Abstract
Cadmium is a human carcinogen with unfavorable health impact probably associated with its DNA methylation property. Recent data suggest that environmental cadmium exposure is associated with incidence of myocardial infarction and peripheral arterial disease. Nonetheless, the effect of chronic cadmium exposure on cardiac contractile function remains elusive.
This study was designed to examine the impact of low-dose cadmium exposure on cardiac contractile function and intracellular Ca2+ homeostasis. Adult male mice were exposed to cadmium for 4 weeks with or without the DNA methylation inhibitor (5-aza-2’-deoxyctidene, 5-AZA). Cardiac contractile and intracellular Ca2+ properties were analyzed including echocardiographic left ventricular parameters, fractional shortening (FS), peak shortening amplitude (PS), maximal velocity of shortening/relengthening (± dL/dt), time-to-PS (TPS), time-to-90% relengthening (TR90), electrically-stimulated increase of intracellular Ca2+ and intracellular Ca2+ decay.
Our results revealed that cadmium exposure depressed FS, PS, ± dL/dt and electrically-stimulated rise in intracellular Ca2+ without affecting TPS, TR90, intracellular Ca2+ level and decay rate, the effects of which were significantly attenuated or nullified by 5-AZA. Cadmium exposure led to overt interstitial fibrosis (collagen deposition), the effect of which was mitigated by 5-AZA. Western blot analysis showed unchanged expression of ICAM-1, TNF-α and Cleaved caspase-3 in response to cadmium exposure and/or 5-AZA treatment, suggesting a relatively minor role of pro-inflammatory cytokines and apoptosis in cadmium- and 5-AZA-induced cardiac responses.
Taken together, our data demonstrated for the first time direct cardiac depressant effect following cadmium exposure, which may be rescued by DNA methylation inhibition.
Keywords: Cadmium, cardiac, contraction, intracellular Ca2+
INTRODUCTION
Cadmium is a ubiquitous environmental toxin with the major known sources of exposure encompassing emissions from industrial activity and waste management, intake of foods (e. g. leafy vegetables, grains, organ meats and crustaceans) and exposure to cigarette smoke.1–3 Accumulation of cadmium leads to tissue toxicity exerting a broad spectrum of biological actions possibly through induction of inflammation and apoptosis. Cadmium toxicity and carcinogenesis has been extensively examined in organs such as testis, liver, lung and kidney.4 For instance, our laboratory recently demonstrated persistent liver and renal damage at more than 40 weeks after a 4-week exposure to low-dose cadmium in rodents. 5–7. Nonetheless, cardiac toxicity, if any, and the underlying mechanisms remain poorly elucidated.
It has been reported that cadmium exerts its adverse cardiovascular effects by promoting atherosclerosis as well as disadvantageous functional and metabolic changes in the heart.1 In particular, high susceptibility of heart to cadmium was reported in a human 15-year following up study,8 in line with the fact that heart is a relatively sensitive organ due to its low antioxidant capacity. Similarly, high susceptibility of the heart to cadmium was also found in experimental animals.9, 10 Prenatal exposure to cadmium is known to lead to change of cardiovascular function during adulthood.1, 11, 12 Environmental exposure to cadmium has been found to be associated with an increased prevalence of heart failure1 although the precise toxic response of cadmium exposure on myocardial function remains elusive. More recent data from the National Health and Nutrition Examination Survey have strongly suggested that cadmium, at substantially low levels of exposure, remains a rather important determinant of all-cause cardiovascular mortality in certain populations of U.S. adults.13 It is thus pertinent to examine the toxic effect of cadmium on myocardial function, if any, and the mechanism of action involved.
Traditional concepts for the mechanisms for cadmium toxicity include its generation of reactive oxygen or nitrogen species (ROS and RNS), inhibition of antioxidants, and impairment of DNA repair enzymes.14–16 However, a recent study has shown that even though a soy-based diet was capable of ameliorating cadmium-induced oxidative stress and damage, it failed to prevent against cadmium-elicited cardiac damage,17 suggesting possible involvement of other mechanisms including epigenetic modulating properties in cadmium-elicited toxicity.18–24
Epigenetics refers to the heritable regulation of gene expression via modification of chromosomal components without an alteration in the nucleotide sequence of the genome. Such modifications include methylation of genomic DNA as well as acetylation, methylation, phosphorylation, ubiquitination, and SUMOylation of core histone proteins. Epigenetic changes in the heart following environmental stress and life style changes have been implicated in the pathogenesis of cardiac remodeling and dysfunction.25–29 Recently we have demonstrated the induction of global DNA hypermethylation in the liver of rats and mice exposed to low-dose cadmium.7 Accordingly, we hypothesize that exposure to low-dose cadmium may cause cardiac global DNA methylation to modify certain key gene expression, leading to cardiac dysfunction.
Therefore, the present study was designed to address the following novel and important questions: (1) whether exposure of low-dose cadmium elicits cardiac functional toxicity and involvement of DNA methylation in such pathological process; (2) the effect of DNA methylation inhibition on cadmium exposure-induced unfavorable cardiac responses, if any.
MATERIALS AND METHODS
Experimental animal model
The animal experimental procedure described in the present study was approved by our Institutional Animal Care and Use Committee (University of Wyoming, Laramie, WY). In brief, forty adult male C57BL/6 mice (6-week-old, ~25 g) were randomly divided into four groups (10 each) as the following: (1) Control group: Mice were given i.p. injection of saline every other day for 4 weeks; (2) Cd treatment group: Mice were given i.p. injection of CdCl2 (20 nmol/kg in saline) every other day for 4 weeks; (3) 5-AZA treatment group: Mice were given i.p. injection of 5-aza-2’-deoxyctidene (5-AZA, Sigma-Aldrich, 0.25 mg/kg in saline) twice weekly for 6 weeks; and (4) CdCl2/5-AZA group: Mice were given both CdCl2 and 5-AZA (20 nmol/kg and 0.25 mg/kg, respectively, in saline, i.p.). All mice were sacrificed at the 56th week following the 4-week Cd exposure (i.e.: 60th week since the initiation of the study) as early appearance of hepatic steatosis and preneoplasia were reported to develop at ~ 60 weeks, leading to induction of hepatic cancers at more advanced stages.30–32
Echocardiographic assessment
Cardiac geometry and function were evaluated in anesthetized mice using a 2-D guided M-mode echocardiography (Sonos 5500, Phillips Medical System, Andover, MA) equipped with a 15-6 MHz linear transducer. Left ventricular (LV) wall thickness, diastolic and systolic dimensions were recorded from the M-mode images. Fractional shortening was calculated from end-diastolic diameter (EDD) and end-systolic diameter (ESD) using the equation of (EDD-ESD)/EDD x100%. Heart rate was measured in 10 consecutive cardiac cycles.33
Isolation of murine cardiomyocytes
After ketamine/xylazine sedation (ketamine 80 mg/kg and xylazine 12 mg/kg, i.p), hearts were removed from mice and perfused with Ca2+-free Tyrode’s solution containing (in mM): NaCl 135, KCl 4.0, MgCl2 1.0, HEPES 10, NaH2PO4 0.33, glucose 10, butanedione monoxime 10, and the solution was gassed with 5% CO2/95% O2. Hearts were digested with Liberase Blendzyme 4 (Hoffmann-La Roche Inc., Indianapolis, IN) for 15 min. Left ventricles were removed and minced before being filtered. Tissue pieces were gently agitated and pellet of cells was resuspended. Extracellular Ca2+ was added incrementally back to 1.20 mM. Cardiomyocytes were used within 8 hrs of isolation. A yield of ~ 60% viable rod-shaped cardiomyocytes with clear sarcomere striations was achieved. Only rod-shaped cells with clear edges were selected for mechanical study.33
Cardiomyocyte shortening and relengthening
Mechanical properties of cardiomyocytes were assessed using an IonOptix Myocam system (IonOptix Inc., Milton, MA). Cells were placed in a chamber mounted on the stage of an inverted microscope and superfused (at 25°C) with a buffer containing (in mM): 131 NaCl, 4 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES, at pH of 7.4. The cells were field stimulated at a frequency of 0.5 Hz. Cell shortening and relengthening were assessed using the following indices: peak shortening (PS), time-to-PS (TPS) and time-to-90% re-lengthening (TR90), maximal velocities of shortening (+ dL/dt) and relengthening (− dL/dt).33
Intracellular Ca2+ fluorescence measurement
A cohort of cardiomyocytes was loaded with fura-2/AM (0.5 µM) for 10 min at 25°C, and fluorescence measurements were recorded with a dual-excitation fluorescence photomultiplier system (Ionoptix) as described.33 Myocytes were imaged through an Olympus IX-70 Fluor oil objective. Cells were exposed to light emitted by a 75W lamp and passed through either a 360 or a 380 nm filter (bandwidths were ±15 nm), while being stimulated to contract at 0.5 Hz. Fluorescence emissions were detected between 480–520 nm by a photomultiplier tube after first illuminating the cells at 360 nm for 0.5 sec then at 380 nm for the duration of the recording protocol (333 Hz sampling rate). The 360 excitation scan was repeated at the end of the protocol and qualitative changes in intracellular Ca2+ concentration was inferred from the ratio of the fura-2 fluorescence intensity (FFI) at the two wavelengths. Fluorescence decay time (single and bi-exponential) was also measured as an indication of intracellular Ca2+ clearing rate.
Western blot analysis
Western blotting was performed as described.34, 35 In brief, tissue samples from mouse ventricles were removed and homogenized in a lysis buffer containing 10% 10 × RIPA, 1% NaF, 1% Na3VO4 and 1% protease inhibitor cocktail. Samples were sonicated on ice for 15 sec and centrifuged at 13,000 × rpm for 20 min at 4°C. Murine cardiomyocytes were collected and sonicated in a lysis buffer. The protein concentration of the supernatant was evaluated using a Protein Assay Reagent (500-0006; Bio-Rad Laboratories, Hercules, CA). Protein samples were then mixed 1:2 with Laemmli sample buffer with 5% 2-mercaptoethanol and heated at 95°C for 5 min. Equal amounts (50 µg protein/lane) of proteins were separated on 7%, 10% or 15% SDS-polyacrylamide gels in a minigel apparatus (Mini-PROTEAN II, Bio-Rad) and transferred electrophoretically to nitrocellulose membranes (0.2 µm pore size, Bio-Rad). Membranes were incubated for 1 hr in a blocking solution containing 5% milk in Tris-buffered saline (TBS). Membranes were washed briefly in TBS and incubated overnight at 4°C with the anti-intercellular adhesion molecule 1 (ICAM-1, 1:500), anti-TNF-α (1:500, Abcam), anti-Cleaved caspase-3 (1:500, Cell Signaling) and anti-β-actin (1:2000, loading control) antibodies. All primary antibodies were purchased from Santa Cruz Biotech Inc. (Santa Cruz, CA) unless otherwise stated. After washing blots to remove excessive primary antibody binding, blots were incubated for 1 h with horseradish peroxidase (HRP)-conjugated secondary antibody (1:2,500). Antigens were detected by the luminescence method. Quantification of band density was determined using Quantity One software (Bio-Rad, version 4.4.0, ChemiDoc XRS) and reported in optical density per square millimeter.
Histopathology examination
Mouse hearts were collected and immersion-fixed in 10% neutral formalin, embedded in paraffin and sectioned into 5-µm-thick sections onto glass slides. After deparaffinization, tissue sections were rehydrated and stained with 0.1% Sirius-red F3BA and 0.25% Fast green FCF. Sirius-red stained sections were assessed for the proportion of fibrosis (collagen) in the heart tissues, as described previously.36
Data analysis
Data were expressed as Mean ± SEM. Statistical significance (p < 0.05, two-sided) for each variable was estimated by two-way analysis of variance (ANOVA) followed by Tukey’s test for post hoc analysis.
RESULTS
Effect of cadmium exposure and 5-AZA treatment on cardiomyocyte contractile function
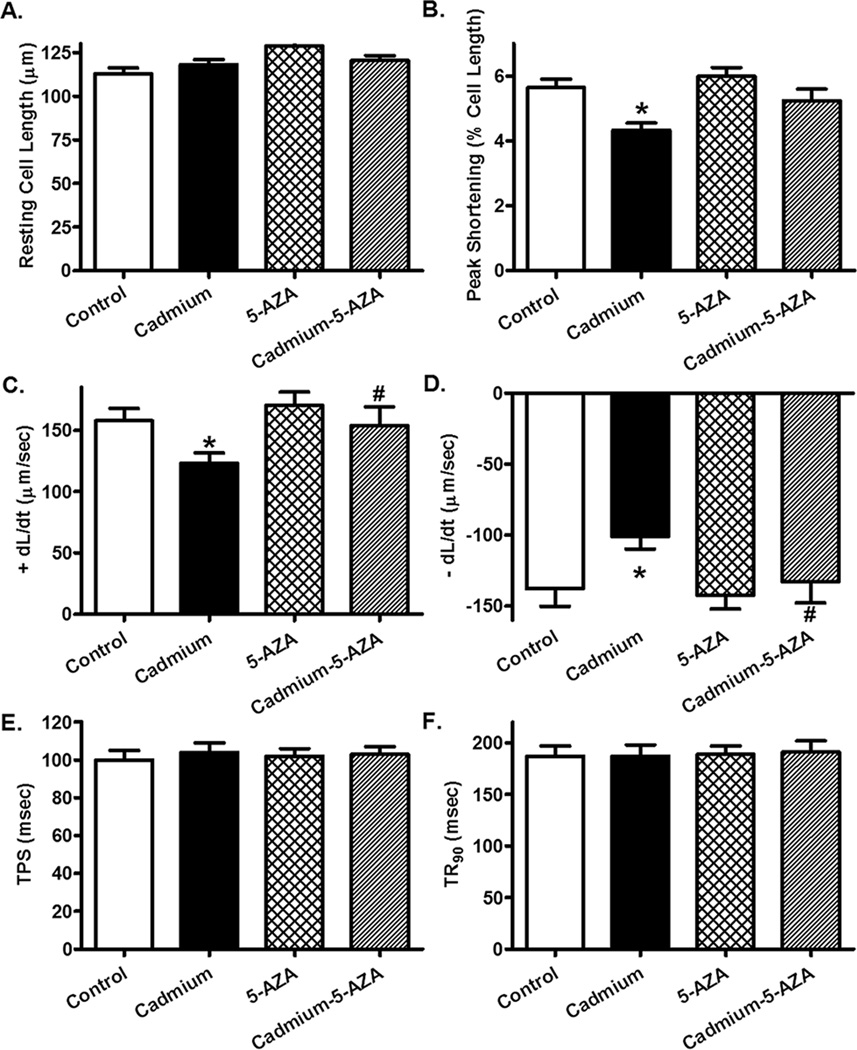
Neither chronic cadmium exposure nor 5-AZA treatment significantly affected blood glucose levels, body and organ (heart, liver, kidney and testis) weights, as well as organ size when normalized to body weight. Our data depicted that cadmium exposure overtly increased LVESD and suppressed fractional shortening without affecting heart rate, left ventricular wall thickness and LVEDD. While 5-AZA itself did not elicit any overt effect on echocardiographic parameters tested, it mitigated cadmium exposure-induced changes in echocardiographic indices (Table 1). Neither cadmium exposure nor 5-AZA significantly affected resting cell length. Chronic cadmium exposure significantly suppressed peak shortening (PS) and maximal velocity of shortening/relengthening (α dL/dt) without affect duration of shortening (TPS) and relengthening (TR90) in murine cardiomyocytes. Although 5-AZA did not elicit any effect on these cardiomyocyte contractile parameters, it significantly attenuated or mitigated cadmium exposure-induced cardiomyocyte contractile defects (Fig. 1).
Table 1.
Biometric parameters of adult mice exposed to cadmium (CdCl2, 20 nmol/kg, every other day for 4 weeks) in the absence or presence of the DNA methylation inhibitor 5-AZA treatment (0.25 mg/kg, i.p. twice weekly for 6 weeks)
| Parameter | Control | Cadmium | 5-AZA | Cadmium-5-AZA |
|---|---|---|---|---|
| Body Weight (g) | 33.3 ± 2.6 | 33.2 ± 3.7 | 29.8 ± 1.3 | 35.7 ± 1.8 |
| Heart Weight (mg) | 138 ± 10 | 148 ± 10 | 156 ± 4 | 166 ± 8 |
| Heart/Body Weight (mg/g) | 4.23 ± 0.42 | 4.57 ± 0.34 | 5.26 ± 0.16 | 4.71 ± 0.37 |
| Liver Weight (g) | 1.84 ± 0.11 | 1.90 ± 0.16 | 1.64 ± 0.10 | 1.64 ± 0.07 |
| Liver/Body Weight (mg/g) | 55.8 ± 2.7 | 58.1 ± 3.5 | 55.0 ± 3.0 | 46.0 ± 1.9 |
| Kidney Weight (mg) | 450 ± 29 | 438 ± 44 | 383 ± 29 | 440 ± 6 |
| Kidney/Body Weight (mg/g) | 13.6 ± 0.4 | 13.3 ± 0.8 | 12.8 ± 0.4 | 12.4 ± 0.5 |
| Testis Weight (mg) | 191 ± 15 | 221 ± 10 | 229 ± 11 | 236 ± 8 |
| Testis/Body Weight (mg/g) | 5.88 ± 0.65 | 6.90 ± 0.58 | 7.69 ± 0.11 | 6.63 ± 0.20 |
| Blood Glucose (mg/dl) | 116.5 ± 5.5 | 125.2 ± 5.9 | 118.3 ± 7.4 | 115.2 ± 6.6 |
| Heart Rate (bpm) | 503 ± 25 | 492 ± 14 | 497 ± 17 | 492 ± 24 |
| LV Wall Thickness (mm) | 1.01 ± 0.04 | 0.98 ± 0.04 | 1.03 ± 0.05 | 1.00 ± 0.04 |
| LV EDD (mm) | 2.30 ± 0.06 | 2.23 ± 0.08 | 2.29 ± 0.07 | 2.29 ± 0.08 |
| LV ESD (mm) | 1.15 ± 0.04 | 1.36 ± 0.05* | 1.20 ± 0.06 | 1.23 ± 0.06# |
| Fractional Shortening (%) | 49.8 ± 2.0 | 38.9 ± 2.1* | 47.7 ± 1.8 | 46.5 ± 1.6# |
LV: left ventricular; LV ESD: LV end systolic diameter; LV EDD: LV end diastolic diameter; Mean ± SEM, n = 5 mice per group
p < 0.05 vs. Control group
p < 0.05 vs. Cadmium group.
Fig. 1.
Murine cardiomyocyte contractile function in response to chronic cadmium exposure with or without 5-AZA treatment. A: Resting cell length; B: Peak shortening (% of cell length); C: Maximal velocity of shortening (+ dL/dt); D: Maximal velocity of relengthening (− dL/dt); E: Time-to-PS (TPS); and F: Time-to-90% relengthening (TR90). Mean α SEM, n = 79–80 cells per group, * p < 0.05 vs. Control, # p < 0.05 vs. Cadmium group.
Effect of cadmium exposure and 5-AZA treatment on intracellular Ca2+ handling
To explore the possible mechanism(s) of action behind chronic cadmium exposure-induced cardiomyocyte contractile anomalies, the membrane permeable intracellular Ca2+ fluorescent dye fura-2 was employed to evaluate intracellular Ca2+ handling in cardiomyocytes. Our results shown in Fig. 2 depicted comparable resting intracellular Ca2+ levels and intracellular Ca2+ transient decay rates (either single or bi-exponential) in cardiomyocytes from mice exposed to cadmium with or without 5-AZA treatment. Interestingly, chronic cadmium exposure overtly suppressed electrically-stimulated rise in intracellular Ca2+ in cardiomyocytes, the effect of which was obliterated by 5-AZA treatment. 5-AZA did not exert any effect on intracellular Ca2+ handling properties. These data favored a role of intracellular Ca2+ handling underscoring chronic cadmium exposure- and 5-AZA-induced cardiac contractile responses.
Fig. 2.
Cardiomyocyte intracellular Ca2+ property in response to chronic cadmium exposure with or without 5-AZA treatment. A: Baseline intracellular Ca2+ fura-2 fluorescence intensity (FFI); B: Electrically-stimulated increase in FFI (ΔFFI); C: Intracellular Ca2+ decay rate (single exponential); and D: Intracellular Ca2+ decay rate (bi-exponential). Mean α SEM, n = 60 cells per group, * p < 0.05 vs. control, # p < 0.05 vs. cadmium group.
Effect of cadmium exposure and 5-AZA treatment on myocardial histology
Myocardial histological examination using Sirius-red staining revealed overt interstitial fibrosis manifested as enhanced collagen deposition following cadmium exposure, the effect of which was negated by 5-AZA treatment. 5-AZA treatment itself did not elicit any notable effect on myocardial histology and collagen content (Fig. 3).
Fig. 3.
Interstitial fibrosis and collagen content in myocardium from mice exposed to cadmium with or without 5-AZA treatment. A: Representative Sirius-red stained myocardial sections used to assess fibrosis (collagen); and B: Collagen content. Mean α SEM, n = 5–6 sections per group, * p < 0.05 vs. control, # p < 0.05 vs. cadmium group.
Protein expression of pro-inflammatory cytokines and apoptosis following chronic cadmium exposure and 5-AZA treatment
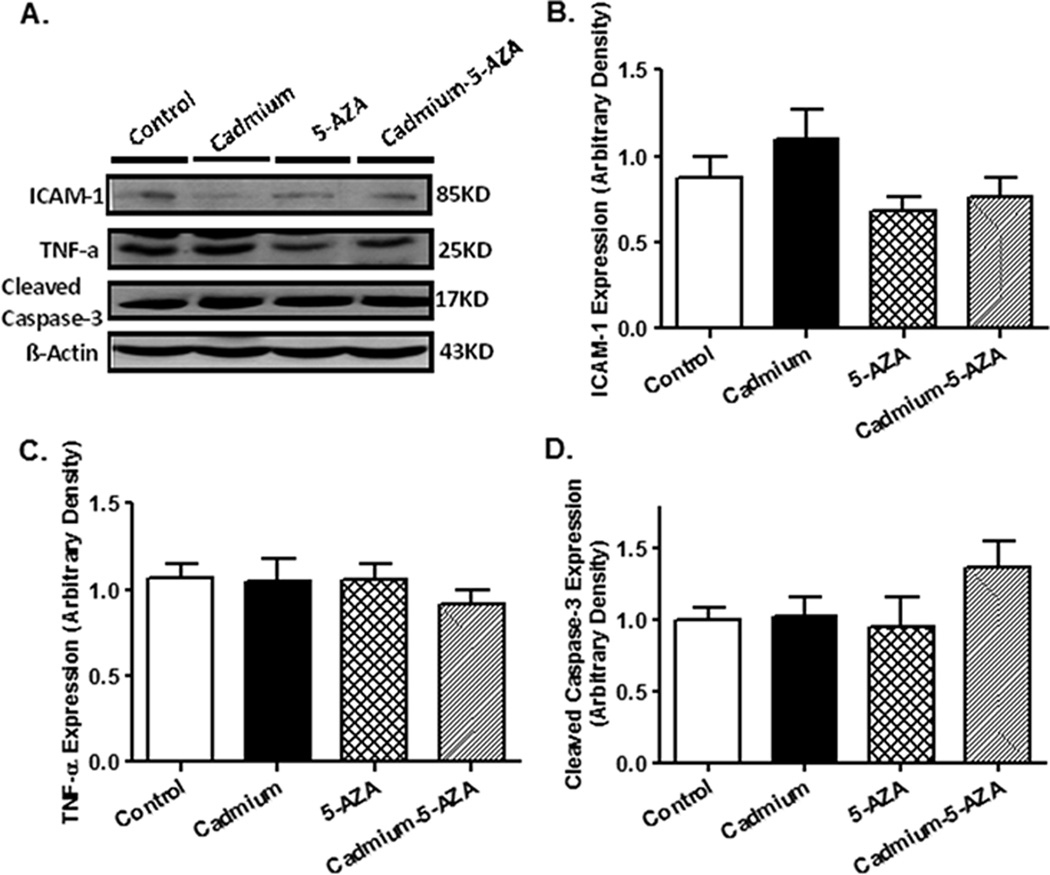
To evaluate if pro-inflammatory cytokines and apoptosis contribute to chronic cadmium exposure and/or 5-AZA-induced responses in the heart, Western blot analysis was performed to assess the expression of ICAM-1, TNF-α and cleaved caspase-3 in murine hearts following cadmium exposure and/or 5-AZA treatment. Our data shown in Fig. 4 indicated that neither cadmium exposure nor 5-AZA treatment, or both, significantly affected the protein expressions of ICAM-1, TNF-α and cleaved caspase-3, not favoring a major role of these pro-inflammatory cytokines and caspase-3-mediated apoptosis in chronic cadmium exposure and 5-AZA-induced cardiac mechanical responses.
Fig. 4.
Levels of ICAM-1, TNF-α and cleaved caspase-3 in myocardium from mice exposed to cadmium with or without 5-AZA treatment. A: Representative gel blots depicting ICAM-1, TNF-α and cleaved caspase-3 using specific antibodies (β-actin used as loading control); B: Quantitative analysis based on gel profiles were presented for ICAM-1 (B), TNF-α (C), and cleaved caspase-3 (D). Mean α SEM, n = 7 – 8 hearts per group.
DISCUSSION
The salient findings from our present study provided evidence for the first time that the human carcinogen cadmium exposure alters myocardial geometry, depresses cardiac contractile function and compromises intracellular Ca2+ handling in murine hearts. More strikingly, our results indicated that inhibition of DNA methylation improves chronic cadmium exposure-induced cardiac mechanical function, favoring a likely role of DNA methylation in cadmium exposure-induced cardiomyocyte mechanical anomalies.
Data from our present study revealed that chronic cadmium exposure led to myocardial and cardiomyocyte contractile dysfunction, altered myocardial geometry (LVESD but not LVEDD or LV wall thickness), intracellular Ca2+ mishandling and overt interstitial fibrosis (as evidenced by collagen deposition), the effect of which was mitigated by 5-AZA. Although the mechanism(s) of action underscoring compromised cardiomyocyte contractile function in response to chronic low-dose cadmium exposure is not clear at this time, several speculations may be considered. First, the observation that cadmium exposure inhibits the electrically-stimulated intracellular Ca2+ release depicts that cadmium exposure may directly interfere with intracellular Ca2+ handling. The reduced intracellular Ca2+ release in response to electrical stimuli is consistent with the compromised myocardial and cardiomyocyte contractile capacity (fractional shortening, ± dL/dt and PS) following chronic cadmium exposure. Second, global DNA methylation caused by chronic exposure to cadmium may play a key role. It is known that the carcinogenic mechanism of cadmium include its abnormal effect on DNA methylation. Three in vitro studies have indicated that prolong exposure to low-dose cadmium results global DNA methylation through stimulation of DNA methyltransferase (MeTase) activity.20, 21, 37 Our very recent in vivo study indicated that chronic cadmium exposure may promote DNA methylation in the liver.7 Ample evidence has suggested a unique role of abnormal DNA methylation in the etiology of cardiomyopathies en route to heart failure.28, 38–43 Movassagh and colleagues have identified at least three loci of angiogenesis-related genes where methylation of which may be correlated with overt differential expression of corresponding genes in myopathic hearts in patients with end-stage heart failure. These findings suggest that altered DNA methylation may be responsible for, at least in part, characteristic gene expression changes in heart failure.28
It is known that sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA2a) plays an essential role in intracellular Ca2+ homeostasis and maintenance of cardiac function. The promoter region of SERCA2a possesses a high level of CpG islands. Therefore, epigenetic modification through inhibition of methylation can enhance SERCA2a expression in cardiomyocytes. Recent study has demonstrated that hydralazine, a potential DNA methylation inhibitor used for the management of heart failure, decreased the expression of MeTase 1 in the heart in association with increased intracellular Ca2+ transients and SR Ca2+ contents along with a decrease in SERCA2a promoter region methylation and an increase the RNA and protein expressions of SERCA2a.44 Moreover, treatment with hydralazine in isoproterenol-induced heart failure suppressed SERCA2a promoter methylation and increased SERCA2a RNA expression. These data favor the notion that hydralazine improves cardiac function through induction of SERCA2a promoter demethylation to increase SERCA2a function and intracellular Ca2+ handling in cardiomyocytes.44 Along the same line, our data indicated comparable resting intracellular Ca2+ levels and intracellular Ca2+ transient decay rates (single or bi-exponential) in cardiomyocytes from mice exposed to cadmium with or without 5-AZA treatment. However, cadmium exposure overtly suppressed electrically-stimulated rise in intracellular Ca2+ in cardiomyocytes, the effect of which was obliterated by 5-AZA, suggesting the likelihood involvement of intracellular Ca2+ homeostasis in cadmium and 5-AZA-induced cardiac mechanical and geometric responses. Nonetheless, whether SERCA2a was reduced by cadmium-induced hypermethylation of SERCA2a promoter, an effect that was prevented by 5-AZA, warrants further investigation.
We did not see any increased level of cleaved caspase-3 in the present study. It is possible that the caspase-3 dependent apoptosis may not be the predominant form of cell death induced by cadmium in the current experimental setting. Earlier studies depicted that cadmium is capable of promoting apoptosis, necrosis, programmed necrosis, or autophagy. For instance, a recent study has indicated that cadmium may turn on a programmed, lysosomal membrane permeabilization-dependent necrosis under certain conditions.45 Data from our current study revealed unchanged levels of ICAM-1 and TNF-α which are expectable since these measurements are often observed in the acute cardiac injury.46–48 These measurements from the heart collected at 56 weeks after mice were exposed to chronic cadmium may be abnormally changed at the time period during cadmium exposure, but not right now. For instance, TNF-α is a common marker in the heart in response to stress such as cadmium.47 An increase in TNF-α may play an important role in cadmium-induced DNA methylation as TNF-α may decrease the SERCA2a RNA and protein levels in the heart with an increased methylation in the SERCA2a promoter region, both of which were attenuated by 5-AZA. In addition, TNF-α may upregulate the expression of MeTase 1 in the heart. Taken together our finding with these previous studies, it suggests that inhibition of hypermethylation may be a novel treatment strategy for cardiac dysfunction.
In conclusion, our study has provided evidence, for the first time, at the levels of whole heart and cardiomyocytes to confirm clinical observations that the carcinogen cadmium may trigger myocardial contractile depression possibly through intracellular Ca2+ dysregulation. These findings not only reveal an essential role of the basic myocardial working element, cardiomyocytes, in chronic cadmium exposure-induced cardiac anomalies but also provide new insights for better therapeutic remedy against chronic cadmium exposure-induced myopathic changes. Assessment of cardiac electromechanical response in multicellular preparations may suffer from the limitation of heterogeneity in the heart, which may not accurately represent changes in the cardiomyocytes. For example, compromised myocardial contractile function seen at our whole heart or in vivo settings may reflect increased ventricular stiffness or poor ventricular compliance due to interstitial fibrosis and a shift in collagen content (as seen in our present study) rather than a loss of mechanical properties of cardiomyocytes. It is noteworthy that the precise nature behind chronic cadmium exposure-induced depression on cardiac contractile function still remains not fully clear. Awaiting future studies include cardiac excitation-contraction coupling and membrane ion channels in response to cadmium exposure. These approaches will be crucial to further our understanding of the pathological profiles of cadmium exposure.
ACKNOWLEDGMENTS
The work was supported in part by grants from Natural Science Foundation of Inner Mongolia Autonomous Region of China [grant: 2011BS1103, to LJM], from the CEGIB Pilot Project of the NIEHS Center for Environmental Genomics and Integrative Biology at the University of Louisville [grant: 2007, to LC] and NIH [RR016474, to JR].
REFERENCES
- 1.Peters JL, Perlstein TS, Perry MJ, McNeely E, Weuve J. Cadmium exposure in association with history of stroke and heart failure. Environ Res. 2010;110:199–206. doi: 10.1016/j.envres.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109:3196–3201. doi: 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]
- 3.Lee MS, Park SK, Hu H, Lee S. Cadmium exposure and cardiovascular disease in the 2005 Korea National Health and Nutrition Examination Survey. Environ Res. 2011;111:171–176. doi: 10.1016/j.envres.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Qu W, Kadiiska MB. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol. 2009;238:209–214. doi: 10.1016/j.taap.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang B, Wang S, Shao C, Wang G, Li Y, Cai L. Proteomic characterization of the late and persistent effects of cadmium at low doses on the rat liver. J Appl Toxicol. 2013;33:546–557. doi: 10.1002/jat.1757. [DOI] [PubMed] [Google Scholar]
- 6.Wang B, Luo Q, Shao C, et al. The late and persistent pathogenic effects of cadmium at very low levels on the kidney of rats. Dose Response. 2013;11:60–81. doi: 10.2203/dose-response.11-046.Wang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang B, Li Y, Tan Y, et al. Low-dose Cd induces hepatic gene hypermethylation, along with the persistent reduction of cell death and increase of cell proliferation in rats and mice. PLoS One. 2012;7:e33853. doi: 10.1371/journal.pone.0033853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishijo M, Nakagawa H, Morikawa Y, et al. Mortality of inhabitants in an area polluted by cadmium: 15 year follow up. Occup Environ Med. 1995;52:181–184. doi: 10.1136/oem.52.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomera JF, Lilford K, Kukulka SP, Friend KD, Harakal C. Divalent cations in hypertension with implications to heart disease: calcium, cadmium interactions. Methods Find Exp Clin Pharmacol. 1994;16:97–107. [PubMed] [Google Scholar]
- 10.Carmignani M, Boscolo P. Cardiovascular responsiveness to physiological agonists of male rats made hypertensive by long-term exposure to cadmium. Sci Total Environ. 1984;34:19–33. doi: 10.1016/0048-9697(84)90038-x. [DOI] [PubMed] [Google Scholar]
- 11.Zepeda R, Castillo P, Saez D, Llanos MN, Ronco AM. Cardiac tissue injury resistance during myocardial infarction at adulthood by developmental exposure to cadmium. Cardiovasc Toxicol. 2012;12:64–72. doi: 10.1007/s12012-011-9139-6. [DOI] [PubMed] [Google Scholar]
- 12.Ronco AM, Montenegro M, Castillo P, et al. Maternal exposure to cadmium during gestation perturbs the vascular system of the adult rat offspring. Toxicol Appl Pharmacol. 2011;251:137–145. doi: 10.1016/j.taap.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Tellez-Plaza M, Navas-Acien A, Menke A, Crainiceanu CM, Pastor-Barriuso R, Guallar E. Cadmium exposure and all-cause and cardiovascular mortality in the U.S. general population. Environ Health Perspect. 2012;120:1017–1022. doi: 10.1289/ehp.1104352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szuster-Ciesielska A, Stachura A, Slotwinska M, et al. The inhibitory effect of zinc on cadmium-induced cell apoptosis and reactive oxygen species (ROS) production in cell cultures. Toxicology. 2000;145:159–171. doi: 10.1016/s0300-483x(00)00144-x. [DOI] [PubMed] [Google Scholar]
- 15.Dailianis S, Piperakis SM, Kaloyianni M. Cadmium effects on ros production and DNA damage via adrenergic receptors stimulation: role of Na+/H+ exchanger and PKC. Free Radic Res. 2005;39:1059–1070. doi: 10.1080/10715760500243765. [DOI] [PubMed] [Google Scholar]
- 16.Son YO, Wang L, Poyil P, et al. Cadmium induces carcinogenesis in BEAS-2B cells through ROS-dependent activation of PI3K/AKT/GSK-3beta/beta-catenin signaling. Toxicol Appl Pharmacol. 2012;264:153–160. doi: 10.1016/j.taap.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferramola ML, Perez Diaz MF, Honore SM, et al. Cadmium-induced oxidative stress and histological damage in the myocardium. Effects of a soy-based diet. Toxicol Appl Pharmacol. 2012;265:380–389. doi: 10.1016/j.taap.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Fujishiro H, Okugaki S, Yasumitsu S, Enomoto S, Himeno S. Involvement of DNA hypermethylation in down-regulation of the zinc transporter ZIP8 in cadmium-resistant metallothionein-null cells. Toxicol Appl Pharmacol. 2009;241:195–201. doi: 10.1016/j.taap.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Vandegehuchte MB, Kyndt T, Vanholme B, Haegeman A, Gheysen G, Janssen CR. Occurrence of DNA methylation in Daphnia magna and influence of multigeneration Cd exposure. Environ Int. 2009;35:700–706. doi: 10.1016/j.envint.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Jiang G, Xu L, Song S, et al. Effects of long-term low-dose cadmium exposure on genomic DNA methylation in human embryo lung fibroblast cells. Toxicology. 2008;244:49–55. doi: 10.1016/j.tox.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 21.Takiguchi M, Achanzar WE, Qu W, Li G, Waalkes MP. Effects of cadmium on DNA-(Cytosine-5) methyltransferase activity and DNA methylation status during cadmium-induced cellular transformation. Exp Cell Res. 2003;286:355–365. doi: 10.1016/s0014-4827(03)00062-4. [DOI] [PubMed] [Google Scholar]
- 22.Traore A, Ruiz S, Baudrimont I, et al. Combined effects of okadaic acid and cadmium on lipid peroxidation and DNA bases modifications (m5dC and 8-(OH)-dG) in Caco-2 cells. Arch Toxicol. 2000;74:79–84. doi: 10.1007/s002040050656. [DOI] [PubMed] [Google Scholar]
- 23.Arita A, Costa M. Epigenetics in metal carcinogenesis: nickel, arsenic, chromium and cadmium. Metallomics. 2009;1:222–228. doi: 10.1039/b903049b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang B, Li Y, Shao C, Tan Y, Cai L. Cadmium and its epigenetic effects. Curr Med Chem. 2012;19:2611–2620. doi: 10.2174/092986712800492913. [DOI] [PubMed] [Google Scholar]
- 25.D'Cruz LG, Baboonian C, Phillimore HE, et al. Cytosine methylation confers instability on the cardiac troponin T gene in hypertrophic cardiomyopathy. J Med Genet. 2000;37:E18. doi: 10.1136/jmg.37.9.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaikwad AB, Sayyed SG, Lichtnekert J, Tikoo K, Anders HJ. Renal failure increases cardiac histone h3 acetylation, dimethylation, and phosphorylation and the induction of cardiomyopathy-related genes in type 2 diabetes. Am J Pathol. 2010;176:1079–1083. doi: 10.2353/ajpath.2010.090528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monkemann H, De Vriese AS, Blom HJ, et al. Early molecular events in the development of the diabetic cardiomyopathy. Amino Acids. 2002;23:331–336. doi: 10.1007/s00726-001-0146-y. [DOI] [PubMed] [Google Scholar]
- 28.Movassagh M, Choy MK, Goddard M, Bennett MR, Down TA, Foo RS. Differential DNA methylation correlates with differential expression of angiogenic factors in human heart failure. PLoS One. 2010;5:e8564. doi: 10.1371/journal.pone.0008564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson TB, Chen T, Richard S. The physiological and pathophysiological role of PRMT1-mediated protein arginine methylation. Pharmacol Res. 2009;60:466–474. doi: 10.1016/j.phrs.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Denda A, Kitayama W, Kishida H, et al. Development of hepatocellular adenomas and carcinomas associated with fibrosis in C57BL/6J male mice given a choline-deficient, L-amino acid-defined diet. Jpn J Cancer Res. 2002;93:125–132. doi: 10.1111/j.1349-7006.2002.tb01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill-Baskin AE, Markiewski MM, Buchner DA, et al. Diet-induced hepatocellular carcinoma in genetically predisposed mice. Hum Mol Genet. 2009;18:2975–2988. doi: 10.1093/hmg/ddp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang B, Majumder S, Nuovo G, et al. Role of microRNA-155 at early stages of hepatocarcinogenesis induced by choline-deficient and amino acid-defined diet in C57BL/6 mice. Hepatology. 2009;50:1152–1161. doi: 10.1002/hep.23100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren J, Privratsky JR, Yang X, Dong F, Carlson EC. Metallothionein alleviates glutathione depletion-induced oxidative cardiomyopathy in murine hearts. Crit Care Med. 2008;36:2106–2116. doi: 10.1097/CCM.0b013e31817bf925. [DOI] [PubMed] [Google Scholar]
- 34.Cai L, Wang Y, Zhou G, et al. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;48:1688–1697. doi: 10.1016/j.jacc.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Zhou G, Li X, Hein DW, et al. Metallothionein suppresses angiotensin II-induced nicotinamide adenine dinucleotide phosphate oxidase activation, nitrosative stress, apoptosis, and pathological remodeling in the diabetic heart. J Am Coll Cardiol. 2008;52:655–666. doi: 10.1016/j.jacc.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 36.Cai L, Wang J, Li Y, et al. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes. 2005;54:1829–1837. doi: 10.2337/diabetes.54.6.1829. [DOI] [PubMed] [Google Scholar]
- 37.Inglot P, Lewinska A, Potocki L, et al. Cadmium-induced changes in genomic DNA-methylation status increase aneuploidy events in a pig Robertsonian translocation model. Mutat Res. 2012;747:182–189. doi: 10.1016/j.mrgentox.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Beltran-Alvarez P, Pagans S, Brugada R. The cardiac sodium channel is post-translationally modified by arginine methylation. J Proteome Res. 2011;10:3712–3719. doi: 10.1021/pr200339n. [DOI] [PubMed] [Google Scholar]
- 39.Kao YH, Chen YC, Chung CC, et al. Heart failure and angiotensin II modulate atrial Pitx2c promotor methylation. Clin Exp Pharmacol Physiol. 2013;40:379–384. doi: 10.1111/1440-1681.12089. [DOI] [PubMed] [Google Scholar]
- 40.Pandya K, Kohro T, Mimura I, et al. Distribution of histone3 lysine 4 trimethylation at T3-responsive loci in the heart during reversible changes in gene expression. Gene Expr. 2012;15:183–198. doi: 10.3727/105221612x13372578119698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Movassagh M, Vujic A, Foo R. Genome-wide DNA methylation in human heart failure. Epigenomics. 2011;3:103–109. doi: 10.2217/epi.10.70. [DOI] [PubMed] [Google Scholar]
- 42.Haddad F, Jiang W, Bodell PW, Qin AX, Baldwin KM. Cardiac myosin heavy chain gene regulation by thyroid hormone involves altered histone modifications. Am J Physiol Heart Circ Physiol. 2010;299:H1968–H1980. doi: 10.1152/ajpheart.00644.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol. 2009;5:401–408. doi: 10.1038/nrendo.2009.102. [DOI] [PubMed] [Google Scholar]
- 44.Kao YH, Cheng CC, Chen YC, et al. Hydralazine-induced promoter demethylation enhances sarcoplasmic reticulum Ca2+ -ATPase and calcium homeostasis in cardiac myocytes. Lab Invest. 2011;91:1291–1297. doi: 10.1038/labinvest.2011.92. [DOI] [PubMed] [Google Scholar]
- 45.Messner B, Ploner C, Laufer G, Bernhard D. Cadmium activates a programmed, lysosomal membrane permeabilization-dependent necrosis pathway. Toxicol Lett. 2012;212:268–275. doi: 10.1016/j.toxlet.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 46.Knoflach M, Messner B, Shen YH, et al. Non-toxic cadmium concentrations induce vascular inflammation and promote atherosclerosis. Circ J. 2011;75:2491–2495. doi: 10.1253/circj.cj-11-0196. [DOI] [PubMed] [Google Scholar]
- 47.Yazihan N, Kocak MK, Akcil E, Erdem O, Sayal A. Role of midkine in cadmium-induced liver, heart and kidney damage. Hum Exp Toxicol. 2011;30:391–397. doi: 10.1177/0960327110372402. [DOI] [PubMed] [Google Scholar]
- 48.Houston MC. The role of mercury and cadmium heavy metals in vascular disease, hypertension, coronary heart disease, and myocardial infarction. Altern Ther Health Med. 2007;13:S128–S133. [PubMed] [Google Scholar]