Abstract
Nonmetastatic gene 23-H1 (NME1, also known as nm23-H1) is a wide-spectrum tumor metastasis suppressor gene that plays an important role in suppressing the proliferation, adhesion and invasion of endometrial stromal cells (ESCs). The present study is undertaken to explore the mechanism by which NME1 in ESCs from endometriosis modulates the angiogenesis and herein participates in the pathogenesis of endometriosis. The expression of NME1 in the primary ESCs from normal endometrium without endometriosis was higher than that from eutopic endometrium and ectopic lesion with endometriosis. Silencing NME1 stimulated the secretion of angiogenic factors interleukin-8 (IL-8) and vascular-endothelial growth factor (VEGF) of the eutopic ESCs from women with endometriosis, and these effects could be abrogated by MAPK/ERK1/2 or AKT inhibitor. In addition, the supernatant of NME1-silenced ESCs increased the expression of angiogenesis-relative molecules CD62E and CD105, and promoted angiogenesis of human umbilical vein endothelial cells (HUVECs). Anti-human IL-8 or VEGF neutralizing antibody reversed the effect on angiogenesis of HUVECs induced by NME1-silenced ESCs. Our current results suggest that the abnormal lower expression of NME1 in ESCs secrete more IL-8 and VEGF through activation of MAPK/ERK1/2 and AKT signal pathways, up-regulate the level of CD62E and CD105, and finally lead to numerous angiogenesis of vascular endothelial cells in the endometriotic milieu, which is beneficial to the origin and development of endometriosis.
Keywords: NME1, ESCs, HUVECs, angiogenesis, endometriosis
Introduction
Endometriosis represents a common gynecological disorder, which is characterized by the presence and growth of endometriotic lesions consisting of endometrial glands and stroma outside the uterine cavity [1]. The mechanisms contributing to the establishment of endometriotic lesions still remains controversial despite extensive research. In fact, similar to tumors and their metastases, there is no doubt that long-term survival and proliferation of these lesions are crucially dependent on the formation of new blood vessels, which guarantee oxygen and essential nutrient supply [2-7]. Several growth factors and cytokines have been recognized as angiogenic factors, such as interleukin-8 (IL-8), vascular endothelial growth factor (VEGF) [8-11].
The nonmetastatic gene 23 (NME, also known as nm23) has been identified as a family of genes encoding different isoforms of nucleoside diphosphate kinase (NDPK) [12]. Ten isotypes of the human NM23 gene have been identified [13]. Among these, only NME1 and NME2 have been studied extensively in human cancers. Based on tumor prognostic and transfection studies, a correlation of increased NME1 expression to low metastatic potential of cancer cells has been established in several malignancies [14-16]. Our previous research has established that NME1 is also expressed in human endometrial stromal cells (ESCs) and plays a significant role in regulating the growth, adhesion and invasion of ESCs [17]. NME1 is also expressed in human first-trimester trophoblasts and plays a significant part in the regulation of human trophoblast invasion [18,19]. Moreover, we have shown that human first-trimester trophoblasts secrete thymic stromal lymphopoietin (TSLP), which induces the proliferation and invasion of human trophoblasts in an autocrine manner through down-regulating the expression of tumor metastasis suppressor NME1. Che et al have reported that transfection of NME1 decreases the expression of VEGF of lung cancer cells [20]. However, there are still questions whether NME1 of ESCs is involved in the angiogenesis in the endometriotic milieu through modulating the expression of angiogenic factors.
Therefore, the present study is undertaken to evaluate the expression of NME1 in ESCs from women with or without endometriosis, and further investigate the effect and mechanism of NME1 in ESCs on the angiogenesis of human umbilical vein endothelial cells (HUVECs).
Materials and methods
Tissue collection
All tissue samples were obtained with informed consent in accordance with the requirements of the Research Ethics Committee in Hospital of Obstetrics and Gynecology, Fudan University Shanghai Medical College. Samples of the endometriotic peritoneal lesion (n=6) were obtained from women age 29-47 years undergoing laparoscopy for pain or other benign indications. The endometrial tissues were obtained from fertile women (age 25-44 years) with (n=18) or without (n=6) endometriosis as control. None of the women had received hormonal medication in the 3 months prior to the surgical procedure. All the samples were confirmed histologically according to established criteria.
Cell isolation and culture
The endometrial tissue and lesion were collected under sterile conditions and transported to the laboratory on ice in DMEM (Dulbecco’s modified Eagle’s medium)/F-12 (Gibco, USA). The ESCs were isolated according to the previous methods [17,21,22]. The HUVECs cell line was maintained as monolayers in Kaighn Modification of DMEM/F-12 Medium supplemented with 10% FCS (Hyclone, Logan, UT, USA) and 10 ng/ml human EGF (R&D Systems, Abingdon, UK). These cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Immunocytochemistry
The NME1 protein level in primary ESCs of the endometriotic and eutopic endometrial tissues from women with or without endometriosis were evaluated by immunocytochemical staining, as previously described [23]. ESCs growing on coverslips were cultured for 48 h. The coverslips were fixed in 4% (vol/vol) paraformaldehyde for 20 min at room temperature, washed in PBS and permeabilized for 10 min with 0.25% (vol/vol) Triton-100 in PBS. The cells were then incubated with 1% BSA in PBS/Tween (PBST) for 30 min to block non-specific binding of antibodies. The anti-human vimentin monoclonal antibody as markers for ESCs, and cytokeratin-7 antibodies as markers for glandular epithelial cells were then added (Golden Bridge International, Inc., Beijing, China). The anti-human NME1 monoclonal antibody (SC-465, 1:100, Santa Cruz Biotechnology, Inc., CA, USA) was used to evaluate NME1 expression in three kinds of ESCs. The cells were incubated with primary antibody or isotypic control overnight at 4°C, and then incubated with a peroxidase-conjugated secondary antibody (Golden Bridge International, Inc.) for 60 min at 37°C. The slides were stained with DAB, and counterstained with hematoxylin. The experiments were repeated five times.
NME1 silence in ESCs
According to the description by our previous method [17], NME1 in ESCs from eutopic endometrium with endometriosis was effective silenced by siRNA transfection. These siRNAs and related non-silencing controls were synthesized by Invitrogen.
Tube formation assay (matrigel assay)
The tube formation was performed as Klein described [24]. In brief, HUVECs were seeded on growth factor-reduced Matrigel in normal growth medium with the supernatant of siRNA transfected ESCs, and cultured with or without anti-VEGF or anti-IL-8 neutralizing antibody (R&D system). Capillary-like tube formation was quantified by counting numbers of junctions/enclosed circles in 5 randomly chosen optical fields using light microscopy after 16 hours.
Flow cytometry
After the siRNA-transfected eutopic ESCs were treated with or without U0126 (30 uM) or LY294002 (PI3 Kinase Inhibitor) (50 uM) (Cell Signaling Technology, MA, USA) for another 24 h, with vehicle as control, the supernatant was collected. Subsequently, we incubated HUVECs with the supernatant for 48 h, and then digested with 0.25% trypsin only for 30-50 s, and blown off gently and washed with phosphate-buffered saline (PBS). After blocking with 10% FBS, the recovered cells were mixed with mouse anti-human CD31-Percp5.5 monoclonal antibody and CD62E-Allophycocyanin (APC) monoclonal antibody or CD105-fluorescein isothiocyanate (FITC) monoclonal antibody (Biolegend, USA), meanwhile, the isotypic control was used. After incubation in darkness for 30 min at room temperature, the cells were analyzed immediately by a flow cytometer (FACS Calibur, BD). The experiments were repeated five times.
ELISA
The siRNA-transfected eutopic ESCs were treated with or without U0126 (30 uM) or LY294002 (50 uM) for another 24 h, with vehicle as control. Then the secretion of VEGF, IL-8, and IL-6 by ESCs were detected by ELISA (R&D system), according to the manufacturer’s instruction.
Statistics
All values were shown in the mean±SD. Data were analyzed by using one-way analysis of variance and least significant difference (equal variances assumed), or Tamhane’s test (equal variances not assumed) was used post hoc for multiple comparisons with Statistical Package for the Social Sciences software version 11.5. Differences were considered as statistically significant at P<0.05.
Results
The expression of NME1 is decreased in the eutopic and ectopic ESCs from women with endometriosis
At first, we isolated normal ESCs from healthy control, eutopic and ectopic ESCs from women with endometriosis, respectively, and further compared the expression of NME1 in these three kinds of ESCs. As shown in Figure 1, immunocytochemistry showed more than 95% vimentin-positive and cytokeratin-negative ESCs (Figure 1). Eutopic and ectopic ESCs were weak stained for NME1, in contrast to stronger staining in normal ESCs (Figure 1). However, there was no difference in NME1 expression between the eutopic and ectopic ESCs (Figure 1). Collectively, these results suggest that abnormal lower expression of NME1 in the ESCs from endometriosis may be associated with the unique biological characteristics of the ESCs in the endometriotic milieu.
Figure 1.
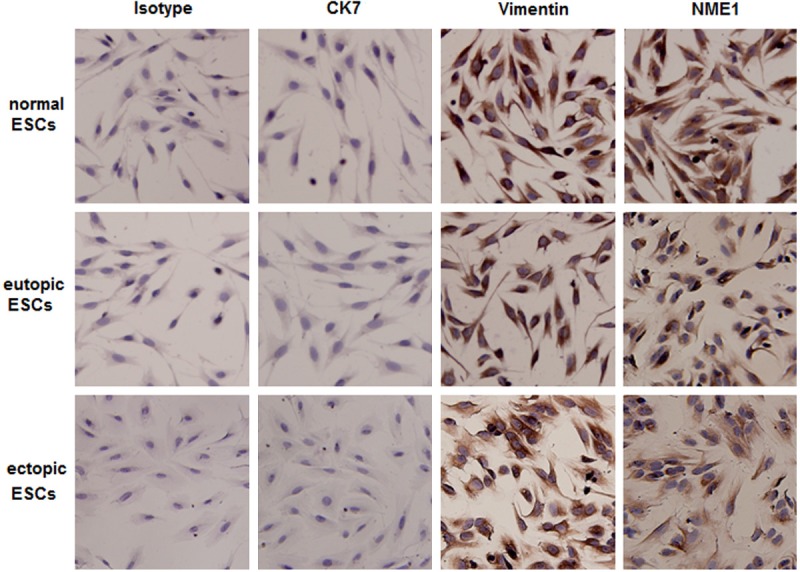
The expression of NME1 is decreased in the eutopic and ectopic ESCs from women with endometriosis. The expression of NME1 in primary cultured ESCs from women with and without endometriosis was analyzed by immunocytochemistry. Here normal ESCs: ESCs from women without endometriosis; eutopic ESCs and ectopic ESC: ESCs of eutopic endometium and endometriotic peritoneal lesion from women with endometriosis. Original magnification: ×200.
Silencing NME1 of eutopic ESCs from women with endometriosis stimulates angiogenesis of HUVECs
Based on the finding by che et al [20], we speculated that NME1 in ESCs regulated the formation of new blood vessels through regulating the production of VEGF. In order to answer this question, we incubated HUVECs with the supernatant of eutopic ESCs transfected with siRNA, and found that the supernatant of NME1-silenced ESCs significantly promotes the tube formation of HUVECs (P<0.01) (Figure 2A and 2B). These data indicated that down-regulation of NME1 in ESCs might stimulate angiogenesis of HUVECs, may further participate in the formation of high vascular density in the endometriotic lesions.
Figure 2.
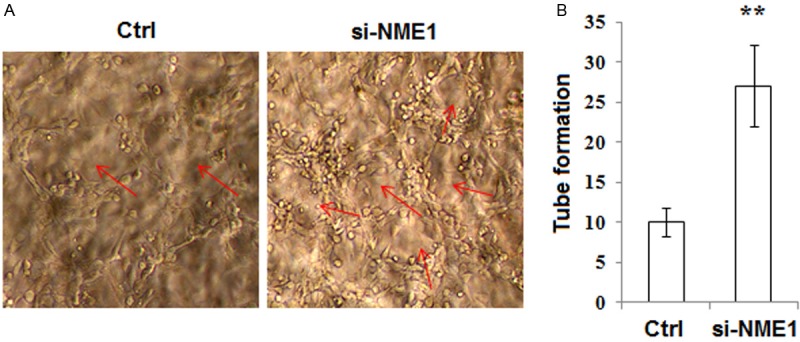
Silencing NME1 of eutopic ESCs from women with endometriosis stimulates angiogenesis of HUVECs. A, B: We collected the ESCs supernatant of transfection for 72 h and added to HUVECs culture system, and then analyzed the pro-angiogenic activity in NME1-silenced ESCs by tube formation assay. Original magnification: ×200. Ctrl: the nontargeting siRNA oligonucleotides; si-NME1: NME1 is knocked down. Results were highly reproducible in three independent experiments. Data are mean±SD. **P<0.01 compared to the negative control.
NME1 silence of ESCs up-regulates the expression of CD62E and CD105 on HUVECs
Next, we analyzed the effect of NME1 in ESCs on the expression of endothelial cell-activating factor CD62E and CD105 on HUVECs. Data were presented in Figure 3 that the expression of both CD62E and CD105 on HUVECs are increased after treatment with the supernatant of NME1-silenced ESCs (P<0.05) (Figure 3A and 3B). Because our recent study showed that NME1 inactivates MAPK/ERK1/2 and AKT signal pathways, we further investigated whether the effect of NME1 on CD62E and CD105 expression of HUVECs is dependent of these signals. As shown, blocking MAPK/ERK1/2 signal with U0126 rather than AKT signal inhibitor LY294002 could abrogate the increase of CD62E on HUVECs induced by the supernatant of NME1-silenced ESCs (Figure 3A and 3B). However, neither U0126 nor LY294002 regulated CD105 expression of HUVECs mediated by NME1 (Figure 3A and 3B). The results above suggest that NME1 silence-mediated up-regulation of CD62E and CD105 on HUVECs is dependent on different signaling pathways.
Figure 3.
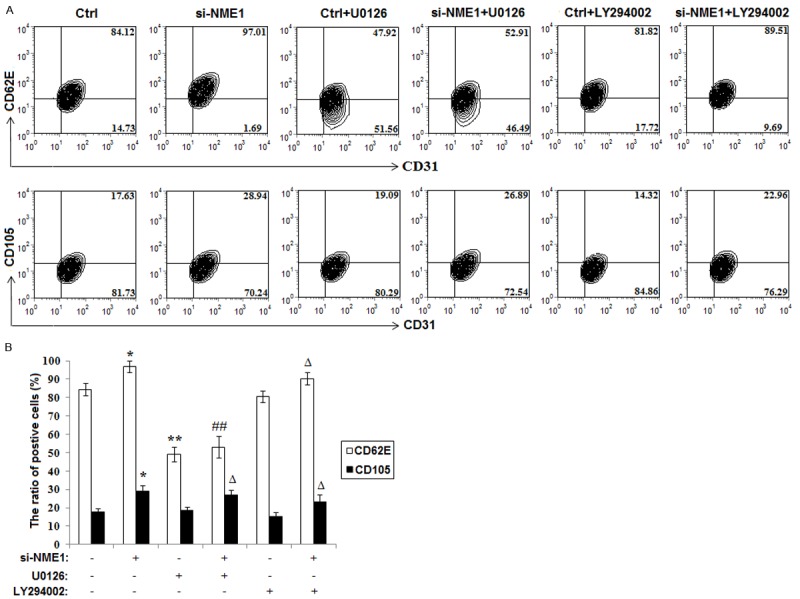
NME1 silence of ESCs up-regulates the expression of CD62E and CD105 on HUVECs. A, B: Flow cytometry was performed to analysis the expression of CD62E and CD105 of HUVECs incubated with the supernatant of siRNA transfected ESCs for 48 h, which treated with or without U0126 and LY294002. Data are mean±SD. *P<0.05, **P<0.01 compared to the negative control. ##P<0.01 compared to NME1 silence. ΔP<0.05 compared to the negative control plus U0126 or LY294002 treatment.
Silencing NME1 stimulates the secretion of VEGF and IL-8 of ESCs
According to the recently reported relevance of NME1 for the angiogenesis-related VEGF expression [20], we next investigated whether NME1 modulates the production of angiogenic factors (VEGF, IL-6 and IL-8) of ESCs. As shown in Figure 4, NME1 silence promoted the secretion of VEGF and IL-8 (P<0.05) (Figure 4), but not influenced the level of IL-6 of ESCs (P>0.05) (Figure 4). In addition, the effect on VEGF and IL-8 could be reversed by U0126 and LY294002 (Figure 4). Therefore, it can be concluded that NME1 down-regulates the secretion of VEGF and IL-8 of ESCs through MAPK/ERK/1/2 and AKT signal pathway, and possibly further participates in the regulation of tube formation of HUVECs.
Figure 4.
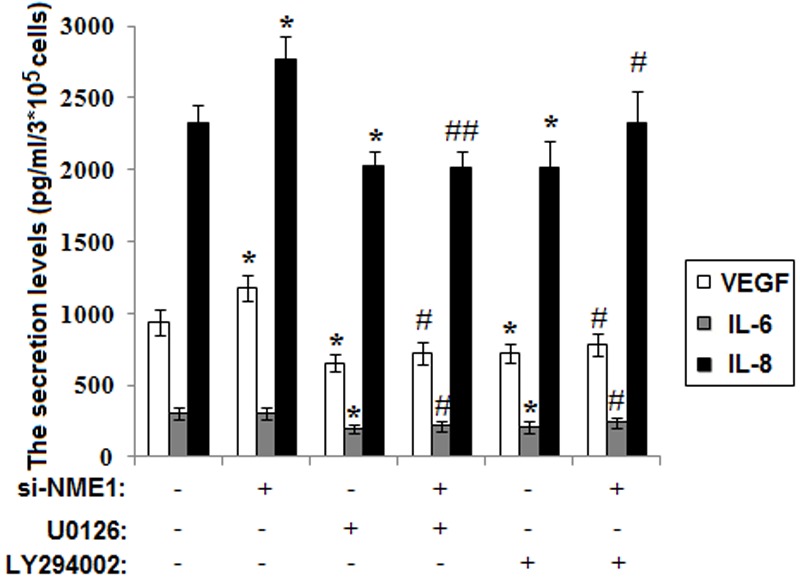
Silencing NME1 stimulates the secretion of VEGF and IL-8 of ESCs. We used ELISA to detect the secretion level of VEGF, IL-6 and IL-8 of siRNA transfected ESCs. Data are mean±SD. *P<0.05 compared to the negative control. #P<0.05, ##P<0.01 compared to NME1 silence.
NME1 silence of ESCs stimulates angiogenesis of HUVECs through promoting the secretion of VEGF and IL-8 of ESCs
To investigate the mechanism of NME1 on tube formation of HUVECs, we incubated HUVECs with the supernatant of transfected ESCs, anti-human VEGF and or IL-8 neutralizing antibody. We found that, both NME1 of ESCs and blocking VEGF or IL-8 with neutralizing antibody reduce the tube formation of HUVECs (P<0.05 or P<0.01) (Figure 5). Moreover, anti-human VEGF or IL-8 neutralizing antibody could abolish the increase of tube formation of HUVECs mediated by NME1 silence (Figure 5). These findings above suggest that NME1 restricts pro-angiogenic activity of human endometrial stromal cells in the endometriotic milieu via AKT and MAPK/ERK1/2 signal pathway, which is dependent on VEGF and IL-8.
Figure 5.
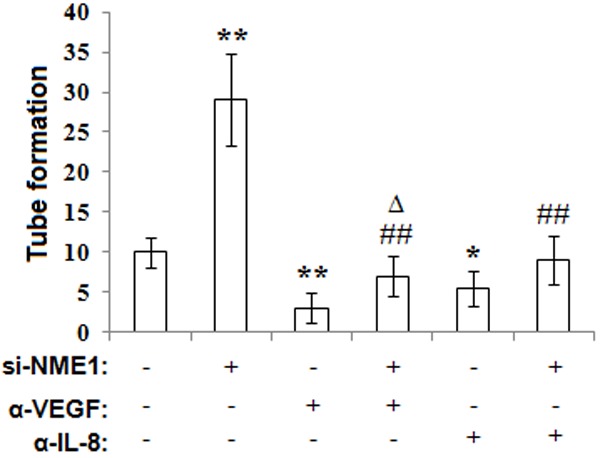
NME1 silence of ESCs stimulates angiogenesis of HUVECs through promoting the secretion of VEGF and IL-8 of ESCs. We added the supernatant of siRNA transfected ESCs to HUVECs culture system, which treated with or without VEGF or IL-8 neutralizing antibody for 48 h, and analyzed the tube formation of HUVECs by tube formation assay. Data are mean±SD. *P<0.05, **P<0.01 compared to the negative control. ##P<0.01 compared to NME1 silence. ΔP<0.05 compared to the negative control plus VEGF neutralizing antibody treatment.
Discussion
The most widely accepted theory for the development of peritoneal endometriotic lesions is the implantation theory of Sampson [25], postulating that endometrial fragments reach the peritoneal cavity by retrograde menstruation. During the last years, numerous studies demonstrated that the establishment and survival of these lesions is crucially dependent on the formation of blood vessels, which guarantee their oxygen supply [2-5]. Thus, endometriosis is an angiogenesis-dependent disorder [6,7,26,27]. A typical clinical feature of endometriotic lesions is dense vascularization [26]. The development of new blood vessels occurs via two principal processes, angiogenesis and vasculogenesis. Angiogenesis is defined as the formation of new microvessels from pre-existing ones and occurs by sprouting angiogenesis and intussusception. Sprouting angiogenesis is a tightly regulated multi-step process with the release of pro-angiogenic growth factors, matrix degradation by proteases, endothelial cell migration and proliferation, sprout and network formation as well as vessel maturation [28,29].
Our previous research has confirmed that TSLP induces the proliferation and invasion of human trophoblasts through decreasing the expression of NME1 expression [19]. TSLP secreted by cervical carcinomas cells is involved in the angiogenesis of cervical cancer in a paracrine manner [11]. Recently, Urata et al had reported that TSLP concentrations in the serum and PF were both higher in women with endometriosis compared with those without endometriosis, and positive immunostaining of TSLP was observed in the stroma of endometrioma tissue. Moreover, IL-1β stimulates the expression of TSLP from ESCs [30]. Therefore, as a down-stream molecule of TSLP, whether NME1 regulates the angiogenesis in the endometriotic milieu was an urgent question to answer.
The key finding of the present study is that down-regulation of NME1 can promote pro-angiogenic activity of ESCs. In addition, we have found that these effects are mediated by the elevated expression of downstream molecules, such as angiogenic factor VEGF, IL-8, endothelial cell-activating factor CD62E and CD105 in MAPK/ERK1/2 and AKT signal dependent or independent manners. These findings suggest that NME1 may play an important role in inhibiting the development and progression of endometriosis through regulating the formation of new blood vessel.
Endometriotic lesions express several angiogenic growth factors and release them into the peritoneal fluid of patients with endometriosis [26]. Increasing evidence indicates that VEGF is involved in both the development and maintenance of peritoneal endometriosis [31,32]. Highly active red endometriotic lesions contain the highest VEGF concentrations when compared with other lesion types [33]. In addition, peritoneal fluid concentrations of VEGF correlate significantly with the stage of endometriosis [34]. Our previous work has shown that IL-8 can promote the growth and restrict the apoptosis of ESCs in an autocrine dependent manner. In the present study, silencing NME1 directly stimulated the secretion of VEGF and IL-8 of ESCs through MAPK/ERK1/2 and AKT signal pathways, and further promoted the tube formation of HUVECs. However, the regulatory mechanism of NME1 on the expression of VEGF and IL-8 need further research.
Endoglin, or CD105 is a type I transmembrane protein that functions as a co-receptor for transforming growth factor-β1 (TGF-β1) to modulate its signaling by binding to the TGF-β receptors [35]. Endoglin is expressed mainly on proliferating endothelial cells and tumor-associated endothelium and is involved in numerous diseases with vascular abnormalities [36]. Up-regulation of endoglin expression is found in tumor vasculature and proliferating cells, suggesting that endoglin is a biomarker for endothelial proliferation or angiogenesis [37,38]. In addition, endoglin staining was found in the microvessels of eutopic endometrium from endometriosis cases [39] but was only increased significantly in the late secretory phase [40]. It suggested that endoglin and TGF-β may be involved in the vascular remodelling in endometriotic angiogenesis and thus maintain growth of endometriotic implants in the peritoneum. Angiogenesis-relative molecule CD62E, is also known as E-selectin and endothelial leukocyte adhesion molecule-1 (ELAM-1). CD62E is found to mediate the adhesion of tumor cells to endothelial cells, by binding to CD62E ligands, which also play a role in cancer metastasis [41]. Our current results showed that NME1 suppression up-regulates the expression of angiogenesis-relative molecule CD62E and CD105 on HUVECs in MAPK/ERK1/2 and AKT dependent or independent manner. Therefore, the abnormal lower expression of NME1 in the eutopic ESCs can also promote the ectopic survival through stimulating angiogenesis in the endometriotic milieu. These results further add to our understanding on the biological function and role manner of NME1 in non-tumor cells.
In conclusion, our findings suggest that down-regulated NME1 is responsible for the up-regulation of angiogenesis in abdominal cavity and that abnormal level of NME1 gives rise to the formation of endometriotic lesions by MAPK/Erk, Akt and other unknown signaling. Our data bring new insight into the regulation and effective mechanisms of NME1 in pathogenesis of endometriosis.
Acknowledgements
This work was partly supported by National Natural Science Foundation of China (NSFC) No. 31270969 (to D-J Li); National and Shanghai Leading Academic Discipline Project 211XK22 (to D-J Li); Program for Outstanding Medical Academic Leader of Shanghai (to D-J Li); NFSC 31170870 to L-P Jin; NSFC 31101064 to Ming-Qing Li; and Program for ZhuoXue of Fudan University to Ming-Qing Li.
Disclosure of conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Galle PC. Clinical presentation and diagnosis of endometriosis. Obstet Gynecol Clin North Am. 1989;16:29–42. [PubMed] [Google Scholar]
- 2.Groothuis PG, Nap AW, Winterhager E, Grümmer R. Vascular development in endometriosis. Angiogenesis. 2005;8:147–156. doi: 10.1007/s10456-005-9005-x. [DOI] [PubMed] [Google Scholar]
- 3.Laschke MW, Menger MD. In vitro and in vivo approaches to study angiogenesis in the pathophysiology and therapy of endometriosis. Hum Reprod Update. 2007;13:331–342. doi: 10.1093/humupd/dmm006. [DOI] [PubMed] [Google Scholar]
- 4.May K, Becker CM. Endometriosis and angiogenesis. Minerva Ginecol. 2008;60:245–254. [PubMed] [Google Scholar]
- 5.Taylor RN, Yu J, Torres PB, Schickedanz AC, Park JK, Mueller MD, Sidell N. Mechanistic and therapeutic implications of angiogenesis in endometriosis. Reprod Sci. 2009;16:140–146. doi: 10.1177/1933719108324893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laschke MW, Giebels C, Menger MD. Vasculogenesis: a new piece of the endometriosis puzzle. Hum Reprod Update. 2011;17:628–636. doi: 10.1093/humupd/dmr023. [DOI] [PubMed] [Google Scholar]
- 7.Laschke MW, Menger MD. Anti-angiogenic treatment strategies for the therapy of endometriosis. Hum Reprod Update. 2012;18:682–702. doi: 10.1093/humupd/dms026. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto J, Sakaguchi H, Aoki I, Tamaya T. Clinical implications of expression of interleukin-8 related to angiogenesis in uterine cervical cancers. Cancer Res. 2000;60:2632–2635. [PubMed] [Google Scholar]
- 10.Lopez-Ocejo O, Viloria-Petit A, Bequet-Romero M, Mukhopadhyay D, Rak J, Kerbel RS. Oncogenes and tumor angiogenesis: the HPV-16 E6 oncoprotein activates the vascular endothelial growth factor (VEGF) gene promoter in a p53 independent manner. Oncogene. 2000;19:4611–4620. doi: 10.1038/sj.onc.1203817. [DOI] [PubMed] [Google Scholar]
- 11.Xie F, Meng YH, Liu LB, Chang KK, Li H, Li MQ, Li DJ. Cervical Carcinoma Cells Stimulate the Angiogenesis through TSLP Promoting Growth and Activation of Vascular Endothelial Cells. Am J Reprod Immunol. 2013;70:69–79. doi: 10.1111/aji.12104. [DOI] [PubMed] [Google Scholar]
- 12.Lacombe ML, Milon L, Munier A, Mehus JG, Lambeth DO. The human Nm23/nucleoside diphosphate kinases. J Bioenerg Biomembr. 2000;32:247–258. doi: 10.1023/a:1005584929050. [DOI] [PubMed] [Google Scholar]
- 13.Bilitou A, Watson J, Gartner A, Ohnuma S. The NM23 family in development. Mol Cell Biochem. 2009;329:17–33. doi: 10.1007/s11010-009-0121-6. [DOI] [PubMed] [Google Scholar]
- 14.Horak CE, Mendoza A, Vega-Valle E, Albaugh M, Graff-Cherry C, McDermott WG, Hua E, Merino MJ, Steinberg SM, Khanna C, Steeg PS. Nm23-H1 suppresses metastasis by inhibiting expression of the lysophosphatidic acid receptor EDG2. Cancer Res. 2007;67:11751–11759. doi: 10.1158/0008-5472.CAN-07-3175. [DOI] [PubMed] [Google Scholar]
- 15.Boissan M, De Wever O, Lizarraga F, Wendum D, Poincloux R, Chignard N, Desbois-Mouthon C, Dufour S, Nawrocki-Raby B, Birembaut P, Bracke M, Chavrier P, Gespach C, Lacombe ML. Implication of metastasis suppressor NM23-H1 in maintaining adherens junctions and limiting the invasive potential of human cancer cells. Cancer Res. 2010;70:7710–7722. doi: 10.1158/0008-5472.CAN-10-1887. [DOI] [PubMed] [Google Scholar]
- 16.Lim J, Jang G, Kang S, Lee G, Nga do TT, Phuong do TL, Kim H, El-Rifai W, Ruley HE, Jo D. Cell-permeable NM23 blocks the maintenance and progression of established pulmonary metastasis. Cancer Res. 2011;71:7216–7225. doi: 10.1158/0008-5472.CAN-11-2015. [DOI] [PubMed] [Google Scholar]
- 17.Li MQ, Shao J, Meng YH, Mei J, Wang Y, Li H, Zhang L, Chang KK, Wang XQ, Zhu XY, Li DJ. NME1 suppression promotes growth, adhesion and implantation of endometrial stromal cells via Akt and MAPK/Erk1/2 signal pathways in the endometriotic milieu. Hum Reprod. 2013;28:2822–2831. doi: 10.1093/humrep/det248. [DOI] [PubMed] [Google Scholar]
- 18.Xie KM, Hou XF, Li MQ, Li DJ. NME1 at the human maternal–fetal interface downregulates titin expression and invasiveness of trophoblast cells via MAPK pathway in early pregnancy. Reproduction. 2010;139:799–808. doi: 10.1530/REP-09-0490. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Fan DX, Du J, Li MQ, Zhu XY, Jin LP. Thymic stromal lymphopoietin downregulates NME1 expression and promotes invasion in human trophoblasts via the activation of STAT3 signaling pathway. Clin Immunol. 2012;143:88–95. doi: 10.1016/j.clim.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Che G, Chen J, Liu L, Wang Y, Li L, Qin Y, Zhou Q. Transfection of nm23-H1 increased expression of beta-Catenin, E-Cadherin and TIMP-1 and decreased the expression of MMP-2, CD44v6 and VEGF and inhibited the metastatic potential of human non-small cell lung cancer cell line L9981. Neoplasma. 2006;53:530–537. [PubMed] [Google Scholar]
- 21.Li MQ, Li HP, Meng YH, Wang XQ, Zhu XY, Mei J, Li DJ. Chemokine CCL2 enhances survival and invasion of endometrial stromal cells in an autocrine manner by activating AKT and MAPK/Erk1/2 Signal Pathway. Fertil Steril. 2012;97:919–929. doi: 10.1016/j.fertnstert.2011.12.049. [DOI] [PubMed] [Google Scholar]
- 22.Li MQ, Luo XZ, Meng YH, Mei J, Zhu XY, Jin LP, Li DJ. CXCL8 enhances proliferation and growth and reduces apoptosis in endometrial stromal cells in an autocrine manner via a CXCR1-triggered PTEN/AKT signal pathway. Hum Reprod. 2012;27:2107–2116. doi: 10.1093/humrep/des132. [DOI] [PubMed] [Google Scholar]
- 23.Li MQ, Tang CL, Du MR, Fan DX, Zhao HB, Xu B, Li DJ. CXCL12 controls over-invasion of trophoblasts via upregulating CD82 expression in DSCs at maternal-fetal interface of human early pregnancy in a paracrine manner. Int J Clin Exp Pathol. 2011;4:276–286. [PMC free article] [PubMed] [Google Scholar]
- 24.Klein D, Demory A, Peyre F, Kroll J, Augustin HG, Helfrich W, Kzhyshkowska J, Schledzewski K, Arnold B, Goerdt S. Wnt2 acts as a cell type-specific, autocrine growth factor in rat hepatic sinusoidal endothelial cells cross-stimulating the VEGF pathway. Hepatology. 2008;47:1018–1031. doi: 10.1002/hep.22084. [DOI] [PubMed] [Google Scholar]
- 25.Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissues into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–469. [Google Scholar]
- 26.Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann N Y Acad Sci. 2002;955:89–100. doi: 10.1111/j.1749-6632.2002.tb02769.x. [DOI] [PubMed] [Google Scholar]
- 27.Laschke MW, Menger MD. In vitro and in vivo approaches to study angiogenesis in the pathophysiology and therapy of endometriosis. Hum Reprod Update. 2007;13:331–342. doi: 10.1093/humupd/dmm006. [DOI] [PubMed] [Google Scholar]
- 28.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 29.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 30.Urata Y, Osuga Y, Izumi G, Takamura M, Koga K, Nagai M, Harada M, Hirata T, Hirota Y, Yoshino O, Taketani Y. Interleukin-1β stimulates the secretion of thymic stromal lymphopoietin (TSLP) from endometrioma stromal cells: possible involvement of TSLP in endometriosis. Hum Reprod. 2012;27:3028–3035. doi: 10.1093/humrep/des291. [DOI] [PubMed] [Google Scholar]
- 31.McLaren J. Vascular endothelial growth factor and endometriotic angiogenesis. Hum Reprod Update. 2000;6:45–55. doi: 10.1093/humupd/6.1.45. [DOI] [PubMed] [Google Scholar]
- 32.Xu H, Becker CM, Lui WT, Chu CY, Davis TN, Kung AL, Birsner AE, D'Amato RJ, Wai Man GC, Wang CC. Green tea epigallocatechin-3-gallate inhibits angiogenesis and suppresses vascular endothelial growth factor C/vascular endothelial growth factor receptor 2 expression and signaling in experimental endometriosis in vivo. Fertil Steril. 2011;96:1021–1028. doi: 10.1016/j.fertnstert.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Donnez J, Smoes P, Gillerot S, Casanas-Roux F, Nisolle M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod. 1998;13:1686–1690. doi: 10.1093/humrep/13.6.1686. [DOI] [PubMed] [Google Scholar]
- 34.Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, Jaffe RB, Taylor RN. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 1996;81:3112–3118. doi: 10.1210/jcem.81.8.8768883. [DOI] [PubMed] [Google Scholar]
- 35.Barbara NP, Wrana JL, Letarte M. Endoglin is an accessory protein that interact with the signaling receptor complex of multiple members of the transforming growth factor-h superfamily. J Biol Chem. 1999;274:584–594. doi: 10.1074/jbc.274.2.584. [DOI] [PubMed] [Google Scholar]
- 36.Ten Dijke P, Goumans MJ, Pardali E. Endoglin in angiogenesis and vascular diseases. Angiogenesis. 2008;11:79–89. doi: 10.1007/s10456-008-9101-9. [DOI] [PubMed] [Google Scholar]
- 37.Wang JM, Kumar S, Pye D, van Agthoven AJ, Krupinski J, Hunter RD. A monoclonal antibody detects heterogeneity in vascular endothelium of tumours and normal tissues. Int J Cancer. 1993;54:363–370. doi: 10.1002/ijc.2910540303. [DOI] [PubMed] [Google Scholar]
- 38.Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 39.Hayrabedyan S, Kyurkchiev S, Kehayov I. FGF-1 and S100A13 possibly contribute to angiogenesis in endometriosis. J Reprod Immunol. 2005;67:87–101. doi: 10.1016/j.jri.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Kim SH, Choi YM, Chae HD, Kim KR, Kim CH, Kang BM. Increased expression of endoglin in the eutopic endometrium of women with endometriosis. Fertil Steril. 2001;76:918–922. doi: 10.1016/s0015-0282(01)02733-9. [DOI] [PubMed] [Google Scholar]
- 41.Tabatabai G, Herrmann C, von Kürthy G, Mittelbronn M, Grau S, Frank B, Möhle R, Weller M, Wick W. VEGF-dependent induction of CD62E on endothelial cells mediates glioma tropism of adult haematopoietic progenitor cells. Brain. 2008;131:2579–2595. doi: 10.1093/brain/awn182. [DOI] [PubMed] [Google Scholar]


