Abstract
Angiogenesis is essential for invasive tumor growth and metastasis. Bevacizumab has been widely used for the treatment of non-small cell lung cancer (NSCLC). Various studies clearly demonstrate the relevance of Id-1 and VEGF in angiogenesis. The aim of this study was to establish the role of Id-1 expression in tumor progression and angiogenesis in relation to VEGF in NSCLC. Seventy five patients underwent surgery for lung cancers. The expressions of Id-1 and VEGF in NSCLC samples were determined by immunohistochemistry. Expression of Id-1 and VEGF showed a close correlation in NSCLC (p < 0.001). In addition, Id-1 strong expression group showed high incidence of metastasis in multivariate analysis (p = 0.028). Id-1 strong expression group had short metastasis-free survival (p = 0.008) and short recurrence-free survival (p = 0.027). Strong Id-1 expression in NSCLC had a poor prognosis in association with VEGF expression. Id-1 may function in tumor growth and progression via angiogenesis. Therefore, Id-1 is considered to be a candidate for new therapeutic target and a prognostic factor in NSCLC.
Keywords: Id proteins, vascular endothelial growth factor (VEGF), non-small cell lung cancer, prognosis, metastasis
Introduction
Non-small cell lung cancer (NSCLC) is one of the major causes of death across the world. Despite critical advances in its treatment, this disease still results in unsatisfactory prognosis and median survival for patients. Vascular endothelial growth factor (VEGF) is one of the key mediators of angiogenesis and its overexpression is associated with poor prognosis in various tumors [1-3]. Angiogenesis is essential for tumor growth and metastasis and is related to poor prognosis of NSCLC. The inhibition of VEGF by molecular-targeting agent is the leading treatment of NSCLC nowadays. Bevacizumab is one of the agents that use this molecular-targeted therapy [4].
The inhibitor of DNA-binding (Id) protein, one of the HLH proteins lacking DNA-binding domain, acts as a negative regulator of cell transcription [5]. During mammalian embryogenesis, Id family of proteins (Id 1, Id 2, Id 3, and Id 4) are expressed in multiple tissues [6,7]. Id-1 plays an important role in angiogenesis and Id-3 is found to be expressed in tumors; there are several articles demonstrating the association between VEGF and Id-1 in angiogenesis [8-12]. Because regardless of histologic type of non-small cell lung cancer, Bevacizumab had been a leading treatment of NSCLC in association of VEGF and angiogenesis. From the point of view of angiogenesis and VEGF, we want to investigate relationship between Id-1 protein expression and poor prognosis. Further, there are many researches that show an association between high level of Id-1 expression and progression of cancer [13]. The aim of this study was to establish the role of Id-1 expression in tumor progression and angiogenesis in relation to VEGF in NSCLC.
Materials and methods
Patients and tissue sample
Formalin-fixed paraffin-embedded specimens were obtained from 75 patients with stage I–IIB NSCLC who underwent surgical resection without adjuvant chemotherapy at Kyungpook National University Hospital, and were recruited between 2003 and 2007. The pathological stages of the tumor were determined according to the TNM classification system of American Joint Committee on Cancer, 7th edition. We intentionally sampled relatively less advanced stage of lung cancer (up to stage IIB) with leaning towards stage of pT1 and pT2 (Table 1, pT1: 34.7%, pT2a: 49.3%, pT2b: 8%, pT3: 8%). There is only two patient underwent lymph node metastasis in our cohort. No patient was lost on follow-up. The median duration of follow-up was 61.67 months for disease-free patients, 38.45 months for relapsed patients, and 24.25 months for patients who showed metastasis. The relevant clinical information was extracted from the medical records (Table 1). The expressions of Id-1 and VEGF were evaluated by immunohistochemical stain. The pathology of each tumor sample was reevaluated by two experienced pathologists (T. I. Park and S. Z. Kim).
Table 1.
Clinical characteristics in 75 primary nonsmall cell lung cancer patients
| Clinicopathologic feature | N | % |
|---|---|---|
| Gender | ||
| Female | 19 | 25.3 |
| Male | 56 | 74.7 |
| Age | ||
| ≤ 60 | 27 | 36 |
| > 60 | 48 | 64 |
| pT stage | ||
| pT1 | 26 | 34.7 |
| pT2a | 37 | 49.3 |
| pT2b | 6 | 8 |
| pT3 | 6 | 8 |
| pN stage | ||
| pN0 | 73 | 97.33 |
| pN1 | 1 | 1.33 |
| pN2 | 1 | 1.33 |
| Diagnosis | ||
| Squamous cell carcinoma | 46 | 61.33 |
| Adenocarcinoma | 28 | 37.33 |
| Adenosquamous cell carcinoma | 1 | 1.33 |
| Distant metastasis (at diagnosis) | ||
| None detected | 75 | 100 |
| Present | 0 | 0 |
| Distant metastasis (Follow-up) | ||
| None detected | 67 | 89.3 |
| Present | 8 | 10.7 |
| Recurrence | ||
| Yes | 11 | 14.7 |
| No | 64 | 85.3 |
Tissue microarray construction
Seventy-five surgically resected specimens were used to prepare the tissue microarray for immunohistochemical stain analysis. The representative tumor areas of each specimen were selected by experienced pathologists (T. I. Park and S. Z. Kim). The tumor core tissues 5 mm in diameter were obtained from formalin-fixed paraffin-embedded tissues and transferred to TMA blocks. One normal lung core tissue was included each TMA blocks.
Immunohistochemical staining
Sections (4 μm) of formalin-fixed paraffin-embedded tissue samples from lung cancers were cut with a microtome and dried overnight at 37°C on a salinized-slide. Immunohistochemical staining using Benchmark XT slide stainer (Ventana Medical System, Inc.) was performed according to the manufacturer’s instructions. Polyclonal anti-Id-1 antibodies (Abcam, San Francisco, CA) were added to TMA slides at a 1:20 dilution. Monoclonal anti-VEGF antibodies (Dako Corporation, Glostrup, Denmark) were mixed with TMA slides at a 1:50 dilution.
Evaluation of immunohistochemical staining results
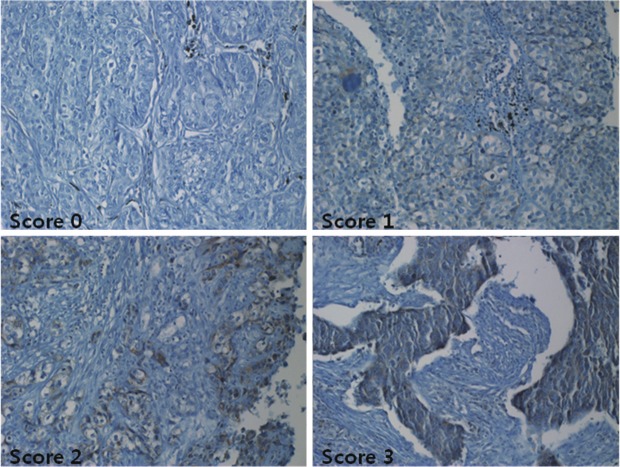
TMA of immunohistochemical staining for Id-1 were evaluated in a semiquantitative fashion according the similar method described by McCarty et al and Maw et al [11]. We consider both the intensity and the percentage of cells stained in each of four intensity categories. Intensities were classified as 0 (no staining), 1 (weak staining), 2 (distinct staining), 3 (strong staining), 4 (very strong staining). A normal lung core was included per each TMA. In normal lung tissue, alveolar macrophages were strong stained; endothelial cells and normal alveolar epitheliums were weak stained (Figure 1). These findings were based in scoring of intensity. The cases stained on the same plane as alveolar macrophages were scored 3 (strong staining) and that of as normal alveolar epitheliums or endothelial cells were scored 1 (weak staining). In cases of more weak stained than scored 1 was scored 0 and more strong stained than score 3 were scored 4. In cases between score 1 and 3 were scored 2 (Figure 2). And then the scores were estimated by following algorithm: score= i × Pi, where i and Pi represent intensity and percentage of cells that stain at each intensity (Table 2). Results were assigned to two groups according to their overall scores: weak ≤ 200; strong > 200. The stained sections were reviewed by two independent observers who had no knowledge of the clinical data of patient cohorts (M. H. Han and J. Y. Jung) and reevaluated by one independent observer (J. S. Kim). The unstained and weak cytoplasmic stained group (score ≤ 200) was called the weak group, and strong cytoplasmic stained group (score > 200) was called the strong group.
Figure 1.
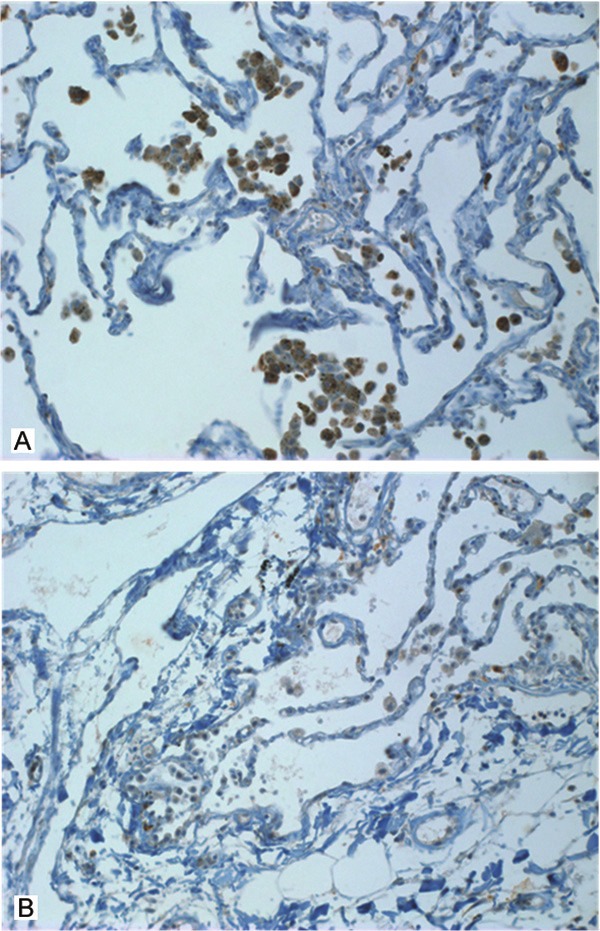
Immunohistochemical stain of Id-1 expression in normal lung specimens. A: Strong Id-1 immunoreactivity was present in the cytoplasm of alveolar macrophages. B: In the normal lung there was weak Id-1 immunoreactivity in the epitheliums of alveolus and endothelial cells.
Figure 2.
Immunohistochemical stain of Id-1 expression in lung tumor cells. Weak to strong Id-1 immunoreactivity was present in the cytoplasm of cancer cells. There was four intensity categories. Intensities were classified as 0 (no staining), 1 (weak staining), 2 (distinct staining), 3 (strong staining), 4 (very strong staining).
Table 2.
Immunohistochemical stain score of Id-1
| Id-1 intensity | Score 0 | Score 1 | Score 2 | Score 3 | Score 4 |
|---|---|---|---|---|---|
| N (%) | 0 (0%) | 34 (45.33%) | 12 (16%) | 29 (38.67%) | 0 |
| Mean (i × Pi) | Not applicable | 85.0 | 135 | 225 | Not applicable |
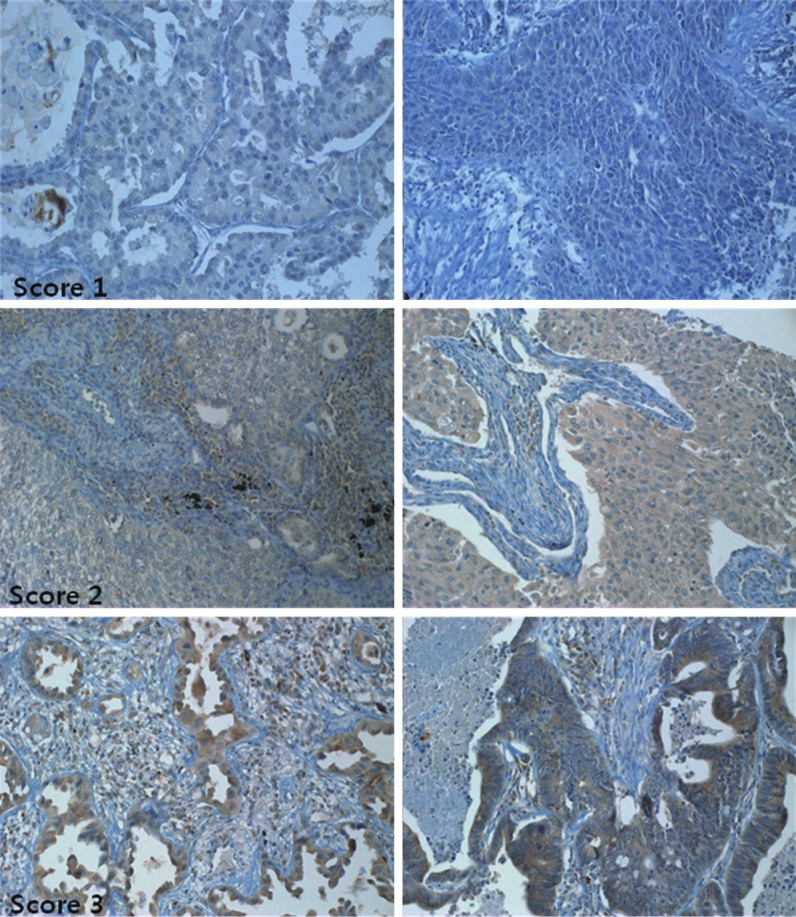
TMA of immunohistochemical staining for VEGF were evaluated according the method described by Lammli J et al [1,2]. Two investigators (M. H. Han and J. Y. Jung) independently read all IHC-stained sections and graded the immunostains as 0 (negative), 1 (< 20% positive cells), 2 (< 50% positive cells), 3 (> 50% positive cells), and 4 (100% positive) (Figure 3, Table 3). The group of lesser than 20% of VEGF+ cells was called the low-VEGF group, whereas specimens with more than 20% VEGF-positive cells was called the high-VEGF group.
Figure 3.
Immunohistochemical stain against VEGF on lung tumor cells. Score 0 ( no positive cells), score 1 (< 20% positive cells), score 2 (< 50% positive cells), score 3 (> 50% positive cells).
Table 3.
Immunohistochemical stain score of VEGF
| VEGF score | Score 0 | Score 1 | Score 2 | Score 3 | Score 4 |
|---|---|---|---|---|---|
| N (%) | 38 (50.67%) | 16 (21.33%) | 6 (8%) | 15 (20%) | 0 (0%) |
Statistical analysis
Statistical analyses were done using SPSS 14.0 software. Student’s t-test, chi-square test, and Fisher’s exact test were employed to analyze the relationship between Id-1 expression (independent variable) and VEGF expression with clinicopathologic characteristics including age, sex, diagnosis, T stage, N stage, and EGFR expression (dependent variable). The same tests were performed to analyze the relationship between VEGF expression (independent variable) and Id-1 expression with clinicopathologic characteristics (dependent variable). Furthermore, to evaluate the significant predictor of metastasis and recurrence, univariate and multivariate logistic regression models were conducted. To estimate the disease-free survival times of both the Id-1 weak and Id-1 strong groups, Kaplan-Meier analysis was performed with regard to metastasis and recurrence. Logrank test was used to compare the survival distribution of two groups in metastasis free survival, and Breslow test was used in recurrence free survival in which two survival curves did not show equal proportional hazards. Furthermore, we calculated odds ratios (ORs) for metastasis and recurrence to evaluate the significant predictors of metastasis and recurrence using univariate and multivariate logistic regression analysis. Logistic regression model included id-1 expression, age, sex, diagnosis, VEGF, T stage, N stage, and EGFR. For the purpose of this article, a p-value of < 0.05 was considered statistically significant.
Results
Relationship between Id-1 expression and VEGF with clinicopathologic characteristics
The mean age of strong Id-1 group (62.6 ± 8.1 years) was higher than that of weak Id-1 group (61.9 ± 9.6 years), but did not reach statistical significance. In addition, independent variables such as sex, T stage, N stage, and EGFR expression in immunohistochemistry did not show statistical significance. A significant independent variable of Id-1 was the tumor type of lung cancer. Of the tumor types, 70% of Id-1 weak expressions were squamous cell carcinoma and 30% of the tumors were adenocarcinoma. Furthermore, 44% of Id-1 strong expressed specimens were squamous cell carcinoma, 52% were adenocarcinoma, and 4% were adenosquamous cell carcinoma (p = 0.031). Especially VEGF expressions showed strong associations with Id-1 cytoplasmic expressions. Further, 90% of weak Id-1 expressions exhibited low-VEGF and 64% of strong Id-1 expressions showed high-VEGF expression with a statistical significance (p < 0.001). Significant relation was observed between cytoplasmic Id-1 expression and metastasis in our cohort of patients. In the group of weak expression, 4% of cohort developed into metastasis, and in the group of strong expression, 24% of cohort developed into metastasis (p = 0.014). In addition, Id-1 expression was related to recurrence. In the study group, 8% of weak Id-1 group and 28% of strong Id-1 group showed recurrence (p = 0.035) (Table 4).
Table 4.
Id-1 expression and clinicopathologic characteristics
| Id1 weak | Id1 strong | p-value | ||
|---|---|---|---|---|
|
|
||||
| N = 50 | N = 25 | |||
| Age | Mean ± S.D. | 61.9 ± 9.6 | 62.6 ± 8.1 | 0.743 |
| Sex | male | 38 (76.0%) | 18 (72.0%) | 0.707 |
| female | 12 (24.0%) | 7 (28.0%) | ||
| Diagnosis | SCC | 35 (70.0%) | 11 (44.0%) | 0.031 |
| AC | 15 (30.0%) | 13 (52.0%) | ||
| ASC | 0 (0.0%) | 1 (4.0%) | ||
| T stage | 1 | 20 (40.0%) | 6 (24.0%) | 0.251 |
| 2 | 25 (50.0%) | 18 (72.0%) | ||
| 3 | 5 (10.0%) | 1 (4.0%) | ||
| T stage 2 | 1 | 20 (40.0%) | 6 (24.0%) | 0.205 |
| 2 + 3 | 30 (60.0%) | 19 (76.0%) | ||
| N stage | 0 | 48 (96.0%) | 25 (100.0%) | > 0.99 |
| 1 | 1 (2.0%) | 0 (0.0%) | ||
| 2 | 1 (2.0%) | 0 (0.0%) | ||
| N stage 2 | 0 | 48 (96.0%) | 25 (100.0%) | 0.550 |
| 1 + 2 | 2 (4.0%) | 0 (0.0%) | ||
| VEGF | weak | 45 (90.0%) | 9 (36.0%) | < 0.001 |
| strong | 5 (10.0%) | 16 (64.0%) | ||
| Metastasis | negative | 48 (96.0%) | 19 (76.0%) | 0.014 |
| positive | 2 (4.0%) | 6 (24.0%) | ||
| Recurrence | negative | 46 (92.0%) | 18 (72.0%) | 0.035 |
| positive | 4 (8.0%) | 7 (28.0%) | ||
SCC: Squamous cell carcinoma; AC: Adenocarcinoma; ASC: Adenosquamous cell carcinoma.
Relationship between VEGF expression and Id-1 expressions with clinicopathologic characteristics
The mean age of low-VEGF group (62.4 ± 9.0 years) was higher than that of high-VEGF group (61.4 ± 9.7 years), but it did not reach statistical significance. In addition, any clinicopathologic parameter did not acquire statistical significance except Id-1 expression. The significant relationship between Id-1 expression and VEGF expression had been observed. Of these, 83.3% of low-VEGF expressions show weak Id-1 expressions and 76.2% of the high-VEGF expressions exhibited strong Id-1 expressions to statistical significance (p < 0.001) (Table 5).
Table 5.
VEGF expression and clinicopathologic characteristics
| VEGF weak | VEGF strong | p-value | ||
|---|---|---|---|---|
|
|
||||
| N = 54 | N = 21 | |||
| Age | Mean ± S.D. | 62.4 ± 9.0 | 61.4 ± 9.7 | 0.691 |
| Sex | male | 40 (74.1%) | 16 (76.2%) | 0.850 |
| female | 14 (25.9%) | 5 (23.8%) | ||
| Diagnosis | SCC | 34 (63.0%) | 12 (57.1%) | 0.412 |
| AC | 20 (37.0%) | 8 (38.1%) | ||
| ASC | 0 (0.0%) | 1 (4.8%) | ||
| T stage | 1 | 20 (37.0%) | 6 (28.6%) | 0.449 |
| 2 | 31 (57.4%) | 12 (57.1%) | ||
| 3 | 3 (5.6%) | 3 (14.3%) | ||
| T stage 2 | 1 | 20 (37.0%) | 6 (28.6%) | 0.489 |
| 2 + 3 | 34 (63.0%) | 15 (71.4%) | ||
| N stage | 0 | 52 (96.3%) | 21 (100.0%) | > 0.99 |
| 1 | 1 (1.9%) | 0 (0.0%) | ||
| 2 | 1 (1.9%) | 0 (0.0%) | ||
| N stage 2 | 0 | 52 (96.3%) | 21 (100.0%) | > 0.99 |
| 1 + 2 | 2 (3.7%) | 0 (0.0%) | ||
| ID | weak | 45 (83.3%) | 5 (23.8%) | < 0.001 |
| strong | 9 (16.7%) | 16 (76.2%) | ||
| Metastasis | negative | 49 (90.7%) | 18 (85.7%) | 0.679 |
| positive | 5 (9.3%) | 3 (14.3%) | ||
| Recurrence | negative | 49 (90.7%) | 15 (71.4%) | 0.063 |
| positive | 5 (9.3%) | 6 (28.6%) | ||
SCC: Squamous cell carcinoma; AC: Adenocarcinoma; ASC: Adenosquamous cell carcinoma.
Independent predictor of metastasis and recurrence
To identify independent predictors for metastasis and recurrence, univariate and multivariate logistic regression analyses were performed. All the clinicopathologic parameters, Id-1 protein and VEGF expression including age, sex, diagnosis, TNM stage, and EGFR by immunohistochemical stain were taken into consideration. We found that strong expression of Id-1 was the only significant predictor of metastasis in univariate analysis (odds ratio: 7.579 (1.404-40.917), p = 0.019). In addition, we found that strong expression of Id-1 (odds ratio: 4.472 (1.166-17.146), p = 0.029) and VEGF (odds ratio: 3.92 (1.047-14.678), p = 0.043), and diagnosis of adenocarcinoma (odds ratio: 5.733 (1.373-23.934), p = 0.017) were significant predictors of recurrence in univariate analysis. In the multivariate analysis, strong Id-1 expression was the only significant predictor of metastasis (odds ratio: 9.85 (1.285-75.510), p = 0.028) and there was no significant independent variable of recurrence (Tables 6 and 7).
Table 6.
Independent predictor of metastasis
| Dependent variable: Metastasis | 8/75 | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Univariate | Multivariate | ||||||
|
| |||||||
| Independent variable | Reference | OR | 95% CIs | p-value | OR | 95% CIs | p-value |
| ID1 = strong | weak | 7.579 | (1.404-40.917) | 0.019 | 9.85 | (1.285-75.510) | 0.028 |
| Age | 1.047 | (0.956-1.146) | 0.325 | 1.058 | (0.937-1.193) | 0.363 | |
| Sex = male | female | 2.571 | (0.295-22.377) | 0.392 | 2.806 | (0.189-41.653) | 0.453 |
| AC | SCC | 0.984 | (0.216-4.478) | 0.983 | 0.314 | (0.027-3.596) | 0.352 |
| ASC | SCC | < 0.001 | (< 0.001 - > 999.999) | 0.989 | < 0.001 | (< 0.001 - > 999.999) | 0.989 |
| VEGF = strong | weak | 1.633 | (0.354-7.542) | 0.530 | 0.860 | (0.111-6.658) | 0.885 |
| T stage = 2 + 3 | T stage = 1 | 4.167 | (0.484-35.877) | 0.194 | 4.423 | (0.388-50.458) | 0.231 |
| N stage = 1 + 2 | N stage = 0 | < 0.001 | (< 0.001 - > 999.999) | 0.984 | < 0.001 | (< 0.001 - > 999.999) | 0.985 |
SCC: Squamous cell carcinoma; AC: Adenocarcinoma; ASC: Adenosquamous cell carcinoma.
Table 7.
Independent predictor of recurrence
| Dependent variable: Recurrence | 11/75 | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Univariate | Multivariate | ||||||
|
| |||||||
| Independent variable | Reference | OR | 95% CIs | p-value | OR | 95% CIs | p-value |
| ID1 = strong | weak | 4.472 | (1.166-17.146) | 0.029 | 1.944 | (0.248-15.221) | 0.527 |
| Age | 1.023 | (0.950-1.102) | 0.546 | 1.004 | (0.913-1.104) | 0.933 | |
| Sex = male | female | 0.336 | (0.089-1.266) | 0.107 | 1.046 | (0.175-6.249) | 0.961 |
| AC | SCC | 5.733 | (1.373-23.934) | 0.017 | 8.677 | (1.001-75.212) | 0.050 |
| ASC | SCC | < 0.001 | (< 0.001 - > 999.999) | 0.986 | < 0.001 | (< 0.001 - > 999.999) | 0.986 |
| VEGF = strong | weak | 3.92 | (1.047-14.678) | 0.043 | 4.371 | (0.511-37.368) | 0.178 |
| T stage = 2+3 | T stage = 1 | 2.7 | (0.538-13.555) | 0.228 | 2.229 | (0.298-16.693) | 0.435 |
| N stage = 1+2 | N stage = 0 | 6.3 | (0.364-109.019) | 0.206 | 71.37 | (1.653 - > 999.999) | 0.026 |
SCC: Squamous cell carcinoma; AC: Adenocarcinoma; ASC: Adenosquamous cell carcinoma.
Disease-free survival with Id-1 expression
By Kaplan-Meier analysis, strong Id-1 expression group did show significant short metastasis-free survival than that of weak Id-1 expression group (p = 0.008). Furthermore, strong Id-1 expression group did show significant short recurrence-free survival compared to the weak Id-1 expression group (p = 0.027) (Figure 4). All these results suggest that strong Id-1 expression group possibly has a poor prognosis compared to the weak Id-1 expression group.
Figure 4.
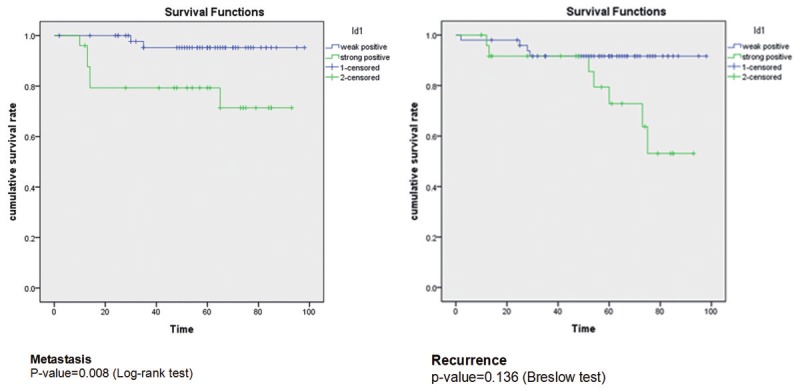
By Kaplan-Meier analysis, strong Id-1 expression group showed significant short metastasis-free survival than that of weak Id-1 expression group (p = 0.008). And there is no significant finding of recurrence between strong Id-1 expression group and weak Id-1 expression group (p = 0.136).
Discussion
The function of helix-loop-helix (HLH) proteins is important in the regulation of gene expressions and cell differentiations. Id (inhibitor of differentiation/DNA synthesis) proteins form a distinct subfamily of HLH proteins. Because of the lack of DNA-binding regions, Id proteins negatively regulate cell differentiation by dimerization with the other basic HLH proteins [5]. Id-1 is a member of the Id protein family (Id-1, Id-2, Id-3, and Id-4), and plays an important role in the control of cell cycle regulations and transcriptions [6-8,13]. Previous studies have shown that the expression of Id-1 and Id-3 in angiogenesis is necessary for the progression of the tumor xenograft [7,8]. Therefore, it is assumed that Id-1 is likely to affect the growth and metastasis of tumor through angiogenesis. In fact, in many human tumors, a high level of Id-1 is related to a poor prognosis [12,13].
Vascular endothelial growth factor was first discovered as an endothelial specific growth factor that induces proliferation, migration, and nitric oxide generation of endothelial cells in vitro [14-19]. Vascular endothelial growth factor also induces angiogenic response in a number of in vivo models including rabbit cornea [14-19]. In situ hybridization studies demonstrated higher levels of VEGF mRNAs in many human tumors than in normal tissues [14-19]. Tumor cells require a persistent supply of nourishment and oxygen and therefore the function of VEGF is necessary in tumor growth and in transition to tumor with an ability to metastasize [14-20].
During murine angiogenesis, expressions of Id-1 and Id-3 genes are observed [7]. Double knockouts of Id-1 and Id-3 lead to vascular malformations and disability of branching of blood vessels into neuroectoderm [8]. Adult mice have single allele of Id-1 and absence of Id-3 gene. Tumor xenograft reveals a defective angiogenesis that induces failure of tumor growth and metastasis [8]. Id1+/-; Id3-/- mutant mice have defective angiogenesis due to impaired mobilization of VEGFR2+ circulating endothelial precursor cells with defective proliferation and corporation of VEGFR1+ cells induced by VEGF [21]. Endothelial progenitor cells are key modulators of reendothelialization after injury [21-23]. Mature endothelial cells possess decreased proliferative potential, and hence circulating endothelial progenitor cells participate in reendothelialization. Id-1 protein facilitates proliferation or survival of endothelial progenitor cells [21-23]. The proliferation and migration of endothelial progenitor cells were found to be increased in the overexpression of exogenous Id-1 in vitro [23]. However, the definite mechanism is not known for the association of Id-1 and endothelial progenitor cells. But there are possible pathways in which Id-1 is linked to phosphatidylinositol 3-kinase/Akt, phosphatidylinositol 3-kinase/Akt/nuclear factor kappa B, and nuclear factor kappa B/survivin [22]. In quiescent endothelial progenitor cells, Id-1 present in low levels and its expression is significantly up-regulated in the stimulation of VEGF in vitro [9,22]. This suggests that there is a close correlation between Id-1 and VEGF in endothelial progenitor cells.
Our statistical analysis results repeatedly show a significant association of Id-1 and VEGF. 90% of weak Id-1 expressions exhibited low-VEGF and 64% of strong Id-1 expressions showed high-VEGF expression with a statistical significance (p < 0.001) (Table 4). And 83.3% of low-VEGF expressions show weak Id-1 expressions and 76.2% of the high-VEGF expressions exhibited strong Id-1 expressions to statistical significance (p < 0.001) (Table 5). To identify independent predictors for metastasis and recurrence, multivariate logistic regression analyses were performed. And then we found that strong Id-1 expression was significant predictor of metastasis (odds ratio: 9.85 (1.285-75.510), p = 0.028) and there was no significant independent variable of recurrence (Tables 6 and 7). But this study has some significant limitations. First, sample size is too small due to low incidence of NSCLC cases and only some of them underwent a surgical resection during relatively short-term period. Second, the sample is lack of the representativeness. Because of heterogeneity in lung tumor, more than three cores were required per cases. But due to limitation of tumor size (Table 1, pT1: 34.7%), we failed to obtain equally 3 cores of 5 mm per cases. Third, absence of molecular studies of Id-1 and VEGF and the lack of microvessel index data in tumors were noted in our study. So this study does not confirm the correlation between the strong Id-1 expression and incidence of metastasis as well as that of Id-1 and VEGF. Further studies using large cohorts are needed to confirm our findings. If the relevance of Id-1 protein and angiogenesis in tumor are identified in future studies, Id-1 protein may be the new target of treatment in NSCLC as well as in its prognosis.
Disclosure of conflict of interest
None.
References
- 1.Lammli J, Fan M, Rosenthal HG, Patni M, Rinehart E, Vergara G, Ablah E, Wooley PH, Lucas G, Yang SY. Expression of Vascular Endothelial Growth Factor correlates with the advance of clinical osteosarcoma. Int Orthop. 2012;36:2307–2313. doi: 10.1007/s00264-012-1629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu L, Deng L, Li J, Zhang Y, Hu L. The prognostic value of vascular endothelial growth factor in ovarian cancer: a systematic review and meta-analysis. Gynecol Oncol. 2013;128:391–396. doi: 10.1016/j.ygyno.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Koo PJ, Morgensztern D, Boyer JL, Herbst RS. Targeting vascular endothelial growth factor in patients with squamous cell lung cancer. J. Clin. Oncol. 2012;30:1137–1139. doi: 10.1200/JCO.2011.40.4053. [DOI] [PubMed] [Google Scholar]
- 4.Das M, Wakelee H. Targeting VEGF in lung cancer. Expert Opin Ther Targets. 2012;16:395–406. doi: 10.1517/14728222.2012.669752. [DOI] [PubMed] [Google Scholar]
- 5.Norton JD. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci. 2000;113:3897–3905. doi: 10.1242/jcs.113.22.3897. [DOI] [PubMed] [Google Scholar]
- 6.Yokota Y. Id and development. Oncogene. 2001;20:8290–8298. doi: 10.1038/sj.onc.1205090. [DOI] [PubMed] [Google Scholar]
- 7.Jen Y, Manova K, Benezra R. Expression patterns of Id1, Id2, and Id3 are highly related but distinct from that of Id4 during mouse embryogenesis. Dev Dyn. 1996;207:235–252. doi: 10.1002/(SICI)1097-0177(199611)207:3<235::AID-AJA1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 8.Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O’Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, Benezra R. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- 9.Song X, Liu S, Qu X, Hu Y, Zhang X, Wang T, Wei F. BMP2 and VEGF promote angiogenesis but retard terminal differentiation of osteoblasts in bone regeneration by up-regulating Id1. Acta Biochim Biophys Sin (Shanghai) 2011;43:796–804. doi: 10.1093/abbs/gmr074. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Chung H, Yoo YG, Kim H, Lee JY, Lee MO, Kong G. Inhibitor of DNA binding 1 activates vascular endothelial growth factor through enhancing the stability and activity of hypoxia-inducible factor-1alpha. Mol Cancer Res. 2007;5:321–329. doi: 10.1158/1541-7786.MCR-06-0218. [DOI] [PubMed] [Google Scholar]
- 11.Maw MK, Fujimoto J, Tamaya T. Overexpression of inhibitor of DNA-binding (ID)-1 protein related to angiogenesis in tumor advancement of ovarian cancers. BMC Cancer. 2009;9:430. doi: 10.1186/1471-2407-9-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling MT, Lau TC, Zhou C, Chua CW, Kwok WK, Wang Q, Wang X, Wong YC. Overexpression of Id-1 in prostate cancer cells promotes angiogenesis through the activation of vascular endothelial growth factor (VEGF) Carcinogenesis. 2005;26:1668–1676. doi: 10.1093/carcin/bgi128. [DOI] [PubMed] [Google Scholar]
- 13.Ling MT, Wang X, Zhang X, Wong YC. The multiple roles of Id-1 in cancer progression. Differentiation. 2006;74:481–487. doi: 10.1111/j.1432-0436.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- 14.McMahon G. VEGF receptor signaling in tumor angiogenesis. Oncologist. 2000;5(Suppl 1):3–10. doi: 10.1634/theoncologist.5-suppl_1-3. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi JH, Claesson-Welsh L, Alitalo K. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci U S A. 1998;95:14389–14394. doi: 10.1073/pnas.95.24.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 18.Hasan J, Byers R, Jayson GC. Intra-tumoural microvessel density in human solid tumours. Br J Cancer. 2002;86:1566–1577. doi: 10.1038/sj.bjc.6600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 20.Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- 21.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Wang H, Kuang CY, Zhu JK, Yu Y, Qin ZX, Liu J, Huang L. An essential role for the Id1/PI3K/Akt/NFkB/survivin signalling pathway in promoting the proliferation of endothelial progenitor cells in vitro. Mol Cell Biochem. 2012;363:135–145. doi: 10.1007/s11010-011-1166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Yu Y, Guo RW, Shi YK, Song MB, Chen JF, Yu SY, Yin YG, Gao P, Huang L. Inhibitor of DNA binding-1 promotes the migration and proliferation of endothelial progenitor cells in vitro. Mol Cell Biochem. 2010;335:19–27. doi: 10.1007/s11010-009-0236-9. [DOI] [PubMed] [Google Scholar]