Abstract
Purpose
Survey questions are commonly used to assess sleep duration because of their low-cost and convenience. Responses to these questions correlate moderately with objectively measured sleep duration in non-pregnant individuals, but little is known about the validity of self-reported sleep measures in pregnancy. The aim of the present study was to determine the extent to which self-reported gestational sleep duration assessed by questionnaire predicted objectively-measured gestational sleep duration via actigraphy.
Methods
We analyzed data from 80 mothers enrolled in an ancillary study of Project BABIES, a prospective cohort study of urban, pregnant women. Sleep measurements were collected in mid-pregnancy and included 7 days of wrist actigraphy, a sleep log, and survey questions about sleep time adapted from the Pittsburgh Sleep Quality Index.
Results
Mean measured gestational sleep duration derived from actigraphy was 6.87 hours (SD 0.87), and questionnaire-assessed nocturnal sleep time averaged 7.29 hours (SD 1.84). While the difference between measures did not reach statistical significance (p = 0.07 for paired samples t-test), over half (62%) of participants reported a habitual average nightly sleep time that differed more than one hour from their average actigraphically measured sleep duration (39% overestimated by more than an hour; 23% underestimated by more than an hour). There was no correlation between measures (r = 0.007, 95% confidence interval [CI]: −0.21, 0.23).
Conclusion
Questionnaire-derived reports of usual sleep hours do not reflect objectively measured sleep time in urban, pregnant women. Actigraphy is preferable to accurately assess gestational sleep duration.
Keywords: Sleep duration, Actigraphy, Pregnancy, Self-report, Objective
Introduction
Mounting experimental and epidemiologic evidence suggest that among non-pregnant adults and children, short sleep duration is an important and novel risk factor for chronic disease and early mortality [1, 2]. A growing number of investigators have also become interested in examining the influence of sleep deprivation on pregnancy-related health outcomes [3–11], as the majority of pregnant women (66% to 94%) report alterations in sleep, largely influenced by the dramatic hormonal, physiologic, and metabolic changes that accompany this period in a woman’s life course [12].
There are several methods for measuring sleep duration. Objective assessments include polysomnography (PSG) and wrist actigraphy. PSG is considered the “gold standard,” providing measures of both sleep and wake time, along with the classification of sleep stages [13]. However, PSG evaluations are labor and cost intensive, often requiring participants to sleep in a laboratory, limiting their usability in studies where the primary focus is solely quantifying sleep time. Wrist actigraphy is now an acceptable alternative objective method to assess sleep duration [14]. Using highly sensitive accelerometers, these devices measure gross motor activity, analyzed to identify sleep periods. When compared to PSG, wrist actigraphy demonstrates a correlation (r) of over 0.8 for sleep duration, including studies of pregnant women [15, 16]. Actigraphy is often preferable for objectively measuring sleep duration because it is unobtrusive, given its similarity to a wristwatch (but with a blank face), ambulatory, and can record for multiple days and nights at a much lower cost than PSG.
Despite the increasing availability and convenience of objective tools to assess sleep duration, the most common method of assessing sleep quantity in epidemiologic studies of pregnant women is through self-report – using one or two survey questions assessing habitual sleep time [3–7, 9–11, 17–19], such as “During the past month, how many hours of actual sleep do you get at night?” (adapted from the widely used Pittsburgh Sleep Quality Index [PSQI]) [20]. These subjective questions provide a cheaper and less burdensome alternative to objective measures. While responses to these questions typically correlate moderately with objectively measured sleep durations in healthy, non-pregnant individuals (r = 0.45) [21], little is known about the validity of self-reported survey assessments of sleep time in pregnancy.
Sleep often becomes increasingly disturbed as pregnancy progresses, characterized by more restless sleep, frequent nocturnal awakenings, and a decline in total sleep time that promotes intractable fatigue and sleepiness [22, 23]. Thus, pregnant women may be more prone to misreport actual time spent sleeping. Data in fact reveal that those individuals with disturbed sleep (e.g., patients with insomnia) have little agreement between objective and subjective reports of total sleep time [24]. If pregnant women behave similarly, there may be significant associations between self-reported pregnancy-related changes in sleep time and health that are not due to actual sleep duration, or alternatively, true associations may be hidden. A better understanding of the relationship between subjective and objective sleep measures is urgently needed as more investigators explore the implications of sleep duration in pregnancy.
The aim of the present study was to determine the extent to which self-reported sleep duration assessed by questionnaire predicted objectively-measured sleep duration via actigraphy in a cohort of urban, pregnant women. We hypothesized that self-reported sleep duration in pregnancy would be longer than objectively measured sleep time, with a lower correlation between the two measures than reported in non-pregnant populations.
Methods
Research subjects were participants in an ancillary study of Project BABIES, a prospective cohort study of urban, low-income pregnant women designed to examine the relationship between bacterial vaginosis and spontaneous preterm birth. Recruitment began in July 2008 at five university-affiliated outpatient prenatal care clinics in Philadelphia, as summarized previously [25]. Eligibility criteria included: < 16 weeks’ gestation at enrollment, singleton pregnancy, English or Spanish fluency, and current residence in Philadelphia. Ninety percent of women seen at the clinics consented to participate in Project BABIES, with a 5% refusal rate and a 5% missed rate. From July 2009 to September 2010, Project BABIES participants aged 18 or older were invited to take part in our ancillary sleep study; 101 of 139 (73%) agreed to do so. All participants provided written informed consent, and all procedures were in accordance with ethical standards for human experimentation. The institutional review board of Temple University approved the study protocol.
Sleep data were collected in mid-pregnancy (mean 21 weeks’ gestation, range 18 to 25 weeks). Participants were asked to wear an actigraph wristwatch (AW-64, Philips Respironics) continuously for seven days and nights. The actigraph device had an event marker that could be pressed to indicate specific times; participants were asked to press this marker when they got into bed at night and when they exited from bed each morning. Data were transferred via interface to a computer and then analyzed by two trained investigators (S.J.H. and K.W.M.) with the use of Actiware Software version 5.5 (Phillips Respironics). Over this same seven day period, participants were also asked to keep a daily sleep log, recording their sleep onset time and sleep end time. Sleep log records were used to aid in scoring the actigraph data when participants forgot to push their event markers. Questionnaires, administered at the completion of the objective sleep assessment, queried participants about their sleep time over the preceding month using questions adapted from the PSQI (“During the past month, on average, how many hours of actual sleep did you get at night? Hours per weekday? Hours per weekend day? This may be different than the number of hours you spent in bed”) [20].
To determine average nocturnal sleep duration for each participant, we first separately averaged nightly weekday (Sunday through Thursday) and weekend (Friday and Saturday) sleep times derived from actigraphy and questionnaire. Because we found statistically significant differences when comparing weekend and weekday sleep durations derived from actigraphy (p = 0.02), we created a single average nightly objective sleep duration variable to use in our analyses that was weighted by day of the week: 5/7*(average weekday sleep duration) + 2/7*(average weekend sleep duration). Using the same formula, we created a variable for average sleep time derived from questionnaire. A similar approach has been used in studies of non-pregnant persons [21].
We also queried participants about sociodemographics, including maternal age, race/ethnicity, parity, employment status, medical insurance (income proxy), and education. We obtained information from the prenatal medical record about delivery date and used mid-pregnancy ultrasound to calculate gestation length.
Statistical analysis
Participant characteristics were summarized by means and standard deviations (SD) for continuous variables, and counts and percentages for categorical variables. We examined differences in actigraph-measured and questionnaire-derived nocturnal sleep duration using paired t-tests; level of agreement was assessed using Pearson’s correlation coefficients. To assess for systematic bias, we constructed Bland-Altman plots that compared objective sleep time to the average difference between measures, treating objective sleep as the gold standard [21, 26]. We used SAS version 9.2 (SAS Institute, Cary, NC) to carry out all analyses.
Results
Of the 101 women enrolled in our ancillary study, we excluded participants from this analysis who had less than two weeknights and one weekend night of actigraphy data (n = 15), were missing questionnaire-derived sleep duration (n = 3), had implausible self-reports of surveyed sleep time (< 3 hours per night or > 20 hours per night, n = 2), or delivered twins (n = 1), leaving a final sample of 80 participants. The majority of women were African-American (84%), under 25 years of age (73%, range 18 years to 42 years), and multiparous (54%). One-third of participants did not complete high school (33%) and few were employed (28%). All participants received Medicaid.
On average, participants wore the actiwatch for 6 nights (range 4 to 7 nights), with nearly two-thirds (65%) wearing the watch for 7 full nights. Mean measured sleep duration derived from actigraphy was 6.87 hours (SD 0.87), and questionnaire-assessed nocturnal sleep time averaged 7.29 hours (SD 1.84). While the difference between measures did not reach statistical significance (p = 0.07 for paired samples t-test), over half (62%) of participants reported a habitual average nightly sleep time that differed more than one hour from their average actigraphically measured sleep duration (39% overestimated by more than an hour; 23% underestimated by more than an hour). There was no correlation between measures (r = 0.007, 95% confidence interval [CI]: −0.21, 0.23).
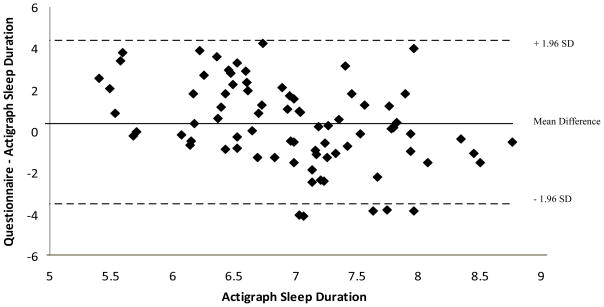
Examining the Bland-Altman plot (Figure 1) comparing questionnaire-derived and actigraph-measured sleep duration revealed a systematic bias towards overestimation among shorter sleepers and underestimation in longer sleepers. The mean difference between measures was 1.85 hours for mothers sleeping approximately 5 hours per night compared to −0.97 hours for mothers sleeping more than 8 hours per night (p < 0.0001).
Figure 1.
Bland-Altman plot comparing questionnaire-derived and actigraph-measured sleep duration in hours (using a criterion variable)
Discussion
Findings from this study revealed a considerable degree of incongruence between questionnaire-derived and actigraph-measured sleep duration in pregnancy. We found no correlation between measures. Further, the majority of women had over one hour discrepancy between self-report and actigraphically measured sleep time. Despite high demand for use of subjective sleep assessments in large epidemiologic studies of pregnant women, our data indicate that actigraphy is preferable to accurately assess gestational sleep duration, particularly among urban women.
Difficulty estimating sleep duration has been reported in several studies of non-pregnant women and men [21, 24, 27, 28]; however, our study is one of only a handful to describe the relationship between self-reported and objective measures of sleep duration among pregnant women, and the only study that includes an urban, low-income population. Moreover, it is the largest study to date in pregnant women. Bei et al. examined the relationship between sleep and mood in a sample of 44 healthy, low-risk, Australian pregnant and postpartum women using questionnaire and actigraphy [29]. Similar to our findings, linear correlation analyses examining total sleep time showed no significant correlation between subjective and objective measures, with self-reports longer than actigraphy. Tsai et al. also found that nocturnal sleep time was over-reported by questionnaire when compared to actigraphy among a sample of 38 well-educated, employed, pregnant Taiwanese women [30]. Further, a recent study comparing a single night of sleep in pregnancy using PSG and questionnaire among a sample of 49 pregnant Australian women found that the two measures were largely discordant in sleep time estimates, consistent with our findings [31]. While we hypothesized that self-reported sleep duration in mid-pregnancy would be longer than objectively measured sleep time, with a lower correlation between the two measures than reported in non-pregnant populations, our finding of zero correlation between measures is striking. One possible explanation is that pregnant women have greater difficulty estimating their nocturnal sleep time over 30-nights due to a large amount of variability in sleep, arising from the physical discomforts or hormonal changes that occur during pregnancy [12]. Because questionnaire assessments address more global perceptions of sleep, these assessments may be particularly flawed in pregnancy when nocturnal sleep duration varies over a short time period [22].
It is important to acknowledge that limitations to this study do exist. Our cohort was comprised of a relatively homogenous sample of low-income, urban mothers, and thus, our findings may not be generalizable to other populations of pregnant women. However, three other studies with very different populations than ours revealed similar findings [29–31]. We also may have introduced some error by comparing an average of 6 nights of actigraphy to questionnaire-derived sleep over 30 days. Because we asked women to self-report habitual sleep immediately after completing their actigraph assessment of sleep, we suspect that most women recalled their most recent nocturnal sleep time, which should have minimized bias. Additionally, the actigraphy measure itself could be a contributing factor to the bias we found, as we did not include an internal validation of actigraphy among pregnant women in our sample. However, sleep duration from actigraphy is well-correlated with PSG [15, 16], and studies examining bias have revealed that actigraphy more commonly overestimates sleep duration [32].
These findings have important implications for studies of sleep duration in diverse samples of pregnant women. Given the absence of a correlation between questionnaire-derived and actigraph-measured sleep time, and the fact that the majority of women in our sample had over one hour discrepancy between measures, relying on questionnaire-derived reports of sleep duration may lead to spurious associations in studies of sleep and health outcomes in pregnancy.
Acknowledgments
This study was supported by grants from the US National Institutes of Health (R01 HD038856) and Temple University Department of Medicine. Dr. Herring was additionally supported by grant K23 HL106231 from the US National Institutes of Health.
Footnotes
The study was conducted in Philadelphia at Temple University.
The authors have no conflicts of interest to disclose.
References
- 1.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Practice & Research Clinical Endocrinology & Metabolism. 2010;24:731–743. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, Stampfer MJ, Hu FB. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 3.Facco FL, Grobman WA, Kramer J, Ho KH, Zee PC. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. American Journal of Obstetrics & Gynecology. 2010;203:142.e141–145. doi: 10.1016/j.ajog.2010.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reutrakul S, Zaidi N, Wroblewski K, Kay HH, Ismail M, Ehrmann DA, Van Cauter E. Sleep disturbances and their relationship to glucose tolerance in pregnancy. Diabetes Care. 2011;34:2454–2457. doi: 10.2337/dc11-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu C, Enquobahrie D, Frederick IO, Abetew D, Williams MA. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: a pilot study. BMC Womens Health. 2010;14:17. doi: 10.1186/1472-6874-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams MA, Miller RS, Qiu C, Cripe SM, Gelaye B, Enquobahrie D. Associations of early pregnancy sleep duration with trimester-specific blood pressures and hypertensive disorders in pregnancy. Sleep. 2010;33:1363–1371. doi: 10.1093/sleep/33.10.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Micheli K, Komninos I, Bagkeris E, Roumeliotaki T, Koutis A, Kogevinas M, Chatzi L. Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology. 2011;22:738–744. doi: 10.1097/EDE.0b013e31822546fd. [DOI] [PubMed] [Google Scholar]
- 8.Lee KA, Gay CL. Sleep in late pregnancy predicts length of labor and type of delivery. American Journal of Obstetrics & Gynecology. 2004;191:2041–2046. doi: 10.1016/j.ajog.2004.05.086. [DOI] [PubMed] [Google Scholar]
- 9.Abeysena C, Jayawardana P. Sleep deprivation, physical activity and low income are risk factors for inadequate weight gain during pregnancy: a cohort study. Journal of Obstetrics & Gynaecology Research. 2011;37:734–740. doi: 10.1111/j.1447-0756.2010.01421.x. [DOI] [PubMed] [Google Scholar]
- 10.Zafarghandi N, Hadavand S, Davati A, Mohseni SM, Kimiaiimoghadam F, Torkestani F. The effects of sleep quality and duration in late pregnancy on labor and fetal outcome. Journal of Maternal-Fetal & Neonatal Medicine. 2012;25:535–537. doi: 10.3109/14767058.2011.600370. [DOI] [PubMed] [Google Scholar]
- 11.Ulman TF, Von Holle A, Torgersen L, Stoltenberg C, Reichborn-Kjennerud T, Bulik CM. Sleep Disturbances and Binge Eating Disorder Symptoms during and after Pregnancy. Sleep. 2012;35:1403–1411. doi: 10.5665/sleep.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;27:1405–1417. doi: 10.1093/sleep/27.7.1405. [DOI] [PubMed] [Google Scholar]
- 13.Sivertsen B, Omvik S, Havik OE, Pallesen S, Bjorvatn B, Nielsen GH, Straume S, Nordhus IH. A comparison of actigraphy and polysomnography in older adults treated for chronic primary insomnia. Sleep. 2006;29:1353–1358. doi: 10.1093/sleep/29.10.1353. [DOI] [PubMed] [Google Scholar]
- 14.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 15.Jean-Louis G, von Gizycki H, Zizi F, Spielman A, Hauri P, Taub H. The actigraph data analysis software: I. A novel approach to scoring and interpreting sleep-wake activity. Perceptual & Motor Skills. 1997;85:207–216. doi: 10.2466/pms.1997.85.1.207. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien LM, Bullough AS, Shelgikar AV, Chames MC, Armitage R, Chervin RD. Validation of Watch-PAT-200 Against Polysomnography During Pregnancy. Journal of Clinical Sleep Medicine. 2012;8:287–294. doi: 10.5664/jcsm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borodulin K, Evenson KR, Monda K, Wen F, Herring AH, Dole N. Physical activity and sleep among pregnant women. Paediatric and Perinatal Epidemiology. 2010;24:45–52. doi: 10.1111/j.1365-3016.2009.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams MA, Aurora SK, Frederick IO, Qiu C, Gelaye B, Cripe SM. Sleep duration, vital exhaustion and perceived stress among pregnant migraineurs and non-migraineurs. BMC Pregnancy & Childbirth. 2010;10:72. doi: 10.1186/1471-2393-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dørheim SK, Bjorvatn B, Eberhard-Gran M. Insomnia and depressive symptoms in late pregnancy: a population-based study. Behavioral Sleep Medicine. 2012;10:152–166. doi: 10.1080/15402002.2012.660588. [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KA, Zaffke ME, McEnany G. Parity and sleep patterns during and after pregnancy. Obstetrics & Gynecology. 2000;95:14–18. doi: 10.1016/s0029-7844(99)00486-x. [DOI] [PubMed] [Google Scholar]
- 23.Bourjeily G, Raker C, Chalhoub M, Miller M. Excessive daytime sleepiness in late pregnancy may not always be normal: results from a cross-sectional study. Sleep and Breathing. 2012 doi: 10.1007/s11325-012-0753-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Means MK, Edinger JD, Glenn DM, Fins AI. Accuracy of sleep perceptions among insomnia sufferers and normal sleepers. Sleep Medicine. 2003;4:285–296. doi: 10.1016/s1389-9457(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 25.Herring SJ, Nelson DB, Davey A, Klotz AA, Dibble LV, Oken E, Foster GD. Determinants of excessive gestational weight gain in urban, low-income women. Women’s Health Issues. 2012;22:e439–446. doi: 10.1016/j.whi.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krouwer JS. Why Bland-Altman plots should use X, not (Y+X)/2 when X is a reference method. Statistics in Medicine. 2008;27:778–780. doi: 10.1002/sim.3086. [DOI] [PubMed] [Google Scholar]
- 27.Van Den Berg JF, Van Rooij FJ, Vos H, Tulen JH, Hofman A, Miedema HM, Neven AK, Tiemeier H. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. Journal of Sleep Research. 2008;17:295–302. doi: 10.1111/j.1365-2869.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 28.Okifuji A, Hare BD. Nightly analyses of subjective and objective (actigraphy) measures of sleep in fibromyalgia syndrome: what accounts for the discrepancy? Clinical Journal of Pain. 2011;27:289–296. doi: 10.1097/AJP.0b013e31820485db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bei B, Milgrom J, Ericksen J, Trinder J. Subjective perception of sleep, but not its objective quality, is associated with immediate postpartum mood disturbances in healthy women. Sleep. 2010;33:531–538. doi: 10.1093/sleep/33.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai SY, Lin JW, Kuo LT, Thomas KA. Daily sleep and fatigue characteristics in nulliparous women during the third trimester of pregnancy. Sleep. 2012;35:257–262. doi: 10.5665/sleep.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson DL, Fung A, Walker SP, Barnes M. Subjective Reports Versus Objective Measurement of Sleep Latency and Sleep Duration in Pregnancy. Behavioral Sleep Medicine. 2012 doi: 10.1080/15402002.2012.670674. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.de Souza L, Benedito-Silva AA, Pires ML, Poyares D, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep. 2003;26:81–85. doi: 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]