Abstract
Plasma cell leukemia is a rare neoplastic proliferation of circulating plasma cells. Clonal proliferations of plasma cells, such as in plasma cell leukemia or plasma cell myeloma, are typically characterized by production of a monoclonal heavy and/or light chain immunoglobulin. We present a case of a secondary plasma cell leukemia arising from plasma cell myeloma with dual expression of lambda and kappa light chains along with aberrant expression of CD33, CD20, and dim CD56. This case emphasizes the importance of recognizing aberrant immunophenotypes in plasma cell leukemias and represents the first reported case of biclonal light chain expression in a secondary plasma cell leukemia.
Keywords: Plasma cell leukemia, biclonal gammopathy, aberrant immunophenotype
Introduction
Plasma cell leukemia (PCL) is a neoplastic clonal proliferation in which plasma cells constitute 20% of circulating leukocytes or exceed 2x109 cells/L in the peripheral blood [1]. PCL typically expresses a monoclonal immunoglobulin but may rarely be a non-producer [2] or have biclonality [3-5]. As a proportion of all forms of plasma cell dyscrasias, biclonal heavy chain production is more common than biclonal light chain production, and in addition, overlapping cases exist [5].
The two basic types of plasma cell leukemia are primary and secondary. Primary plasma cell leukemias have circulating neoplastic plasma cells at the initial presentation whereas secondaryplasma cell leukemias arise from a pre-existing plasmacytoma or plasma cell myeloma [6]. Clinically, primary PCL tends to present in slightly younger patients and have a more prominent extramedullary involvement (i.e. ymphadenopathy, hepatosplenomegaly) than secondary forms, which tend to present in older patients with the disease process more limited to the bone marrow and circulation [7]. Secondary PCL has been estimated to occur in 1-4% of myelomas [8-11] and the incidence of primary PCL relative to plasma cell myeloma was 3.8% in one study [12]. Although uniformly dismal, secondary PCL has worse survival rates than primary PCL [7].
There are scant reports on biclonal light chain production in plasma cell leukemias in the literature. We present the case of a secondary plasma cell leukemia with biclonal expression of IgG kappa and free lambda as well as aberrantexpression of CD33, CD20 and dim CD56.
Case report
A 68 yo female presented to her physician in 2006 for back pain which reportedly began after doing heavy lifting at work. Initially diagnosed with sciatica, her pain continued to worsenand additional work-up in April 2007 revealed an elevated total serum protein, elevated creatine,and multiple lytic bone lesions. Serum immunofixation was positive for an IgG kappa and free lambda light chain. Urine immunofixation confirmed the presence of free lambda and kappa light chains. Staging marrows confirmed a diagnosis of plasma cell myeloma and the patient was treated with thalidomide, dexamethasone, and bisphosphonates for 3 years. From December 2008 to July 2009, serial quantitative serum light chain measurements had consistently elevated free kappa light chains and free lambda levels that fluctuated from the high end of the normal range to abnormally elevated levels. The kappa:lambda ratio stayed between 2-4:1.
In January 2010, the patient developed chest congestion with a productive cough and worsening altered mental status. A bone marrow biopsy revealed greater than 90% cellularity with over 95% designated as immature/atypical plasma cells with plasmablastic morphology.The peripheral blood was significant for numerous circulating atypical plasma cells and plasmablasts. Serum clonality studies and in situ hybridization indicated continued biclonality with expression of both an IgG kappa immunoglobulin and a free lambda light chain (Figure 1) and flow cytometry demonstrated aberrant expression of CD33 and CD20 with dim CD56 expression in a subset population. The quantitative serum light chains again showed elevatedf ree kappa consistent with previous levels as well as a marked increase in free lambda with a kappa:lambda ratio of 0.01:1. The patient passed away within 2 weeks of her admission after a continued decline resulting in multiorgan dysfunction and failure.
Figure 1.
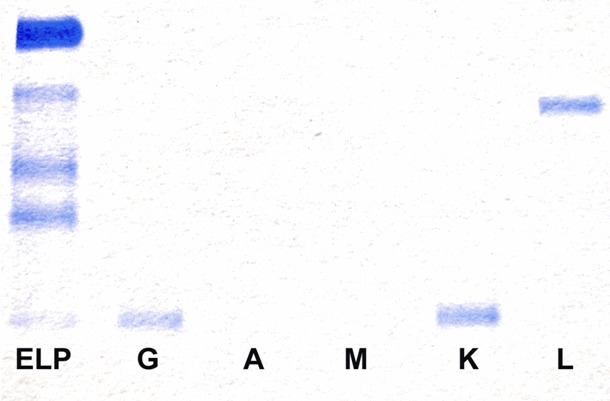
Immunofixation of the serum demonstrating IgG Kappa and free Lambda. The first lane is the patient’s serum electrophoresis. Lanes 2, 3, 4, 5, and 6 represent the patient’s serum mixed with antibodies to heavy chains G, A, and M and to light chains K and L, respectively.
Microscopic examination of a bone marrow biopsy obtained in May 2007 revealed a 50-60% cellular marrow with multilineage hematopoiesis.33% of the cells were plasma cells by a manual differential count and were characterizedby round, eccentrically located nuclei with mature, clumped chromatin and a biphasic cytoplasm with perinuclear clearing and surrounding cytoplasmic basophilia (Figure 2A). A karyotype from the same bone marrow sample was a normal 46, XX but fluore scence in situ hybridization (FISH) revealed 3 copies of the ATM and CCND1 probes which was suggestive of an extra chromo some 11. In situ hybridization with probes for kappa and lambda RNA were both positive; however only the lambda antibody had positive staining by immunohistochemistry.
Figure 2.

Evolution of the plasma cell dyscrasia morphology from (A) atypical mature plasma cells in 2007 to (B) plasmablastic cells with increased nuclear to cytoplasmic ratios, immature chromatin, and prominent nucleoli in 2010. (Wright-Giemsa stain, magnification 600x).
Microscopic examination of the bone marrow obtained in January 2010 revealed a hypercellular marrow (approaching 100%) composed predominantly of atypical plasmablastic mononuclear cells characterized by high nuclear to cytoplasmic ratios, dispersed chromatin, prominent nucleoli, scant basophilic cytoplasm, and scattered cytoplasmic vacuoles (Figure 2B). In situ hybridization of the paraffin embedded block revealed dual expression of kappa and lambda light chains in the plasmablastic cell population. Flow cytometry analysis of the bone marrow aspirate identified an abnormal population comprising 82% of all cells that expressed CD38, CD138, CD20, CD33, and dim CD56 and had dual expression of intracellular kappa and lambda immunoglobulins without expression of CD19 or surface kappa and lambda light chains (Figure 3). Cytogenetic examination of 20 metaphase cells identified multiple chromosomal abnormalities and overall hyperdiploidy, consistent with a plasma cell neoplasm. The karyotype was 53,XX,del(1)(p22p24),add(2)(q31)add(4)(q37),+3,del(3)(p22),-6,+9,+11,+15,+18,+21,+mar[cp20] (Figure 4). Again, the RNA in situ hybridization for kappa and lambda were both positive but only the lambda immunohistochemical study was positive. The peripheral blood had 32% plasmablasts and additional atypical plasma cells.
Figure 3.
Flow cytometry of the bone marrow. A: CD45 positivity vs side scatter of all cells. The neoplastic plasma cells are gated red. B: The subsequent graphs show the plasma cells are positive for CD33, CD38, CD138, CD20, CD56 (dim) and cytoplasmic kappa and lambda, and negative for CD7 (PC5, phycoerythrin-cyanine5; PE, phycoerythrin; FITC, fluorescein).
Figure 4.

Karyotype of a representative neoplastic plasma cell in metaphase (2010). Obtained from the bone marrow.
Discussion
There are a scant number of biclonal plasma cell dyscrasia cases that have been reported in the literature. Most of these plasma cell dyscrasias are plasma cell myeloma and involve two classes of heavy chain immunoglobulin [5]. To our knowledge this is the first reported example of kappa/lambda dual expression in a secondary plasma cell leukemia. One reported case exists of a biclonal primary plasma cell leukemia with dual kappa/lambda expression [3]. However, in that case, the neoplastic cells were not evaluated for cytogenetic features of plasma cell dyscrasia nor for the plasma cell markers CD38 and CD138, calling into question the exact nature of this neoplastic process. The malignant cells in that case were diagnosed as plasmablasts based off of morphology aloneand the reported immunophenotype was IgM+ (weak), IgG+(weak), kappa+, lambda+, CD45S+, HLA-DR+, CD45-RO, CD68+, and pancytokeratin+ and was negative for additional hematopoietic markers.
In our case, the circulating plasma cells had the typical bright expression of CD138 and CD38 immunophenotypically. Additionally, these cells expressed CD20, CD33 and dim CD56. CD20 is normally expressed on B-lymphocytes in various stages of maturation and can be found in plasmacytomas but has a higher percentage expression in plasma cell leukemias [6]. CD33, however, is generally expressed only by immature myeloid lineage cells as well as monocytes and macrophages [13] but has been reported in as many as 5-35% of plasma cell myeloma cases in some studies [14-16]. Data concerningthe expression of CD33 in plasma cell leukemiasis sparse. One study with an extremely limited sample size of 4 found no aberrant expression of CD33 [17]. There is some evidence to suggest that CD33 expressing plasmacytomas have a worse prognosis than those without such expression [15]. Unlike other plasmacell myelomas, the lack of aberrant CD56 expression is typically observed in PCL. However in our case dim expression of CD56 was detected in a subset of the neoplastic plasma cell population.
The mechanism by which a cell prevents productionof multiple light chain classes or heavy chain classes is known generally as allelic exclusion but the actual cellular mechanisms are still poorly understood. The locus encoding for the heavy chain portion of the immunoglobulin undergoes rearrangement first, and if successful, is followed by rearrangement of the kappa light chain DNA locus. Only if the kappa light chain rear rangement for both alleles is ineffective does lambda light chain rearrangementoccur. Generally, the formation of a viable heavy chain or light chain product prevents additional heavy or light chain rearrangements.
In this case, three separate methods of light chain detection independently verified the presence of kappa and lambda light chains. First, serological studies repeatedly showed an IgG kappa and a free lambda. Secondly, flow cytometric analysis detected dual expression of kappa and lambda light chains. In situ hybridizationof light chain specific RNA was also positive for cytoplasmic kappa and lambda in the same cell population. The fact that immunohistochemical studies of kappa and lambda were consistently positive for only lambda epitopes may be explained by the use of a monoclonal immunohistochemical light chain antibody in our case. Thus, the negative result via immunohistochemistry may be due to loss or sequestration of the antigenic epitope on the kappa light chain that corresponds with the monoclonal antibody. This seems likely, given the positivity of the other tests over the course of multipleyears and multiple tissue and blood samples. Other possibilities include 2 separate monoclonal populations of plasma cells. However, this seems less likely given that the light chain ISH did not highlight two distinct cell populations on tissue sections.
Plasma cell leukemias are an aggressive clonal proliferation of plasma cells with extension into the blood and carry a poor prognosis. To our knowledge, this is the first reported case of biclonal light chain gammopathy in a secondary plasma cell leukemia aberrantly coexpressing CD33, CD20 and dim CD56 with the morphologic and cytogenetic evidence of clonal evolution of the disease.
Disclosure of conflict of interest
The authors declare no conflicts of interest.
References
- 1.Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003 Jun;121:749–57. [PubMed] [Google Scholar]
- 2.Dadu T, Rangan A, Handoo A, Bhargava M. Primary non-secretory plasma cell leukemia with atypical morphology - a case report. Indian J Hematol Blood Transfus. 2009 Jun;25:81–3. doi: 10.1007/s12288-009-0019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tóth Z, Sipos J. Biclonal primary plasma cell leukemia. Pathol Oncol Res. 1998;4:48–51. doi: 10.1007/BF02904696. [DOI] [PubMed] [Google Scholar]
- 4.Chattopadhyay A, Nath UK, De R, Singh A, Sanyal S, Chatterjee SK, Chaudhuri U. Primary plasma cell leukemia with initial cutaneous involvement and IgA biclonal gammopathy. Ann Hematol. 2008 Mar;87:249–51. doi: 10.1007/s00277-007-0383-6. Epub 2007 Oct 2. [DOI] [PubMed] [Google Scholar]
- 5.Kyle RA, Robinson RA, Katzmann JA. The clinical aspects of biclonal gammopathies. Review of 57 cases. Am J Med. 1981 Dec;71:999–1008. doi: 10.1016/0002-9343(81)90326-0. [DOI] [PubMed] [Google Scholar]
- 6.García-Sanz R, Orfão A, González M, Tabernero MD, Bladé J, Moro MJ, Fernández-Calvo J, Sanz MA, Pérez-Simón JA, Rasillo A, Miguel JF. Primary plasma cell leukemia: clinical, immunophenotypic, DNA ploidy, and cytogenetic characteristics. Blood. 1999;93:1032–1037. [PubMed] [Google Scholar]
- 7.Noel P, Kyle RA. Plasma cell leukemia: an evaluation of response to therapy. Am J Med. 1987 Dec;83:1062–8. doi: 10.1016/0002-9343(87)90942-9. [DOI] [PubMed] [Google Scholar]
- 8.Tiedemann RE, Gonzalez-Paz N, Kyle RA, Santana- Davila R, Price-Troska T, Van Wier SA, Chng WJ, Ketterling RP, Gertz MA, Henderson K, Greipp PR, Dispenzieri A, Lacy MQ, Rajkumar SV, Bergsagel PL, Stewart AK, Fonseca R. Genetic aberrations and survival in plasma cell leukemia. Leukemia. 2008 May;22:1044–52. doi: 10.1038/leu.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bichel J, Effersoe P, Gormsen H, Harboe N. Leukemic myelomatosis (plasma cell leukemia); a review with report of four cases. Acta radiol. 1952 Mar-Apr;37:196–207. doi: 10.3109/00016925209139868. [DOI] [PubMed] [Google Scholar]
- 10.Creyssel R, Groulade J, Fine JM, Betuel H. Etude immunochimique de 463 serums contenant une paraproteine. In: Peeters H, editor. Protides of the biological fluids; proceeding of the 10th and 11th Colloquium, Bruges, 1962-1963. Amsterdam: Elsevier Publishing; 1964. pp. 97–99. [Google Scholar]
- 11.Kyle RA, Maldonado JE, Bayrd ED. Plasma cell leukemia. Report on 17 cases. Arch Intern Med. 1974 May;133:813–8. doi: 10.1001/archinte.133.5.813. [DOI] [PubMed] [Google Scholar]
- 12.García-Sanz R, Orfão A, González M, Tabernero MD, Bladé J, Moro MJ, Fernández-Calvo J, Sanz MA, Pérez-Simón JA, Rasillo A, Miguel JF. Primary plasma cell leukemia: clinical, immunophenotypic, DNA ploidy, and cytogenetic characteristics. Blood. 1999 Feb 1;93:1032–7. [PubMed] [Google Scholar]
- 13.Crocker PR, Varki A. Siglecs, sialic acids and innate immunity. Trends Immunol. 2001 Jun;22:337–42. doi: 10.1016/s1471-4906(01)01930-5. [DOI] [PubMed] [Google Scholar]
- 14.Robillard N, Wuillème S, Lodé L, Magrangeas F, Minvielle S, Avet-Loiseau H. CD33 is expressed on plasma cells of a significant number of myeloma patients, and may represent a therapeutic target. Leukemia. 2005 Nov;19:2021–2. doi: 10.1038/sj.leu.2403948. [DOI] [PubMed] [Google Scholar]
- 15.Sahara N, Ohnishi K, Ono T, Sugimoto Y, Kobayashi M, Takeshita K, Shigeno K, Nakamura S, Naito K, Tamashima S, Nara K, Tobita T, Takeshita A, Ohno R. Clinicopathological and prognostic characteristics of CD33-positive multiple myeloma. Eur J Haematol. 2006 Jul;77:14–8. doi: 10.1111/j.1600-0609.2006.00661.x. [DOI] [PubMed] [Google Scholar]
- 16.Mateo G, Castellanos M, Rasillo A, Gutiérrez NC, Montalbán MA, Martín ML, Hernández JM, López-Berges MC, Montejano L, Bladé J, Mateos MV, Sureda A, de la Rubia J, Díaz-Mediavilla J, Pandiella A, Lahuerta JJ, Orfao A, San Miguel JF. Genetic abnormalities and patterns of antigenic expression in multiple myeloma. Clin Cancer Res. 2005 May 15;11:3661–7. doi: 10.1158/1078-0432.CCR-04-1489. [DOI] [PubMed] [Google Scholar]
- 17.Kraj M, Kopec-Szlezak J, Poglod R, Kruk B. Flow cytometric immunophenotypic characteristics of 36 cases of plasma cell leukemia. Leuk Res. 2011;35:169–176. doi: 10.1016/j.leukres.2010.04.021. [DOI] [PubMed] [Google Scholar]



