Abstract
Evidence is accumulating to suggest that our indigenous microbial communities (microbiota) may play a role in modulating allergic and immune disorders of the skin (Gallo and Nakatsuji, 2011; Macia et al., 2012). To examine the link between the microbiota and atopic dermatitis, we examined a mouse model of defective cutaneous barrier function with an atopic dermatitis-like disease due to loss of Notch signaling. Comparisons of conventionally-raised (CONV-R) and germ-free (GF) mice revealed a similar degree of allergic skin inflammation, systemic atopy, and airway hypersensitivity. GF mutant animals expressed significantly higher levels of thymic stromal lymphopoietin (TSLP), a major proinflammatory cytokine released by skin with defective barrier function, resulting in a more severe B-lymphoproliferative disorder that persisted into adulthood. These findings suggest a role for the microbiota in ameliorating stress signals released by keratinocytes in response to perturbation in cutaneous barrier function.
Keywords: Microbiota, Germ-free mice, TSLP, Asthma, Atopic dermatitis, Notch, B-cells
Introduction
Atopic Dermatitis (AD) is a common allergic disease that affects about 10% of children in the USA and generally resolves by puberty (Spergel, 2010). However, up to 70% of individuals with a history of AD go on to develop asthma and other atopic disorders later in life, a phenomenon called the “atopic march” (Spergel, 2010; Spergel and Paller, 2003). AD flare-ups are known to be associated with overgrowth of bacterial species, including Staphylococcus aureus, in barrier-defective lesional skin (Boguniewicz and Leung, 2011; Kong et al., 2012). Paradoxically, increased AD prevalence correlates with improvements in human hygiene in the last century. One explanation for this correlation is that improved hygiene and increased use of antibiotics have led to decreases in host exposure to microorganisms (Kalliomaki et al., 2001; Macia et al., 2012; Romagnani, 2004; Umetsu et al., 2002; Yazdanbakhsh et al., 2002), which may in turn alter immune responses leading to an increase in atopy.
In this study, we used a mouse model of AD generated by removing the floxed alleles of RBPj, the DNA binding partner of Notch, in the skin via expression of a Msx2-Cre transgene (Demehri et al., 2008; Dumortier et al., 2010). RBPj and Notch signaling are essential for coordinating terminal differentiation of epidermal keratinocytes and formation of a competent barrier (Blanpain et al., 2006). The Msx2-Cre transgene (Demehri et al., 2008; Dumortier et al., 2010) deletes RBPjflox in a mosaic (calico) pattern along the back and stomach of the mouse, allowing juxtaposed patches of RBPj-deficient and wild type skin to be compared and contrasted (Demehri et al., 2008). These mice exhibit many of the hallmarks of AD including T helper (Th)2-mediated skin inflammation, high serum IgE levels, and greater susceptibility to asthma (Demehri et al., 2009; Dumortier et al., 2010). In addition, these mice develop a marked, transient B-cell expansion early in life and a myeloproliferative disorder in adulthood (Demehri et al., 2008; Demehri et al., 2009; Dumortier et al., 2010).
Epidermal-derived thymic stromal lymphopoeitin (TSLP), an IL-7-like cytokine, is responsible for driving inflammation, B-cell expansion, and allergen sensitization in these animals (Demehri et al., 2009; Han et al., 2012; Zhang et al., 2009). Moreover, TSLP is both necessary and sufficient to drive asthma in another mouse model, and it is upregulated in several models displaying cutaneous barrier defects (Comeau and Ziegler, 2010; Demehri et al., 2008; Ziegler and Artis, 2010).
Germ-free mice are widely accepted as being an invaluable tool to characterize the impact of indigenous microbial communities on host physiology. For example, comparisons of germ-free mice and mice colonized with gut microbiota harvested from conventionally-raised mouse donors that have been subjected to deliberately manipulated host and environmental factors, or from human donors representing a variety of physiological or disease states, have provided important insights about the causal relationship between the microbiota and obesity (e.g., (Liou et al., 2013; Turnbaugh et al., 2006; Turnbaugh et al., 2009)), immune development (e.g., (Ivanov et al., 2009)), metabolic syndrome (Vijay-Kumar et al., 2010), cardiovascular disease (Wang et al., 2011), and severe acute malnutrition (Smith et al., 2013). To investigate the role that indigenous microbes play in modulating local as well as systemic inflammation, we generated germ-free Msx2-Cre/+;RBP-jflox/flox and wild-type littermate controls. Germ-free (GF) status did not affect allergic skin inflammation and asthma susceptibility in mutant or wild-type mice contrary to the prediction of the hygiene hypothesis. However, GF mutant mice produced significantly higher epidermal TSLP levels than their microbe-laden, conventionally-raised (CONV-R) counterparts, leading to a persistent expansion of immature B cells. These results suggest a role for indigenous microbial communities in controlling TSLP levels and helping mitigate some deleterious effects such as B-lymphoproliferative disorder (B-LPD). They also highlight the role of the microbiota in modulating cytokine production by keratinocytes.
RESULTS
Morphogenesis and maintenance of the epidermis and hair follicle in GF mice is unaltered
To assess the contribution of indigenous microbial communities to the phenotypes documented in our animals, we re-derived Msx2-Cre/+;RBP-jflox/flox and wild-type littermate control embryos into pseudopregnant sterile dams using an embryo transfer technique described in an earlier publication (Faith et al, 2010). The GF status of the resulting mice was confirmed by aerobic and anaerobic culture as well as by PCR assays targeting conserved regions of the bacterial 16S rDNA gene. Skin morphology and histology in wild-type GF and CONV-R animals were indistinguishable (Fig. 1A,C). At postnatal day 9 (P9), Msx2-Cre/+;RBP-jflox/flox (RBPjCKO) mutant skin had a thickened epidermis, a highly cellular dermis and hair-follicle derived epidermal cysts (Fig 1A). These changes became more pronounced at P30 (Fig 1C) and occurred independently of the presence or absence of the microbiota. Immunocytochemical staining for filaggrin (FLG), involucrin (INV), and keratin-14 (K14) revealed that GF and CONV-R wild-type mice expressed these differentiation markers in the granular, spinous, and basal epidermis, respectively (Fig 1B,D). Conversely, RBPjCKO mice showed expansion of K14 into suprabasal cells at P9 (Fig 1B). At P30, FLG and INV expression overlapped, an indication of abnormal differentiation (Fig 1D). Trans-epidermal water loss (TEWL) measurements detected a barrier defect of similar magnitude in P9 GF and CONV-R RBPjCKO mice (Fig S1). Collectively, these observations indicate that morphological and functional characteristics of the skin, and the dysfunction caused by the absence of Notch signaling, are not modulated by the microbiota. This allowed us to ask if the microbiota plays a role in skin inflammation and atopy in the RBPjCKO model.
Figure 1. Epidermal and hair follicle structure are unaffected in germ-free (GF) mice compared to their conventionally-raised (CONV-R) counterparts.
H&E staining at P9 (A) and P30 (C) shows skin morphology and inflammation levels in GF and CONV-R RBPjCKO and wild-type (Wt) mice. Scale bar, 50μm. Immunocytochemical staining for Keratin-14 (K14), Involucrin (INV), and Filaggrin (FLG) at P9 (B) and P30 (D) shows skin architecture in GF and CONV-R wild-type and RBPjCKO animals. Both GF and CONV-R RBPjCKO animals exhibit similar barrier defects. Scale bar, 10μm.
Skin inflammation and AD-like disease persist in GF RBPjCKO mice
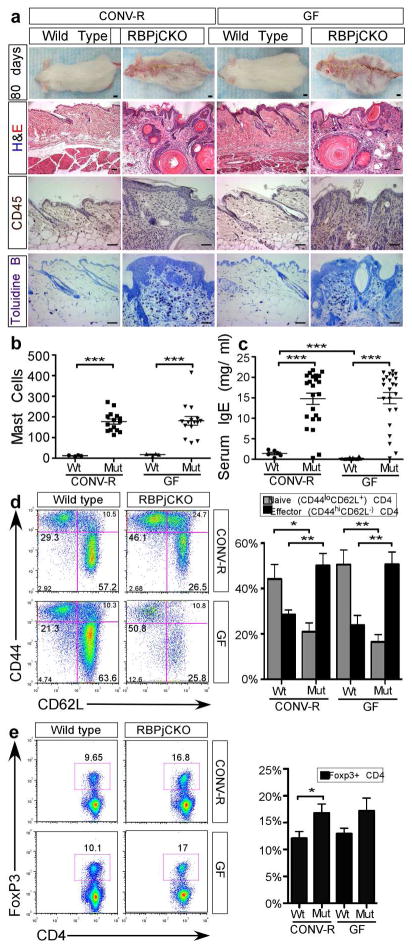
To better understand the role that indigenous microbial communities play in skin inflammation and the systemic diseases associated with cytokine production by skin with a chronic barrier defect, we analyzed RBPjCKO mice at 10–13 weeks, the age at which the AD-like phenotype develops (Demehri et al., 2009; Dumortier et al., 2010). GF RBPjCKO mice were morphologically and histologically indistinguishable from their CONV-R RBPjCKO counterparts (Fig 2A) with all mice displaying the same macroscopic signature of AD, including excoriation and scaling. Histological analysis of H&E stained sections revealed similar levels of hyperkeratosis, parakeratosis and acanthosis. Staining for CD45 (a pan haematopoietic cell marker) and Toluidine blue staining revealed similar levels of skin inflammation and mast cell infiltration, respectively, in both GF and CONV-R animals (Fig 2A,B). Tumor resistance and loss of mutant keratinocytes are TSLP and T cell-dependent consequences of the severe inflammatory response that develops in RBPjCKO mice over a period of 75–200 days (Demehri et al., 2012; Di Piazza et al., 2012). The absence of a microbiota did not affect this inflammatory, Th2-mediated response.
Figure 2. Skin inflammation and AD-like disease persist in GF RBPjCKO animals.
(A) CONV-R and GF P80 mice. Scale bar, 1cm. H&E, CD45 immunohistochemistry, and Toluidine blue staining reveal skin inflammation and mast cell infiltration in both CONV-R and GF wild-type (Wt) and RBPjCKO (Mut) mice. Scale bar, 50μm. (B) Quantification of mast cells viewed in three randomly selected microscopic fields at 200x (CONV-R Wt, n=4; CONV-R Mut, n=15; GF Wt, n=4; GF Mut, n=15). (C) Serum IgE levels in P80 Wt mice (CONV-R, n=10; GF, n=6) and RBPjCKO animals (CONV-R, n= 23; GF, n=23). (D) FACS gated on CD4+ T cells in the spleen to detect effector (CD44hi CD62L−) and naïve (CD44lo CD62L+) cells in RBPjCKO mice (CONV-R, n=9; GF, n=9) and in Wt mice (CONV-R, n=4; GF, n=4). A compilation of results from four independent experiments is shown. (E) FACS gated on CD4+ T cells detects regulatory T-cells (Treg, Foxp3+) in RPBjCKO (GF and CONV-R, n=6) and Wt mice (CONV-R and GF, n=3). A compilation of three independent experiments is shown. Asterisks marks statistical significance; * p<0.05, ** p<0.01, *** p<0.001(unpaired Students t-test).
Finally, systemic atopy, as measured by serum IgE levels, was also not significantly different between the GF and CONV-R RBPjCKO mice (Fig 2C); effector CD4+ T cells (CD44hiCD62L-) were elevated to similar levels in RBPjCKO mice in the spleen, skin-draining lymph nodes, and mesenteric lymph nodes (Fig 2D and Fig. S2). RBPjCKO animals had higher percentages of FoxP3+ Treg cells among CD4+ T cells compared to their wild-type littermates in the mesenteric and skin draining lymph nodes as well as in the spleen (Fig 2E and Fig S2).
GF RBPjCKO mice have elevated TSLP levels but similar asthma susceptibility
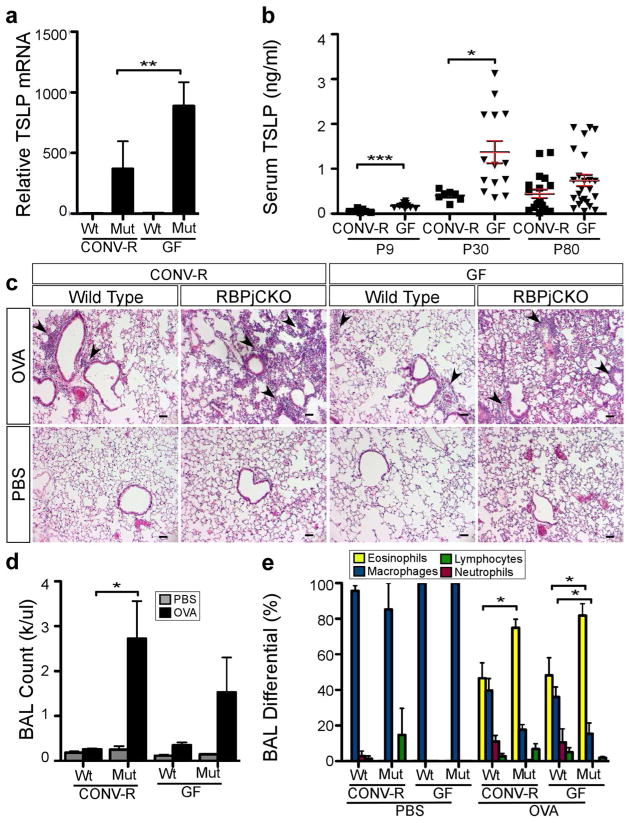
TSLP produced by the perturbed epidermis is a significant driver of skin inflammation and many of the systemic effects in this mouse model (Demehri et al., 2008; Demehri et al., 2009; Demehri et al., 2012). Given the similar degree of disruption of epidermal differentiation and cutaneous barrier function (Fig 1, Fig 2 and Fig S1), we were surprised to find two-fold higher epidermal Tslp mRNA levels in the GF RBPjCKO mice compared to CONV-R controls at P9 (p<0.05; n=6–10 mice/group; Fig 3A). Elevated Tslp mRNA levels in the skin were associated with elevated serum TSLP levels at P9 and P30 (Fig 3B).
Figure 3. Asthma in GF RBPjCKO mice is not more severe but TSLP levels are higher than in their CONV-R counterparts.
(A) Tslp mRNA levels in P9 CONV-R Wt (n=6), CONV-R RBPjCKO (n=7), GF Wt (n=10) and GF RBPjCKO (n=10) mice. (B) Serum TSLP levels in P30 RBPjCKO (CONV-R, n=7; GF,n=14) and P80 (CONV-R, n=19; GF, n=24). (C) H&E staining of lungs after OVA-challenge at 6–8 weeks. Scale bar, 50μm (D) WBC count in bronchial alveolar lavage fluid (BAL) of Wt (CONV-R, n=6; GF, n=10) and RBPjCKO (CONV-R, n=8; GF, n= 8) mice. (E) The percentage of eosinophils, macrophages, lymphocytes, or neutrophils in BAL from RBPjCKO (CONV-R, n=11; GF, n=9) or Wt (CONV-R, n=3; GF, n=4) mice. The differences between RBPjCKO and wild-type animals remain evident in a GF state. * p<0.05, ** p<0.01, *** p<0.001 (unpaired Students t-test).
To determine whether the microbiota ameliorated the severity of asthma in CONV-R mice, we subjected CONV-R and GF mice to Ovalbumin (OVA) sensitization and intranasal challenge. Wild-type GF and CONV-R littermates displayed similar mild responses to OVA challenge (Fig. 3). Similarly, no significant differences in the severity of the asthmatic response were detected between GF and CONV-R-RBPjCKO animals in the severity of the asthmatic response, as judged by lung histology and total and total counts of leukocytes and other cellular elements in lung washings obtained by brocheoalveolar lavage (BAL) (Fig. 3E–G). These data suggest that although the microbiota might well regulate skin and serum levels of TSLP in the absence of Notch signaling in the skin, the microbiota does not modulate atopic dermatitis and asthma in this model.
GF RBPjCKO mice have elevated WBCs and persistent B-cell expansion
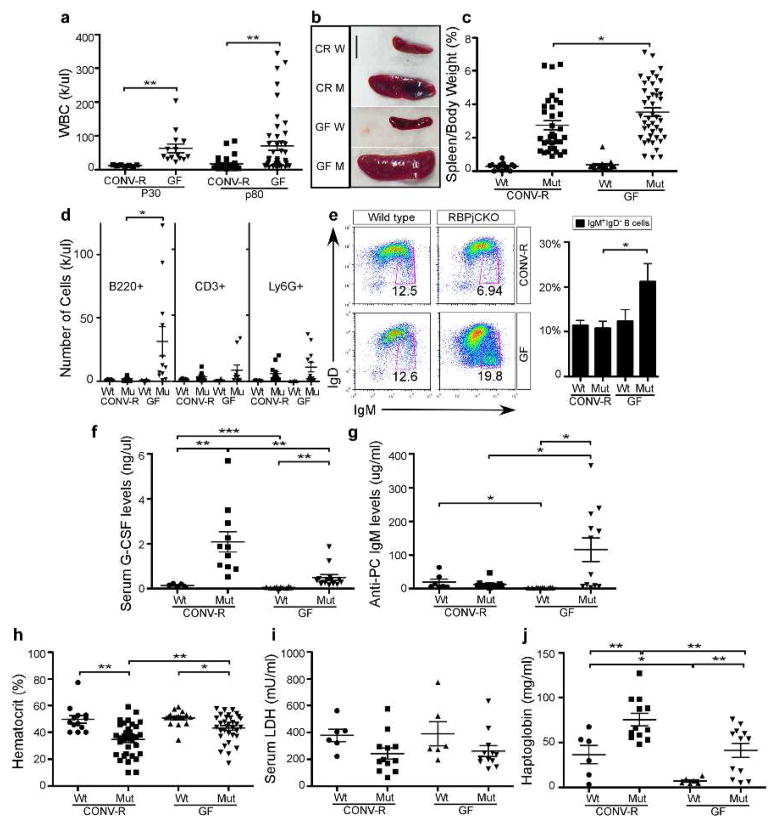
Epidermal-derived TSLP directly drives B-cell lymphoproliferative disease (B-LPD) in newborns. B-LPD severity mirrors TSLP levels early in life but is resolved after P30 despite persistently high TSLP levels (Demehri et al., 2008). Myeloproliferative disorder (MPD) replaces B-LPD, persisting into adulthood (Demehri et al., 2009; Dumortier et al., 2010). Surprisingly, white blood cell (WBC) counts did not normalize in young (P30-P45) GF RBPjCKO animals (Fig 4A). At P80, WBC counts in GF RBPjCKO mice remained significantly higher than in CONV-R animals, splenomegaly was more severe (Fig 4A–C), B-cell number were elevated (CD45+B220+; Fig 4D) and T-cell numbers were unchanged (CD45+CD3+; Fig 4D) in the blood. As previously reported, MPD in RBPjCKO animals coincided with an increase in granulocyte colony stimulating factor (G-CSF). Although G-CSF levels were significantly lower in GF RBPjCKO mice (Fig. 4F), MPD (CD45+Ly6G+) persisted to the same extent as it did in CONV-R RBPjCKO animals (Fig 4D).
Figure 4. B cell expansion persists in GF RBPjCKO animals.
(A) WBC counts in P30 RBPjCKO mice (CONV-R, n=7; GF, n=14) and P80 (CONV-R, n=33; GF, n=43). Spleen size mirrored WBC levels (B-C), reported as a percentage of body weight (Wt, CONV-R n=13; GF, n=19; Mut CONV-R, n=34; GF, n=43). Scale bar = 1cm. (D) B- cell (B220+ CD45+), myeloid (Ly6G+) and T cell (CD3+) expansion in 10–12 week old mice, quantified by flow cytometry (Wt CONV-R, n=5; GF, n=4; RBPjCKO CONV-R, n=13, GF, n=12). (E) The percentage of immature B220+ cells (IgM+ IgDlo) in GF RPBjCKO animals. Data compiled from five independent experiments (Wt CONV-R, n=5, GF, n=4; RBPjCKO CONV-R & GF, n=11). (F) G-CSF levels in serum from GF and CONV-R mice (Wt CONV-R, n=5, GF, n=10; RBPjCKO CONV-R, n=11; GF n=14). (G) Anti-phosphorylcholine (anti-PC) IgM levels (Wt CONV-R, n=6; GF, n-10; RBPjCKO CONV-R, n=9; GF n=12). (H) RBPjCKO mice show lower hemotcrit (HCT) than Wt counterparts (Wt CONV-R, n=13; GF, n=17; RBPjCKO CONV-R, n=34; GF n=43). (I) Serum LDH levels (Wt CONV-R & GF, n=6; RBPjCKO CONV-R and GF, n=12). (J) Serum haptoglobin levels (Wt CONV-R and GF, n=6; RBPjCKO CONV-R and GF, n=12;). * p<0.05, ** p<0.01, *** p<0.001 (unpaired Students t-test).
The hematocrit (HCT) in both the GF and CONV-R RBPjCKO mice was significantly lower than in their wild-type littermates. We hypothesized that epidermal-derived TSLP may drive a B-cell-mediated autoimmune hemolytic anemia, which could cause low HCT (Han et al., 2012). To test if autoimmune hemolytic anemia (AIHA) occurs in our mice, we measured the levels of lactate dehyrogenase (LDH) and haptoglobin. In AIHA, release of LDH from lysed RBCs elevates its level in serum, and because haptoglobin binds to hemoglobin released from lysed RBCs, its level plummets (Tabbara, 1992). We detected no significant difference in LDH levels between wild-type and RBPjCKO (Fig 4I, S3A), and serum haptoglobin levels were significantly higher in both GF and CONV-R RBPjCKO compared to wild-type mice (Fig 4J, S3B). We concluded that it is unlikely that the anemia is due to AIHA. An alternative explanation for the low HCT is that an expanded B-cell and granulocytic compartment may cause a reactive contraction in other cell types without autoimmunity.
Analysis of P80 GF RBPjCKO mice identified both mature (B220+ CD23+ CD93−; Fig S2) and immature (IgD− IgM+) B cells (Fig 4C). High levels of TSLP in newborn GF RBPjCKO animals could affect neonatal B1 cell production dynamics or the activities of skin B1 cells in adults (Geherin et al., 2012; Vosshenrich et al., 2004). Accordingly, we detected a significantly elevated level of resting anti-phosphorylcholine IgM (anti-PC IgM), most of which is made by B1 cells, in the sera of GF RBPjCKO mice (Fig 4F). This is not solely a result of losing microbial antigens, since GF wild-type mice have significantly lower levels of anti-PC IgM than their CONV-R counterparts (Fig 4G). Collectively, these data establish that the microbiota has effects on B-cells but not T-cells in mice with chronic cutaneous barrier defects.
Discussion
The role of indigenous microbial communities in skin development and function is only beginning to be explored (Grice and Segre, 2011; Lai et al., 2009; Naik et al., 2012). In this study, mice deficient in Notch signaling in their skin that develop severe, chronic skin inflammation and systemic atopy were re-derived as germ-free. Morphologically and histologically, skin pathology was unaffected by the presence of a microbiota, and lack of a microbiota did not modulate skin inflammation in these animals. Surprisingly, despite these similarities, we observed an increase in the production of TSLP by the skin and the amount of TSLP in the serum of GF animals. These results indicate that the microbiota plays a role in suppressing TSLP production in the barrier-defective skin.
TSLP is emerging as an essential cytokine in the development of allergic responses (Demehri et al., 2009; Han et al., 2012; Jiang et al., 2012; Yoo et al., 2005; Zhang et al., 2009; Zhou et al., 2005). Local TSLP expression in the skin is sufficient for the progression from atopic dermatitis to asthma (Han et al., 2012). Consistent with the finding that TSLP is upregulated in utero (Demehri et al., 2008), microbial products are not necessary to induce TSLP expression in barrier defective skin but may instead limit its expression level or duration.
In contrast to the transient B-cell lymphoproliferative disorder (B-LPD) that is apparent in neonatal CONV-R RBPjCKO mice and transitions to myeloproliferative disease (MPD) in adulthood, both B-LPD and MPD persist to adulthood in GF animals (Demehri et al., 2008). Previously, we proposed that fetal B cells are responsive to TSLP but that marrow-derived B cells are not (Demehri et al., 2008). The present study demonstrates that marrow-derived B cells continue to be responsive to TSLP in GF mice. It is possible that in GF mice, B cell maturation is delayed or altered in the presence of TSLP, establishing a persistent B-LPD and expansion of B1 cells. One mechanistic explanation may relate to the lower G-CSF levels that we document in GF RBPjCKO animals; G-CSF has been shown to suppress B cells in the bone marrow (Dumortier et al., 2010; Winkler et al., 2012) and could thus contribute to resolving B-LPD. These observations also indicate that the link between TSLP and G-CSF (Dumortier et al., 2010) does not require the presence of a microbiota.
Despite an increase in TSLP and WBC counts, we see no evidence for a more severe allergic disease in GF RBPjCKO animals. These findings do not support the hypothesis that complete lack of a microbiota will create a runaway Th2-skewed immune response in this model, but understanding the generality of this observation will depend on the results of similar experiments with other AD models. While our findings clearly demonstrate that the microbiota modulates expression of at least one proinflammatory cytokine in barrier defective skin, a task ahead is to identify which microbial species or species consortia from which body habitat-associated communities mediate these observed effects and through which signaling pathways. Colonization of the GF mice we describe in this report with components of the skin and/or gut microbiota should provide a path ahead for addressing these questions.
Materials & Methods
Mice
All experiments involving mice were conducted using protocols approved by the Animal Studies Committee of Washington University. Msx2-Cre/+;RBP-jflox/flox (RBPjCKO) mice were generated as previously described (Pan et al., 2004). GF mice were derived by embryo extraction, sterilization and transfer into a pseudopregnant sterile dam (Faith et al., 2010) and maintained in flexible film gnotobiotic isolators under a strict 12h light cycle on a standard plant polysaccharide-rich low fat chow diet fed ad libitum (B&K Universal; England). Maintenance of GF status in the colony was confirmed using culture-independent methods (i.e. negative PCR assays of fecal DNA using universal primers directed against bacterial 16S rRNA genes), or culture-based approaches (culturing feces in brain heart infusion (BHI) broth, nutrient broth or Sabouraud dextrose broth under anaerobic conditions). We analyzed age- and sex- matched GF wild-type littermates and their CONV-R siblings (i.e. mice derived from the same founders but reared in a mouse barrier facility in a specified pathogen-free state, and acquiring microbes from their environment beginning at birth).
OVA Treatment
Airway challenge was performed on GF and CONV-R mice as outlined previously (Zhou et al., 2005). Six to eight week-old female mice were sensitized by intraperitoneal injection of 250μL of a solution containing 50 μg OVA (Sigma A5503) dissolved in PBS and 1.3 mg aluminum hydroxide gel (Sigma; St Louis, MO) on experimental days 1 (d1) and d14. Mice were challenged intra-nasally with 150 μg of OVA dissolved in 40 μL of PBS on d21, d22, and d23. Controls were given the same regimen without the addition of OVA antigen. Mice were sacrificed on d24.
Histology, Immunohistochemistry, and Immunofluorescence
After sacrifice, lungs were inflated with 4% PFA at 25 cm water pressure. Dorsal skin and lung tissues were fixed in 4% PFA overnight, dehydrated in ethanol and embedded in paraffin. 5μm thick sections were used for all analyses. To assess inflammation and skin morphology, sections were stained with hematoxylin and eosin (H&E) or toluidine blue. Mast cell infiltration in the skin was quantified by counting the average number of toluidine blue positive cells in three randomly selected microscopic fields at 200x magnification. Paraffin embedded sections were rehydrated and stained with biotinlylated anti-keratin 14 (clone LL002, Neomarkers), chick anti-filaggrin, and rabbit anti-involucrin, and antigen-antibody complexes were visualized with Cy5-conjugated streptavidin, Cy3-conjugated anti-rabbit Ig, and Alexa488-conjugated anti-chicken Ig (all from Jackson ImmunoResearch Laboratory). CD45 staining was performed as previously described (Demehri et al., 2012).
BAL and WBC Analyses
Bronchoalveolar (BAL) fluid was collected by infusing 1 mL of PBS into the lung at sacrifice using a Surflo catheter (Terumo Medical; Somerset, NJ) (Demehri et al., 2009). BAL fluid was spun down, the cell pellet was stained with Giemsa and at least 100 cells were counted to determine the differential leukocyte cell counts. Blood was diluted 1:1 with 10mM EDTA in PBS and total white blood cell count and hematocrit were measured using a Hemavet 950 analyzer.
Serology
Serum IgE was determined using Mouse IgE ELISA (Immunology Consultants Laboratory). Serum TSLP and G-CSF levels were measured using Mouse TSLP ELISA (Biolegend; San Diego, CA) and Mouse G-CSF ELISA (R&D Systems; Minneapolis, MN), respectively. Serum anti-phosphorylcholine IgM (anti-PC) was measured by coating 96-well flat-bottom EIA/RIA plates (Costar; Tewksbury, MA) overnight at 4°C with 2 μg/mL of PC-BSA and performing ELISA assays (Foote and Kearney, 2009). To test for hemolytic anemia, serum LDH was determined using LDH cytotoxicity assay (Cayman Chemical Company; Ann Arbor, MI), and serum haptoglobin was measured using Mouse Haptoglobin Elisa Test Kit (Life Diagnostics, Inc; West Chester, PA).
Flow Cytometry
This method was applied to single cell suspensions prepared from spleen, skin-draining lymph nodes, or mesenteric lymph nodes. Cells were surface stained with the reagents listed in Supplementary Materials and Methods, fixed overnight at 4°C using FoxP3 Fixation/Permabilization buffer (eBioscience; San Diego, CA), and stained intracellularly using FoxP3 Permeabilization buffer as per the manufacturer’s instructions. Samples were acquired on a BD FACScan or BD LSR II flow cytometer. Data were analyzed using FlowJo software (TreeStar; Ashland, OR).
PCR and qRT-PCR
To genotype mice, PCR was performed on DNA extracted from toes (Demehri et al., 2009). For gene expression analysis, mice were euthanized on P9, the skin was removed, placed on dry ice. The epidermis was scraped off using a scalpel, homogenized, and RNA was isolated using RNeasy Mini Kit (Qiagen; Germantown, MD). cDNA was generated using Superscrpipt RT-II kit (Invitrogen; Grand Island, NY). Quantitative RT-PCR was performed for mouse Tslp and Hprt mRNA (control) as previously described (Demehri et al., 2008; Lee et al., 2007).
Statistical Analysis
An unpaired Students t-test was used to test statistical significance; p<0.05 was considered significant. Mean values ± SEM are presented in all graphs.
Supplementary Material
Acknowledgments
We would like to thank Michael Holtzman for helpful discussions and many valuable reagents and the members of the Kopan laboratory for their suggestions during the course of this study. We would like to thank Gail Martin for providing Msx2-Cretg/+ mice, Tasuku Honjo for Rbpjflox/flox mice, plus Dave O’Donnell and Maria Karlsson and other members of the Gordon lab for their assistance with and advice about gnotobiotic husbandry. This work was supported with funds from the American Asthma Foundation (Grant 09-0234) to RK. AT was supported by NIH AI070489, J.K by NIAID AI14782-33, and J.I.G by NIH grant P30DRO52574 (Murine Model Core of the Washington University Digestive Disease Research Core Center, DDRCC) and by the Crohn’s and Colitis Association of America.
Footnotes
Conflict of interest: The authors state no conflict of interest
References
- Blanpain C, Lowry WE, Pasolli HA, Fuchs E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006;20:3022–3035. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau MR, Ziegler SF. The influence of TSLP on the allergic response. Mucosal Immunol. 2010;3:138–147. doi: 10.1038/mi.2009.134. [DOI] [PubMed] [Google Scholar]
- Demehri S, Liu Z, Lee J, Lin MH, Crosby SD, Roberts CJ, et al. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. 2008;6:e123. doi: 10.1371/journal.pbio.0060123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demehri S, Morimoto M, Holtzman MJ, Kopan R. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067. doi: 10.1371/journal.pbio.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demehri S, Turkoz A, Manivasagam S, Yockey LJ, Turkoz M, Kopan R. Elevated Epidermal Thymic Stromal Lymphopoietin Levels Establish An Anti-Tumor Environment In The Skin. Cancer Cell. 2012;4:494–505. doi: 10.1016/j.ccr.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Piazza M, Nowell CS, Koch U, Durham AD, Radtke F. Loss of Cutaneous TSLP-Dependent Immune Responses Skews the Balance of Inflammation from Tumor Protective to Tumor Promoting. Cancer Cell. 2012;22:479–493. doi: 10.1016/j.ccr.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Dumortier A, Durham AD, Di Piazza M, Vauclair S, Koch U, Ferrand G, et al. Atopic dermatitis-like disease and associated lethal myeloproliferative disorder arise from loss of Notch signaling in the murine skin. PLoS One. 2010;5:e9258. doi: 10.1371/journal.pone.0009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith JJ, Rey FE, O’Donnell D, Karlsson M, McNulty NP, Kallstrom G, et al. Creating and characterizing communities of human gut microbes in gnotobiotic mice. ISME J. 2010;4:1094–1098. doi: 10.1038/ismej.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote JB, Kearney JF. Generation of B cell memory to the bacterial polysaccharide alpha-1,3 dextran. J Immunol. 2009;183:6359–6368. doi: 10.4049/jimmunol.0902473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol. 2011;131:1974–1980. doi: 10.1038/jid.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geherin SA, Fintushel SR, Lee MH, Wilson RP, Patel RT, Alt C, et al. The skin, a novel niche for recirculating B cells. J Immunol. 2012;188:6027–6035. doi: 10.4049/jimmunol.1102639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9:244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Xu W, Headley MB, Jessup HK, Lee KS, Omori M, et al. Thymic stromal lymphopoietin (TSLP)-mediated dermal inflammation aggravates experimental asthma. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Hener P, Li J, Li M. Skin thymic stromal lymphopoietin promotes airway sensitization to inhalant house dust mites leading to allergic asthma in mice. Allergy. 2012;67:1078–1082. doi: 10.1111/j.1398-9995.2012.02857.x. [DOI] [PubMed] [Google Scholar]
- Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076–1079. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 2009;15:1377–1382. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Basak JM, Demehri S, Kopan R. Bi-compartmental communication contributes to the opposite proliferative behavior of Notch1-deficient hair follicle and epidermal keratinocytes. Development. 2007;134:2795–2806. doi: 10.1242/dev.02868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou AP, Paziuk M, Luevano JM, Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra141. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia L, Thorburn A, Binge L, Marino E, Rogers K, Maslowski K, et al. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunological reviews. 2012;245:164–240. doi: 10.1111/j.1600-065X.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, et al. Compartmentalized Control of Skin Immunity by Resident Commensals. Science. 2012 doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Lin M, Tian X, Cheng H, Gridley T, Shen J, et al. g-Secretase functions through Notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Dev Cell. 2004;7:731–743. doi: 10.1016/j.devcel.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Romagnani S. The increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both? Immunology. 2004;112:352–363. doi: 10.1111/j.1365-2567.2004.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339:548–554. doi: 10.1126/science.1229000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel JM. From atopic dermatitis to asthma: the atopic march. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2010;105:99–106. doi: 10.1016/j.anai.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112:S118–127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Tabbara IA. Hemolytic anemias. Diagnosis and management. Med Clin North Am. 1992;76:649–668. doi: 10.1016/s0025-7125(16)30345-5. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshenrich CA, Cumano A, Muller W, Di Santo JP, Vieira P. Pre-B cell receptor expression is necessary for thymic stromal lymphopoietin responsiveness in the bone marrow but not in the liver environment. Proc Natl Acad Sci U S A. 2004;101:11070–11075. doi: 10.1073/pnas.0402919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler IG, Bendall LJ, Forristal CE, Helwani FM, Nowlan B, Barbier V, et al. B-lymphopoiesis is stopped by mobilizing doses of G-CSF and is rescued by overexpression of the anti-apoptotic protein Bcl2. Haematologica. 2012 doi: 10.3324/haematol.2012.069260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Hener P, Frossard N, Kato S, Metzger D, Li M, et al. Thymic stromal lymphopoietin overproduced by keratinocytes in mouse skin aggravates experimental asthma. Proc Natl Acad Sci U S A. 2009;106:1536–1541. doi: 10.1073/pnas.0812668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.