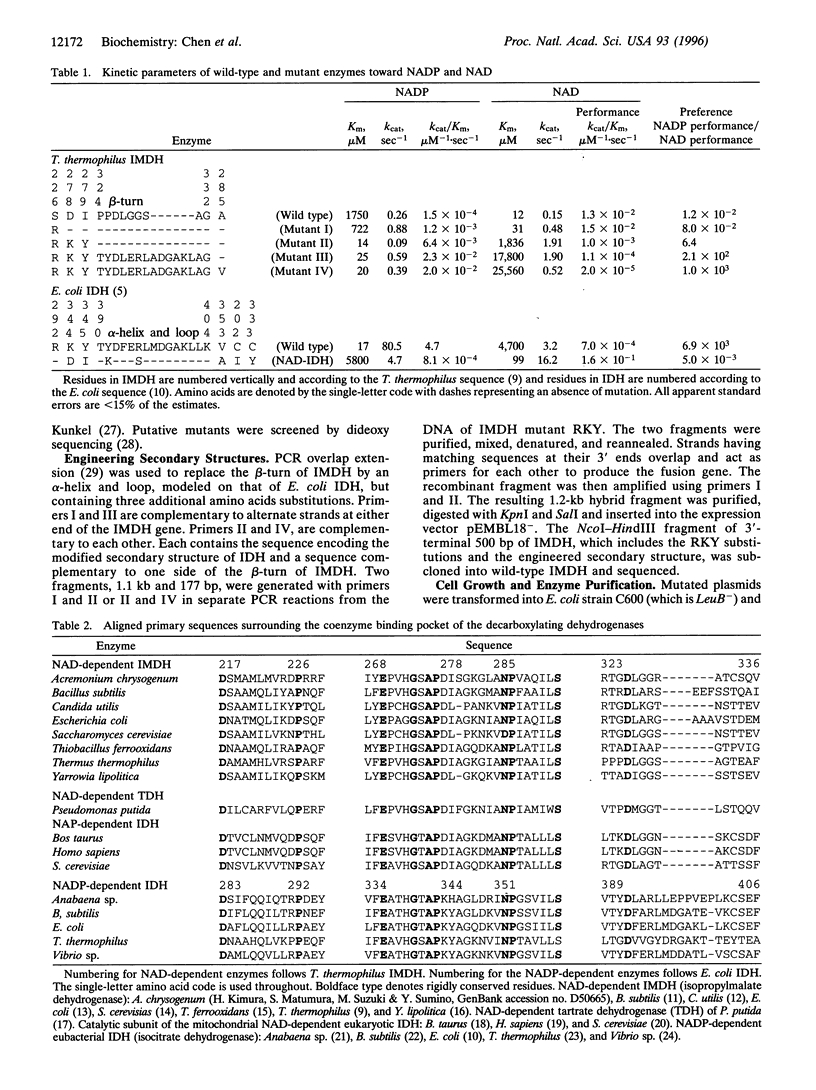
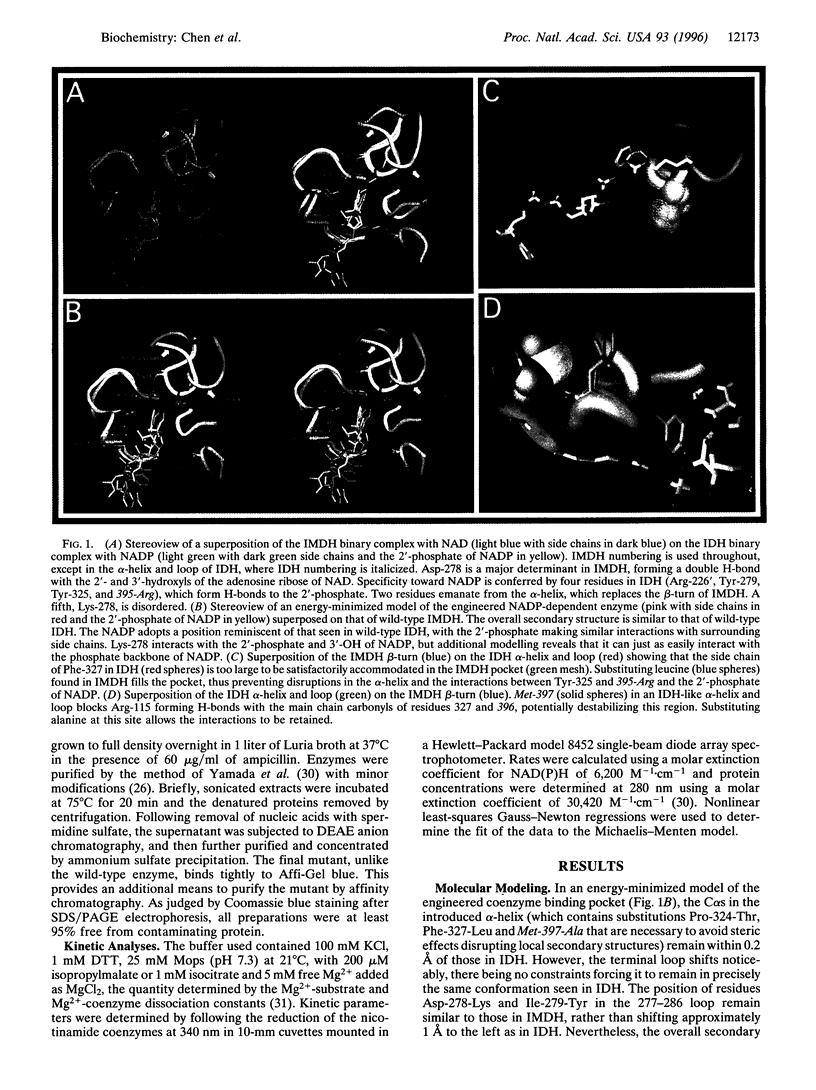
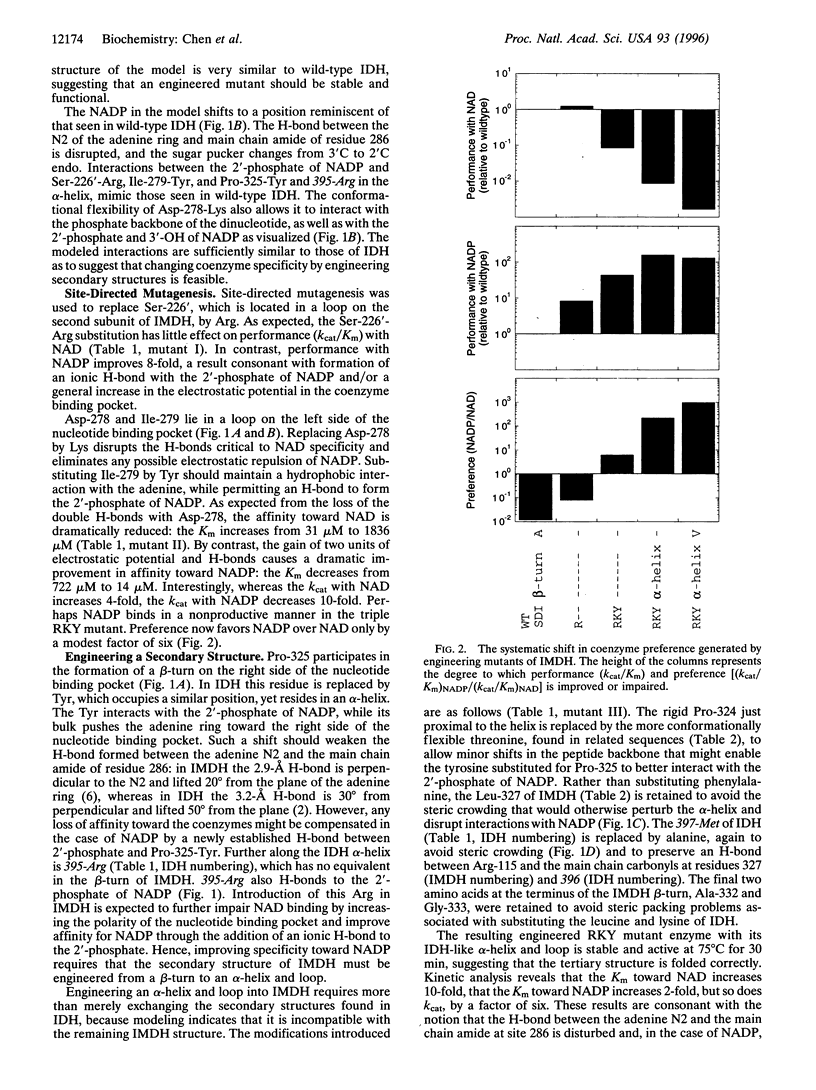
Abstract
Rational engineering of enzymes involves introducing key amino acids guided by a knowledge of protein structure to effect a desirable change in function. To date, all successful attempts to change specificity have been limited to substituting individual amino acids within a protein fold. However, the infant field of protein engineering will only reach maturity when changes in function can be generated by rationally engineering secondary structures. Guided by x-ray crystal structures and molecular modeling, site-directed mutagenesis has been used to systematically invert the coenzyme specificity of Thermus thermophilus isopropylmalate dehydrogenase from a 100-fold preference for NAD to a 1000-fold preference for NADP. The engineered mutant, which is twice as active as wild type, contains four amino acid substitutions and an alpha-helix and loop that replaces the original beta-turn. These results demonstrate that rational engineering of secondary structures to produce enzymes with novel properties is feasible.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreadis A., Hsu Y. P., Hermodson M., Kohlhaw G., Schimmel P. Yeast LEU2. Repression of mRNA levels by leucine and primary structure of the gene product. J Biol Chem. 1984 Jul 10;259(13):8059–8062. [PubMed] [Google Scholar]
- Bocanegra J. A., Scrutton N. S., Perham R. N. Creation of an NADP-dependent pyruvate dehydrogenase multienzyme complex by protein engineering. Biochemistry. 1993 Mar 23;32(11):2737–2740. doi: 10.1021/bi00062a001. [DOI] [PubMed] [Google Scholar]
- Bolduc J. M., Dyer D. H., Scott W. G., Singer P., Sweet R. M., Koshland D. E., Jr, Stoddard B. L. Mutagenesis and Laue structures of enzyme intermediates: isocitrate dehydrogenase. Science. 1995 Jun 2;268(5215):1312–1318. doi: 10.1126/science.7761851. [DOI] [PubMed] [Google Scholar]
- Chen R., Greer A., Dean A. M. A highly active decarboxylating dehydrogenase with rationally inverted coenzyme specificity. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11666–11670. doi: 10.1073/pnas.92.25.11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupp J. R., McAlister-Henn L. Kinetic analysis of NAD(+)-isocitrate dehydrogenase with altered isocitrate binding sites: contribution of IDH1 and IDH2 subunits to regulation and catalysis. Biochemistry. 1993 Sep 14;32(36):9323–9328. doi: 10.1021/bi00087a010. [DOI] [PubMed] [Google Scholar]
- Davidow L. S., Kaczmarek F. S., DeZeeuw J. R., Conlon S. W., Lauth M. R., Pereira D. A., Franke A. E. The Yarrowia lipolytica LEU2 gene. Curr Genet. 1987;11(5):377–383. doi: 10.1007/BF00378180. [DOI] [PubMed] [Google Scholar]
- Dean A. M., Dvorak L. The role of glutamate 87 in the kinetic mechanism of Thermus thermophilus isopropylmalate dehydrogenase. Protein Sci. 1995 Oct;4(10):2156–2167. doi: 10.1002/pro.5560041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean A. M., Koshland D. E., Jr Kinetic mechanism of Escherichia coli isocitrate dehydrogenase. Biochemistry. 1993 Sep 14;32(36):9302–9309. doi: 10.1021/bi00087a007. [DOI] [PubMed] [Google Scholar]
- Hamasawa K., Kobayashi Y., Harada S., Yoda K., Yamasaki M., Tamura G. Molecular cloning and nucleotide sequence of the 3-isopropylmalate dehydrogenase gene of Candida utilis. J Gen Microbiol. 1987 Apr;133(4):1089–1097. doi: 10.1099/00221287-133-4-1089. [DOI] [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989 Apr 15;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Hurley J. H., Dean A. M., Koshland D. E., Jr, Stroud R. M. Catalytic mechanism of NADP(+)-dependent isocitrate dehydrogenase: implications from the structures of magnesium-isocitrate and NADP+ complexes. Biochemistry. 1991 Sep 3;30(35):8671–8678. doi: 10.1021/bi00099a026. [DOI] [PubMed] [Google Scholar]
- Hurley J. H., Dean A. M. Structure of 3-isopropylmalate dehydrogenase in complex with NAD+: ligand-induced loop closing and mechanism for cofactor specificity. Structure. 1994 Nov 15;2(11):1007–1016. doi: 10.1016/s0969-2126(94)00104-9. [DOI] [PubMed] [Google Scholar]
- Hurley J. H., Thorsness P. E., Ramalingam V., Helmers N. H., Koshland D. E., Jr, Stroud R. M. Structure of a bacterial enzyme regulated by phosphorylation, isocitrate dehydrogenase. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8635–8639. doi: 10.1073/pnas.86.22.8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada K., Sato M., Tanaka N., Katsube Y., Matsuura Y., Oshima T. Three-dimensional structure of a highly thermostable enzyme, 3-isopropylmalate dehydrogenase of Thermus thermophilus at 2.2 A resolution. J Mol Biol. 1991 Dec 5;222(3):725–738. doi: 10.1016/0022-2836(91)90508-4. [DOI] [PubMed] [Google Scholar]
- Imai R., Sekiguchi T., Nosoh Y., Tsuda K. The nucleotide sequence of 3-isopropylmalate dehydrogenase gene from Bacillus subtilis. Nucleic Acids Res. 1987 Jun 25;15(12):4988–4988. doi: 10.1093/nar/15.12.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A., Suzuki M., Sahara T., Takada Y., Sasaki S., Fukunaga N. Genes encoding two isocitrate dehydrogenase isozymes of a psychrophilic bacterium, Vibrio sp. strain ABE-1. J Bacteriol. 1993 Nov;175(21):6873–6880. doi: 10.1128/jb.175.21.6873-6880.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Sonenshein A. L. Identification of two distinct Bacillus subtilis citrate synthase genes. J Bacteriol. 1994 Aug;176(15):4669–4679. doi: 10.1128/jb.176.15.4669-4679.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi H., Inagaki K., Kuwata Y., Tanaka H., Tano T. 3-Isopropylmalate dehydrogenase from chemolithoautotroph Thiobacillus ferrooxidans: DNA sequence, enzyme purification, and characterization. J Biochem. 1993 Sep;114(3):370–377. doi: 10.1093/oxfordjournals.jbchem.a124183. [DOI] [PubMed] [Google Scholar]
- Kim Y. O., Oh I. U., Park H. S., Jeng J., Song B. J., Huh T. L. Characterization of a cDNA clone for human NAD(+)-specific isocitrate dehydrogenase alpha-subunit and structural comparison with its isoenzymes from different species. Biochem J. 1995 May 15;308(Pt 1):63–68. doi: 10.1042/bj3080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino H., Aoki M., Aoshima M., Hayashi Y., Ohba M., Yamagishi A., Wakagi T., Oshima T. Hydrophobic interaction at the subunit interface contributes to the thermostability of 3-isopropylmalate dehydrogenase from an extreme thermophile, Thermus thermophilus. Eur J Biochem. 1994 Feb 15;220(1):275–281. doi: 10.1111/j.1432-1033.1994.tb18623.x. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Eguchi H., Yamagishi A., Wakagi T., Oshima T. Molecular cloning of the isocitrate dehydrogenase gene of an extreme thermophile, Thermus thermophilus HB8. Appl Environ Microbiol. 1992 Jan;58(1):93–98. doi: 10.1128/aem.58.1.93-98.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K., Oshima T. Co-enzyme specificity of 3-isopropylmalate dehydrogenase from Thermus thermophilus HB8. Protein Eng. 1994 Mar;7(3):401–403. doi: 10.1093/protein/7.3.401. [DOI] [PubMed] [Google Scholar]
- Muro-Pastor M. I., Florencio F. J. NADP(+)-isocitrate dehydrogenase from the cyanobacterium Anabaena sp. strain PCC 7120: purification and characterization of the enzyme and cloning, sequencing, and disruption of the icd gene. J Bacteriol. 1994 May;176(9):2718–2726. doi: 10.1128/jb.176.9.2718-2726.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama M., Birktoft J. J., Beppu T. Alteration of coenzyme specificity of malate dehydrogenase from Thermus flavus by site-directed mutagenesis. J Biol Chem. 1993 Mar 5;268(7):4656–4660. [PubMed] [Google Scholar]
- Rossmann M. G., Moras D., Olsen K. W. Chemical and biological evolution of nucleotide-binding protein. Nature. 1974 Jul 19;250(463):194–199. doi: 10.1038/250194a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Kawano N., Oshima T. Cloning of 3-isopropylmalate dehydrogenase gene of an extreme thermophile and partial purification of the gene product. J Biochem. 1981 Feb;89(2):677–682. doi: 10.1093/oxfordjournals.jbchem.a133245. [DOI] [PubMed] [Google Scholar]
- Thorsness P. E., Koshland D. E., Jr Inactivation of isocitrate dehydrogenase by phosphorylation is mediated by the negative charge of the phosphate. J Biol Chem. 1987 Aug 5;262(22):10422–10425. [PubMed] [Google Scholar]
- Tipton P. A., Beecher B. S. Tartrate dehydrogenase, a new member of the family of metal-dependent decarboxylating R-hydroxyacid dehydrogenases. Arch Biochem Biophys. 1994 Aug 15;313(1):15–21. doi: 10.1006/abbi.1994.1352. [DOI] [PubMed] [Google Scholar]
- Yamada T., Akutsu N., Miyazaki K., Kakinuma K., Yoshida M., Oshima T. Purification, catalytic properties, and thermal stability of threo-Ds-3-isopropylmalate dehydrogenase coded by leuB gene from an extreme thermophile, Thermus thermophilus strain HB8. J Biochem. 1990 Sep;108(3):449–456. doi: 10.1093/oxfordjournals.jbchem.a123220. [DOI] [PubMed] [Google Scholar]
- Zeng Y., Weiss C., Yao T. T., Huang J., Siconolfi-Baez L., Hsu P., Rushbrook J. I. Isocitrate dehydrogenase from bovine heart: primary structure of subunit 3/4. Biochem J. 1995 Sep 1;310(Pt 2):507–516. doi: 10.1042/bj3100507. [DOI] [PMC free article] [PubMed] [Google Scholar]




