Abstract
It is well recognized that patients with immunodeficiency have a high risk of development of lymphoproliferative disorders (LPDs), and Epstein-Barr virus (EBV) is associated with the occurrence of LPDs. Methotrexate (MTX) is one of the common cause of iatrogenic-associated LPD, and approximately 40-50% of MTX-related LPD cases occur in extranodal sites. However, the occurrence of MTX-related LPD in the gingiva is extremely rare. Herein, we report the fourth documented case of MTX-related EBV-associated LPD occurring in the gingiva of a patient with rheumatoid arthritis (RA). A 76-year-old Japanese female with a 10-year history of RA, who was treated with MTX and infliximab, presented with a tumorous lesion in the gingiva. Biopsy of the gingiva tumor revealed diffuse proliferation of large-sized lymphoid cells with cleaved nuclei containing conspicuous nucleoli. These lymphoid cells were CD20- and EBER-positive. Therefore, a diagnosis of MTX-related EBV-associated LPD showing features of diffuse large B-cell lymphoma (DLBCL) that occurred in the gingiva was made. Although the occurrence of LPD in the oral region, as seen in the present case, is rare, the prevalence of this disorder may be on the rise due to the increased number of patients undergoing immunosuppression therapy. Moreover, immunosenescence can also be a cause of EBV-associated LPD. Therefore, recognition of the occurrence of this disorder in the oral cavity and consideration of the clinical history can facilitate the correct diagnosis.
Keywords: Lymphoproliferative disorder, Epstein-Barr virus, methotrexate, gingiva
Introduction
It is well recognized that patients with immunodeficiency have a higher risk of lymphoproliferative disorders (LPDs) compared with those who are immunocompetent. Patients with iatrogenic immunodeficiency, who are treated with immunosuppressive drugs for autoimmune diseasesor allograft/autograft transplant show a high prevalence of LPDs [1]. Methotrexate (MTX) is an effective immunosuppressive agent that is widely administered to patients with autoimmune diseases including rheumatoid arthritis (RA). This leads to an immunosuppression state and can provide a basis for the development of LPD [2]. Epstein-Barr virus (EBV) is associated with development of LPDs, and approximately 30-50% of LPDs in RA patients treated with MTX are EBV-positive [3,4]. Moreover, approximately 40-50% of MTX-related LPD cases occur in extranodal sites, such as the gastrointestinal tract, skin, liver, lung, and kidney [3,4]. However, the occurrence of MTX-related EBV-associated LPD occurring in the gingiva is extremely rare, and only three cases have been reported in the English literature [5-7]. Herein, we report the fourth documented case of MTX-related EBV-associated LPD occurringin the gingiva of a patient with RA and review the clinicopathological features.
Case report
A 76-year-old Japanese female with a 10-year history of RA presented with a tumorous lesion with hemorrhage in her left upper gingiva. Physical examination revealed that there was a tumorous lesion with central ulceration and hemorrhage in her gingiva, and no swelling of superficial lymph nodes was detected. Computed tomography demonstrated no lymph node swelling and hepatosplenomegaly. She had been administered MTX for 10 years and infliximab for 6 years for RA. Moreover, she was also diagnosed with autoimmune hepatitis 10 years earlier and had been treated with steroid and ursodeoxycholic acid.
Laboratory tests revealed mild anemia (hemoglobin10.3 g/dL; range 11.3-15.0), elevated white blood cell count (15.0 x 109/L; range 3.8-4.8 x 109/L), and elevated soluble interleukin-2 receptor (626 U/mL; range 124-466). Lactate dehydrogenase was within normal range (193 U/L; range 119-229).
Biopsy of the gingiva tumor was performed. Subsequently, upper gastrointestinal endoscopic examination revealed an ulcerative lesion at the lesser curvature of the antrum, and biopsy of the ulcerative lesion was also performed. Colonoscopy demonstrated no ulcerative or tumorous lesions.
Gingiva tumor
Histopathological study of the gingiva tumor revealed diffuse proliferation of large-sized lymphoid cells accompanied by ulceration of the squamous mucosa (Figure 1A). These large-sized lymphoid cells had large cleaved nuclei with conspicuous nucleoli, and mitotic figures were scattered (Figure 1B). Infiltrationof small-sized lymphocytes and macrophages were also observed (Figure 1B). Neither Hodgkin cells nor Reed-Sternberg cells were present.
Figure 1.
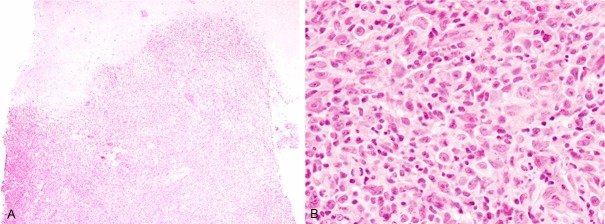
Histopathological features of the gingiva tumor. A: Diffuse proliferation of lymphoid cells with ulceration, HE, x 40. B: Proliferation of large-sized lymphoid cells with large convoluted nuclei containing conspicuous nucleoli accompanied by infiltration of small lymphocytes and histiocytes, HE, x 400.
Immunohistochemical staining and in situ hybridization were performed using an autostainer(Ventana) by the same method as previously reported [8-12]. The large-sized lymphoid cells were positive for CD20, bcl-6, and MUM-1 (Figure 2A), but negative for CD3, CD5, CD10, CD15, CD30, CD138, bcl-2, and cyclin D1. Moreover, in situ hybridization for EBV-encoded small RNA (EBER) demonstrated marked positivity in the majority of these large-sized lymphoid cells (Figure 2B).
Figure 2.
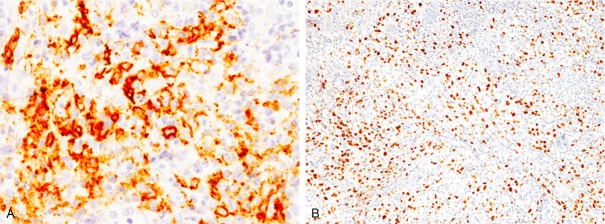
Immunohistochemical and in situ hybridization findings of the gingiva tumor. A: CD20 is expressed in the large-sized lymphoid cells, x 400. B: Many EBER-positive cells are detected by in situ hybridization, x 100.
Gastric ulcer
A few atypical lymphoid cells were present in the lamina propria of the regenerative gastric mucosa (Figure 3). Immunohistochemical and in situ hybridization studies revealed that these atypical lymphoid cells were CD20- and EBER-positive (Figure 3, inset).
Figure 3.
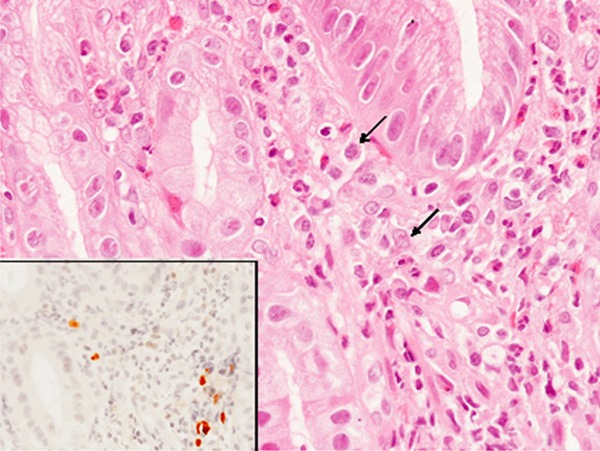
Histopathological and in situ hybridization findings of the gastric ulcer. A few atypical lymphoid cells are observed in the lamina propria (arrows), HE, x 400. A few EBER-positive cells are detected (inset, x 400).
According to these findings, an ultimate diagnosis of MTX-related EBV-associated LPD showing features of diffuse large B-cell lymphoma (DLBCL) was made.
After the diagnosis, administration of MTX and infliximab was discontinued, and R-THP-COP (rituximab, pinorubin, oncovin, endoxan, and prednisolone) therapy was initiated. No tumor recurrence was observed during medical follow-up. She died of rupture of a thoracic aortic aneurysm 30 months after the initial presentation.
Discussion
After primary infection of EBV at an early age, this virus persistently infects B lymphocytes of most adults. In physiological circumstances, the number of EBV-infected B cells is inhibited at very low level due to complex immunological interactions. However, immunosuppression can affect immune homeostasis and surveillance for EBV-infected B cells, which may permit the emergence of EBV-induced LPDs. The spectrum of immunosuppressive etiologies includes primary immunodeficiency, human immunodeficiency virus infection, post-transplantation,and iatrogenic immunodeficiency.
MTX is one of the common causes of iatrogenic immunodeficiency-associated LPD. Although approximately 40-50% MTX-related LPD cases occur in extranodal sites [3,4], oral cavity is one of the rarest location of this disorder [5-7]. In this report, we describe the fourth documented case of MTX-related EBV-associated LPD occurring in the gingiva of a RA patient. Table 1 summarizes the clinicopathological features of the previously reported cases of MTX-related EBV-associated LPD occurring in the gingiva as well as the present case. All patients were middle-aged to elderly, and females were predominantly affected (male/female 1:3). The main complaint was pain, ulceration or tumor of the gingiva. Three of four cases of this type of disorder experienced reduction of the lesion by discontinuation of MTX therapy [5-7].
Table 1.
Clinicopathological features of MTX-related EBV-associated LPD occurring in the gingiva
| Case No. | Age/Gender | Complaint | Underlying disease | Drugs | Histopathology | Reference |
|---|---|---|---|---|---|---|
| 1 | 44/Male | Pain | RA, Still’s disease | MTX, betamethasone | DLBCL | [6] |
| 2 | 70/Female | Tumor | RA | MTX, prednisolone | DLBCL | [7] |
| 3 | 69/Female | Ulcer | RA | MTX | Hodgkin-like | [5] |
| Present case | 76/Female | Tumor | RA, autoimmune hepatitis | MTX, infliximab, prednisolone | DLBCL |
DLBCL, Diffuse large B-cell lymphoma; EBV, Epstein-Barr virus; LPD, lymphoproliferative disorder; MTX, methotrexate; RA, rheumatoid arthritis.
A variety of histopathological subtypes can occur in patients with MTX-related LPDs [1,3]. However, the characteristic feature of MTX-related LPDs is an increase in the frequency of Hodgkin lymphoma and lymphoid proliferation of Hodgkin-like features [1]. According to the analysis of 135 reported cases of MTX-related LPDs with detailed histopathological features, the most common subtype is DLBCL (64/135 cases), followed by Hodgkin lymphoma and lymphoid proliferation of Hodgkin-like features (33/135 cases) [1]. The third most common subtype is polymorphic/lymphoplasmacytic LPD (11/135 cases), and the frequency of follicular lymphoma, Burkitt lymphoma, and marginal-zone lymphoma is low [1]. Among the MTX-related LPDs occurring in the gingiva, the most common histopathological subtype is DLBCL (3 cases) [6,7], and only one case of Hodgkin-like lesion has been reported (Table 1) [5].
In addition, only a few cases of LPDs occurring in the gingiva and tongue caused by other immunosuppressive agents have been reported[13]. Dojcinov et al. reported the clinicopathological features of EBV-positive mucocutaneous ulcers [13]. This series included cases of MTX-related LPD of the tongue or oral mucosa in RA patients, one case of azathioprine-related LPD of the oral mucosa, and one case of cyclosporine A-related LPD of the tongue in a patient with lupus erythematosus [13]. Moreover, albeit extremely rare, EBV-associated LPD occurring in the gingiva or tongue in patients with post-transplantation has been reported [14,15]. Interestingly, 26 cases of EBV-positive mucocutaneous ulcers reported by Dojcinov et al. included 17 cases of age-related immunosenescence,who had no evidence of immunosuppression other than advanced age beyond 60 [13]. Immunosenescence owing to ageing has recently been recognized as a cause of EBV-positive diffuse large-B-cell lymphoma in elderlypatients [16,17]. This condition can occur in the oral region [13].
Recently, the occurrence of LPDs in patients with RA receiving anti-TNF agents has also been reported, although the odds ratio for LPD in patients who received anti-TNF therapy compared with patients who did not receive it was 1.0. Moreover, the odds ratio for LPD in patients who received anti-TNF therapy plus MTX comparedwith patients who received MTX alone was 1.1 [2]. In the present case, a diagnosis of MTX-related EBV-associated LPD was made, however, the occurrence of LPD may also be related to infliximab therapy because this patient had been treated with both MTX and anti-TNF agent.
In conclusion, we describe a case of MTX-related EBV-associated LPD occurring in the gingiva of a RA patient. The occurrence of EBV-associated LPD may be on the rise due to the increased number of patients undergoing immunosuppression therapy [18,19]. More-over, immunosenescence can also be a cause of EBV-associated LPD. Therefore, recognition of the occurrence of this disorder in the oral cavity and consideration of the clinical history can facilitate the correct diagnosis.
Disclosure of conflict of interest
None.
References
- 1.Gaulard P, Swerdlow SH, Harris NL, Jaffe ES, Sundström C. Other iatrogenic immunodeficiency-associated lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. pp. 350–351. [Google Scholar]
- 2.Wolfe F, Michaud K. The effect of methotrexate and anti-tumor necrosis factor therapy on the risk of lymphoma in rheumatoid arthritis in 19,562 patients during 89,710 person-years of observation. Arthritis Rheum. 2007;56:1433–1439. doi: 10.1002/art.22579. [DOI] [PubMed] [Google Scholar]
- 3.Hoshida Y, Xu JX, Fujita S, Nakamichi I, Ikeda J, Tomita Y, Nakatsuka S, Tamaru J, Iizuka A, Takeuchi T, Aozasa K. Lmyphoproliferative disorders in rhematoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medicaton. J Rheumatol. 2007;34:322–331. [PubMed] [Google Scholar]
- 4.Salloum E, Cooper DL, Howe G, Lacy J, Tallini G, Crouch J, Schultz M, Murren J. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rhematoid arthritis and other rheumatic diseases. J. Clin. Oncol. 1996;14:1943–1949. doi: 10.1200/JCO.1996.14.6.1943. [DOI] [PubMed] [Google Scholar]
- 5.Kikuchi K, Miyazaki Y, Tanaka A, Shigematu H, Kojima M, Sakashita H, Kusama K. Methotrexate-related Epstein-Barr virus (EBV)-associated lymphoproliferative disorder- so called “Hodgkin-like lesion”- of the oral cavity in a patient with rheumatoid arthritis. Head Neck Pathol. 2010;4:305–311. doi: 10.1007/s12105-010-0202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka A, Shigematsu H, Kojima M, Sakashita H, Kusama K. Methotrexate-associated lymphoproliferative disorder arising in a patient with adult Still’s disease. J Oral Maxillofac Surg. 2008;66:1492–1495. doi: 10.1016/j.joms.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Uneda S, Sonoki T, Nakamura Y, Matsuoka H, Nakakuma H. Rapid vanishing of tumor by withdrawal of methotrexate in Epstein-Barr virus-related B cell lymphoproliferative disorder. Intern Med. 2008;47:1445–1456. doi: 10.2169/internalmedicine.47.0989. [DOI] [PubMed] [Google Scholar]
- 8.Ishida M, Iwai M, Yoshida K, Kagotani A, Okabe H. Sarcomatoid carcinoma with small cell carcinoma component of the urinary bladder: A case report with review of the literature. Int J Clin Exp Pathol. 2013;6:1671–1676. [PMC free article] [PubMed] [Google Scholar]
- 9.Ishida M, Mori T, Umeda T, Kawai Y, Kubota Y, Abe H, Iwai M, Yoshida K, Kagotani A, Tani T, Okabe H. Pleomorphic lobular carcinoma in a male breast: case report with review of the literature. Int J Clin Exp Pathol. 2013;6:1441–1444. [PMC free article] [PubMed] [Google Scholar]
- 10.Nakanishi R, Ishida M, Hodohara K, Yoshida T, Yoshii M, Okuno H, Horinouchi A, Iwai M, Yoshida K, Kagotani A, Okabe H. Prominent gelatinous bone marrow transformation presenting prior to myelodysplastic syndrome: a case report with review of the literature. Int J Clin Exp Pathol. 2013;6:1677–1682. [PMC free article] [PubMed] [Google Scholar]
- 11.Ishida M, Mochizuki Y, Iwai M, Yoshida K, Kagotani A, Okabe H. Pigmented squamous intraepithelial neoplasia of the esophagus. Int J Clin Exp Pathol. 2013;6:1868–73. [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshii M, Ishida M, Hodohara K, Okuno Y, Nakanishi R, Yoshida T, Okabe H. Systemic Epstein-Barr virus-positive T-cell lymphoproliferative disease of childhood: Report of a case with review of the literature. Oncol Lett. 2012;4:381–384. doi: 10.3892/ol.2012.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES. EBV positive mucocutaneous ulcers- a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34:405–417. doi: 10.1097/PAS.0b013e3181cf8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leon JE, Takahama Junior A, Vassallo J, Soares FA, de Almeida OP, Lopes MA. EBV-associated polymorphic posttransplant lymphoproliferative disorder presenting as gingival ulcers. Int J Surg Pathol. 2011;19:241–246. doi: 10.1177/1066896909353599. [DOI] [PubMed] [Google Scholar]
- 15.Bruce AJ, Subtil A, Rogers RS III, Castro LA. Monomorphic Epstein-Barr virus (EBV)-associated large B-cell posttransplant lymphoproliferative disorder presenting as a tongue ulcer in a pancreatic transplant patient. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:e24–28. doi: 10.1016/j.tripleo.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Oyama T, Yamamoto K, Asano N, Oshiro A, Suzuki R, Kagami Y, Morishima Y, Takeuchi K, Izumo T, Mori S, Ohshima K, Suzumiya J, Nakamura N, Abe M, Ichimura K, Sato Y, Yoshino T, Naoe T, Shimoyama Y, Kamiya Y, Kinoshita T, Nakamura S. Age-related EBV-assocaited B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: a study of 96 patients. Clin Cancer Res. 2007;13:5124–5132. doi: 10.1158/1078-0432.CCR-06-2823. [DOI] [PubMed] [Google Scholar]
- 17.Shimoyama Y, Yamamoto K, Asano N, Oyama T, Kinoshita T, Nakamura S. Age-related Epstein-Barr virus-associated B-cell lymphoproliferative disorders: special references to lymphomas surrouding this newly recognized clinicopathologic disease. Cancer Sci. 2008;99:1085–1091. doi: 10.1111/j.1349-7006.2008.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izumiya S, Ishida M, Hodohara K, Yoshida T, Okabe H. Epstein-Barr virus-assocaited lymphoproliferative disorder developed following autologous peripheral blood stem cell transplantation for relapsing Hodgkin’s lymphoma. Oncol Lett. 2012;3:1203–1206. doi: 10.3892/ol.2012.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohta M, Taga T, Nomura A, Kato H, Takano T, Maruo Y, Takeuchi Y, Ishida M, Ohta S. Epstein-Barr virus-related lymphoproliferative disorder, cytomegalovirus reactivation, and varicella zoster virus encephalitis during treatement of medulloblastoma. J Med Virol. 2011;83:1582–1584. doi: 10.1002/jmv.22136. [DOI] [PubMed] [Google Scholar]


