing more subtle and incremental improvements in behavior resulting from an approach that focuses on building communication and problem-solving skills. Additionally, timing of the intervention may also be relevant when considering the non-significant findings on the measures of behavioral functioning. Recent findings have reported behavioral and social competence deficitsthat emerge with greater time since injury (Chapman et al., 2010). In the animal literature, research suggests that brain injured rodents often exhibit long-term, persistent neurocognitive and behavioral deficits and continuing neurodegeneration, indicating that the effects of neural insult not only fail to fully resolve over time, but may create vulnerability for future decline (Pierce, Smith, Trojanowski, & McIntosh, 1998). Therefore, some adolescents may evidence greater behavioral deficits with time and thus may benefit from interventions at a time farther from injury. One of the study’s limitations was the limited knowledge of pre-injury behavior functioning. While some studies have relied on retrospective reports for pre-TBI functioning (Yeates et al., 2010; Chapman et al., 2010; Karver et al., 2012), the accuracy of such reports in the current study would likely been biased by the time from injury to baseline assessment (several weeks to months). Parental reports of the child’s functioning in the study may have also been inflated by social desirability biases, therefore reducing reliability. Participant refusal and exclusionary criteria, such as English as the primary language and availability of internet access, may limit generalizability of the findings. As noted above, potential participants that either chose not to participate were more likely than participants to be non-white and have sustained less severe injuries than participants. It is also notable that the median family income of the obtained sample is above the national median despite substantial variability in income levels. These limitations in recruitment should be considered when interpreting the current results. Additionally, because Vocabulary was assessed after the injury and a control group without TBI was not included, we cannot be certain of the extent to which Vocabulary scores were affected by TBI. However, prior literature suggests that verbal intelligence may be less affected in adolescence during the post-acute injury period than other cognitive skills (Schmand, Smit, Geerlings, & Lindeboom, 1997; Anderson, Catroppa, Morse, Haritou, & Rosenfeld, 2000). Finally, it is acknowledged that there are numerous other factors, including age at injury, socio-demographic factors, family functioning, and parental stress/distress, which may influence response to post-TBI interventions; while the current study sought to specifically examine the role of cognitive reserve, future studies could consider these factors when seeking to comprehensively describe moderators of response to post-TBI behavioral interventions.
Despite these limitations, the current study makes a unique contribution to the literature by addressing the understudied issue of the relationship between neurocognitive ability and response to intervention. The results of this study afford clinicians greater ability to tailor post-injury interventions to the individual, increasing ability to offer personally relevant mental health services. Specifically, a problem-solving intervention that targets meta-cognitive skills may be more efficacious for youth with lower verbal intelligence. These findings are also among the first to support the concept of cognitive reserve as a potential moderator of the effects of interventions for pediatric TBI and thus will require replication. One consideration in conducting future studies in this area is to include other measures of cognitive reserve and to focus on measures of cognition that may be more sensitive to the effects of TBI. This approach would allow a fuller investigation of both indices of pre-injury cognitive status and post-injury cognitive changes as moderators of treatment effects. Measures of social cognition and executive function may be especially useful in view of the associations of these skills with behavioral outcomes after TBI (Yeates et al., 2007).
Acknowledgments
This work was supported in part by (1) grant R01-MH073764 from the National Institute of Mental Health, (2) a grant from the Colorado Traumatic Brain Injury Trust Fund Research Program, Colorado Department of Human Services, Division of Vocational Rehabilitation, Traumatic Brain Injury Program, and (3) the University of Cincinnati, University Research Counsel. The authors wish to acknowledge the contributions of Kendra McMullen, Robert Blaha, Elizabeth Hagesfeld, Michelle Jacobs, Daniel Maier, and Nina Fox in data collection and entry and John Stullenberger in website support. We would also like to acknowledge the contributions of the therapists JoAnne Carey, Psy. D., Britt Nielsen, Psy. D., and Brad Jackson, Ph. D.
Contributor Information
Christine L. Karver, University of Cincinnati, Department of Psychology, and Cincinnati Children’s Hospital Medical Center, Department of Physical Medicine and Rehabilitation, Cincinnati, Ohio
Shari L. Wade, Cincinnati Children’s Hospital Medical Center, Department of Physical Medicine and Rehabilitation, and the University of Cincinnati, College of Medicine, Cincinnati, Ohio
Amy Cassedy, Cincinnati Children’s Hospital Medical Center, Division of Biostatistics, Cincinnati, Ohio.
H. Gerry Taylor, Case Western Reserve University and Rainbow Babies & Children’s Hospital, University Hospitals Case Medical Center, Cleveland, Ohio.
Tanya M. Brown, Mayo Clinic College of Medicine, Department of Psychiatry and Psychology
Michael W. Kirkwood, Children’s Hospital Colorado, Department of Physical Medicine & Rehabilitation
Terry Stancin, Case Western Reserve University and MetroHealth Medical Center, Department of Psychiatry, Cleveland, Ohio.
References
- Achenbach TM, Rescorla LA. Child Behavior Checklist for Ages 1 1/2 - 5. Burlington, VT: The University of Vermont; 2000. [Google Scholar]
- Achenbach TM, Rescorla LA. Child Behavior Checklist for Ages 6–18. Burlington, VT: The University of Vermont; 2001. [Google Scholar]
- Allison PD. Logistic Regression Using the SAS system: Theory and Application. Cary, N.C.: SAS Institute; 1999. [Google Scholar]
- Anderson VA, Catroppa C, Morse S, Haritou F, Rosenfeld J. Recovery of intellectual ability following traumatic brain injury in childhood: Impact of severity and age of injury. Pediatric Neurosurgery. 2000;32(6):282–290. doi: 10.1159/000028956. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Baum KT, Byers AW, deGrauw TJ, Dunn DW, Bates JE, Howe SR, Chiu C-YP, Austin JK. The effect of temperament and neuropsychological functioning on behavior problems in children with new-onset seizures. Epilepsy & Behavior. 2010;17(4):467–473. doi: 10.1016/j.yebeh.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierman KL, Domitrovich CE, Nix RL, Gest SD, Welsh JA, Greenberg MT, Blair C, Nelson KE, Gill S. Promoting academic and social-emotional school readiness: The head start REDI program. Child Development. 2008;79(6):1802–1817. doi: 10.1111/j.1467-8624.2008.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom DR, Levin HS, Ewing-Cobbs L, Saunders AE, Song J, Fletcher JM, Kowatch RA. Lifetime and novel psychiatric disorders after pediatric traumatic brain injury. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40(5):572–579. doi: 10.1097/00004583-200105000-00017. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Anderson VA. Recovery in memory function, and its relationship to academic success, at 24 months following pediatric TBI. Child Neuropsychology. 2007;13(3):240–261. doi: 10.1080/09297040600837362. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Anderson VA, Morse SA, Haitou F, Rosenfeld JV. Outcome and predictors of functional recovery 5 years following pediatric traumatic brain injury (TBI) Journal of Pediatric Psychology. 2008;33(7):707–718. doi: 10.1093/jpepsy/jsn006. [DOI] [PubMed] [Google Scholar]
- Chevignard M, Toure H, Brugel DG, Poirier J, Laurent-Vannier A. A comprehensive model of care of rehabilitation of children with acquired brain injuries. Child Care Health and Development. 2010;36:31–43. doi: 10.1111/j.1365-2214.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- Chapman LA, Wade SL, Walz NC, Taylor HG, Stancin T, Yeates KO. Clinically significant behavior problems during the initial 18 months following early childhood traumatic brain injury. Rehabilitation Psychology. 2010;55:48–57. doi: 10.1037/a0018418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong J, Donders J. A confirmatory factor analysis of the California Verbal Learning Test- Second Edition (CVLT-II) in a traumatic brain injury sample. Assessment. 2008;16(4):328–336. doi: 10.1177/1073191109336989. [DOI] [PubMed] [Google Scholar]
- Donders J. Sensitivity of the WISC-III to injury severity in children with traumatic brain injury. Assessment. 1997;4:107–109. [Google Scholar]
- Donders J, Janke K. Criterion validity of the Wechsler Intelligence Scale for Children – Fourth Edition after pediatric Traumatic Brain Injury. Journal of the International Neuropsychological Society. 2008;14:651–655. doi: 10.1017/S1355617708080752. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Fletcher JM, Levin HS, Francis DJ, Davidson K, Miner ME. Longitudinal neuropsychological outcomes in infants and children with traumatic brain injury. Journal of the International Neuropsychological Society. 1997;3:581–591. [PubMed] [Google Scholar]
- Farmer JE, Kanne SM, Haut JS, Williams J, Johnstone B, Kirk K. Memory functioning following traumatic brain injury in children with premorbid learning problems. Developmental Neuropsychology. 2002;22:455–469. doi: 10.1207/S15326942DN2202_2. [DOI] [PubMed] [Google Scholar]
- Fay TB, Yeates KO, Taylor HG, Bangert B, Dietrich A, Nuss KE, Rusin J, Wright M. Cognitive reserve as a moderator of post-concussive symptoms in children with complicated and uncomplicated mild traumatic brain injury. Journal of the International Neuropsychological Society. 2010;16:94–105. doi: 10.1017/S1355617709991007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesalingam K, Sanson A, Anderson V, Yeates KO. Self-regulation and social and behavioral functioning following childhood traumatic brain injury. Journal of the International Neuropsychological Society. 2006;12:609–621. doi: 10.1017/S1355617706060796. [DOI] [PubMed] [Google Scholar]
- Ganesalingam K, Sanson A, Anderson V, Yeates KO. Self regulation as a mediator of the effects of childhood traumatic brain injury on social and behavioral functioning. The Journal of the International Neuropsychological Society. 2007;13(2):298–31. doi: 10.1017/S1355617707070324. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive function. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- Grados MA, Vasa RA, Riddle MA, Slomine BS, Salorio C, Christensen J, Gerring J. New onset obsessive-compulsive symptoms in children and adolescents with severe traumatic brain injury. Depression and Anxiety. 2008;25(5):398–407. doi: 10.1002/da.20398. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP. Moderators and mediators of treatment outcome for youth with ADHD: Understanding for whom and how interventions work. Ambulatory Pediatrics. 2007;1S:91–100. doi: 10.1016/j.ambp.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Hodges K. Child and Adolescent Functional Assessment Scale (CAFAS) In: Maruish ME, editor. The Use of Psychological Testing for Treatment Planning and Outcome Assessment. 2. Mahwah, NJ: Lawrence Erlbaum; 1999. [Google Scholar]
- Jacbos M, Donders J. Performance discrepancies on the California Verbal Learning Test- Second Edition (CVLT-II) after traumatic brain injury. Achieves of Clinical Neuropsychology. 2008;23:113–118. doi: 10.1016/j.acn.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Karver CL, Wade SL, Cassedy A, Taylor HG, Stancin T, Yeates KO, Walz NC. Age at injury and long-term behavior problems following traumatic brain injury in young children. Rehabilitation Psychology. 2012;57(3):256–265. doi: 10.1037/a0029522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Adams HF, Blasey CM, Bigler ED. Premorbid intellectual functioning, education, and brain size in Traumatic Brain Injury: An investigation of the Cognitive Reserve Hypothesis. Applied Neuropsychology. 2003;10(3):153–162. doi: 10.1207/S15324826AN1003_04. [DOI] [PubMed] [Google Scholar]
- Levi RB, Drotar D, Yeates KO, Taylor HG. Posttraumatic stress symptoms in children following orthopedic or traumatic brain injury. Journal of Clinical Child Psychology. 1999;28(2):232–243. doi: 10.1207/s15374424jccp2802_10. [DOI] [PubMed] [Google Scholar]
- Levin HS, et al. Prediction of cognitive sequelae based on abnormal tomography findings in children following mild traumatic brain injury. Journal of Neurosurgery: Pediatrics. 2008;1:461–470. doi: 10.3171/PED/2008/1/6/461. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4. New York: Oxford University Press; 2004. [Google Scholar]
- Max JE, et al. Posttraumatic stress symptomology after childhood traumatic brain injury. Journal of Nervous & Mental Disease. 1998;186(10):589–596. doi: 10.1097/00005053-199810000-00001. [DOI] [PubMed] [Google Scholar]
- McCauley SR, Wilde EA, Anderson VA, Bedell G, Beers SR, Campbell TF, Chapman SB, Ewing-Cobbs L, Gerring JP, Gioia GA, Levin HS, Michaud LJ, Prasad MR, Swaine BR, Turkstra LS, Wade SL, Yeates KO. Recommendations for the use of common outcome measures in pediatric traumatic Brain injury research. Journal of Neurotrauma. 2011;4:678–705. doi: 10.1089/neu.2011.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K, Glang A, Beaver SV, Gau GM, Keen S. Web-based training in family advocacy. Journal of Head Trauma Rehabilitation. doi: 10.1097/HTR.0b013e31824e1d43. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AB, Lilenfeld SO. A meta-analysis of the relation between antisocial behavior and neuropsychological measures of executive function. Clinical Psychology Review. 2000;20:113–136. doi: 10.1016/s0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- Mottram L, Donders J. Construct validity of the California Verbal Learning Test – Children’s Version (CVLT-C) after pediatric traumatic brain injury. Psychological Assessment. 2005;17(2):212–217. doi: 10.1037/1040-3590.17.2.212. [DOI] [PubMed] [Google Scholar]
- Pierce JES, Smith DH, Trojanowski JQ, McIntosh TK. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience. 1998;2(20):359–369. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. Wechsler Abbreviated Scales of Intelligence. San Antonio, TX: Author; 1999. [Google Scholar]
- Reitman D, Murphy MA, Hupp SDA, O’Callaghan PM. Behavior change and perceptions of change: Evaluating the effectiveness of a token economy. Child & Family Behavior Therapy. 2004;26(2):17–36. [Google Scholar]
- Reyno SM, McGrath PJ. Predictors of parent training efficacy for child externalizing behavior problems – A meta-analytic review. Journal of Child Psychology and Psychiatry. 2006;47:99–111. doi: 10.1111/j.1469-7610.2005.01544.x. [DOI] [PubMed] [Google Scholar]
- Roe CM, Xiong C, Miller JP, Morris JC. Education and Alzheimer disease without dementia: Support for cognitive reserve. Neurology. 2007;68(3):223–228. doi: 10.1212/01.wnl.0000251303.50459.8a. [DOI] [PubMed] [Google Scholar]
- Schmand B, Smit JH, Geerlings MI, Lindeboom J. The effect of intelligence and education on the development of dementia: A test of brain reserve hypothesis. Psychological Medicine. 1997;27:1337–1344. doi: 10.1017/s0033291797005461. [DOI] [PubMed] [Google Scholar]
- Schwartz L, Taylor HG, Drotar D, Yeates KO, Wade SL, Stancin T. Long- term behavior problems following pediatric traumatic brain injury: Prevalence, predictors, and correlates. Journal of Pediatric Psychology. 2003;28:251–263. doi: 10.1093/jpepsy/jsg013. [DOI] [PubMed] [Google Scholar]
- Staff RT, Murray AD, Deary IJ, Whalley LJ. What provides cerebral reserve? Brain. 2004;127(5):1191–1199. doi: 10.1093/brain/awh144. [DOI] [PubMed] [Google Scholar]
- Stern RA, Silva SG, Chaisson N, Evans DL. Influence of cognitive reserve on neuropsychological functioning in asymptomatic human immunodeficiency virus-1 infection. Achieves of Neurology. 1996;53(2):148–153. doi: 10.1001/archneur.1996.00550020052015. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2003;8:448–460. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer’s disease. Alzheimer’s Disease and Associated Disorders. 2006;20:S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5. New York: Allyn and Bacon; 2007. [Google Scholar]
- Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term outcomes after traumatic brain injury in children: Behavior and achievement. Neuropsychology. 2002;16:15–27. doi: 10.1037//0894-4105.16.1.15. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Yeates KO, Wade SL, Drotar D, Klein SK, Stancin T. Influences on first-year recovery from traumatic brain injury in children. Neuropsychology. 1999;13:76–89. doi: 10.1037//0894-4105.13.1.76. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Tremont G, Mittenberg W, Miller LJ. Acute intellectual effects of pediatric head trauma. Child Neuropsychology. 1999;5(2):104–114. [Google Scholar]
- van der Heijden P, Donders J. WAIS-III factor index score patterns after traumatic brain injury. Assessment. 2003;10(2):115–122. doi: 10.1177/1073191103010002001. [DOI] [PubMed] [Google Scholar]
- Vasa RA, Gerring JP, Grados M, Slomine B, Christensen JR, Rising W, Denckla MB, Riddle MA. Anxiety after severe pediatric closed head injury. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(2):148–156. doi: 10.1097/00004583-200202000-00008. [DOI] [PubMed] [Google Scholar]
- Verger K, et al. Correlation of atrophy measures on MRI with neuropsychological sequelae in children and adolescents with traumatic brain injury. Brain Injury. 2001;15(3):211–221. doi: 10.1080/02699050010004059. [DOI] [PubMed] [Google Scholar]
- Wade SL, Carey J, Wolfe CR. The efficacy of an online cognitive–behavioral family intervention in improving child behavior and social competence following pediatric brain injury. Rehabilitation Psychology. 2006;51(3):179–189. [Google Scholar]
- Wade SL, Carey J, Wolfe CR. An online family intervention to reduce parental stress following pediatric brain injury. Journal of Counseling and Clinical Psychology. 2006b;74(3):445–454. doi: 10.1037/0022-006X.74.3.445. [DOI] [PubMed] [Google Scholar]
- Wade SL, Stancin T, Kirkwood M, Brown TM, McMullen KM, Taylor HG. Counselor-Assisted Problem Solving (CAPS) improves behavioral outcomes in older adolescents with complicated mild to severe TBI. Journal of Head Trauma rehabilitation. doi: 10.1097/HTR.0b013e31828f9fe8. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade SL, Wolfe C, Brown TM, Pestian JP. Putting the pieces together: Preliminary efficacy of a web-based family intervention for children with traumatic brain injury. Journal of Pediatric Psychology. 2005;30(5):437–442. doi: 10.1093/jpepsy/jsi067. [DOI] [PubMed] [Google Scholar]
- Wade SL, Walz NC, Carey J, Williams KM, Cass J, Herren L, Mark E, Yeates KO. A randomized trial of Teen Online Problem Solving for improving execution function deficits following pediatric traumatic brain injury. Journal of Head Trauma Rehabilitation. 2010;25(6):409–415. doi: 10.1097/HTR.0b013e3181fb900d. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Bigler ED, Dennis M, Gerhardt CA, Rubin KH, Stancin T, Taylor HG, Vannatta K. Social outcomes in childhood brain disorder: A heuristic integration of social neuroscience and developmental psychology. Psychological Bulletin. 2007;133(3):535–556. doi: 10.1037/0033-2909.133.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term neuropsychological outcomes after traumatic brain injury in children. Neuropsychology. 2002;16:514–523. doi: 10.1037//0894-4105.16.4.514. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Walz NC, Stancin T, Wade SL. The family environment as a moderator of psychosocial outcomes following traumatic brain injury in young children. Neuropsychology. 2010;24(3):345–356. doi: 10.1037/a0018387. [DOI] [PMC free article] [PubMed] [Google Scholar]


