Abstract
Tau gene has been consistently associated with the risk of Parkinson disease in recent genome wide association studies. Additionally, alterations of the levels of total tau, phosphorylated tau [181P], and amyloid beta 1–42 in cerebrospinal fluid have been reported in patients with sporadic Parkinson disease and asymptomatic carriers of leucine rich repeat kinase 2 mutations, in patterns that clearly differ from those typically described for patients with Alzheimer disease. To further determine the potential roles of these molecules in Parkinson disease pathogenesis and/or in tracking the disease progression, especially at early stages, the current study assessed all three proteins in 403 Parkinson disease patients enrolled in the DATATOP (Deprenyl and tocopherol antioxidative therapy of parkinsonism) placebo-controlled clinical trial, the largest cohort to date with cerebrospinal fluid samples collected longitudinally. These initially drug-naive patients at early disease stages were clinically evaluated, and cerebrospinal fluid was collected at baseline and then at endpoint, defined as the time at which symptomatic anti-Parkinson disease medications were determined to be required. General linear models were used to test for associations between baseline cerebrospinal fluid biomarker levels or their rates of change and changes in the Unified Parkinson Disease Rating Scale (total or part III motor score) over time. Robust associations among candidate markers are readily noted. Baseline levels of amyloid beta were weakly but negatively correlated with baseline Unified Parkinson Disease Rating Scale total scores. Baseline phosphorylated tau/total tau and phosphorylated tau/amyloid beta were significantly and negatively correlated with the rates of the Unified Parkinson Disease Rating Scale change. While medications (deprenyl and or tocopherol) did not appear to alter biomarkers appreciably, a weak but significant positive correlation between the rate of change in total tau or total tau/amyloid beta levels and the change of the Unified Parkinson Disease Rating Scale was observed. Notably, these correlations did not appear to be influenced by APOE genotype. These results are one of the very first pieces of evidence suggesting that tau and amyloid beta are critically involved in early Parkinson disease progression, potentially by a different mechanism than that in Alzheimer disease, although their applications as Parkinson disease progression markers will likely require the addition of other proteins.
Keywords: amyloid beta, APOE, cerebrospinal fluid, Longitudinal study, Parkinson disease progression, tau
Introduction
Parkinson disease (PD) is the second most common serious age-related neurodegenerative disease after Alzheimer disease (AD) and, although non-motor symptoms are often identified, devastating motor symptoms including rigidity, akinesia, and tremor are more pronounced clinical manifestations of the disease, especially at relatively early stages [19]. Recently, several genome wide association studies [10,51,59] have found that the MAPT gene (encoding tau protein) is consistently associated with the risk of sporadic PD. This discovery has spurred the field to investigate tau concentrations in human cerebrospinal fluid (CSF), which is in direct contact with brain tissue. We and others have reported that the CSF concentrations of total tau (t-tau) and phosphorylated tau (p-tau) are lower in PD patients compared to healthy controls [3,36,54]. This is quite different from AD cases, including prodromal AD, i.e., those with mild cognitive impairment (MCI), where both tau species are drastically increased in CSF [16,18,32,53]. Additionally, several independent investigations have reported that CSF concentrations of amyloid beta peptide 1–42 (Aβ42), a traditional marker for AD, are also lower, though to a lesser extent than in AD, in patients with PD [7,36,44,54,58]. More recently, in a cohort of early PD patients enrolled in the Parkinson’s Progression Markers Initiative (PPMI), decreased Aβ42, t-tau and p-tau were also observed [22]. Remarkably, even in asymptomatic carriers of PD-causing genetic mutations of leucine rich repeat kinase 2 (LRRK2), all three markers decreased, in concordance with a decline in striatal dopamine as assessed by positron emission tomography imaging [1].
Notably, however, association studies between the tau gene (MAPT) and CSF protein levels in patients with AD have produced inconsistent results. For example, significant association between CSF tau levels and a MAPT single-nucleotide polymorphism (SNP) rs242557 was reported in one study [27], while another study failed to detect this association, but instead found significant associations with other SNPs [23]. There is also evidence for the influence of a few other genes, APOE in particular (APOE genotype, specifically the ε4 allele, is an established risk factor for late onset AD [13]), on CSF t-tau, p-tau, and/or Aβ42 concentrations [25,46,53]. Whether these associations are applicable to PD cases, however, needs to be validated.
It should be emphasized that almost all of the PD studies discussed above have been carried out using cross-sectional methods. To further evaluate the roles of Aβ42, t-tau and p-tau in PD pathogenesis or PD severity or progression, in this study, we took advantage of a large cohort with longitudinally collected CSF samples. The DATATOP (deprenyl and tocopherol antioxidative therapy for Parkinsonism) study, initiated in 1987, was a multicenter, placebo-controlled, double-blind randomized clinical trial of a cohort of 800 patients with early, untreated PD [34,40,57]. Clinical information including Unified Parkinson Disease Rating Scale (UPDRS) total and motor scores and CSF from each patient were obtained at baseline and endpoint, defined as the time at which it was determined that the patient required levodopa therapy. To date, DATATOP is the largest PD cohort, with longitudinal CSF samples collected in a significant portion of the subjects, along with comprehensive clinical assessments. Given that the study was initiated about three decades ago, with many years follow up and multiple investigations published [20,24,34,40–43,56,57], the dataset has been extensively cleaned and most atypical parkinsonian cases (e.g., those with progressive supranuclear palsy or PSP and multiple system atrophy or MSA) have already been identified and can be excluded from the cohort. Another advantage of this cohort is that none of the cases were treated at baseline, i.e., potential drug effects on biomarkers can be excluded in the baseline measurements.
Materials and methods
Participants
The DATATOP study was designed to examine the impact of the monoamine oxidase type B inhibitor deprenyl (selegiline) (DEP) and the antioxidant α-tocopherol (vitamin E) (TOC) on the progression of disability in 800 patients with early PD (Hoehn and Yahr stage 1 or 2; none had any evidence of severe tremor or met the study criteria for significant dementia, defined as a Mini Mental State Examination [MMSE] score <23) who did not yet require anti-PD medication [20,34,39,40,57]. It was initially planned to follow subjects for 24 months using a 2 × 2 factorial design where participants were randomly assigned to placebo, DEP, TOC, or both DEP and TOC. The subjects were enrolled between September 1987 and November 1988 (“baseline”) and the inclusion and exclusion criteria have been published elsewhere [40]. The study’s primary outcome measure was the time until an “endpoint”, defined as the development of disability necessitating the introduction of levodopa therapy as judged by the blinded evaluating clinician, was reached. After an average 14 months of follow-up (“Period I”, original DATATOP), in the fall of 1989, preliminary analysis indicated unexpectedly striking effects of DEP in postponing PD disability [41]. After this disclosure, all active study subjects were placed on open-label DEP for about 18 months, from Fall 1989 to Spring 1991 (“Period II”). Blinded TOC treatment assignments were maintained for about 3 years after the initial randomization. Clinical data (e.g., UPDRS) and CSF samples were collected at the baseline and final (endpoint or the end of Period II, after 1–2 month washout) time points.
In this study, we excluded 110 patients whose follow-up time was less than 6 months (including 76 who reached endpoint within 6 months and 34 who withdrew), 45 whose PD diagnoses were unlikely to be correct, 30 who withdrew after 6 months, and 212 who had missing CSF or UPDRS at the baseline (63) or final (189) time point. The 6-month cut-off was applied mainly because, in chronically progressive diseases, sufficient time is needed for changes in biochemical markers to be appreciated. The demographic information at baseline of the remaining 403 subjects included in this study can be found in Table 1.
Table 1.
Demographics and CSF marker values at baseline
| Number of cases | 403 |
|---|---|
| Sex, F/M (% of male) | 142/261 (54.4) |
| Age, yr | |
| Mean ± SD | 60.93 ± 9.18 |
| Range | 34 – 79 |
| Duration of disease, year | |
| Mean ± SD | 2.04 ± 1.40 |
| Range | 0 – 7 |
| Baseline MMSE | |
| Mean ± SD | 28.87 ± 1.45 |
| Range | 23 – 30 |
| Baseline UPDRS total | |
| Mean ± SD | 24.34 ± 11.72 |
| Range | 0 – 63 |
| Baseline UPDRS motor | |
| Mean ± SD | 16.31 ± 8.81 |
| Range | 0 – 50 |
| Baseline Hoehn and Yahr | |
| Mean ± SD | 1.6 ± 0.5 |
| Range | 1 – 3 |
| Baseline CSF Aβ42 (pg/mL) | |
| Mean ± SD | 236.14 ± 74.99 |
| Range | 52.14 – 670.08 |
| Baseline CSF t-tau (pg/mL) | |
| Mean ± SD | 47.03 ± 25.50 |
| Range | 11 – 214 |
| Baseline CSF p-tau (pg/mL) | |
| Mean ± SD | 23.34 ± 11.89 |
| Range | 3.99 – 93.11 |
| Baseline CSF p-tau/t-tau | |
| Mean ± SD | 0.54 ± 0.21 |
| Range | 0.14 – 1.06 |
| Baseline CSF t-tau/Aβ42 | |
| Mean ± SD | 0.21 ± 0.12 |
| Range | 0.08 – 1.21 |
| Baseline CSF p-tau/Aβ42 | |
| Mean ± SD | 0.11 ± 0.06 |
| Range | 0.02 – 0.55 |
Aβ42, amyloid beta peptide 1–42; CSF, cerebrospinal fluid; MMSE, Mini Mental State Examination; p-tau, phosphorylated tau; t-tau, total tau; UPDRS, Unified Parkinson Disease Rating Scale.
CSF specimen collection, storage, and quality control
The CSF collection protocol for the DATATOP subjects was described elsewhere [42]. Briefly, the lumbar puncture was carried out in the decubitus position between 6 and 10 AM, and CSF was collected sequentially in measured aliquots and then immediately placed on ice before freezing at −70 °C. Polypropylene tubes (Sarstedt. Nümbrecht, Germany) were used for CSF collection and sample storage; all the sites involved in the study used the same collection supplies. The CSF samples were only thawed immediately before the Luminex assay. An aliquot was also taken to measure hemoglobin levels (as an index of the degree of red blood cell contamination of CSF) as previously described [17]. Note that although the DATATOP CSF samples were frozen for more than two decades, we found that the concentrations of CSF markers (such as Aβ and tau) in these samples were comparable with those in the CSF samples collected recently [54], which is in line with previous findings by others [4,61].
Luminex assays
CSF Aβ42, t-tau, and p-tau[181P] (p-tau) levels were measured using the INNO-BIA AlzBio3 kit obtained from Innogenetics (Gent, Belgium) following the manufacturer’s instructions as described previously [54]. All CSF samples were analyzed using a LiquiChip Luminex 200™ Workstation (Qiagen, Valencia, CA, USA). The assay platform and quality control procedures have been utilized in our laboratory extensively and described in previous publications [1,17,54].
Statistical analysis
All analyses were performed with SPSS Statistics 20.0 (IBM, Chicago, IL, USA). Six CSF markers were analyzed: Aβ42, t-tau, p-tau, plus the ratios p-tau/t-tau, t-tau/Aβ42, and p-tau/Aβ42. Except for Aβ42 and p-tau/t-tau (which were normally distributed), all other CSF markers were log(10) transformed to obtain normal distribution. Group differences between males and females were assessed using Mann-Whitney U test; all other between-group differences were tested by using ANOVA or ANCOVA controlling for covariates. Potential confounding variables were assessed by performing bivariate analysis (p<0.15 was used as a cutoff for selection). Pearson’s correlation was employed to analyze the age dependence of CSF markers. All other correlations were analyzed by using general linear models (GLM). Age and sex were used as covariates for correlations between baseline parameters. For correlations related to prediction and progression analyses, rate of clinical score (UPDRS) change (average change per month) between baseline and final time points was tested as a dependent variable, controlling for treatment group, baseline UPDRS and MMSE scores in addition to age and sex of the subject. Kaplan-Meier survival and Cox proportional hazards regression tests were also used to test for associations between baseline CSF biomarkers and the time needed to reach endpoint. All analyses were conducted at a 2-sided α=0.05 significance level; however, multiple comparisons need to be addressed since six markers are being analyzed, hence a p-value of >0.008 (uncorrected, i.e., 0.05/6; Bonferroni correction) should be interpreted with caution.
Results
Effects of age, sex, and cross-sectional analyses at baseline and final time points
Table 1 lists the demographic information and summary of CSF biomarker concentrations at baseline. As is typical for most published PD cohorts, there were more males than females in the DATATOP study, but age and other parameters including UPDRS scores are not significantly different between males and females (Mann-Whitney U test, p>0.05). The cohort overall demonstrated significant progression from baseline (UPDRS total [mean ± SD], 24.34 ± 11.72; motor, 16.31 ± 8.81) to final time point (UPDRS total, 39.91 ± 15.69; motor, 26.84 ± 11.46). The effects of age and sex of the subjects on the CSF protein concentrations (Aβ42, t-tau, and p-tau) and p-tau/tau, t-tau/Aβ42, and p-tau/Aβ42 ratios (the last two are often used in AD diagnosis) were first evaluated using the baseline samples from all 403 patients included in this study. Consistent with previous studies in patients with PD and healthy controls by us and others [7,35,54,60], CSF t-tau and p-tau were significantly correlated with age, but Aβ42 was not (Supplementary Fig. 1). Although all CSF marker concentrations increased as a function of age, the p-tau/t-tau ratio was lower in older patients. Additionally, differences between males and females in p-tau or its combinations with other markers were identified (Mann-Whitney U test: p-tau, p=0.027; p-tau/t-tau, p=0.036, p-tau/Aβ42, p=0.024). Similar to what we reported previously in a cross-sectional study [54], the baseline CSF Aβ42 (p=0.088), t-tau (p=0.125) and p-tau (p=0.389) concentrations did not correlate with CSF hemoglobin levels, suggesting the effects of potential blood contamination in CSF on these markers were minimal. Additionally, it appears that the effects of APOE genotype (available in 279 subjects) on the baseline levels of these CSF markers were also minimal, except that the CSF Aβ42 levels were slightly lower in subjects with at least one ε4 allele than in those without (Supplementary Table 1). These observations were largely confirmed when analyzing CSF marker concentrations at the final time point. Given the age- and sex-dependence differences in CSF markers, age and sex were controlled as covariates in further analyses.
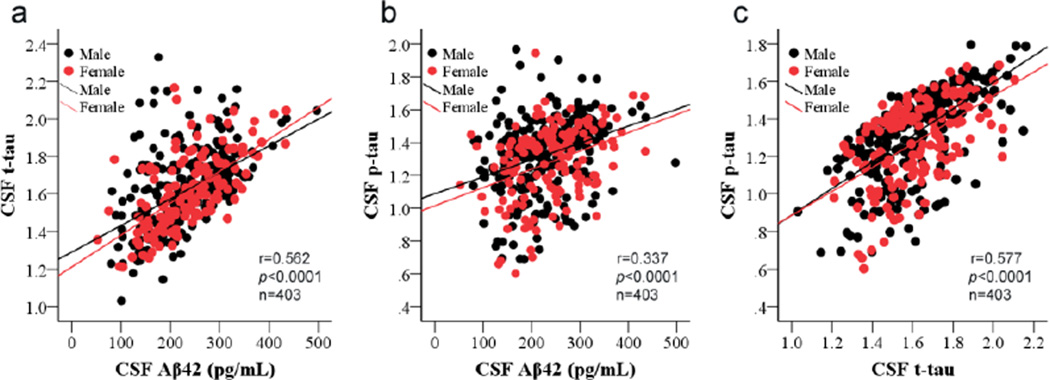
The correlations between CSF marker levels at baseline and final time points were analyzed using GLM. Remarkably, all baseline CSF marker levels were positively correlated with each other when controlling for age and sex of subjects (Aβ42 vs. t-tau, r= 0.562, p <0.0001; Aβ42 vs. p-tau, r=0.337, p <0.0001; t-tau vs. p-tau, r=0.577, p <0.0001) (Fig. 1). For example, lower CSF Aβ42 concentrations in a given subject are typically associated with lower t-tau and p-tau concentrations. Similar correlations were also observed between the CSF marker concentrations at the final time point (data not shown). Note that there are only 17 subjects (4%) at baseline and 29 (7%) at final time point had abnormally elevated p-tau/Aβ42 (using an upper cutoff value defined previously [36]), which is comparable to typical age-matched control populations reported in various investigations [12,29,36]. In other words, there were no apparent molecular CSF signatures of Alzheimer’s changes in this population during two years of investigation.
Figure 1. Correlations of CSF Aβ42, t-tau, and p-tau levels at baseline.
CSF amyloid beta 1–42 (Aβ42), total tau (t-tau) and phosphorylated tau [181P] (p-tau) concentrations were measured in 403 DATATOP subjects at the baseline using Luminex assays. CSF t-tau and p-tau were log transformed due to non-normal distribution. (a) CSF Aβ42 and t-tau (r=0.562, p<0.0001); (b) CSF Aβ42 and p-tau (r=0.337, p<0.0001); (c) CSF t-tau and p-tau (r=0.577, p<0.0001).
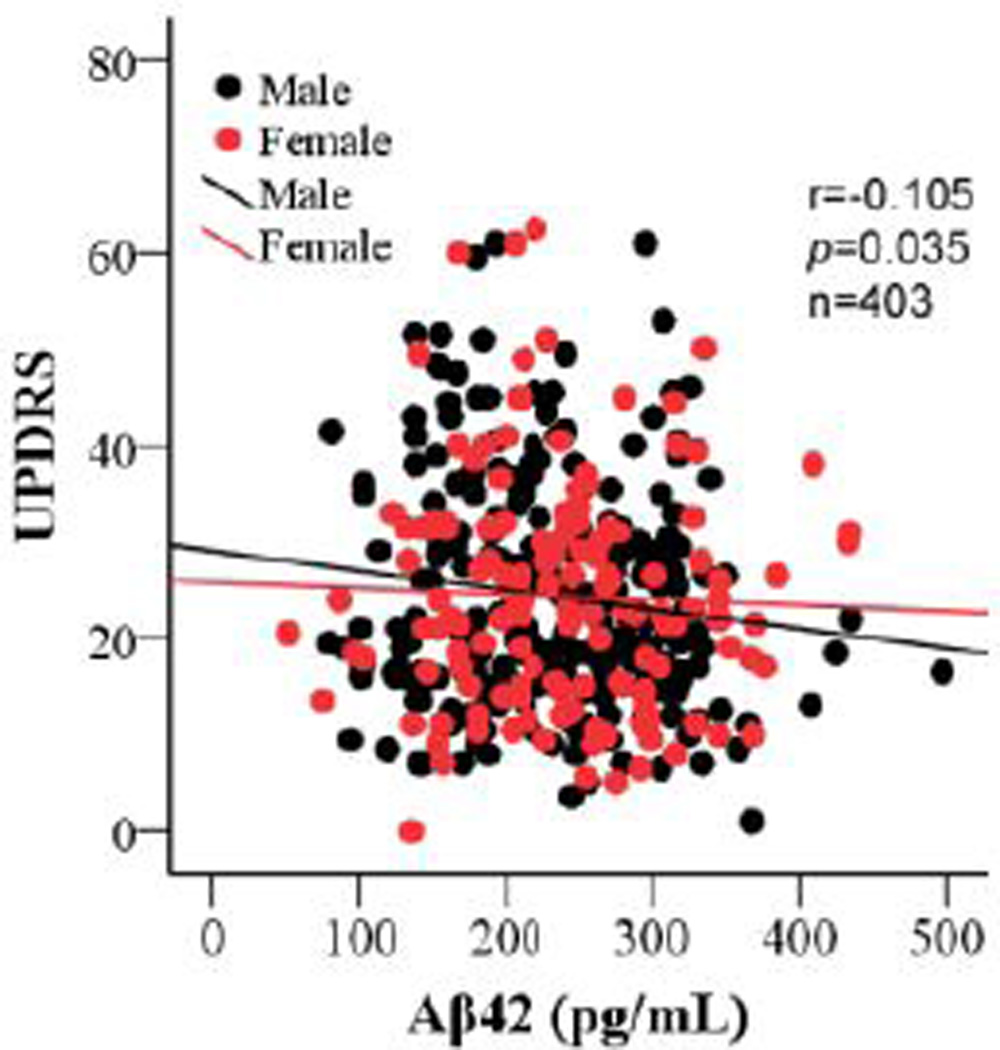
To determine if CSF concentrations of Aβ42, t-tau, p-tau, p-tau/t-tau, t-tau/Aβ42, and p-tau/Aβ42 are related to disease severity, correlation was analyzed between baseline concentrations of the CSF markers and baseline UPDRS scores or duration of disease. We found no associations except for Aβ42 concentration, which weakly and negatively correlated with total UPDRS score at baseline when controlling for age and sex of the subjects (Fig. 2; r= −0.105, p=0.035), i.e., Aβ42 tended to be lower when UPDRS was higher. The significance of the correlation was not notably changed when APOE ε4 carriers were compared to noncarriers, i.e., no substantial effects of APOE ε4 status were observed. Intriguingly, no correlations were observed between any of the CSF marker concentrations, including Aβ42, and UPDRS scores at the final time point (p>0.05). There were also no correlations between any of the CSF markers and the MMSE score (index of general cognitive impairment) at baseline (p>0.1).
Figure 2. Correlations of CSF Aβ42 with UPDRS total score at baseline.
The baseline CSF amyloid beta 1–42 (Aβ42) concentrations correlated with the baseline UPDRS total scores when controlling for age and sex of the subjects (r=−0.105, p=0.035).
Prediction of disease progression using baseline CSF marker levels
The nature of this longitudinal study allows us to determine whether baseline CSF markers are predictive of PD progression in terms of the change in UPDRS score from baseline to final time points. Because all subjects who didn’t reach endpoint by the end of the first period (original DATATOP) were treated with DEP in the second period, this cohort closely mimics a so-called “two-period, delayed-start” model that is being actively employed in clinical trials currently for PD and other neurodegenerative disorders [38,39,43,49]. Note that those subjects who reached endpoint by the end of the first period (before the “delayed start”) were considered as failed subjects but still a part of such model, and were thus included in the analyses in this study.
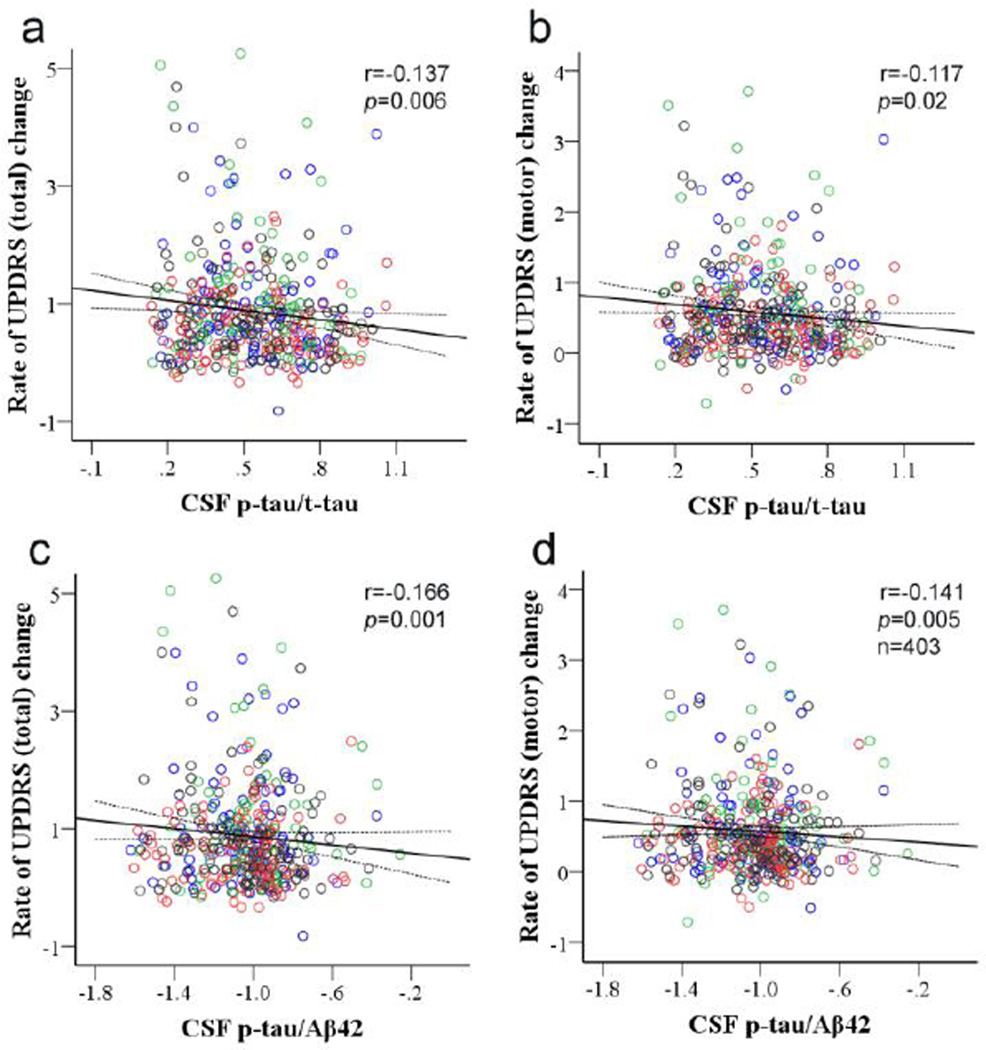
When controlling for baseline UPDRS score, treatment group, length of time exposed to DEP, age and sex of the subject, analyses performed using GLM indicated that the baseline CSF p-tau/t-tau significantly correlated with rate of change between baseline and final time points of total (Fig. 3a; r=−0.137, p=0.006) and motor UPDRS (Fig. 3b; r=−0.117, p=0.02). The correlations were improved slightly or remained the same when baseline MMSE score was added to or baseline UPDRS score was removed from the model (Supplementary Table 2). Similarly, the baseline p-tau/Aβ42 ratio was significantly correlated with the rate of total (r=−0.166, p=0.001) and motor UPDRS changes (r=−0.141, p=0.005) (Fig. 3c and 3d). These results were also not altered appreciably with or without controlling for baseline UPDRS and baseline MMSE scores (Supplementary Table 2). The correlation of p-tau/t-tau and p-tau/Aβ42 with total UPDRS remained statistically significant even after adjusting for multiple comparisons (Bonferroni correction). Furthermore, the significance of the correlations was not influenced by APOE genotype (ε4 status).
Figure 3. Correlation of baseline CSF p-tau/t-tau or p-tau/Aβ42 and rate of UPDRS changes between baseline and final time points.
CSF amyloid beta 1–42 (Aβ42), total tau (t-tau) and phosphorylated tau [181P] (p-tau) concentrations were measured in 403 DATATOP subjects using Luminex assays. CSF p-tau/Aβ42 was log transformed due to non-normal distribution. The baseline p-tau/t-tau significantly correlated with the rate of total (a; r= −0.137, p=0.006) or motor (b; r= −0.117, p=0.02) UPDRS change (average change per month) between baseline and final time points. Similar correlations were also observed between the baseline p-tau/Aβ42 and the rate of total (c; r=−0.166, p=0.001) or motor (d; r=−0.141, p=0.005) UPDRS change. Subjects in the placebo group are marked in blue, subjects in the deprenyl (DEP)-treated group are in red, subjects in the α-tocopherol (TOC)-treated group are in green, and subjected treated with both drugs (TOC/DEP) are in black. The treatments did not show significant effects on the correlations.
As mentioned, in the DATATOP study, the endpoint is defined as the time at which “the patient developed sufficient disability to require dopaminergic therapy”. As a secondary analysis, the effects of the baseline CSF marker values on the time needed to reach endpoint were also tested using a survival time analysis, with or without controlling for age and sex of the subject, baseline UPDRS total score, and treatment group. Only the baseline CSF p-tau/t-tau (p=0.013) or Aβ42 (p=0.082) showed borderline effects on the time taken to reach endpoint, but the significance was lost after controlling for covariates (p-tau/t-tau, p=0.635; Aβ42, p=0.324).
Tracking of disease progression using rate of CSF marker changes
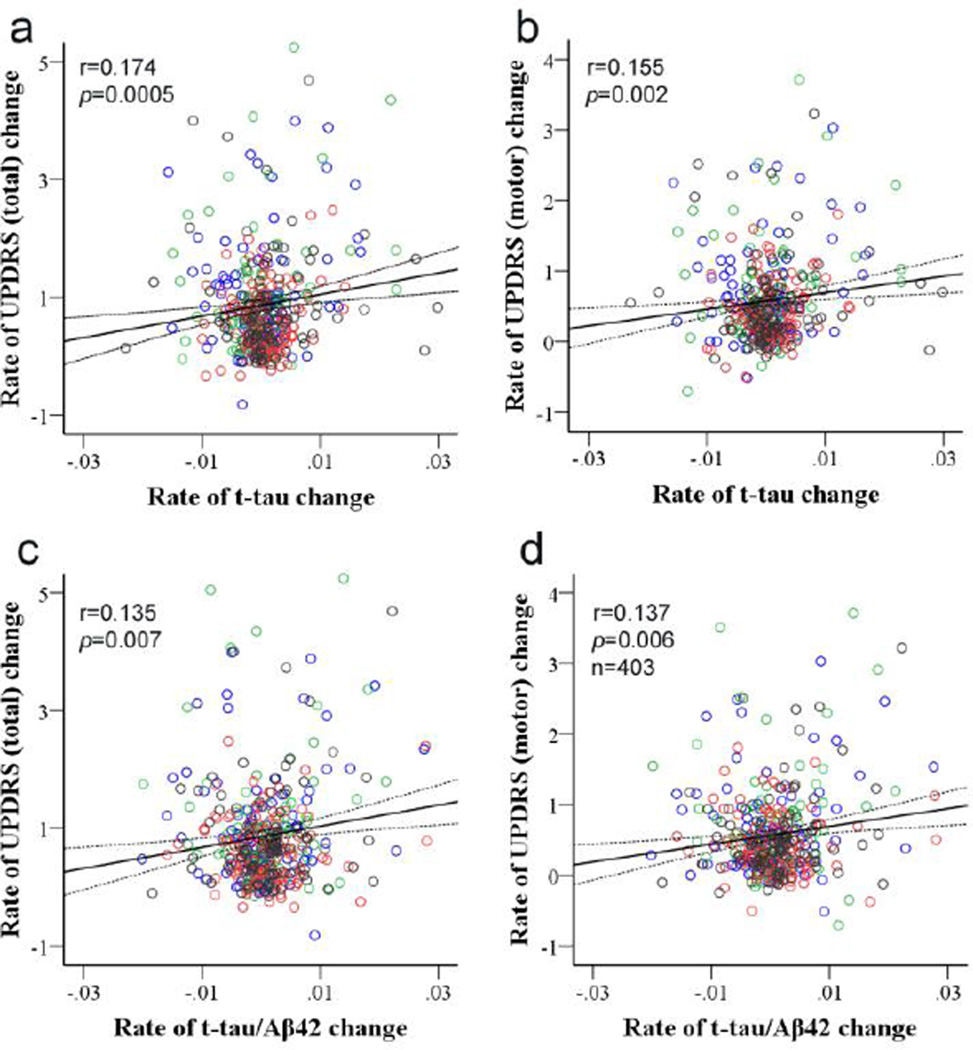
We next investigated whether the change in CSF markers corresponded to changes in UPDRS scores. First, we assessed whether different treatments had an effect on observed alterations in biomarker levels compared to placebo. As shown in Supplementary Fig. 2, there were no significant differences in the rate of change for any of the CSF markers investigated among the treatment groups (ANOVA, p>0.05). Nevertheless, using GLM analysis, we considered length of time exposed to DEP as a covariate, in addition to baseline UPDRS score, treatment group, and age and sex of the subject. The results demonstrate that the rate of change of t-tau was significantly correlated with the rate of change in UPDRS total (r=0.174, p=0.0005) or motor scores (r=0.155, p=0.002) (Fig. 4a and 4b). Significant correlations were also observed for the t-tau/Aβ42 ratio (UPDRS total, r=0.135, p=0.007; motor, r=0.137, p=0.006) (Fig. 4c and 4d), with or without adjusting for multiple comparisons. The significances of these correlations were largely the same with or without controlling for baseline UPDRS and MMSE scores (Supplementary Table 2). Additionally, the correlations did not differ by APOE ε4 status.
Figure 4. Correlation of rate of CSF t-tau or t-tau/Aβ42 changes and rate of UPDRS changes between baseline and final time points.
CSF amyloid beta 1–42 (Aβ42), total tau (t-tau) and phosphorylated tau [181P] (p-tau) concentrations and UPDRS total and motor scores were measured in 403 DATATOP subjects at baseline and final time points. CSF t-tau and t-tau/Aβ42 were log transformed due to non-normal distribution. The rate of change (average change per month) in CSF t-tau significantly correlated with the rate of total (a; r=0.174, p=0.0005) or motor (b; r=0.155, p=0.002) UPDRS change. Similar correlations were also observed between the change in CSF t-tau/Aβ42 and the change in total (c; r=0.135, p=0.007) or motor (d; r=0.137, p=0.006) UPDRS. Subjects in the placebo group are marked in blue, subjects in the deprenyl (DEP)-treated group are in red, subjects in the α-tocopherol (TOC)-treated group are in green, and subjected treated with both drugs (TOC/DEP) are in black. The treatments did not show significant effects on the correlations.
Discussion
This is the first description of the relationship between CSF biomarkers Aβ42, t-tau, and p-tau and PD severity and progression in the largest cohort of PD patients studied. Using GLM models and controlling for age, sex and other potential confounding factors, several significant observations are made. Specifically, all baseline CSF marker levels were strongly correlated with each other, and the levels of Aβ42 were slightly lower in subjects with higher total UPDRS score at baseline, but not at the final point. In addition, the baseline CSF p-tau/t-tau and p-tau/Aβ42 ratio notably correlated with the change in UPDRS total and motor scores. Furthermore, the rate of change in both t-tau and t-tau/Aβ42 ratio significantly, though weakly, paralleled the change in UPDRS scores, suggesting that longitudinal alterations in these CSF biomarkers might be used to assist monitoring disease progression, in addition to indicating the participation of these key proteins in PD pathogenesis. Finally, it is clear that none of the medications substantially affected the CSF biomarkers.
Cross-sectional correlations among CSF markers
The baseline concentrations of Aβ42, t-tau, and p-tau in CSF strongly correlated with each other in this study (Fig. 1). In the AD field, the correlation between CSF p-tau and t-tau is well known, and the importance of tau in mediating Aβ toxicity and the involvement of Aβ in tau phosphorylation and proteolytic cleavage have also been demonstrated experimentally [62,66]. Similarly, in PD, recent studies have reported that Aβ, tau, and α-synuclein might act synergistically to accelerate the accumulation and aggregation of each other and promote cognitive decline in transgenic mice [6], suggesting that Aβ and tau may indeed interact with each other in PD patients. However, the interactions between Aβ and tau are likely different in PD compared to AD, given that the profiles of these CSF proteins are quite different in these two diseases (see further discussion below). Furthermore, although tau and Aβ could be secreted from cells by the same mechanisms (e.g., exosomes) [48,50], potentially contributing to the correlation of their CSF concentrations, it would be very challenging to argue that higher tau in AD CSF and lower tau in PD CSF result from the same mechanism(s). In other words, it is more than likely that tau secretion or clearance in CSF is regulated differently in PD, even if the same pathway is involved in both AD and PD.
Cross-sectional correlation between Aβ42 and UPDRS total
A weak negative correlation between baseline levels of CSF Aβ42 and UPDRS total was observed in this study (Fig. 2), suggesting that lower Aβ42 may be associated with more severe disease (higher UPDRS). This is also indicated in our Kaplan-Meier survival analysis, where lower CSF Aβ42 was weakly associated with shorter time to reach endpoint in this study (data not shown). Additionally, we as well as others have demonstrated that CSF Aβ42 is significantly lower in PD compared to controls [7,36,44,54]. Changes in the levels of CSF Aβ42 are not well understood, but their decreases observed in AD are conventionally thought to arise by the aggregation and deposition of Aβ42 in neuritic plaques or trapped in brain parenchyma as soluble or insoluble oligomers [15,37,55]. This could also be true in PD patients because neuritic and diffuse plaques are occasionally observed in PD, although most reported results have been obtained in autopsy cases where aging is a major confounder, and Aβ plaques are often found in aged individuals without clinical dementia [8,63]. Nonetheless, this potential Aβ deposition and the interaction between Aβ, tau, and α-synuclein in PD brain and their possible contribution to the development of PD, particularly the motor symptoms, remain largely hypothetical. On the other hand, low baseline CSF levels of Aβ42 are reported to be strongly associated with a greater likelihood for cognitive decline in PD [58]. Recent imaging studies also suggest that neocortical Pittsburgh compound B binding correlated robustly with measures of cognitive impairment in subjects with PD at risk for dementia, though elevated cerebral Aβ-amyloid deposition at levels seen in AD is uncommon [14,21,45]. Because cognitive impairment is observed in most advanced PD cases, our results demonstrating a weak correlation between baseline CSF levels of Aβ42 and total UPDRS scores may be attributable, at least in part, to PD patients who have developed subtle cognitive impairment at early stages or are at risk to develop cognitive impairment later (the DATATOP cohort consists of PD patients who are at early stages without apparent dementia). That said, the weak correlation between Aβ42 and UPDRS observed at baseline was lost at final assessment when most subjects had already been treated with DEP, which might still have a subtle but important effect on UPDRS scores after the washout period. To state it differently, studies in subjects without any medications are needed to further investigate the roles of Aβ42 in PD. Another potential limitation is that direct evidence of the stability of CSF Aβ42 after long freezing periods (>20 years) is still lacking, so the results need to validated in a recently collected sample set (e.g., PPMI [31]).
Predictive values of baseline p-tau/tau and p-tau/Aβ42
PD is a well-known synucleinopathy. However, postmortem studies have also identified tau pathology with p-tau changes in PD brains, though, unlike the global tau pathology observed in AD, PD related tau pathology was more restricted to the striatum [65]. Tau is a microtubule-associated protein and hyperphosphorylated tau is a known component of the neurofibrillary tangles in AD pathology [5]. In PD, a few studies have also shown that the status of tau phosphorylation as well as stability of microtubules can also be affected (e.g., by α-synuclein) [11,47]. It is known that pathological hyperphosphorylation of the tubulin-binding domains on tau prevents tubulin binding and thereby results in the destabilization of microtubules [9,33], but the role of tau phosphorylation on the flanking areas (e.g., T181P, the species measured in the current investigation) is largely unknown. Given that higher p-tau/tau and p-tau/Aβ42 predict slower progression, our results seem to suggest that increasing p-tau [181P] (as a proportion of total tau and/or with accompanying decrease of Aβ42) might be a beneficial or protective change secondary to primary PD pathology. These results, obtained in a sizable un-medicated cohort, might be the first piece of evidence to suggest that tau species are involved in PD progression even at relatively early stages.
Tracking PD progression with longitudinal changes of tau and t-tau/Aβ42
One of the most intriguing results of the current study is the observation that an increase in t-tau and t-tau/Aβ42, i.e., a phenomenon largely driven by t-tau, from baseline to final time points significantly corresponds to a faster rate of change in UPDRS scores. The observation is counterintuitive because, as previously indicated, the CSF levels of tau species were lower in patients with PD compared to healthy controls in most well controlled studies where a minimum of 50 cases or controls are included [3,36,54]. Thus, one would expect PD progression to be associated with a further decrease, rather than an increase, in tau. In AD, the significantly higher CSF tau levels compared to controls have been assumed to be secondary to neuronal damage and cell death (i.e., tau being released from damaged cells), but this explanation would lead to the prediction that all neurodegenerative disorders featuring prominent neuronal cell death are associated with higher CSF tau levels, which has clearly not been supported, and thus the underlying mechanisms still remain to be investigated. For PD, a recent study demonstrated that soluble tau levels decreased in the substantia nigra in human PD brain and the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model, and loss of tau caused iron retention and facilitated neurodegeneration in mice [28]. This is in line with other studies reporting that overexpression of wild type tau exhibited protective effects on mitochondrial functions in cells [52]. While decreased tau in PD tissue is consistent with human CSF cross-sectional data (i.e., CSF tau levels might reflect the soluble tau levels in cells), the trend of increasing CSF tau in faster progressing patients in the DATATOP study cannot be explained readily by this mechanism. One potential testable hypothesis could be that, in contrast to AD pathology, pathological processes associated with PD promote reactive change(s) that results in a decrease in CSF tau (for example, tau being actively retained in cells to maintain iron clearance or other critical cellular mechanisms), and with gradual loss of this protective mechanism as PD progresses, tau increases in CSF. That said, a direct correlation of CSF and brain levels of tau cannot be assumed, and disease stages of the DATATOP cohort (purposely selected to be at early disease stages) and those obtained at autopsy may be quite different. Nonetheless, our results suggest that CSF t-tau may serve as a progression marker for PD patients in early disease stages as typically included in the DATATOP study.
The DEP and TOC treatments did not show significant effects on the changes of CSF markers investigated in this study (Supplementary Fig. 2) or affect the predictive or tracking ability of these CSF markers in relationship to the rate of UPDRS changes (data not shown). Nevertheless, these results do not necessarily mean that DEP and or TOC have no effects on other CSF markers, such as α-synuclein and DJ-1, or that they have no protective effects against PD progression. Another important caveat of this study is that the rates are calculated from only two time points (baseline and final) and a linear rate is therefore assumed. However, changes in biomarker level and UPDRS scores are not necessarily linear. Without data points between baseline to final, we cannot know the nature of the curve of progression, and therefore approximations of the rates of change are made using a linear model.
It should also be emphasized that, while the correlations between CSF markers and UPDRS changes identified in this study are statistically impressive, i.e., these disease related proteins are likely to be important in biological PD progression, the rate versus rate correlations should be interpreted with caution because these correlations are between indices that have a common component (study period) [26]. Additionally, it is clear that the correlations between any of the markers assessed with UPDRS scores, total or motor, are not robust enough to be practically useful in a clinical setting, at least in the time frame assessed (about two years). On the other hand, as discussed in several previous studies [54,64], there are several reasons why the correlation between the clinical evaluation (UPDRS) and a CSF marker may not be strong. First, the relationship between worsening UPDRS scores and progressive degeneration of the nigrostriatal system and other brain regions is unlikely to be linear because of compensatory increases in the levels of dopaminergic terminals or D-2 receptors per cell in surviving cells when nigrostriatal dopaminergic neurons degenerate in the basal ganglia. This is indeed one of the reasons that certain biochemical markers may more accurately reflect nigrostriatal degeneration. In the same line of argument, while the DEP treatment is known to mask PD symptoms (UPDRS scores) [41,57], it did not affect CSF markers investigated in this study. Note that although the final UPDRS measurements and CSF samples were taken after a washout period to minimize DEP masking effects, it is unclear how long the DEP effects last. Finally, a significant amount of pathology in PD affects brain areas other than the nigrostriatal system as PD advances [19]. The clinical evaluation, particularly the UPDRS motor score, best reflects disease progression in the nigrostriatal dopaminergic system, while the CSF biomarkers are usually used as indices of pathology in the whole brain. In other words, in the absence of a “Gold Standard” of pathological progression of PD, it is likely to be difficult to discover CSF progression markers that are robustly correlated with UPDRS scores that are predominantly a measure of progression of movement disabilities.
Finally, it should be noted that the DATATOP trial was not set up for tracking the natural history of disease progression and CSF protein marker changes and thus has inherent limitations. However, using a large human cohort (the largest to date), our data are likely some of the first evidence to suggest that tau is involved in PD development and progression, even in the early disease stages at which DATATOP subjects were enrolled. Indeed, tau has been listed as one of the Tier 1 markers (along with α-synuclein) in the currently active large prospective PPMI study [31]. In other words, our results will certainly help other investigators to interpret the data to be gathered in the PPMI cohort (a validation, not discovery, cohort as designed) in future studies. Another limitation is that very limited data is currently available to describe the status of known PD-related gene mutations in DATATOP subjects. However, a few observations strongly suggest that the DATATOP cohort is largely a sporadic cohort; these include: 1) PD-causing gene mutations are usually rather rare in typical clinical cohorts like the DATATOP; 2) in DATATOP subjects, the frequencies of some relatively common gene mutations (e.g., LRRK2 G2019S and Y1699C) are almost identical to those reported previously in Western European “sporadic PD” cohorts [30]; and 3) among the 403 subjects included in the current study, there are only 62 early-onset patients (age at onset <51) - a rate that is not very different from those reported in regular PD clinics. That said, there is data suggesting that in such early-onset patients, a population that may have a higher proportion of familial PD compared to the general PD population, 16.6% of them may carry one of the known PD-related mutations [2]. Thus, without complete genotyping data, we cannot exclude the possibility that the presence of certain gene mutations, though rare in the cohort, may further confound the results.
Taken together, in this investigation, using the largest longitudinal PD cohort, CSF biomarkers Aβ42, t-tau, and p-tau were evaluated as a function of PD severity and progression. The main results are higher baseline CSF p-tau/t-tau and p-tau/Aβ42 associated with slower rates of change in UPDRS total and motor scores. Additionally, increased changes in t-tau and t-tau/Aβ42 ratio tracked faster disease progression estimated by UPDRS scores, suggesting that at least tau may play important mechanistic roles in early PD progression and longitudinal alterations in these CSF biomarkers might contribute to assist monitoring disease progression independently. These results, if validated in another large longitudinal cohort, could have a profound impact on the current understanding of PD pathogenesis and novel PD therapies. On the other hand, the changes in all biochemical markers measured over two years of period, though statistically significant (and likely pathologically important), are relatively small, indicating that CSF tau and Aβ species will have to be used in combination with other markers to clinically assess PD progression.
Supplementary Material
Acknowledgements
This work was supported by the Michael J. Fox Foundation, the Parkinson Study Group; and the National Institutes of Health [AG033398, ES004696-5897, ES007033-6364, ES016873, ES019277, NS057567, NS060252, NS062684-6221, and NS082137 to J.Z.]. We thank Dr. Hunter R. Underhill for his kind assistance in the initial data analysis, Dr. Karen Marder, Dr. Lorraine N. Clark, and the Parkinson’s Disease Foundation Weill Family Fund for providing the APOE data for this study. We also deeply appreciate the patients for their generous participation and sample donations.
Footnotes
Contributors
J.Z.: project supervising, study design, data analysis, data interpretation, and writing; H.A.M.: data analysis and writing; C.L.: data analysis and figures; C.G.: sample handling and data collection; P.A.: study design, data collection, and data analysis; M.P.M.: study design, data analysis, and data interpretation; T.S.: manuscript editing; U.J.K.: study design; Parkinson Study Group DATATOP investigators: sample and data collection; K.C.C.: data analysis and data interpretation; M.S.: study design, data analysis, data interpretation, writing, and figures. All authors critically reviewed the manuscript.
Conflicts of interest
The authors declare that they have no conflict of interest.
Literature Cited
- 1.Aasly JO, Shi M, Sossi V, Stewart T, Johansen KK, Wszolek ZK, Uitti RJ, Hasegawa K, Yokoyama T, Zabetian CP, Kim HM, Leverenz JB, Ginghina C, Armaly J, Edwards KL, Snapinn KW, Stoessl AJ, Zhang J. Cerebrospinal fluid amyloid beta and tau in LRRK2 mutation carriers. Neurology. 2012;78:55–61. doi: 10.1212/WNL.0b013e31823ed101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcalay RN, Caccappolo E, Mejia-Santana H, Tang MX, Rosado L, Ross BM, Verbitsky M, Kisselev S, Louis ED, Comella C, Colcher A, Jennings D, Nance MA, Bressman SB, Scott WK, Tanner C, Mickel S, Andrews H, Waters C, Fahn S, Cote L, Frucht S, Ford B, Rezak M, Novak K, Friedman JH, Pfeiffer R, Marsh L, Hiner B, Siderowf A, Ottman R, Marder K, Clark LN. Frequency of known mutations in early-onset Parkinson disease: implication for genetic counseling: the consortium on risk for early onset Parkinson disease study. Arch Neurol. 2010;67:1116–1122. doi: 10.1001/archneurol.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alves G, Bronnick K, Aarsland D, Blennow K, Zetterberg H, Ballard C, Kurz MW, Andreasson U, Tysnes OB, Larsen JP, Mulugeta E. CSF amyloid-beta and tau proteins, and cognitive performance, in early and untreated Parkinson's disease: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2010;81:1080–1086. doi: 10.1136/jnnp.2009.199950. [DOI] [PubMed] [Google Scholar]
- 4.Andreasen N, Minthon L, Davidsson P, Vanmechelen E, Vanderstichele H, Winblad B, Blennow K. Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol. 2001;58:373–379. doi: 10.1001/archneur.58.3.373. [DOI] [PubMed] [Google Scholar]
- 5.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 6.Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30:7281–7289. doi: 10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Compta Y, Marti MJ, Ibarretxe-Bilbao N, Junque C, Valldeoriola F, Munoz E, Ezquerra M, Rios J, Tolosa E. Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson's disease. Mov Disord. 2009;24:2203–2210. doi: 10.1002/mds.22594. [DOI] [PubMed] [Google Scholar]
- 8.Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging. 1992;13:179–189. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- 9.Drechsel DN, Hyman AA, Cobb MH, Kirschner MW. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992;3:1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards TL, Scott WK, Almonte C, Burt A, Powell EH, Beecham GW, Wang L, Zuchner S, Konidari I, Wang G, Singer C, Nahab F, Scott B, Stajich JM, Pericak-Vance M, Haines J, Vance JM, Martin ER. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann Hum Genet. 2010;74:97–109. doi: 10.1111/j.1469-1809.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esposito A, Dohm CP, Kermer P, Bahr M, Wouters FS. alpha-Synuclein and its disease-related mutants interact differentially with the microtubule protein tau and associate with the actin cytoskeleton. Neurobiol Dis. 2007;26:521–531. doi: 10.1016/j.nbd.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 13.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 14.Foster ER, Campbell MC, Burack MA, Hartlein J, Flores HP, Cairns NJ, Hershey T, Perlmutter JS. Amyloid imaging of Lewy body-associated disorders. Mov Disord. 2010;25:2516–2523. doi: 10.1002/mds.23393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 16.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 17.Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Leverenz JB, Baird G, Montine TJ, Hancock AM, Hwang H, Pan C, Bradner J, Kang UJ, Jensen PH, Zhang J. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain. 2010;133:713–726. doi: 10.1093/brain/awq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hulstaert F, Blennow K, Ivanoiu A, Schoonderwaldt HC, Riemenschneider M, De Deyn PP, Bancher C, Cras P, Wiltfang J, Mehta PD, Iqbal K, Pottel H, Vanmechelen E, Vanderstichele H. Improved discrimination of AD patients using beta-amyloid(1–42) and tau levels in CSF. Neurology. 1999;52:1555–1562. doi: 10.1212/wnl.52.8.1555. [DOI] [PubMed] [Google Scholar]
- 19.Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 20.Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40:1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 21.Johansson A, Savitcheva I, Forsberg A, Engler H, Langstrom B, Nordberg A, Askmark H. [(11)C]-PIB imaging in patients with Parkinson's disease: preliminary results. Parkinsonism Relat Disord. 2008;14:345–347. doi: 10.1016/j.parkreldis.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Kang JH, Caspell C, Coffey C, Taylor P, Frasier M, Marek K, Trojanowski JQ, Shaw LM. Association between CSF biomarkers and clinical phenotype of early Parkinson’s disease in the Parkinson’s Progression Marker Initiative (PPMI) Mov Disord. 2012;27:S34–S35. [Google Scholar]
- 23.Kauwe JS, Cruchaga C, Mayo K, Fenoglio C, Bertelsen S, Nowotny P, Galimberti D, Scarpini E, Morris JC, Fagan AM, Holtzman DM, Goate AM. Variation in MAPT is associated with cerebrospinal fluid tau levels in the presence of amyloid-beta deposition. Proc Natl Acad Sci U S A. 2008;105:8050–8054. doi: 10.1073/pnas.0801227105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kieburtz K, McDermott M, Como P, Growdon J, Brady J, Carter J, Huber S, Kanigan B, Landow E, Rudolph A, et al. The effect of deprenyl and tocopherol on cognitive performance in early untreated Parkinson's disease. Parkinson Study Group. Neurology. 1994;44:1756–1759. doi: 10.1212/wnl.44.9.1756. [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Swaminathan S, Shen L, Risacher SL, Nho K, Foroud T, Shaw LM, Trojanowski JQ, Potkin SG, Huentelman MJ, Craig DW, DeChairo BM, Aisen PS, Petersen RC, Weiner MW, Saykin AJ. Genome-wide association study of CSF biomarkers Abeta1-42, t-tau, and p-tau181p in the ADNI cohort. Neurology. 2011;76:69–79. doi: 10.1212/WNL.0b013e318204a397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kronmal RA. Spurious Correlation and the Fallacy of the Ratio Standard Revisited. Journal of the Royal Statistical Society Series A (Statistics in Society) 1993;156:379–392. [Google Scholar]
- 27.Laws SM, Friedrich P, Diehl-Schmid J, Muller J, Eisele T, Bauml J, Forstl H, Kurz A, Riemenschneider M. Fine mapping of the MAPT locus using quantitative trait analysis identifies possible causal variants in Alzheimer's disease. Mol Psychiatry. 2007;12:510–517. doi: 10.1038/sj.mp.4001935. [DOI] [PubMed] [Google Scholar]
- 28.Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, Wong BX, Adlard PA, Cherny RA, Lam LQ, Roberts BR, Volitakis I, Egan GF, McLean CA, Cappai R, Duce JA, Bush AI. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med. 2012;18:291–295. doi: 10.1038/nm.2613. [DOI] [PubMed] [Google Scholar]
- 29.Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, Kaye JA, Raskind MA, Zhang J, Peskind ER, Montine TJ. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 30.Marder KS, Clark LN, McDermott M, Uc E PSG_DATATOP_Investigators. Genetic Risk Factors for Cognitive Impairment in the DATATOP Cohort. Neurology. 2010;74:A254. [Google Scholar]
- 31.Marek K, Jennings D, Lasch S, Siderowf A, Tanner C, Simuni T, Coffey C, Kieburtz K, Flagg E, Chowdhury S, Poewe W, Mollenhauer B, Klinik PE, Sherer T, Frasier M, Meunier C, Rudolph A, Casaceli C, Seibyl J, Mendick S, Schuff N, Zhang Y, Toga A, Crawford K, Ansbach A, De Blasio P, Piovella M, Trojanowski J, Shaw L, Singleton A, Hawkins K, Eberling J, Brooks D, Russell D, Leary L, Factor S, Sommerfeld B, Hogarth P, Pighetti E, Williams K, Standaert D, Guthrie S, Hauser R, Delgado H, Jankovic J, Hunter C, Stern M, Tran B, Leverenz J, Baca M, Frank S, Thomas CA, Richard I, Deeley C, Rees L, Sprenger F, Lang E, Shill H, Obradov S, Fernandez H, Winters A, Berg D, Gauss K, Galasko D, Fontaine D, Mari Z, Gerstenhaber M, Brooks D, Malloy S, Barone P, Longo K, Comery T, Ravina B, Grachev I, Gallagher K, Collins M, Widnell KL, Ostrowizki S, Fontoura P, Ho T, Luthman J, Brug Mvd, Reith AD, Taylor P. The Parkinson Progression Marker Initiative (PPMI) Progress in Neurobiology. 2011;95:629–635. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka SK, van der Flier WM, Blankenstein MA, Ewers M, Rich K, Kaiser E, Verbeek M, Tsolaki M, Mulugeta E, Rosen E, Aarsland D, Visser PJ, Schroder J, Marcusson J, de Leon M, Hampel H, Scheltens P, Pirttila T, Wallin A, Jonhagen ME, Minthon L, Winblad B, Blennow K. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. Jama. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 33.Mazanetz MP, Fischer PM. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat Rev Drug Discov. 2007;6:464–479. doi: 10.1038/nrd2111. [DOI] [PubMed] [Google Scholar]
- 34.McDermott MP, Jankovic J, Carter J, Fahn S, Gauthier S, Goetz CG, Golbe LI, Koller W, Lang AE, Olanow CW, et al. Factors predictive of the need for levodopa therapy in early, untreated Parkinson's disease. The Parkinson Study Group. Arch Neurol. 1995;52:565–570. doi: 10.1001/archneur.1995.00540300037010. [DOI] [PubMed] [Google Scholar]
- 35.Mollenhauer B, Bibl M, Esselmann H, Steinacker P, Trenkwalder C, Wiltfang J, Otto M. Tauopathies and synucleinopathies: do cerebrospinal fluid beta-amyloid peptides reflect disease-specific pathogenesis? J Neural Transm. 2007;114:919–927. doi: 10.1007/s00702-007-0629-4. [DOI] [PubMed] [Google Scholar]
- 36.Montine TJ, Shi M, Quinn JF, Peskind ER, Craft S, Ginghina C, Chung KA, Kim H, Galasko DR, Jankovic J, Zabetian CP, Leverenz JB, Zhang J. CSF Abeta(42) and tau in Parkinson's disease with cognitive impairment. Mov Disord. 2010;25:2682–2685. doi: 10.1002/mds.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy MP, LeVine H., 3rd Alzheimer's disease and the amyloid-beta peptide. J Alzheimers Dis. 2010;19:311–323. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olanow CW, Rascol O, Hauser R, Feigin PD, Jankovic J, Lang A, Langston W, Melamed E, Poewe W, Stocchi F, Tolosa E. A double-blind, delayed-start trial of rasagiline in Parkinson's disease. N Engl J Med. 2009;361:1268–1278. doi: 10.1056/NEJMoa0809335. [DOI] [PubMed] [Google Scholar]
- 39.Olanow CW, Wunderle KB, Kieburtz K. Milestones in movement disorders clinical trials: advances and landmark studies. Mov Disord. 2011;26:1003–1014. doi: 10.1002/mds.23727. [DOI] [PubMed] [Google Scholar]
- 40.Parkinson_Study_Group. DATATOP: a multicenter controlled clinical trial in early Parkinson's disease. Parkinson Study Group. Arch Neurol. 1989;46:1052–1060. doi: 10.1001/archneur.1989.00520460028009. [DOI] [PubMed] [Google Scholar]
- 41.Parkinson_Study_Group. Effect of deprenyl on the progression of disability in early Parkinson's disease. N Engl J Med. 1989;321:1364–1371. doi: 10.1056/NEJM198911163212004. [DOI] [PubMed] [Google Scholar]
- 42.Parkinson_Study_Group. Cerebrospinal fluid homovanillic acid in the DATATOP study on Parkinson's disease. Arch Neurol. 1995;52:237–245. doi: 10.1001/archneur.1995.00540270025015. [DOI] [PubMed] [Google Scholar]
- 43.Parkinson_Study_Group. A controlled, randomized, delayed-start study of rasagiline in early Parkinson disease. Arch Neurol. 2004;61:561–566. doi: 10.1001/archneur.61.4.561. [DOI] [PubMed] [Google Scholar]
- 44.Parnetti L, Tiraboschi P, Lanari A, Peducci M, Padiglioni C, D'Amore C, Pierguidi L, Tambasco N, Rossi A, Calabresi P. Cerebrospinal fluid biomarkers in Parkinson's disease with dementia and dementia with Lewy bodies. Biol Psychiatry. 2008;64:850–855. doi: 10.1016/j.biopsych.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 45.Petrou M, Bohnen NI, Muller ML, Koeppe RA, Albin RL, Frey KA. Abeta-amyloid deposition in patients with Parkinson disease at risk for development of dementia. Neurology. 2012;79:1161–1167. doi: 10.1212/WNL.0b013e3182698d4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prince JA, Zetterberg H, Andreasen N, Marcusson J, Blennow K. APOE epsilon4 allele is associated with reduced cerebrospinal fluid levels of Abeta42. Neurology. 2004;62:2116–2118. doi: 10.1212/01.wnl.0000128088.08695.05. [DOI] [PubMed] [Google Scholar]
- 47.Qureshi HY, Paudel HK. Parkinsonian neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and alpha-synuclein mutations promote Tau protein phosphorylation at Ser262 and destabilize microtubule cytoskeleton in vitro. J Biol Chem. 2011;286:5055–5068. doi: 10.1074/jbc.M110.178905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer's disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rascol O, Fitzer-Attas CJ, Hauser R, Jankovic J, Lang A, Langston JW, Melamed E, Poewe W, Stocchi F, Tolosa E, Eyal E, Weiss YM, Olanow CW. A double-blind, delayed-start trial of rasagiline in Parkinson's disease (the ADAGIO study): prespecified and post-hoc analyses of the need for additional therapies, changes in UPDRS scores, and non-motor outcomes. Lancet Neurol. 2011;10:415–423. doi: 10.1016/S1474-4422(11)70073-4. [DOI] [PubMed] [Google Scholar]
- 50.Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee AC, Alvarez VE, Lee NC, Hall GF. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem. 2012;287:3842–3849. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, Tomiyama H, Nakashima K, Hasegawa K, Obata F, Yoshikawa T, Kawakami H, Sakoda S, Yamamoto M, Hattori N, Murata M, Nakamura Y, Toda T. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- 52.Schulz KL, Eckert A, Rhein V, Mai S, Haase W, Reichert AS, Jendrach M, Muller WE, Leuner K. A new link to mitochondrial impairment in tauopathies. Mol Neurobiol. 2012;46:205–216. doi: 10.1007/s12035-012-8308-3. [DOI] [PubMed] [Google Scholar]
- 53.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi M, Bradner J, Hancock AM, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Kim HM, Leverenz JB, Montine TJ, Ginghina C, Kang UJ, Cain KC, Wang Y, Aasly J, Goldstein D, Zhang J. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol. 2011;69:570–580. doi: 10.1002/ana.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi M, Zhang J. Cerebrospinal fluid α-synuclein, tau and amyloid β in Parkinson’s disease. Lancet Neurology. 2011;10:681. doi: 10.1016/S1474-4422(11)70130-2. [DOI] [PubMed] [Google Scholar]
- 56.Shoulson I. An interim report of the effect of selegiline (L-deprenyl) on the progression of disability in early Parkinson's disease. The Parkinson Study Group. Eur Neurol. 1992;32(Suppl 1):46–53. doi: 10.1159/000116869. [DOI] [PubMed] [Google Scholar]
- 57.Shoulson I, Oakes D, Fahn S, Lang A, Langston JW, LeWitt P, Olanow CW, Penney JB, Tanner C, Kieburtz K, Rudolph A. Impact of sustained deprenyl (selegiline) in levodopa-treated Parkinson's disease: a randomized placebo-controlled extension of the deprenyl and tocopherol antioxidative therapy of parkinsonism trial. Ann Neurol. 2002;51:604–612. doi: 10.1002/ana.10191. [DOI] [PubMed] [Google Scholar]
- 58.Siderowf A, Xie SX, Hurtig H, Weintraub D, Duda J, Chen-Plotkin A, Shaw LM, Van Deerlin V, Trojanowski JQ, Clark C. CSF amyloid {beta} 1–42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75:1055–1061. doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sjogren M, Davidsson P, Tullberg M, Minthon L, Wallin A, Wikkelso C, Granerus AK, Vanderstichele H, Vanmechelen E, Blennow K. Both total and phosphorylated tau are increased in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001;70:624–630. doi: 10.1136/jnnp.70.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sunderland T, Linker G, Mirza N, Putnam KT, Friedman DL, Kimmel LH, Bergeson J, Manetti GJ, Zimmermann M, Tang B, Bartko JJ, Cohen RM. Decreased beta-amyloid1–42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. Jama. 2003;289:2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 62.Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer's disease. J Alzheimers Dis. 2006;9:309–317. doi: 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- 63.Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain Abeta distinguish Alzheimer's disease from normal and pathologic aging. Exp Neurol. 1999;158:328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Shi M, Chung KA, Zabetian CP, Leverenz JB, Berg D, Srulijes K, Trojanowski JQ, Lee VM, Siderowf AD, Hurtig H, Litvan I, Schiess MC, Peskind ER, Masuda M, Hasegawa M, Lin X, Pan C, Galasko D, Goldstein DS, Jensen PH, Yang H, Cain KC, Zhang J. Phosphorylated alpha-Synuclein in Parkinson's Disease. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3002566. 121ra120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wills J, Jones J, Haggerty T, Duka V, Joyce JN, Sidhu A. Elevated tauopathy and alpha-synuclein pathology in postmortem Parkinson's disease brains with and without dementia. Exp Neurol. 2010;225:210–218. doi: 10.1016/j.expneurol.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wray S, Noble W. Linking amyloid and tau pathology in Alzheimer's disease: the role of membrane cholesterol in Abeta-mediated tau toxicity. J Neurosci. 2009;29:9665–9667. doi: 10.1523/JNEUROSCI.2234-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.