Abstract
Background: Hibernoma is a rare benign fat-forming soft tissue tumor that differentiates similar to brown fat, hence an origin from remnants of fetal brown adipose tissue has been proposed. Mainly young adults are affected, usually without significant clinical symptoms. Material and methods: We report on four patients with hibernomas, who were treated at our hospital during the last 10 years. The clinicopathologic and immunohistochemical features are presented and treatment and follow-up data discussed. Results: Patients were 2 women and 2 men aged 21-67 years (mean: 45 yrs) who presented with a slowly growing, painless mass. The anatomic location was the thigh, upper arm, lateral thoracic wall and paravertebral soft tissue. Two of them were diagnosed preoperatively through a percutaneous core needle biopsy and the other two underwent surgery because of high clinical and radiological suspicion of liposarcoma. The tumor’s size ranged from 7 cm to 15.5 cm (mean: 11 cm). All were deep-seated subfascial intramuscular masses. Histologically, all four tumors were of the typical variant. All patients underwent a R0-surgical resection of the tumor and they were recurrence-free at last follow-up (mean: 47 months; range: 25-87). Conclusion: Hibernoma may present as huge deep intramuscular soft tissue mass in adults, closely mimicking well differentiated liposarcoma and should be considered in the differential diagnosis of fatty soft tissue tumors in any location. Surgical excision is the treatment of choice. The tumor has no malignant or recurrence potential.
Keywords: Hibernoma, brown tissue, soft tissue tumor, deep-seated
Introduction
Hibernoma is a rare benign adipocytic tumor that differentiates similar to brown fat, hence an origin from remnants of fetal brown adipose tissue has been proposed. Since its first description by Merkel in 1906 [1], and proposal of the term hibernoma by Gery in 1914 [2], more than 500 cases have been reported in the literature [3]. The tumor’s name reflects the morphologic similarity of the tumor cells to the cells of hibernating animals.
Unlike benign tumors originated from white adipose tissue (lipomas), hibernomas are strictly uncommon. Their peculiar brownish color reflects the presence of numerous cytoplasmic mitochondria, with high concentration of cytochrome pigments [3]. Histologically, hibernomas are composed mainly of brown fat cells with granular, multivacuolated cytoplasm in addition to varying amounts of white fat cells, spindled cells and myxoid stroma. This highly variable composition makes hibernomas similarly variable on imaging and was the base for recognition of main four histological subtypes (typical, lipoma-like, myxoid and spindle cell variants) [4].
Hibernomas occurs predominantly in young adults in a wide variety of locations, particularly at sites where brown fat persists beyond fetal life. The most common locations are the thigh, trunk, upper extremity, shoulder, axilla, back, and the head and neck area [3]. Symptoms are mainly related to the presence of a mass or impingement upon adjacent structures. If completely excised, hibernomas have not been reported to recur, and malignant transformation has not been unequivocally documented [3]. Herein, we report our experience with 4 cases of hibernomas that presented as huge deep-seated subfascial soft tissue masses closely mimicking well differentiated liposarcoma/atypical lipomatous tumor on preoperative assessment. We discuss their diagnosis, management and prognosis in the light of the literature.
Material and methods
We retrospectively reviewed all patients treated for hibernoma at our institution over the last 10 years. All patients had magnetic resonance imaging (MRI) prior to biopsy and surgery, the MRI protocol contained T1-w, T2-w and T1-w images after contrast material (Gadolinium-chelates) with or without fat suppression. Diagnosis was verified according to currently applied diagnosis criteria [5]. Clinical and pathological records were reviewed and data from cooperating hospitals and general practitioners were included. Patient’s age and sex, past medical history, preoperative symptoms, diagnostic work-up, tumor localization and characteristics, surgical therapy, morphological variant (typical, lipoma-like myxoid, spindle cell) and selected immunohistochemical markers were assessed. Follow-up information was obtained from the patients’ medical records, the patients’ physicians and the patients themselves. Immunohistochemical staining was performed on freshly cut 2-μm paraffin sections using a Ventana automated system (VANTAGE) and the following antibodies: protein S-100 (polyclonal), CD34, CDK4 and MDM2 (sources of antibodies, reagents and staining protocols are available upon request).
Results
Clinical features
The clinical features of the cases are summarized in Table 1. There were 2 females and 2 males with an age ranged between 21 and 69 years (mean: 45.5). None had positive drug or tobacco history, history of irradiation of the tumor site or other relevant clinical history. The anatomic locations were the thigh, upper arm, lateral thoracic wall and paravertebral soft tissue. The majority of tumors presented as slowly enlarging painless masses. Laboratory data, including complete blood count, blood chemistry and coagulation profiles were normal in all cases.
Table 1.
Clinicopathological features of the hibernoma cases
| n | gender | age | localisation | treatment | tumor size (mm) | follow-up (months)/outcome |
|---|---|---|---|---|---|---|
| 1 | female | 21 | paravertebral | surgical excision | 75 | 87 ANED |
| 2 | male | 69 | lateral thoracic wall | surgical excision | 70 | 50 ANED |
| 3 | male | 47 | thigh | surgical excision | 155 | 25 ANED |
| 4 | female | 46 | upper arm | surgical excision | 135 | 25 ANED |
ANED=alive with no evidence of disease.
MRI (performed in all cases) demonstrated well circumscribed lesions with predominantly fat equivalent signal intensity (SI). The lesions showed high SI on T1-w and T2-w TSE (turbo-spin echo) images and marked SI loss with fat suppression. In contrast to subcutaneous fat or lipoma, the lesions contained a variable amount of streaks and septations of intermediate SI that demonstrated increased SI after contrast material injection (Figure 1A, 1B). Inhomogeneity within the texture of the adipose tissue could also be detected. Two patients with extremity tumors underwent a preoperative ultrasound-guided core needle biopsy (Figure 1C). Diagnosis on core needle biopsy was “hibernoma” in one case and “lipomatous neoplasm, suspicious for atypical lipomatous tumor” in the other case. The tumor size ranged from 7 cm to 15.5 cm (mean: 11 cm). Surgical margins were free of tumor tissue (R0) in all cases. Postoperative course was uneventful. Follow-up was obtained for all cases over a mean interval of 47 months (range 25-87); all were alive and free of disease with no evidence of local recurrence or metastases.
Figure 1.
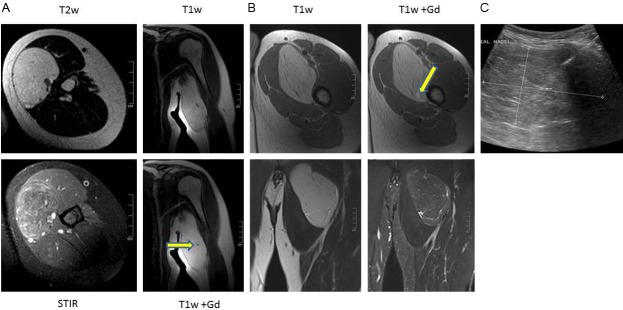
A: 46 year old woman with hibernoma of the left arm. T1-w and T2-w images show a well circumscribed lesion with a high SI similar to the subcutaneous fat. Within the lesion fine septa can be delineated and a small contrast enhanced vessel (arrow T1-w Gd axial). On STIR images, the hibernoma has higher and more inhomogeneous SI as compared to subcutaneous fat. B: 47 year old man with hibernoma of the left thigh. T1-w and T2-w images show a well circumscribed lesion with a high SI similar to the subcutaneous fat. Within the lesion fine septa can be delineated with a small spotty area of contrast enhancement (arrow T1-w Gd axial). On STIR images, this hibernoma demonstrates similar SI like subcutaneous fat with high signal intensity streaks of fibrovascular tissue. C: The ultrasound image demonstrates a well circumscribed slightly inhomogeneous mass with a biopsy needle in place. The small arrow points to a vessel within a septum.
Pathological findings
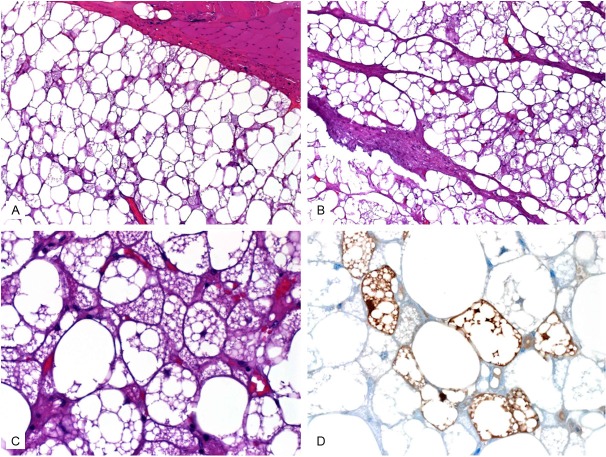
Grossly, all tumors presented as large well circumscribed encapsulated soft tissue masses of soft fleshy consistency (Figure 2A). The cut-surface was brown-yellow with lobulated appearance (Figure 2B). Infiltrative growth and/or satellite nodules were not seen at the periphery. Histologically, the tumor showed an intimate admixture of palely staining univacuolated fat cells and eosinophilic multivacuolated hibernoma cells (Figure 3). The eosinophilic cells comprised at least 30 of the tumor cells but they were generally dominating. They displayed deeply eosinophilic granular cytoplasm with well defined cell borders. Occasional multivacuolated fat cells were reminiscent of lipoblasts but true lipoblasts were not seen. There was no atypical bizarre stromal cell or multinucleated giant cells and mitotic figures were not detected. Immunohistochemistry showed expression of protein S100. The tumor cells were negative consistently for CD34, CDK4 and MDM2 in all cases.
Figure 2.
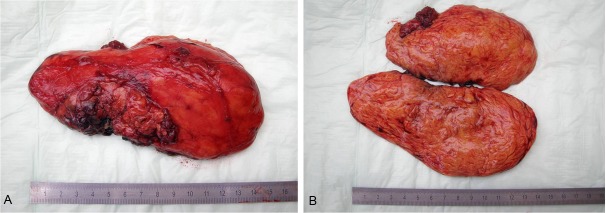
Example of the gross features of resected hibernoma of the left upper arm. A: Huge encapsulated fleshy mass with yellow-brown color. B: Cut-surface showed prominent lobulation and brown-yellowish color.
Figure 3.
Histological features of hibernoma. A: Tumor (lower) is well demarcated from adjacent skeletal muscle (upper) by a thin fibrous capsule. Note admixture of pale and granular eosinophilic cells. B: Prominent fibrovascular septa were seen in this case. C: Higher magnification of eosinophilic cells with lipoblast-like appearance. D: Protein S-100 mainly highlighted the microvesicular brown cells.
Discussion
Hibernomas are rare neoplasms of adults that account for ~1.6% of benign lipomatous tumors [3]. The peak incidence occurs during the 3rd and 4th decade of life, with a wide age range (2-75 years). The reported gender distribution varied from a slight female predilection in older reports to a clear-cut overrepresentation of men in the Armed forces Institute of Pathology (AFIP) series (99 of 170 cases) [3]. Although the location of these tumors was generally thought to parallel the distribution of brown fat in humans, almost one third of the AFIP cases occurred in the thigh and not in sites well known to harbor residual embryonic brown fat. Furthermore, hibernoma is exceptionally rare in children (a single pediatric case reported prior to 2001 [6] and only 9 of 170 AFIP cases occurred before 18 years of age [3]), thus sharply contrasting with the high frequency of brown fat in childhood. These observations make an altered differentiation pathway in the fatty cells of hibernoma rather than true origin from brown fat more plausible [3]. Thus, hibernoma should be defined as a “benign adipocytic tumor with differentiation similar to brown fat” and not as a tumor arising from brown fat. It has been proposed that brown fat is most closely related to the skeletal muscle rather than the white adipose tissue [7,8], which could explain the more common location of hibernomas in the thigh and frequent intramuscular cases at this site. Interestingly, only 2 of 170 cases in the AFIP series were in the breast in sharp contrast to the first description of the entity as a breast tumor [1].
Clinically, the typical presentation is that of a slow growing, progressive, painless soft-tissue mass with a mean size of 9 cm (range, 1-24 cm). The average duration of the tumor prior to diagnosis was 30.6 months [3]. Localized tenderness is rare. Symptoms are present only when compression of adjacent structures exists. Some cases represented incidental findings during investigations for other diseases [3,4,9]. Physical examination often suggests a lipoma. Significant weight loss was also described in rare cases and was attributed to excessive thermogenesis by the tumor tissue [10]. Rare cases displayed a rapid increase in size (as large as 24 cm) [3].
Deep-seated (intramuscular) hibernomas are uncommon (19/170 in the AFIP series) [3]. A majority of intramuscular cases corresponded to the classical variant and only 4 were lipoma-like but none was of spindle cell-type. Our cases were all deep-seated intramuscular masses and showed typical hibernoma histology. Based on the observation that a majority of subfascial soft tissue masses turn out to be malignant, it is this uncommon intramuscular hibernoma variant that closely mimics liposarcoma preoperatively. Conventional radiography often demonstrates a radiolucent mass or swelling with no osseous abnormalities or mineralization. On ultrasonography (first diagnostic step for most of palpable soft-tissue masses), hibernomas are described as well-circumscribed lesions with an echotexture ranging from hypo- to hyperechoic appearance, depending on the content of regular fat. Large feeding vessels on the surface and within septa of the hibernoma may be noted on Doppler ultrasound [11,12].
On computed tomography (CT), they are seen as well-defined intermuscular, intramuscular, subcutaneous or retroperitoneal heterogeneous low-density lesions, with predominantly negative HU values. Internal septations and sometimes a intratumoral vessel can be depicted after intravenous contrast administration [13].
On MRI, fat equivalent SI is characteristic with high SI on both T1- and T2-weighted TSE images. Fat suppression techniques usually result in a marked SI reduction, but depending on the tissue composition and the ratio of regular (yellow) to brown fat, this SI reduction is less than that of subcutaneous fat. After contrast material injection, spotty areas of high SI as well as vessels can be depicted [12,14]. Prominent fibrovascular septa are most probably responsible for the heterogeneity of MRI signal within the lesion [7,8,15]. Using these imaging characteristics, the diagnosis of hibernoma may be suggested [16-20], but they overlap with those of well differentiated liposarcoma. Lipoma on the other hand should demonstrate an almost complete signal loss when fat suppression techniques are used and have no or only minimal internal septations.
Although it is rare, hibernoma should be included in the differential diagnosis of lipomatous soft-tissue tumors. The imaging findings of hibernoma are not specific, other differential diagnostic considerations for a mass with signal similar to fat or containing large intratumoral vessels include angiolipoma, intramuscular hemangioma with fat, spindle cell lipoma, pleomorphic lipoma, lipoblastoma, hemangiopericytoma and hemangioblastoma [21-25], as well as malignant processes including lipoma-like well differentiated liposarcoma and myxoid liposarcoma [15].
Histologically, 4 hibernoma variants have been described. The typical variant is the most frequent and displays a distinct lobular pattern. It is composed of eosinophilic granular cells and multivacuolated pale cells (adipocytes) with a clear appearance in variable proportions. The myxoid variant showed a variable proportion of loose acellular myxoid stroma. This variant causes the most problems for surgical pathologists, because multivacuolated hibernoma cells might be mistaken for lipoblasts, and these tumors may be confused with myxoid liposarcomas. The spindle cell variant shows similar histological features of spindle cell lipoma admixed with multivacuolated cells, often occurring in the neck or scalp. The lipoma-like variant comprises a few scattered hibernoma cells among a predominance of white-fat cells [26,27].
Immunohistochemistry is not contributory, but S-100 protein is usually positive in both eosinophilic and pale cells and staining pattern of S-100-positive cells varies from focal to diffuse. Most tumors are CD34 negative [27]. By definition, hibernoma should be negative for CDK4 and MDM2, two markers that characterize well differentiated liposarcoma. In equivocal cases, immunohistochemistry can be supplemented by fluorescence in-situ hybridization (FISH) to exclude amplification of MDM2 and hence exclude atypical lipomatous tumor/liposarcoma. Cytogenetic analyses of hibernomas have consistently revealed structural rearrangements of chromosome 11q13 and 11q21 [28-32], but these are not unique to hibernomas and have been also described in lipoma [30,32].
Curative treatment of hibernoma is complete excision. Because the tumor usually has a well-defined capsule and does not show infiltrative growth pattern, preserving vital structures is recommended. The vascular supply is considerably more prominent in hibernoma than in lipoma, therefore it should be treated with care to avoid postoperative bleeding or hematoma formation [3]. Incomplete excision of hibernoma has been reported to result in regrowth of the tumor [33]. None of 66 cases with extended follow-up (mean, 7.7 yrs) in the series reported by Furlong et al from the AFIP recurred, even those tumors that were intramuscular [3].
In conclusion, hibernomas are benign rare tumors that show similarity to brown fat and occur at diverse soft tissue sites with a predilection for the thigh. They should be considered in the differential diagnosis of large and deeply seated fatty soft tissue tumors in any location. Due to their uniformly benign nature, distinction from atypical lipomatous tumor/well differentiated liposarcoma and from hibernoma-like myxoid liposarcoma is mandatory.
References
- 1.Merkel H. On a pseudolipoma of the breast (peculiar fat tumor) Beitr Pathol Anat. 1906;39:152–157. [Google Scholar]
- 2.Gery L. In discussion of MF Bonnel’s paper. Bull Mem Soc Anat (Paris) 1914;89:111–112. [Google Scholar]
- 3.Furlong MA, Fanburg-Smith JC, Miettinen M. The morphologic spectrum of hibernoma: a clinicopathologic study of 170 cases. Am J Surg Pathol. 2001;25:809–814. doi: 10.1097/00000478-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen MM, Fanburg-Smith JC, Mandahl N. Hibernoma. In: Fletcher CDM, Unni KK, Mertens F, editors. Pathology and genetics of tumours of soft tissue and bone. WHO Classification of tumours. Lyon, France: IARC Press; 2002. pp. 33–34. [Google Scholar]
- 5.Coindre JM. New WHO classification of tumours of soft tissue and bone. Ann Pathol. 2012 Nov;32(Suppl 5):S115–6. doi: 10.1016/j.annpat.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Baskurt E, Padgett DM, Matsumoto JA. Multiple hibernomas in a 1-month-old female infant. Am J Neuroradiol. 2004;25:1443–1445. [PMC free article] [PubMed] [Google Scholar]
- 7.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Park KW, Halperin DS, Tontonoz P. Before they were fat: adipocyte progenitors. Cell Metab. 2008;8:454–457. doi: 10.1016/j.cmet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Colville J, Feigin K, Tang L, Keating D, Cohen MA. Mammary hibernoma. Breast J. 2006;12:563–565. doi: 10.1111/j.1524-4741.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 10.Essadel A, Bensaid Alaoui S, Mssrouri R, Mohammadine E, Benamr S, Taghy A, Lahlou MK, Chad B, Belmahi A. L’Hibernome: une rare cause d’amaigrissement massif. Ann Chir. 2002;127:215–217. doi: 10.1016/s0003-3944(01)00715-5. [DOI] [PubMed] [Google Scholar]
- 11.Seemayer TA, Kannck J, Wang N, Ahmed MN. On the ultrastructure of hibernoma. Cancer. 1975;36:1785–1793. doi: 10.1002/1097-0142(197511)36:5<1785::aid-cncr2820360533>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 12.Anderson SE, Schwab C, Stauffer E, Banic A, Steinbach LS. Hibernoma: imaging characteristics of a rare benign soft tissue tumor. Skeletal Radiol. 2000;30:590–595. doi: 10.1007/s002560100405. [DOI] [PubMed] [Google Scholar]
- 13.Dursun M, Agayev A, Bakir B, Ozger H, Eralp L, Sirvanci M, Guven K, Tunaci M. CT and MR characteristics of hibernoma: six cases. Clin Imaging. 2008;32:42–47. doi: 10.1016/j.clinimag.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Ong SY, Maziak DE, Shamji FM, Matzinger FR, Perkins DG. Intrathoracic hibernoma. Can J Surg. 2002;45:145–146. [PMC free article] [PubMed] [Google Scholar]
- 15.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JC, Gupta A, Saifuddin A, Flanagan A, Skinner JA, Briggs TW, Cannon SR. Hibernoma: MRI features in eight consecutive cases. Clin Radiol. 2006;61:1029–1034. doi: 10.1016/j.crad.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Peer S, Kuhberger R, Dessl A, Judmaier W. MR imaging findings in hibernoma. Skeletal Radiol. 1997;26:507. doi: 10.1007/s002560050276. [DOI] [PubMed] [Google Scholar]
- 18.Gaskin CM, Helms CA. Lipomas, lipoma variants, and well-differentiated liposarcomas (atypical lipomas): results of MRI evaluations of 126 consecutive fatty masses. Am J Roentgenol. 2004;182:733–739. doi: 10.2214/ajr.182.3.1820733. [DOI] [PubMed] [Google Scholar]
- 19.Ritchie DA, Aniq H, Davies AM, Mangham DC, Helliwell TR. Hibernoma--correlation of histopathology and manetic-resonance-imaging features in 10 cases. Skeletal Radiol. 2006;35:579–589. doi: 10.1007/s00256-006-0114-4. [DOI] [PubMed] [Google Scholar]
- 20.Atilla S, Eilenberg SS, Brown JJ. Hibernoma: MRI appearance of a rare tumor. Magn Reson Imaging. 1995;13:335–337. doi: 10.1016/0730-725x(94)00115-j. [DOI] [PubMed] [Google Scholar]
- 21.Mugel T, Ghossain MA, Guinet C, Buy J, Bethoux J, Texier P, Vadrot D. MR and CT findings in a case of hibernoma of the thigh extending into the pelvis. Eur Radiol. 1998;8:476–478. doi: 10.1007/s003300050419. [DOI] [PubMed] [Google Scholar]
- 22.Kallas KM, Vaughan L, Haghighi P, Resnick D. Hibernoma of the left axilla; a case report and review of MR imaging. Skeletal Radiol. 2003;32:290–294. doi: 10.1007/s00256-002-0533-9. [DOI] [PubMed] [Google Scholar]
- 23.Suh JS, Cho J, Lee SH, Shin KH, Yang WI, Lee JH, Cho JH, Suh KJ, Lee YJ, Ryu KN. Alveolar soft part sarcoma: MR and angiographic findings. Skeletal Radiol. 2000;29:680–689. doi: 10.1007/s002560000285. [DOI] [PubMed] [Google Scholar]
- 24.Suh JS, Cho J, Lee SH, Shin KH, Yang WI, Lee JH, Cho JH, Suh KJ, Lee YJ, Ryu KN. MR imaging of clear cell sarcoma (malignant melanoma of the soft parts): a multicenter correlative MRI-pathology study of 21 cases and literature review. Skelet Radiol. 2000;29:187–195. doi: 10.1007/s002560050592. [DOI] [PubMed] [Google Scholar]
- 25.Chu BC, Terae S, Hida K, Furukawa M, Abe S, Miyasaka K. MR findings in pinal hemangioblastoma: correlation with symptoms and with angiographic and surgical findings. Am J Neuroradiol. 2001;22:206–217. [PMC free article] [PubMed] [Google Scholar]
- 26.Gaffney EF, Hargreaves HK, Semple E, Vellios F. Hibernoma: distinctive light and electron microscopic features and relationship to brown adipose tissue. Hum Pathol. 1983;14:677–87. doi: 10.1016/s0046-8177(83)80139-7. [DOI] [PubMed] [Google Scholar]
- 27.Levine GD. Hibernoma. An electron microscopic study. Hum Pathol. 1972;3:351–359. doi: 10.1016/s0046-8177(72)80036-4. [DOI] [PubMed] [Google Scholar]
- 28.Dal Cin P, Van Damme B, Hoogmartens M, Van Den Berghe H. Chromosome changes in a case of hibernoma. Genes Chromosomes Cancer. 1992;5:178–180. doi: 10.1002/gcc.2870050212. [DOI] [PubMed] [Google Scholar]
- 29.Meloni AM, Spanier SS, Bush CH, Stone JF, Sandberg AA. Involvement of 10q22 and 11q13 in hibernoma. Cancer Genet Cytogenet. 1994;72:59–64. doi: 10.1016/0165-4608(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 30.Mertens F, Rydholm A, Brosjo O, Willen H, Mitelman F, Mandahl N. Hibernomas are characterized by rearrangements of chromosome bands 11q13-21. Int J Cancer. 1994;58:503–505. doi: 10.1002/ijc.2910580408. [DOI] [PubMed] [Google Scholar]
- 31.Maire G, Forus A, Foa C, Bjerkehagen B, Mainguené C, Kresse SH, Myklebost O, Pedeutour F. 11q13 alterations in two cases of hibernoma: large heterozygous deletions and rearrangement breakpoints near GARP in 11q13.5. Genes Chromosomes Cancer. 2003;37:389–395. doi: 10.1002/gcc.10223. [DOI] [PubMed] [Google Scholar]
- 32.Dei Tos AP, Dal Cin P. The role of cytogenetics in the classification of soft tissue tumours. Virchows Arch. 1997;431:83–94. doi: 10.1007/s004280050073. [DOI] [PubMed] [Google Scholar]
- 33.Lele SM, Chundru S, Chaljub G, Adegboyega P, Haque AK. Hibernoma: a report of 2 unusual cases with a review of the literature. Arch Pathol Lab Med. 2002;126:975–978. doi: 10.5858/2002-126-0975-H. [DOI] [PubMed] [Google Scholar]