Abstract
Immune complex-mediated complement activation through the classic pathway plays a key role in the pathogenesis of lupus nephritis (LN). C4d deposition in renal tissue reflects the prognosis of systemic lupus erythematosus (SLE). The aim of the current study is to investigate the pathogenesis and clinicopathologic significance of glomerular C4d deposition in LN. We retrospectively analyzed clinical and histopathological data of 20 SLE patients with renal biopsy-proven LN and 10 non-SLE renal biopsy samples as control. LN biopsies showed varying degrees of glomerular C4d staining associated with immune complex deposits, IgG (p = 0.015), C1q (p = 0.032) and C3 (p = 0.049). 7 LN biopsies had all of C4d, C1q and C3 deposits in their glomeruli, indicative of the activation of the classical pathway, whereas 2 LN biopsies had C4d and C3 deposits without accompanying C1q deposits, indicating the activation of the lectin pathway. Glomerular C4d deposition was correlated with the LN subtype (p < 0.001). In particular, a diffusely intense and coarsely granular pattern of C4d deposition in all glomeruli was detected in class V membranous LN. However, glomerular C4d deposition was correlated with neither disease activity of SLE nor histological activity and chronicity of LN. In conclusion, the activation of the lectin pathway as well as the classical pathway seems to play a crucial role in the pathogenesis of LN. Glomerular C4d staining could be helpful for diagnosing class V membranous LN, although glomerular C4d deposition does not reflect SLE disease activity and histological activity and chronicity.
Keywords: Lupus nephritis, C4d, classical pathway, lectin pathway, disease activity
Introduction
SLE is a prototypic autoimmune disease characterized by loss of self-tolerance and the production of autoantibodies to double-stranded DNA and nucleosomes [1]. Immune complex-mediated complement activation via the classical pathway plays a key role in the tissue injury leading to LN, a serious complication of SLE [1]. However, the pathogenesis of LN remains unclear.
A complement split product C4d is produced by breakdown of C4b into C4c and C4d through the classical pathway or lectin pathway after being activated by immune complexes [2]. C4d deposition in the peritubular capillaries (PTC) is widely accepted as a sensitive and specific marker of antibody-mediated rejection in the transplanted kidney [2]. A few recent studies suggest that glomerular C4d deposition in LN is associated with immune complex deposits and the lectin and alternative pathways may be involved in the glomerular injury of LN [3].
Serum complement component C3 and C4 levels are well recognized as valuable markers for SLE disease activity [4]; however, some studies have suggested that complement split products can be more sensitive indicators of SLE disease activity than C3 and C4 levels [5-9]. With regard to renal tissue, there are a few conflicting studies on the association of C4d deposition with disease activity of SLE. C4d deposition in the peritubular capillaries is correlated with disease activity of SLE [10], whereas C4d deposition in the glomeruli is not correlated with disease activity of SLE [5-11].
Therefore, the aims of this study are to (1) determine what complement pathway is involved in the pathogenesis of LN, to (2) examine whether glomerular C4d deposition is helpful for classifying LN subtypes through evaluation of the pattern of glomerular C4d deposition, and to (3) identify whether glomerular C4d deposition is correlated with disease activity of SLE and histological activity and chronicity of LN.
Materials and methods
Patients
We retrospectively studied 20 SLE patients with biopsy-proven LN at Daegu Catholic Medical Center from January 2010 to March 2013. All patients met the diagnostic criteria for SLE according to the American College of Rheumatology. The medical records of all patients were reviewed independently of the analysis of pathologic features. The demographic and clinical data are summarized in Table 1.
Table 1.
Overview of clinical and laboratory data of 20 patients with lupus nephritis
| Patient with LN | Gender/Age (years) | Serum Cr (mg/dL) | Urine protein (mg/24 hr) | Urine RBCs (per HPF) | Anti-ds DNA IgG (IU/mL) | Serum C3/C4 (mg/dL) | ESR (mm/hr) | CRP (mg/L) | Platelet (x103/μL) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F/41 | 1.3 | 6873.6 | > 30 | 424.00 | 49.6/7.8 | 43 | 3.4 | 200 |
| 2 | F/10 | 0.6 | 1124.2 | 1-3 | 486.70 | 24.8/1.6 | 62 | 5.5 | 145 |
| 3 | F/50 | 2.2 | 2775.6 | 5-10 | 1.09 | 83.2/24.4 | 13 | 4.8 | 48 |
| 4 | F/58 | 0.6 | 1445.4 | 20-30 | 21.06 | 74.0/12.1 | 23 | 4.8 | 129 |
| 5 | F/32 | 1.2 | 286.2 | > 30 | 46.56 | 37.1/2.3 | 15 | 4.8 | 197 |
| 6 | F/14 | 1.0 | 180.0 | 10-20 | 258.20 | 116.1/28.5 | 101 | 4.8 | 164 |
| 7 | F/34 | 0.7 | 7386.6 | > 30 | 499.10 | 21.6/5.5 | 19 | 3.8 | 120 |
| 8 | F/18 | 0.7 | 3504.0 | 20-30 | > 500.00 | 67.8/11.1 | 45 | 5.8 | 190 |
| 9 | F/24 | 0.8 | 1638.0 | 10-20 | 122.70 | 72.0/8.4 | 47 | 4.8 | 186 |
| 10 | F/25 | 0.7 | 4956.0 | > 30 | > 500.00 | 18.3/4.1 | 3 | 4.5 | 159 |
| 11 | F/26 | 0.6 | 1537.8 | 0-1 | 3.07 | 49.9/11.6 | 10 | 4.8 | 154 |
| 12 | M/39 | 0.9 | 2337.0 | 1-3 | > 300.00 | 29.8/3.1 | 2 | 54.4 | 141 |
| 13 | F/52 | 0.8 | 1203.6 | 0-1 | 3.82 | 91.2/34.7 | 24 | 8.4 | 212 |
| 14 | F/27 | 0.7 | 2540.4 | 10-20 | < 1.00 | 36.7/5.8 | 6 | 0.1 | 225 |
| 15 | F/29 | 0.7 | 291.0 | > 30 | 43.00 | 34.7/17.3 | 2 | 0.5 | 239 |
| 16 | F/28 | 0.7 | 922.6 | 3-5 | 150.00 | 24.5/1.0 | 39 | 0.3 | 167 |
| 17 | F/28 | 0.6 | 880.1 | 10-20 | > 300.00 | 16.1/2.1 | 84 | 17.5 | 259 |
| 18 | F/54 | 0.6 | 716.1 | > 30 | 5.00 | 20.4/2.4 | 105 | 4.8 | 207 |
| 19 | F/37 | 0.6 | 4330.0 | > 30 | > 379 | 40.2/4.1 | 53 | 8.7 | 79 |
| 20 | M/29 | 1.5 | 1431.4 | 10-20 | 197.00 | 7.5/3.2 | 90 | 79.6 | 75 |
LN, lupus nephritis; F, female; M, male; Cr, creatinine (reference range: 0.6-1.5 mg/dL); Urine protein (reference range: 28-141 mg/24 hr); RBC, red blood cell; HPF, high power field; Anti-ds DNA, anti-double stranded DNA antibodies (reference range: < 5.3 IU/mL); Ig, immunoglobulin; C3, complement C3 (reference range: 90-180 mg/dL); C4, complement C4 (reference range: 10-40 mg/dL); ESR, erythrocyte sedimentation rate (reference range: < 20 mm/hour); CRP, C-reactive protein (reference range: < 5 mg/L); platelet (reference range: 140-380 x103/uL).
The control sample was composed of negative and positive control groups. The negative control group included 3 renal biopsy specimens with minimal change disease and 1 renal biopsy specimen with thin basement membrane disease. The positive control group included 3 renal biopsy specimens with grade III IgA nephropathy and 3 renal biopsy specimens with membranous glomerulopathy.
Renal tissue and histological evaluation
Percutaneous renal biopsies were performed in 20 SLE and 10 non-SLE patients under ultrasonographic guidance. 10% formalin-fixed tissue was embedded in paraffin. Four μm-thick sections were stained with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), periodic acid methenamine silver (PAM), masson-trichrome and polyclonal rabbit anti-human C4d antibody (Biomedia, Austria) using an autostainer (Bond-Max, Leica, USA). Renal biopsy specimens of SLE patients were assessed using the most recent modification of the World Health Organization (WHO) classification proposed by a joint committee of the International Society of Nephrology and the Renal Pathology Society (2003 ISN/RPS) [12,13]. Activity and chronicity indexes of renal lesions were evaluated according to the NIH scoring system [14,15].
C4d immunohistochemical staining
As in the previously described method, renal C4d staining was performed with polyclonal rabbit anti-human C4d antibody (Biomedia, Austria) using an autostainer (Bond-Max, Leica, USA). Glomerular C4d deposition was defined as glomerular capillary wall and/or mesangial C4d staining. The pattern of glomerular C4d staining was documented, and C4d positivity was semi-quantitatively graded as follows: -, no glomerular staining; +, mild glomerular staining; ++, moderate glomerular staining; and +++, intense glomerular staining. Images were captured by a digital camera with OLYMPUS (Tokyo, Japan) BX51 microscopy.
Immunofluorescence evaluation
Fresh frozen tissue sections were stained with fluorescein isothiocyanate-conjugated polyclonal antibodies directed against human IgG, IgA, IgM, C3, C1q, kappa and lambda, respectively (Dako, Denmark). The pattern of immunofluorescent staining in the glomeruli was evaluated. Intensity of glomerular staining for IgG was also semi-quantitatively graded as +, ++ or +++, and for IgA and C3 as -, + or ++. Staining intensity for IgM and C1q was assessed as - or +, where - represented the absence of immunofluorescent staining, and + represented the presence of immunofluorescent staining.
Electron microscopic evaluation
Renal biopsy tissues was fixed in 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer, post-fixed in 1% osmium tetroxide (OsO4) in 0.1 M sodium phosphate buffer, and embedded in epoxy-resin. Ultrathin sections using an Ultramicrotome were stained with uranyl acetate and lead citrate, and examined with a transmission electron microscope (H-7000, HITACHI Japan). The presence and location of electron dense deposits in the glomeruli were examined.
Statistical analysis
The data were expressed as mean ± standard deviation for continuous variables and frequency and percentage for categorical variables. Mann-Whitney tests were used to compare the mean differences between patient groups. The differences in proportions between various groups were assessed by Fisher’s exact tests. A value of P < 0.05 was considered statistically significant. All statistical analyses were performed by software IBM SPSS Statistics 19.0 (IBM Corp., Armonk, NY, USA).
Results
Relationship between glomerular C4d deposits and immune complex deposits
C4d deposit was absent in the negative control group, whereas C4d deposits in the mesangium or along the pericapillary loops were detected in the positive control group. In LN, glomerular deposition of immune complex was present with varying degrees of staining intensity. The staining intensities of glomerular C4d and immune complex in LN are summarized in Table 2. Immunofluorescent staining of glomerular IgG was detected in 100%, IgA in 75.0%, IgM in 40.0%, C1q in 60.0% and C3 in 70.0% of the 20 cases. Table 2 shows possible complement pathways related to the pathogenesis of LN, based on the relative deposition of C4d, C1q and C3. Glomerular C4d deposition was correlated with deposition of immune complexes, especially IgG (p = 0.015), C1q (p = 0.032) and C3 (p = 0.049) (Table 3).
Table 2.
Overview of C4d and immune complex deposition in the glomeruli with possible complement pathway related to the pathogenesis of lupus nephritis
| Patients with LN | ISN/RPS Class | Immunohistochemical C4d staining | Immunofluorescent staining | Complement pathway | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| IgG | IgA | IgM | C1q | C3 | ||||
| 1 | IV | ++ | +++ | + | + | + | ++ | Classical |
| 2 | II | - | ++ | + | + | + | + | Classical |
| 3 | V | +++ | + | - | + | - | - | Lectin |
| 4 | V | +++ | +++ | + | - | - | - | Lectin |
| 5 | IV | ++ | ++ | + | + | + | + | Classical |
| 6 | IV | + | + | + | - | - | - | Lectin |
| 7 | IV | + | + | + | - | - | + | Lectin |
| 8 | IV | ++ | ++ | + | - | + | + | Classical |
| 9 | IV | + | + | - | - | - | + | Lectin |
| 10 | IV | + | ++ | + | + | + | + | Classical |
| 11 | II | + | + | - | - | - | - | Lectin |
| 12 | III | - | ++ | ++ | + | + | ++ | Classical |
| 13 | II | - | + | - | - | - | - | Unknown |
| 14 | V | ++ | +++ | + | - | + | ++ | Classical |
| 15 | IV | ++ | ++ | + | + | + | + | Classical |
| 16 | III | - | ++ | ++ | - | + | ++ | Classical |
| 17 | III | - | ++ | + | - | + | ++ | Classical |
| 18 | II | - | ++ | ++ | + | - | - | Unknown |
| 19 | IV | + | + | - | - | + | + | Classical |
| 20 | III | - | + | + | - | + | ++ | Classical |
LN, lupus nephritis; ISN/RPS Class, the most recent modification of the previous WHO classification proposed by a joint committee of the International Society of Nephrology and the Renal Pathology Society; II, mesangial proliferative LN; III, focal LN; IV, diffuse LN; V, membranous LN. Symbols: for C4d and IgG: -, negative; +, mild; ++, moderate; +++, intense; for IgA, C3 and C1q: -, negative; +, trace; ++, positive; for IgM: -, negative; +, positive.
Table 3.
Relationship between glomerular C4d deposition and the pathologic classes and immune complex deposition in 20 patients with lupus nephritis
| Variable | C4d positivity | p-value | |||
|---|---|---|---|---|---|
|
|
|||||
| - (n = 7) | + (n = 6) | ++ (n = 5) | +++ (n = 2) | ||
| ISN/RPS Class, n (%) | < 0.001* | ||||
| Class II mesangial proliferative LN | 3 (42.9%) | 1 (16.7%) | 0 (0%) | 0 (0%) | |
| Class III focal LN | 4 (57.1%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Class IV diffuse LN | 0 (0%) | 5 (83.3%) | 4 (80.0%) | 0 (0%) | |
| Class V membranous LN | 0 (0%) | 0 (0%) | 1 (20.0%) | 2 (100.0%) | |
| Glomerular C3 staining, n (%) | 0.049* | ||||
| - | 2 (28.6%) | 2 (33.3%) | 0 (0%) | 2 (100.0%) | |
| + | 1 (14.3%) | 4 (66.7%) | 3 (60.0%) | 0 (0%) | |
| ++ | 4 (57.1%) | 0 (0%) | 2 (40.0%) | 0 (0%) | |
| Glomerular C1q staining, n (%) | 0.032* | ||||
| - | 2 (28.6%) | 4 (66.7%) | 0 (0%) | 2 (100.0%) | |
| + | 5 (71.4%) | 2 (33.3%) | 5 (100.0%) | 0 (0%) | |
| Glomerular IgG staining, n (%) | 0.015* | ||||
| + | 2 (28.6%) | 5 (83.3%) | 0 (0%) | 1 (50.0%) | |
| ++ | 5 (71.4%) | 1 (16.7%) | 3 (60.0%) | 0 (0%) | |
| +++ | 0 (0%) | 0 (0%) | 2 (40.0%) | 1 (50.0%) | |
| Glomerular IgA staining, n (%) | 0.098 | ||||
| - | 1 (14.3%) | 3 (50.0%) | 0 (0%) | 1 (50.0%) | |
| + | 3 (42.9%) | 3 (50.0%) | 5 (100.0%) | 1 (50.0%) | |
| ++ | 3 (42.9%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Glomerular IgM staining, n (%) | 0.564 | ||||
| - | 4 (57.1%) | 5 (83.3%) | 2 (40.0%) | 1 (50.0%) | |
| + | 3 (42.9%) | 1 (16.7%) | 3 (60.0%) | 1 (50.0%) | |
| Activated complement pathway, n (%) | 0.003* | ||||
| Classical pathway | 5 (71.4%) | 2 (33.3%) | 5 (100.0%) | 0 (0%) | |
| Lectin pathway | 0 (0%) | 4 (66.7%) | 0 (0%) | 2 (100.0%) | |
ISN/RPS, International Society of Nephrology and the Renal Pathology Society; n, number; LN, lupus nephritis; C, complement; Ig, immunoglobulin.
Statistically significant by Fisher’s exact tests.
Relationship between pattern of glomerular C4d staining and LN subtype
Out of the 20 cases of LN, glomerular C4d deposits were detected in 13 cases. 1 case of class II LN and 5 cases of class IV LN showed mild C4d deposition along the glomerular capillary loops and in the mesangium (Figure 1). 4 cases of class IV LN and 1 case of class V LN showed moderate C4d deposition along the glomerular capillary loops (Figure 2). 2 cases of class V LN showed a diffusely intense and coarsely granular pattern of C4d deposition along the glomerular capillary loops, involving the entire capillary circumference (Figure 3). As well as glomerular staining of C4d, peritubular capillary staining was observed in 4 cases (case No. 3, 9, 15 and 16) and tubular basement membrane staining in 2 cases (case No. 3 and 18). Although glomerular C4d deposits were detected in various subtypes of LN, a uniformly strong and diffusely granular pattern of C4d deposition in all glomeruli was found exclusively in class V membranous LN (Figure 3). Moreover, glomerular C4d deposition was significantly correlated with ISN/RPS Class (p < 0.001) (Table 3).
Figure 1.
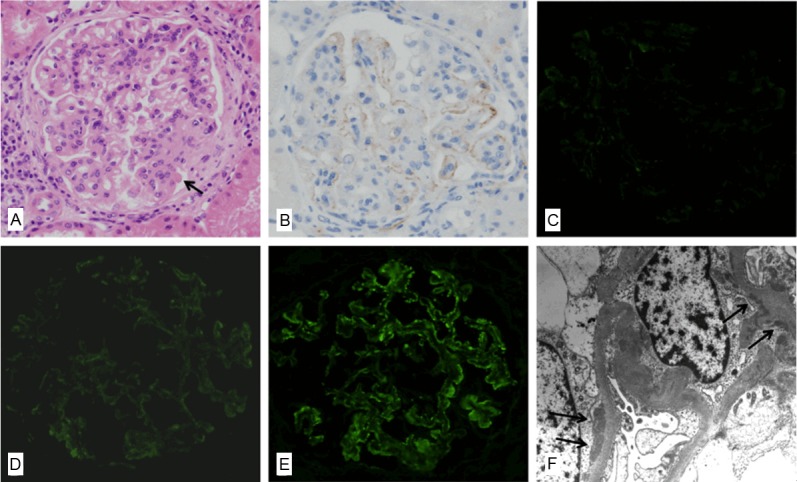
A representative renal biopsy specimen showing activation of the lectin pathway in lupus nephritis (case No. 9). A: Light microscopy in class IV diffuse LN shows increased mesangial cellularity with focally lobular accentuation, hyaline thrombi (arrow) and a few areas of glomerular leukocytic infiltration (hematoxylin and eosin, x 400). B: Mild glomerular C4d staining in the same case is observed along the glomerular capillary loops, represented by +1 (anti-C4d, x 400). C: No C1q deposition in the same case is observed in the glomerulus (anti-C1q immunofluorescence, x 400). D: Immunofluorescence microscopy in the same case reveals trace staining of C3 in the peripheral capillary walls (anti-C3 immunofluorescence, x 400). E: Immunofluorescence microscopy in the same case reveals a weak and granular pattern of IgG deposition in the peripheral capillary walls (anti-IgG immunofluorescence, x 400). F: Electron microscopy in the same case reveals subendothelial deposits (arrow) with effacement of foot processes (transmission electron microscopy, x 8,000).
Figure 2.
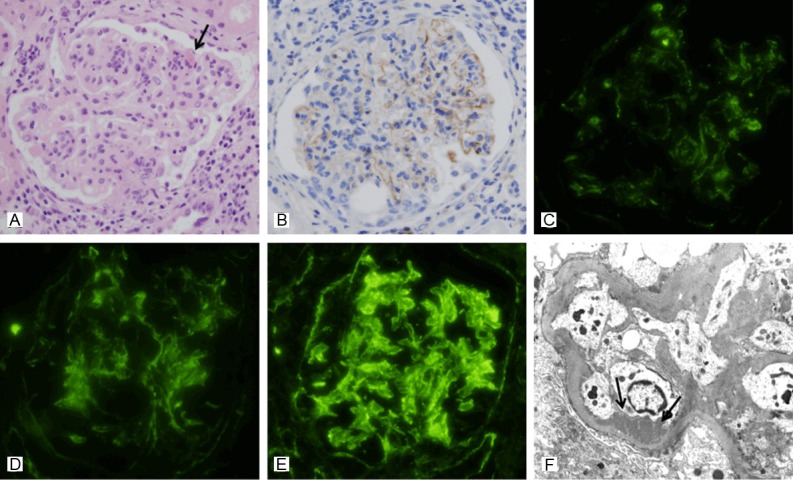
A representative renal biopsy specimen showing activation of the classical pathway in lupus nephritis (case No. 1). A: Light microscopy in class IV LN shows increased mesangial cellularity with focal lobular accentuation, hyaline thrombi (arrow) and glomerular leukocytic infiltration. A greater degree of periglomerular interstitial inflammation is also noted (hematoxylin and eosin, x 400). B: Moderate glomerular C4d staining in the same case is observed along the glomerular capillary loops and in the mesangium (anti-C4d, x 400). C-E: Immunofluorescence microscopy in the same case reveals granular deposition of C1q (C), C3 (D) and IgG (E) both in the measangium and in the peripheral capillary walls (original magnification x 400). F: Electron microscopy in the same case shows subendothelial deposits along the capillary loops. Loss of foot processes is also observed (transmission electron microscopy, x 8,000).
Figure 3.
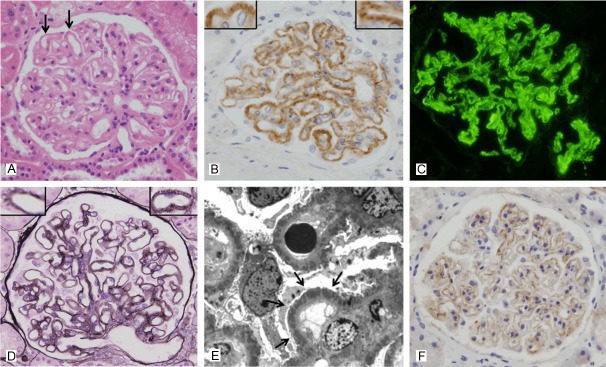
Glomerular C4d staining in class V membranous lupus nephritis (case No. 4). A: Light microscopy in class V membranous LN shows diffuse thickening of the capillary walls or “wire loop” lesions (arrow) (hematoxylin and eosin, x 400). B: Intense glomerular C4d staining in the same case shows a uniformly strong, diffusely intense and coarsely granular pattern along the glomerular capillary loops, represented by +++. Inset: C4d deposits along the glomerular capillary loops resemble the spikes (anti-C4d, x 400). C: Immunofluorescence microscopy in the same case reveals a granular pattern of IgG deposition in the peripheral capillary walls (anti-IgG immunofluorescence, x 400). D: Markedly thickened glomerular basement membrane and small spikes are seen. Inset: Well-developed spikes are present (periodic acid methanemine silver, x 400). E: Electron microscopy in the same case reveals numerous subepithelial deposits (arrow) scattered throughout the capillary loops (transmission electron microscopy, x 8,000). F: The pattern of glomerular C4d staining in membranous nephropathy (positive control) is similar to the C4d staining pattern in case No. 5 class V membranous LN (anti-C4d, x 400).
Relationship between glomerular C4d staining and clinical features of SLE
We divided LN patients into two groups based on the glomerular C4d deposition, and compared the various indexes affecting disease activity of SLE between the two groups. However, there was no significant difference between the two groups, except that LN patients with glomerular C4d deposition showed a lower frequency of increased C-reactive protein (CRP) (p = 0.022). That is, the presence of glomerular C4d deposition was not correlated with the disease activity of SLE (Table 4).
Table 4.
Relationship between glomerular C4d deposition and clinicopathological parameters in 20 patients with lupus nephritis
| Variable | LN with C4d deposition (n = 13) | LN without C4d deposition (n = 7) | p-value† |
|---|---|---|---|
| Female | 13 (65.0%) | 5 (25.0%) | 0.111 |
| Age (years, mean ± SD) | 31.9 ± 12.3 | 34.3 ± 15.4 | 0.579 |
| Serum Cr > 1.5 mg/dL | 1 (5.0%) | 0 (0%) | 1.000 |
| Proteinuria (≥ 3.5 g / 24 hr / 1.73 m2) | 5 (25.0%) | 0 (0%) | 0.114 |
| Hematuria (urine RBC ≥ 10/HPF) | 11 (55.0%) | 3 (15.0%) | 0.122 |
| Anti-ds DNA Ab | 10 (50.0%) | 5 (25.0%) | 1.000 |
| Low C3 (< 90 mg/dL) | 12 (60.0%) | 6 (30.0%) | 1.000 |
| Low C4 (< 10 mg/dL) | 7 (35.0%) | 6 (30.0%) | 0.329 |
| Increased ESR (> 20 mm/hour) | 6 (30.0%) | 6 (30.0%) | 0.158 |
| Increased CRP (≥ 5 mg/L) | 2 (10.0%) | 5 (25.0%) | 0.022* |
| Activity Index (AI) | 5.00 ± 3.08 | 2.86 ± 2.73 | 0.163 |
| Endocapillary hypercellularity | 8 (40.0%) | 3 (15.0%) | 0.642 |
| Cellular crescent | 2 (10.0%) | 0 (0%) | 0.521 |
| Fibrinoid necrosis present | 8 (40.0%) | 2 (10.0%) | 0.350 |
| Hyaline thrombi present | 11 (55.0%) | 3 (15.5%) | 0.122 |
| Glomerular leukocytic infiltration present | 10 (50.0%) | 2 (10.0%) | 0.062 |
| Interstitial inflammation | 11 (55.0%) | 5 (25.0%) | 0.587 |
| Chronicity Index (CI) | 1.92 ±1.32 | 1.14 ± 0.00 | 0.215 |
| Glomerulosclerosis present | 6 (30.0%) | 2 (10.0%) | 0.642 |
| Fibrous crescents | 2 (10.0%) | 1 (5.0%) | 1.000 |
| Interstitial fibrosis present | 12 (60.0%) | 4 (20.0%) | 0.101 |
| Tubular atrophy present | 5 (25.0%) | 1 (5.0%) | 0.354 |
Data were expressed as mean ± SD for continuous variables and n (%) for categorical variables; LN, lupus nephritis;
Calculated by Mann-Whitney tests for continuous variables and Fisher’s exact tests for categorical variables;
Cr, creatinine; RBC, red blood cell; HPF, high power field; Anti-ds DNA, anti-double stranded DNA antibodies; C3, complement C3; C4, complement C4; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
Statistically significant by Fisher’s exact tests.
Relationship between the glomerular C4d staining and histological features of LN
We compared parameters of activity and chronicity of LN between the two groups (Table 4). There was no significant difference between the two groups. The histological features indicating the activity and chronicity of LN are summarized in Table 5.
Table 5.
Summary of histological features of activity and chronicity in lupus nephritis
| Patients with LN | No. of glomeruli | Activity index | Chronicity index | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| ECH | CC | FN | HT | GLI | II | Score | GS | FC | IF | TA | Score | ||
| 1 | 17 | + | - | + | + | + | ++ | 7 | - | - | + | - | 1 |
| 2 | 41 | - | - | - | - | - | - | 0 | - | - | - | - | 0 |
| 3 | 40 | - | - | - | + | - | + | 2 | + | - | + | + | 3 |
| 4 | 22 | - | - | - | + | - | - | 1 | - | - | + | - | 1 |
| 5 | 45 | + | - | + | + | + | +++ | 8 | + | - | + | + | 3 |
| 6 | 27 | + | - | - | - | + | + | 3 | + | - | + | + | 3 |
| 7 | 24 | - | - | + | + | + | + | 5 | - | - | + | - | 1 |
| 8 | 50 | + | + | + | + | + | + | 8 | + | + | + | + | 4 |
| 9 | 28 | + | - | + | + | + | ++ | 7 | + | - | + | - | 2 |
| 10 | 43 | + | - | + | + | + | + | 6 | - | - | + | - | 1 |
| 11 | 29 | - | - | - | - | - | + | 1 | - | - | + | - | 1 |
| 12 | 23 | - | - | - | + | - | ++ | 3 | + | + | + | - | 3 |
| 13 | 12 | - | - | - | + | - | + | 2 | + | - | + | - | 2 |
| 14 | 41 | - | - | - | + | - | - | 1 | - | - | - | - | 0 |
| 15 | 18 | + | - | + | + | + | + | 6 | - | - | + | - | 1 |
| 16 | 13 | + | - | - | - | - | + | 2 | - | - | + | - | 1 |
| 17 | 18 | + | - | + | - | + | +++ | 7 | - | - | + | + | 2 |
| 18 | 33 | - | - | - | - | - | - | 0 | - | - | - | - | 0 |
| 19 | 27 | + | + | + | + | + | +++ | 10 | + | + | + | + | 4 |
| 20 | 34 | + | - | + | + | + | + | 6 | - | - | - | - | 0 |
LN, lupus nephritis; No, number; ECH, endocapillary hypercellularity; CC, cellular crescents; FN, fibrinoid necrosis; HT, hyaline thrombi; GLI, glomerular leukocytic infiltration; II, interstitial inflammation; GS, glomerular sclerosis; FC, fibrous crescents; IF, interstitial fibrosis; TA, tubular atrophy. Symbols: for all index: -, negative; +, mild; ++, moderate; +++, severe.
Discussion
Immune complex-mediated complement activation via the classical pathway plays a key role in the pathogenesis of LN [1]. The classical pathway is initiated by binding of the complement C1 complex to the antigen-antibody complex or CRP, which is followed by the cleavage of the complement component C4 into C4a and C4b, and then C4b is cleaved into C4c and C4d [2]. The presence of C4d and C1q deposits with C3 deposits in the glomerulus is considered to be the evidence for the activation of the classical pathway. In the present study, glomerular C4d deposition, accompanied by C1q deposits, was detected in 7 of 20 LN patients (case No. 1, 5, 8, 10, 14, 15 and 19). Glomerular C3 deposition was also observed in all of them (Table 2), indicating that activation of the classical pathway might be involved in the progression of LN.
C4d is also produced by activation of the lectin pathway [2]. The lectin pathway is activated by binding of mannose-binding lectin (MBL) or ficolins to the carbohydrate ligand on the microbial surface, leading to activation of MBL-associated serine protease (MASP) and cleavage of C4 [2,16]. In addition, the microbial surface directly cleaves C3, leading to activation of the alternative pathway, and factor B, factor D and properdin are involved in the cascade event [2,16]. Recently, Sato et al. [3] reported that activation of the lectin and alternative pathways was involved in the glomerular injury of LN. Glomerular deposition of C4d and C3 without accompanying C1q deposits can be considered to be the evidence for the activation of the lectin pathway. In the present study, 2 LN patients with glomerular C4d deposition showed C3 deposits without C1q deposits (case No. 7 and 9), which indicates the possibility of the activation of the lectin pathway in the pathogenesis of LN. In addition, glomerular C4d deposition without accompanying C1q deposition was found in another 4 LN patients. However, this was not accompanied by C3 deposition (case No. 3, 4, 6 and 11), which indicates there is needed for further evaluation using MBL or L-ficolin staining to check for activation of the lectin pathway in these patients.
Glomerular C3 deposition without C4d and C1q deposits could be considered as a marker for the activation of the alternative pathway. In the present study, we could not find any case showing activation of an alternative pathway. Moreover, no deposits for C1q, C4d and C3 were detected in 2 LN patients, who were classified as class II mesangial proliferative LN (case No. 13 and 18). We thought the reason for the absence of deposition of these complements in class II mesangial proliferative LN is that deposition of these complements is associated with deposition of immune complexes: the relatively small numbers of stable immune complexes prevents the mesangial clearing system from becoming overloaded and allows the complexes to be sequestered in the mesangium [17]. The sequestered immune complexes are apt to be degraded and removed rather than remaining at the sites where they could initiate complement activation, resulting in the absence of deposits of C1q, C4d and C3 in the glomeruli of class II mesangial proliferative LN.
Immunohistochemical staining for C4d on paraffin-embedded tissue is less sensitive than immunofluorescent staining on frozen tissue [18]. In the present study, 5 LN patients had glomerular C1q and C3 deposits without accompanying C4d deposits. Among these patients, 4 are classified as having class III focal LN (case No. 12, 16, 17 and 20) except for one who is having class II mesangial proliferative LN (case No. 2). The activation of the classical pathway could be also involved in the progression of LN in these patients, considering the lower sensitivity of immunohistochemical staining for C4d on paraffin-embedded tissue compared to immunofluorescent staining for C1q and C3 on fresh frozen tissue.
Glomerular C4d deposition was associated with immune complex deposition detected by immunofluorescence or electron microscopy, and significantly correlated with immune complexes, especially IgG (p = 0.015), C1q (p = 0.032) and C3 (p = 0.049) deposits (Table 3). In addition, the pattern of glomerular C4d deposition varied with the LN subtype (p < 0.001) (Table 3): a uniformly strong and granular pattern of C4d deposition in the glomeruli was found exclusively in class V membranous LN (Figure 3), but this pattern was less strong in some class IV diffuse LN (Figures 1 and 2) and class III focal LN. In addition, the pattern of glomerular C4d staining with thickened glomerular basement membrane and spikes in class V membranous LN, was reminiscent of the findings in membranous nephropathy. Thus, glomerular C4d immunohistochemical staining can be a useful method for confirmation of class V membranous LN when there is absence of glomeruli in the frozen renal section.
The pathogenesis of LN varies with the class of the lesion [17]. The pattern of glomerular C4d deposition also varied with the LN subtype. The reason that the pattern of glomerular C4d deposition varies with the LN subtype may be due to the size, quantity or stability of the immune complexes and location of the deposited immune complexes. Large numbers of intermediate-size complexes or large complexes formed by high-affinity antibodies, which are characteristic of class III focal LN and class IV diffuse LN, tend to overcome the mesangial ability to clear these macromolecules, leading to their accumulation in a paramesangial subendothelial location, and then ultimately in the peripheral capillary loops [17]. Immune complexes localized to the subendothelial region have easy access to plasma inflammatory mediators, causing inflammation and injury of the endothelium, thus allowing the permeability of the capillary wall to increase, resulting in deposition of immune complexes in the subendothelial area [17] and immune complex-mediated complement activation.
In contrast, small, unstable, and circulating immune complexes formed by low-avidity and low-affinity antibodies, which are characteristic of class V membranous LN, may dissociate with the antigen or antibody lodging in the glomerular capillaries [17]. Subsequently, complexes may be formed by in situ attachment to the target protein in the outer aspect of the GBM [17], where the complexes may induce activation and deposition of complements. Thus, the different pathogenesis of each LN class seems to result in varying patterns of glomerular C4d deposition according to the LN subtype.
The complement split product C4d can covalently bind to endothelial surfaces and basement membranes via a thioester moiety [19]. C4d deposition on endothelial cells induces insertion of sublytic levels of membrane attack complex (MAC), which is followed by von Willebrand factor release from endothelial cells, and then platelet aggregation [2]. This event might bring about a decrease in platelet levels and renal microthrombi. In the present study, intensity of glomerular C4d deposition was marginally significant by platelet levels (p = 0.056, data not shown). However, only 1 of 13 LN patients with glomerular C4d deposition had microthrombi (case No. 19), and another 1 LN patient had microthrombi without glomerular C4d deposition (case No. 17). Thus we thought that glomerular C4d deposition was not an indicator of thrombotic microangiopathy in LN, which was different the results of Cohen et al. [20]. Furthermore, we divided the LN patients into two groups based on glomerular C4d deposition and compared parameters of SLE disease activity and histological features of activity and chronicity between the two groups. However, glomerular C4d deposition showed a poor correlation with SLE disease activity and histological activity and chronicity, as reported by Batal et al. [5] and Kim et al. [11] (Table 4). In addition, regardless of which complement pathway was activated, there was no significant difference in SLE disease activity and histological activity and chronicity (data not shown).
In conclusion, this small but creative study represents the first attempt to describe the immunopathogenesis of glomerular C4d deposition according to LN subtype. Deposition of immune complexes that exceeds the ability of the mesangial clearing system to clear the macromolecules leads to activation of the complement pathway. The activation of the lectin pathway as well as the classical pathway in the glomerulus might be implicated in the pathogenesis of LN. Furthermore, glomerular C4d deposition is correlated with immune complex deposition, which shows different patterns of deposition according to the LN subtypes. A uniformly strong and granular pattern of glomerular C4d deposition can be a useful for diagnosing class V membranous LN, although glomerular C4d deposition does not reflect disease activity of SLE and histological activity and chronicity of LN.
Acknowledgements
This work was supported by the grant of Research Institute of Medical Science, Catholic University of Daegu, Republic of Korea (2012).
References
- 1.Tang S, Lui SL, Lai KN. Pathogenesis of lupus nephritis: an update. Nephrology (Carlton) 2005;10:174–179. doi: 10.1111/j.1440-1797.2005.00392.x. [DOI] [PubMed] [Google Scholar]
- 2.Murata K, Baldwin WM 3rd. Mechanisms of complement activation, C4d deposition, and their contribution to the pathogenesis of antibody-mediated rejection. Transplant Rev (Orlando) 2009;23:139–150. doi: 10.1016/j.trre.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato N, Ohsawa I, Nagamachi S, Ishii M, Kusaba G, Inoshita H, Toki A, Horikoshi S, Ohi H, Matsushita M, Tomino Y. Significance of glomerular activation of the alternative pathway and lectin pathway in lupus nephritis. Lupus. 2011;20:1378–1386. doi: 10.1177/0961203311415561. [DOI] [PubMed] [Google Scholar]
- 4.Manzi S, Ahearn JM, Salmon J. New insights into complement: a mediator of injury and marker of disease activity in systemic lupus erythematosus. Lupus. 2004;13:298–303. doi: 10.1191/0961203303lu1016oa. [DOI] [PubMed] [Google Scholar]
- 5.Batal I, Liang K, Bastacky S, Kiss LP, McHale T, Wilson NL, Paul B, Lertratanakul A, Ahearn JM, Manzi SM, Kao AH. Prospective assessment of C4d deposits on circulating cells and renal tissues in lupus nephritis: a pilot study. Lupus. 2012;21:13–26. doi: 10.1177/0961203311422093. [DOI] [PubMed] [Google Scholar]
- 6.Manzi S, Rairie JE, Carpenter AB, Kelly RH, Jagarlapudi SP, Sereika SM, Medsger TA Jr, Ramsey-Goldman R. Sensitivity and specificity of plasma and urine complement split products as indicators of lupus disease activity. Arthritis Rheum. 1996;39:1178–1188. doi: 10.1002/art.1780390716. [DOI] [PubMed] [Google Scholar]
- 7.Liu CC, Manzi S, Kao AH, Navratil JS, Ruffing MJ, Ahearn JM. Reticulocytes bearing C4d as biomarkers of disease activity for systemic lupus erythematosus. Arthritis Rheum. 2005;52:3087–3099. doi: 10.1002/art.21305. [DOI] [PubMed] [Google Scholar]
- 8.Muso E, Sekita K, Doi T, Kuwahara T, Yoshida H, Tamura T, Kawai C, Hamashima Y. Immunopathological correlation between mesangial C3d-deposition and C3d-fixing circulating immune complexes in lupus nephritis. Clin Immunol Immunopathol. 1984;32:351–358. doi: 10.1016/0090-1229(84)90278-2. [DOI] [PubMed] [Google Scholar]
- 9.Kao AH, Navratil JS, Ruffing MJ, Liu CC, Hawkins D, McKinnon KM, Danchenko N, Ahearn JM, Manzi S. Erythrocyte C3d and C4d for monitoring disease activity in systemic lupus erythematosus. Arthritis Rheum. 2012;62:837–844. doi: 10.1002/art.27267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li SJ, Liu ZH, Zen CH, Wang QW, Wang Y, Li LS. Peritubular capillary C4d deposition in lupus nephritis different from antibody-mediated renal rejection. Lupus. 2007;16:875–880. doi: 10.1177/0961203307083279. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Jeong HJ. Glomerular C4d deposition indicates in situ classic complement pathway activation, but is not a marker for lupus nephritis activity. Yonsei Med J. 2003;44:75–80. doi: 10.3349/ymj.2003.44.1.75. [DOI] [PubMed] [Google Scholar]
- 12.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–250. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 13.Markowitz GS, D’Agati VD. Classification of lupus nephritis. Curr Opin Nephrol Hypertens. 2009;18:220–225. doi: 10.1097/mnh.0b013e328327b379. [DOI] [PubMed] [Google Scholar]
- 14.Austin HA 3rd, Muenz LR, Joyce KM, Antonovych TA, Kullick ME, Klippel JH, Decker JL, Balow JE. Prognostic factors in lupus nephritis. Contribution of renal histologic data. Am J Med. 1983;75:382–391. doi: 10.1016/0002-9343(83)90338-8. [DOI] [PubMed] [Google Scholar]
- 15.Austin HA 3rd, Muenz LR, Joyce KM, Antonovych TT, Balow JE. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int. 1984;25:689–695. doi: 10.1038/ki.1984.75. [DOI] [PubMed] [Google Scholar]
- 16.Monticielo OA, Mucenic T, Xavier RM, Brenol JC, Chies JA. The role of mannose-binding lectin in systemic lupus erythematosus. Clin Rheumatol. 2008;27:413–419. doi: 10.1007/s10067-008-0838-8. [DOI] [PubMed] [Google Scholar]
- 17.Fogo AB, Kashgarian M. Diagnostic Atlas of Renal Pathology. Second edition. Elsevier Saunders; 2012. Lupus nephritis; pp. 222–223. [Google Scholar]
- 18.Chantranuwat C, Qiao JH, Kobashigawa J, Hong L, Shintaku P, Fishbein MC. Immunoperoxidase staining for C4d on paraffin-embedded tissue in cardiac allograft endomyocardial biopsies: comparison to frozen tissue immunofluorescence. Appl Immunohistochem Mol Morphol. 2004;12:166–171. doi: 10.1097/00129039-200406000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Feucht HE, Opelz G. The humoral immune response towards HLA class II determinants in renal transplantation. Kidney Int. 1996;50:1464–1475. doi: 10.1038/ki.1996.460. [DOI] [PubMed] [Google Scholar]
- 20.Cohen D, Koopmans M, Kremer Hovinga IC, Berger SP, Roos van Groningen M, Steup-Beekman GM, de Heer E, Bruijn JA, Bajema IM. Potential for glomerular C4d as an indicator of thrombotic microangiopathy in lupus nephritis. Arthritis Rheum. 2008;58:2460–2469. doi: 10.1002/art.23662. [DOI] [PubMed] [Google Scholar]


