Abstract
Objectives
We assessed the situation of academic publications on access to and use of medicines (ATM) in low-income and middle-income countries (LMICs) of the Eastern Mediterranean Region (EMR). We aimed to inform priority setting for research on ATM in the region.
Design
Bibliographic review of published studies.
Setting
LMICs in EMR.
Inclusion criteria
Publications on ATM issues originating from or focusing on EMR LMICs covering the period 2000–2011. Publications involving multinational studies were included if at least one eligible country had been included in the study.
Information sources and data extraction
We conducted comprehensive searches of the PubMed, Social Science Citation Index and Science Citation Index. We used the WHO ATM framework for data extraction and synthesis. We analysed the data according to the ATM issues, health system levels, year of publication and the countries of origin or focus of the studies.
Results
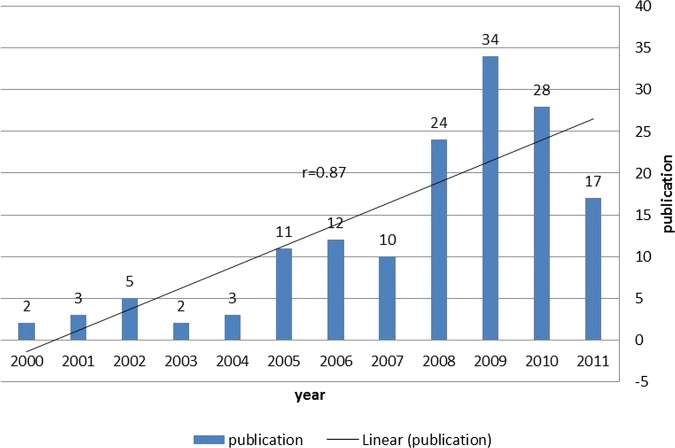
151 articles met the inclusion criteria. Most articles (77%) originated from LMICs in EMR, suggesting that the majority of evidence on ATM in the region is home-grown. Over 60% of articles were from Iran, Pakistan, Jordan and Lebanon (in order of volume), while we found no studies assessing ATM in Somalia, Djibouti and South Sudan, all low-income countries. Most studies focused on the rational use of medicines, while affordability and financing received limited attention. There was a steady growth over time in the number of ATM publications in the region (r=0.87).
Conclusions
There is a growing trend, over the years, of more studies from the region appearing in international journals. There is a need for further research on the financing and affordability aspects of ATM in the region. Cross-border issues and the roles of non-health sectors in access to medicines in the region have not been explored widely.
Keywords: PUBLIC HEALTH, HEALTH SERVICES ADMINISTRATION & MANAGEMENT, EPIDEMIOLOGY
Article summary.
Strengths and limitations of this study
This is the first study conducted in this region that has collated Access To and appropriate use of Medicines (ATM)-related published literature to identify research priorities.
We conducted systematic searches of the main international databases for identifying ATM articles.
Although we followed rigorous methods, the search should not be considered exhaustive. Further studies might have been published in locally indexed or non-indexed journals, which might not have been captured by our methods.
Introduction
The provision of reliable access to affordable, appropriate and high-quality medicines is a key component of a functioning health system.1 According to the WHO Framework for Access To and appropriate use of Medicines (ATM), access has been defined as having four parameters: that the available medicines are effective, of consistently good quality and are used rationally (rational use), that there is no financial obstacle to a patient receiving it (affordability), that the financing mechanisms are sustainable to ensure that access to quality medicines and affordability are ensured over time (sustainable financing), and that the health system provides the required infrastructure, knowledge and guidance for proper use of medicines (health system and availability). The Framework stipulates that any isolated efforts on one ATM aspect would not result in adequate and lasting improvement in the ATM situation.2
Unfortunately, ATM is often poor in low-income and middle-income countries (LMICs). WHO estimated that about one-third of the world’s population had limited access to the medicines they needed. Many factors determine access. They include, for example, tax and tariff policies, pricing and affordability of medicines, price mark-up policies, cost-sharing and copayment for medicines, and healthcare regulation policies and financial protection systems for healthcare users. WHO estimates that, on average, the availability of essential drugs in LMICs is 35% in the public sector facilities and 66% in the private sector.3 The quality of pharmaceutical products and the rational use of medicines (RUMs) also affect the effectiveness of the medicines and health outcomes.4 In many LMICs, there is limited access to information that might help clinical decision-making on the use of medicines.5 6
Medicines account for a high proportion of health spending in LMICs, between 20% and 60% (compared with an average of around 18% in high-income countries).7 Moreover, more than half of the expenditure on medicines in most LMICs is out of the pocket.8 This inequitable financing situation, whether due to a lack of effective general revenue financing or social insurance financing or other mechanisms, creates significant access barriers for the poor and may lead to catastrophic household expenditures. The poor and other population groups often rely on the private informal sector for medicines, particularly in rural areas. Overprescribing and inappropriate prescribing and dispensing of medicines are prevalent.9
Despite some progress in some areas—such as price and availability7—data on ATM are often weak. Even where data are available, there is often limited systematic research that enables the interpretation of the data and using it in identifying priorities and developing policy options to improve access to medicines in LMICs. The application of health systems research tools and methods in the field of access to medicines will help in understanding the weaknesses and barriers of access to medicines and generating useful evidence to formulate policies.10
This study was part of the ATM Policy Research project, funded by the WHO Alliance for Health Policy and Systems Research with the ultimate goal of “increasing access to and improve the use of medicines in low and middle income countries, particularly for the poor (Millennium Development Goal no. 8)”.
We aimed to assess the situation of academic publications on ATM in LIMCs in the Eastern Mediterranean Region (EMR), and the distribution of such publications in terms of geographical coverage and issues of interest. The ultimate objective of the study was to inform priority setting for research in the area of access to medicines in the region and globally.
Methods
Study design
We conducted a bibliographic review of research in EMR, involving comprehensive searches of the literature.
The study involved an extensive search of the national, regional and international literature in the EMR’s LMICs in 2000–2011 and mapping of research to identify the geographical and research gaps that may not have been covered in previous research.
Literature searches
EMR included 16 LMICs according to the World Bank categories which comprised the geographical focus of the study. The country-specific searches were conducted both in relation to author affiliations as well as the titles and abstracts of the articles.
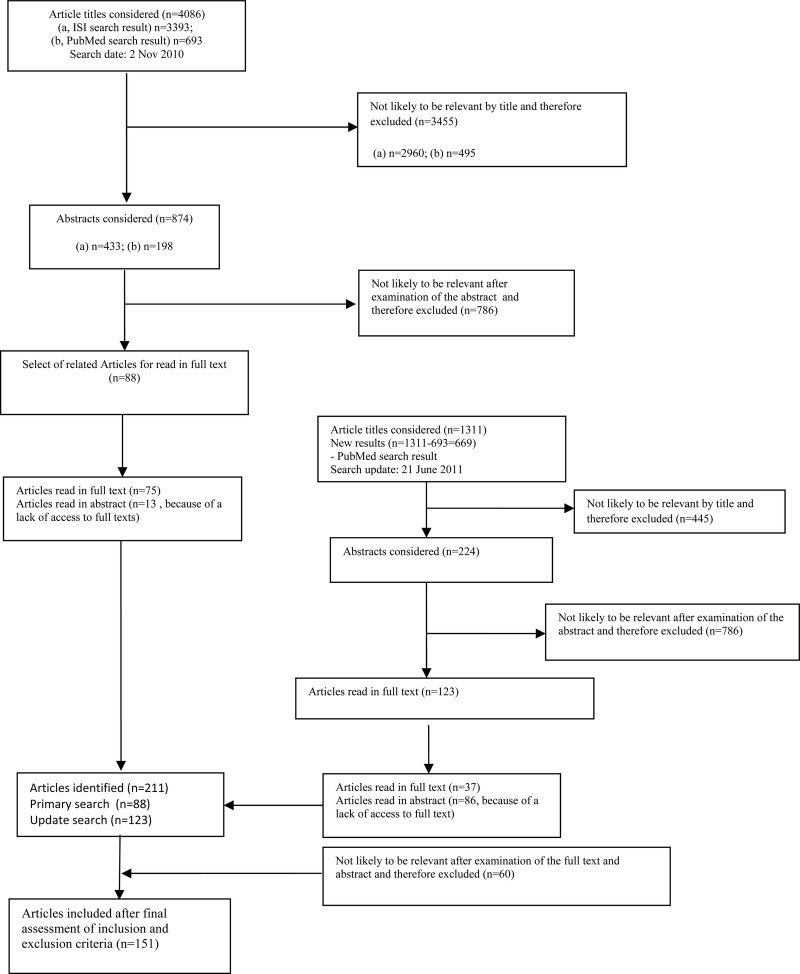
A set of specific ATM terms were developed in two brainstorming meetings and used to devise the search strategy. The initial search strategy was tested in a number of limited searches and was compared with a list of previously known publications. The results of the assessment were used to finalise the search strategy (box 1). The main terms selected for this study included drugs, medicines, medications and pharmaceuticals and their variations. These were suitably linked with ATM-related terms. We conducted comprehensive searches of three major electronic databases (PubMed, Social Science Citation Index and Science Citation Index). Initial searches were conducted in January 2011 and all the searches were updated in June 2011 (figure 1). Additionally, we searched the WHO websites and contacted a few topic experts for additional publications.
Box 1. Search strategy for regional literature.
PubMed example:
#1- ((((((((((((((((Iran[Affiliation]) OR Pakistan[Affiliation]) OR Lebanon[Affiliation]) OR Egypt[Affiliation]) OR Afghanistan[Affiliation]) OR Sudan[Affiliation]) OR Yemen[Affiliation]) OR Jordan[Affiliation]) OR Tunisia[Affiliation]) OR Morocco[Affiliation]) OR Syria[Affiliation]) OR Palestine[Affiliation]) OR Iraq [Affiliation]) OR Djibouti[Affiliation]) OR Libya$[Affiliation]) OR Somalia[Affiliation])
#2- (((((((((((((((((((middle east[Title/Abstract]) OR Iran[Title/Abstract]) OR low income countries[Title/Abstract]) OR middle income countries[Title/Abstract]) OR Pakistan[Title/Abstract]) OR Lebanon[Title/Abstract]) OR Egypt[Title/Abstract]) OR Afghanistan[Title/Abstract]) OR Sudan[Title/Abstract]) OR Yemen[Title/Abstract]) OR Jordan[Title/Abstract]) OR Tunisia[Title/Abstract]) OR Morocco[Title/Abstract]) OR EMRO[Title/Abstract]) OR Syria[Title/Abstract]) OR Palestine[Title/Abstract]) OR eastern Mediterranean[Title/Abstract]) OR Iraq [Title/Abstract]) OR Djibouti[Title/Abstract]) OR Libya$[Title/Abstract]) OR Somalia[Title/Abstract])
#3- (#1) OR (#2)
#4- ((((drug$[Title/Abstract]) OR medicines[Title/Abstract]) OR medication$[Title/Abstract]) OR pharmac$[Title/Abstract])
#5- ((((((((((((((((((use[Title/Abstract]) OR access[Title/Abstract]) OR available[Title/Abstract]) OR availability[Title/Abstract]) OR affordable[Title/Abstract]) OR affordability[Title/Abstract]) OR utilisation[Title/Abstract]) OR utilization[Title/Abstract]) OR essential [Title/Abstract]) OR counterfeit$[Title/Abstract]) OR price[Title/Abstract]) OR pricing[Title/Abstract]) OR licensing[Title/Abstract]) OR licencing[Title/Abstract]) OR labeling[Title/Abstract]) OR labelling[Title/Abstract]) OR formularies[Title/Abstract]) OR generic[Title/Abstract])
#6- ((((((((prescription$ [Title/Abstract]) OR prescrib$ [Title/Abstract]) OR "drug policy"[Title/Abstract]) OR "pharmaceutical policy"[Title/Abstract]) OR formulary[Title/Abstract]) OR pharmacy[Title/Abstract]) OR pharmacies[Title/Abstract]) OR pharmacist$[Title/Abstract])
#7- (#3) AND (#6)
#8- (#3) AND (#4) AND (#5)
#9- (#7) OR (#8)
Figure 1.
Flow chart of search strategies in electronic databases for access to and appropriate use of medicines.
Inclusion process and criteria
Articles published from 2000 onwards were considered. To ensure accuracy, two separate samples of 100 titles were reviewed by two researchers and disagreements were discussed and clarified. All the remaining titles and the abstracts of the identified articles via the search were initially reviewed by one author. After the initial screenings, the abstracts (and subsequently the full text articles) were read by two authors.
We used the following criteria for the inclusion and exclusion of the articles
No research design limit was used. However, letters to the editors and abstract-only publications were excluded;
Studies that were directly relevant to ATM issues were included. For example, for RUM studies, we considered studies that assessed RUM in a certain setting or studies that sought to improve the use of medicines specifically. However, broader studies of improving the quality of clinical care (which might have involved prescribing issues) were not included. (eg, a clinical practice guideline development project may not be included). Although prescribing is a part of the majority of the guidelines, if the purpose of the guideline is not prescribing per se; then the article may not be included in here. This criterion was required to ensure that we remained focused on ATM issues. The same logic was applied to other aspects of ATM;
Studies of drug resistance that did not elaborate on the health system or ATM implications, studies of herbal medicines alone, studies of drug misuse, studies of use of contraceptive medicines that focused on family planning issues only and studies focusing only on education methods and curriculum development for pharmacy courses were not included.
Data extraction and analysis
After agreeing on the inclusion of a study, the full texts of the studies were retrieved. We developed a data extraction tool based on the study conceptual framework. We extracted data on the title, authors, year of publication, corresponding author's country of origin, countries of focus, research design and sample, summary of the main findings, ATM issues considered in the study, ‘levels’ of barrier studied and the research topics recommended by the authors. If a publication discussed more than one ATM issue, we noted as many issues as applied to that publication.
We categorised the ‘levels’ of the health system barriers to ATM as: ‘household and community’, ‘health service providers’, ‘health sector as a whole’, ‘other related sectors’ and ‘cross border issues’.11 12 We defined ATM issues based on the WHO Framework for ATM, which included four aspects: affordability, sustainability of financing, rational use, and health systems and availability of medicines.13
One author extracted data from all the included studies, and another author assessed all the data extractions for accuracy and completeness. Then we used not only descriptive methods and presentational graphs and diagrams pertaining to the study questions, but also Pearson r estimates to assess the publication trends over time.
Results
In total, 4755 titles were retrieved as a result of the searches and were reviewed (figure 1). In total, 151 articles were identified (figure 1) that focused entirely or partially on ATM issues in one or more of EMR's LMICs.
As the search strategy was sensitive to identify studies that were conducted in LMICs, we also identified an additional 12 international studies that had important implications about ATM in LMICs of the region.1 14–24 Among these 12 studies, 8 had been published in 2011 alone. We used these studies to discuss and highlight some of the identified issues but did not include them in the analyses.
Six articles were published in languages other than English: French (two each from Tunisia and Morocco and one from Lebanon) and Czech (on Yemen).
Countries of origins of the studies
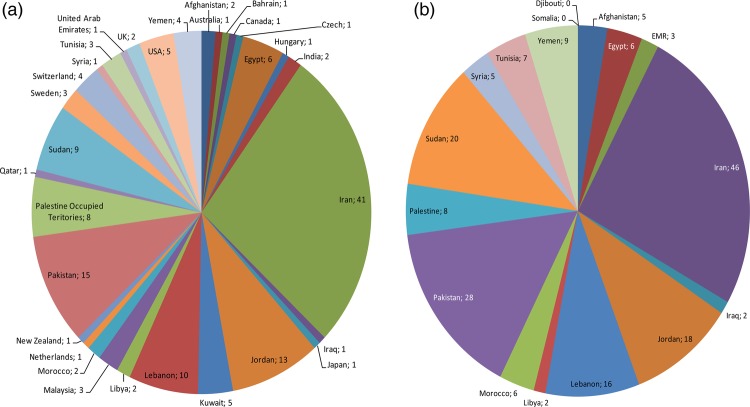
Most of these articles (117 articles, 77%) originated (based on the corresponding author's address) from the LMICs in EMR, while eight (5%) originated from high-income countries of the region and 26 (17%) from other countries (figure 2A). There was a wide variation in the number of publications per country (figure 2A). The countries that produced at least 10 articles in the journals indexed in international databases were Iran (41, 27%), Pakistan (15, 10%), Jordan (13, 9%) and Lebanon (10, 7%). These were followed by Sudan (9), Palestine Occupied Territories (8), Egypt (6), the USA (5) and Kuwait (5).
Figure 2.
Distribution of the country of origin (A) and the country of focus* (B) for publications on access to and appropriate use of medicines issues in the Eastern Mediterranean Region's low-income and middle-income countries.
We found several comparative international studies that used data from EMR or discussed issues relevant to EMR countries. In total, 17% of the identified literature originated from countries outside EMR. Eleven (out of 26) of these studies were multicountry studies that included one or more EMR countries alongside others.
Countries of focus of the studies
In total, 14 EMR LMICs have been the focus of at least one ATM research article in the past decade. The countries that were the focus of at least 10 articles were Iran (46, 30%), Pakistan (28, 19%), Sudan (20, 13%), Jordan (18, 12%) and Lebanon (16, 11%).
Two countries (Iraq, Libya) were discussed in only two articles each (figure 2B). We found no studies on Somalia and Djibouti. There were also no studies on South Sudan, that is, none of the publications discussing Sudan had specific attention or data from South Sudan, which is now an independent country.
Research designs
The majority of the included studies involved cross-sectional studies of various designs. Questionnaire surveys—of facilities, providers and students (38 articles), patients and users (16 articles) and households (7 articles)—were the most common research designs and were used in 41% of the articles. This was followed by prescription audits and medicines utilisation reviews that were observed in 35 articles (23%). Qualitative studies (mainly as case studies) were observed in 10 articles. Similarly, 15 articles (10%) focused on policy-related issues in review articles, policy briefs or advocacy articles, although pinpointing the research designs in these studies was not straightforward and some studies did not employ a formal research approach.
We also identified 13 interventional studies (including 2 randomised controlled trials), 2 economic studies and 2 consensus development studies. These articles, except for one, were all published since 2008. We included nine secondary data analyses (mostly involving multinational data analyses), one systematic review, one bibliometric study, one cohort and one case–control study.
The studies had substantial variations in their designs, from well-designed trials or large-sample national and multinational studies to small-scale studies of convenient samples or with unclear research designs.
Growth in ATM publications
We observed a relatively steady growth in the number of publications per year on ATM in EMR within the last decade (Pearson r=0.87; see figure 3). While in the first 3 years of this period there were only about 10 publications from the region, 80 studies had been published in the last 3 years of the study period. We also observed a modest increase in the proportion of studies originated from outside EMR during the 2006–2010 period (20%) compared with the 2000–2004 period (13%).
Figure 3.
The increasing number of access to and appropriate use of medicines publications per year and Eastern Mediterranean Region. Note: 2011 publications cover only the first half of this year.
ATM issues of focus
To understand the ATM issues on which the published article focused, we assigned each article to one or more of the four large components in the WHO ATM framework. We noted the ATM issues of focus for the articles: affordability (25, 17%), financing (18, 12%), rational use (106, 70%), and health system and availability (63, 42%; table 1). RUM studies were the main bulk of the studies conducted in the region, while very limited attention had been devoted to the financing aspects of access to medicines.
Table 1.
ATM issues and health system levels discussed each year in EMR publications*
| Year | Affordability | Sustainable financing | RUM | Health system and availability | Household and community | Health service public or private | National health sector | National beyond health sector | Cross-border issues |
|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2 | 1 | 2 | ||||||
| 2001 | 1 | 1 | 2 | 1 | 2 | 1 | |||
| 2002 | 1 | 1 | 5 | 3 | 3 | 2 | |||
| 2003 | 1 | 2 | 2 | 2 | |||||
| 2004 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 1 |
| 2005 | 10 | 1 | 4 | 8 | 8 | ||||
| 2006 | 2 | 8 | 5 | 3 | 10 | 5 | 2 | 1 | |
| 2007 | 1 | 1 | 8 | 6 | 3 | 6 | 8 | 1 | |
| 2008 | 4 | 5 | 16 | 9 | 9 | 18 | 13 | 1 | |
| 2009 | 7 | 7 | 20 | 15 | 9 | 22 | 16 | 1 | 1 |
| 2010 | 4 | 20 | 10 | 6 | 20 | 14 | 1 | 1 | |
| 2011 | 3 | 3 | 13 | 10 | 5 | 13 | 8 | 2 | 3 |
| Total | 25 | 18 | 106 | 63 | 44 | 108 | 79 | 7 | 9 |
*The totals add up to more than 151 as an article might be assigned to more than one ATM issue or health system level.
ATM, access to and appropriate use of medicines; EMR, Eastern Mediterranean Region; RUM, rational use of medicines.
Similarly, the articles were assigned to one or more ‘health system levels’ that were the focus of attention for the study (the total adds up to more than 151): household and community (44, 29%), health service (108, 72%), national health sector (79, 52%), national beyond health sector (7, 5%) and cross border issues (9, 6%; table 1). Despite the importance of cross-border issues and the role of sectors beyond the health sector in facilitating or impeding ATM, scarce attention was devoted to those issues in the region.
On the whole, the majority of the studies were limited to the health services level, mainly assessing RUM issues at the level of providers. We found many studies of availability and RUM issues (table 1), including studies that focused on adverse drug reaction (ADR) reporting in several countries.25 Many studies focused on reporting simple RUM indicators for prescribing behaviours (eg, average number of medicines per prescription, or the proportion of prescriptions containing injectables), and most reported an unfavourable picture.26–32 Others focused on self-medication issues from different countries and areas and different subgroups of population.
The articles occasionally offered research recommendations for future studies. Examples of such research recommendations are offered in box 2.
Box 2. A few examples of research recommendations for future studies reported in the included articles.
The cost-effectiveness of different respiratory care services on tuberculosis case detection and health system strengthening33
The cost-effectiveness of using community health workers or outreach clinics for dispersed rural populations34
The effect of various policy interventions to make medicine more affordable7
The impact of the national pharmaceutical policies on the financial capacity of health systems, out-of-pocket health expenditure and the domestic pharmaceutical industry35
Identifying valid indicators for the assessment of physicians’ prescribing performance36
The outcomes of unnecessary ‘stat’ (urgent) prescribing orders on adverse drug events in hospitals37
Using longer term routine data to assess the effects of essential medicines lists and universal health coverage policies on rational use of medicines, out-of-pocket health expenditures and health outcomes38
The impact of legislative and market changes to improve the quality of retail pharmacy in low-income and middle-income countries39
Formulating clinical guidelines for use of benzodiazepines in different indications40
The effectiveness of policies to improve access to medicines in resource-poor countries based on research evidence from such countries41
As a further analysis, we focused attention on the countries for which there were 10 or more ATM publications (Iran, Jordan, Lebanon, Pakistan and Sudan) to see the proportion of these publications that discussed different health system levels (table 2). Very few studies considered cross-border issues and other relevant sectors beyond the health sector. In terms of the ATM issues considered in the studies, ATM research in Iran was heavily biased towards RUM studies with 38 articles, followed by health system issues (15 articles; table 2). Other countries gave a more balanced picture, albeit with a smaller number of articles: 9–13 articles on RUM, 6–14 articles on the health system and availability issues, and 3–10 articles on affordability issues. Similarly, in all five countries, the health service and national health sector levels of the health system had attracted the majority of the published ATM articles.
Table 2.
Distribution of the levels of barriers or issues considered in the ATM publications* in five countries with at least 10 publications†
| Levels of barriers considered |
ATM issues considered |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Household and community | Health service public or private | National health sector | National beyond health sector | Cross-border issues | Affordability | Sustainable financing | RUM | Health system and availability | |
| Iran | 12 | 33 | 25 | 3 | 3 | 6 | 6 | 38 | 15 |
| Jordan | 6 | 14 | 9 | 0 | 0 | 3 | 2 | 10 | 9 |
| Lebanon | 3 | 14 | 9 | 0 | 0 | 4 | 2 | 9 | 10 |
| Pakistan | 7 | 21 | 15 | 3 | 4 | 10 | 5 | 13 | 14 |
| Sudan | 6 | 16 | 11 | 1 | 1 | 6 | 4 | 13 | 6 |
*Each article may have considered more than one barrier or issue.
†The numbers are based on the country of focus of the publications.
ATM, access to and appropriate use of medicines.
Discussion
The results of the study showed that in 16 LMICs (now 17 countries including South Sudan) in EMR, with a total population of over 537 million people, 151 articles were published that focused entirely or in some parts on ATM issues. These studies focused on 14 countries with no publications assessing ATM issues in Djibouti, Somalia or South Sudan. The countries that produced the most publications were Iran, Pakistan, Jordan and Lebanon.
The number of publications for some countries was proportionately very low. For example, if we had excluded the studies from Egypt that were published because of the presence of the WHO regional office in Cairo, then very few studies from Egypt would have remained in our sample. This was surprising given that Egypt is one of the most populous countries in the region which enjoys an expanded academic sector.
The last decade demonstrates a growth in the number of publications per year on ATM in EMR. This is a good sign that with the further development of health systems, the number of publications is growing. This may provide a good opportunity for evidence-based decision-making based on evidence from the region.42 However, the publications were focused mainly on RUM issues and most studies only assessed challenges at the service provider level.43 There was limited attention to affordability and financing issues in the EMR region, and the impact of cross-border factor and sectors other than the health sector on ATM were rarely studied.
Lack of attention to the financing and affordability aspects of ATM in EMR studies is despite the fact that several studies have demonstrated the importance of these aspects.7 In an international study using household survey data from the World Health Surveys, it was clear that affordability was a major barrier to access at the level of households. The study concluded that between 41% and 56% of households in LMICs spent almost all of their healthcare expenditures on medicines.44 The study called for expanded benefit packages and further coverage of medicines in insurance plans in low-income and lower middle-income countries. Whether or not this was taken up in the EMR region is another matter. Among the LMICs in the region, Jordan, Iran, Egypt, Morocco and Tunisia have healthcare insurance systems developed and established by the government.45 46 These schemes have varied successes and limitations that may impede access to medicines.47 Despite this system's development in these countries, still limited attention to insurance-related issues was observed in the ATM publications. For other LMICs in the region, without such nationally funded programmes, it will be more difficult to adopt and implement Wagner et al's44 advice.
Another issue for the affordability as well as financing of medicines in LMICs is the pricing of medicines.43 Wagner et al noted that there was an array of reasons and manifestations of price differences for medicines in different countries. Despite the importance of the issue, they noted that the literature from developing countries has shown very little attention to pricing issues.14 Our findings support their conclusion.
The over-representation of RUM studies might be linked to the availability of widely used instruments to measure RUM, developed by the WHO and other organisations. For example, the “investigating medicine use” tools have existed since the early 1980s. Although in its current edition it covers all areas of ATM,48 its original versions paid special attention to the measurement of RUM aspects of medicine. Pricing and availability tools (WHO-HAI project) have been developed more recently.49 Also, recently growing attention on financing mechanisms and universal health coverage seems to be slowly attracting attention to these aspects applied to the area of medicines. Note also that the standard household survey and healthcare utilisation tools (eg, the World Health Survey tool) that usually allow one to look at health financing aspects or out-of-pocket expenditures often do not discriminate between medicines and other items of expenditure. To assess households’ pharmaceutical expenditure, primary data collection is usually needed, which may be expensive and time consuming and therefore limited only to countries that have the resources to conduct such studies. Also, it seems that the indicators and instruments on RUM are more widely accepted than pricing indicators, for example, there may be disagreement on measuring retail price, procurement price, with or without mark-up.7 50 Still, one might hope that with the emergence of further attention and better tools to assess the financing aspects of ATM, more articles will be published on such issues in the near future.
RUM research in the region has been mainly in the shape of prescription audits, the majority of it showing that there are important problems in prescriptions.32 43 51–53 There are two important patterns to note in here. First, the RUM research, although forming the majority of ATM research, is yet to show a substantial effect in improving the drug utilisation patterns. The prescribing problems of focus that existed 10 years ago remain unresolved today, if not joined by new challenges (eg, non-generic prescribing). For example, one study that assessed ADR repots for artemisinin-based antimalarial treatments did not find a single report of ADR for artemisinin-based medicines from EMR countries with hyperendemic and mesoendemic malaria problems.54 Also, studies of pharmacy service quality demonstrated a low quality of service and a room for improvement in provision of care at the pharmacies.55 In a way, maybe it is safe to conclude that even RUM studies have not been that effective in improving access, or in tackling the main issues.
Second, it seems that a change in research strategies is required and future studies should focus further on assessing the impacts of relevant interventions. Fortunately, in recent years, there is a shift towards interventional studies assessing the impact of interventions on improving prescribing outcomes.33 56–59 Also, further demand side (eg, why the public still seems to be fascinated with antibiotics), supply side (eg, how physicians might be encouraged to follow evidence-based prescribing) and health systems angle (eg, what are the financial and organisational barriers to improving prescribing patterns) research is required.
Other sectors’ (other than the healthcare sector) effects on access to medicines are barely considered in the publications from the region. Studies have generally demonstrated that the general socioeconomic status of a country is linked to ATM, while a few studies have elaborated on this in the region.60 Despite the numerous studies that assessed the impact of the health system and provision issues on ATM, there are very few studies that discuss important policy directions for improving ATM. For example, we did not find any study that had assessed the impact of essential drug list initiatives on access to medicines in an EMR country. This is despite the fact that, according to Mirza,45 all the LMICs in the EMR region, except Libya and Lebanon, had a policy of essential drug lists in place at the time.
Ritz and Laing41 reported the findings of a bibliometric study of ATM literature in LMICs. Compared with our study, they underestimated the number of articles produced from the EMR region by a wide margin. They suggested that medicine selection, intellectual property rights and monitoring and quality assurance were among the top ATM topics studied in developing countries, which is different from what we observed in EMR. Interestingly, Ritz and Laing observed that the corresponding authors residing in high-income countries represented around 50% of all publications relative to LMICs. Our study demonstrated that most articles (77%) in the region originated (as per the corresponding author’s address) from LMICs in the region.
There are several limitations to the current bibliography study. Using the affiliation of the corresponding author to identify the study's country of origin has several limitations. For example, it may be teamwork, in which case authors from different countries contribute. Also, several identified studies were from research students in high-income countries’ institutions running research in their own countries. Many such students are also funded by their own countries, but may use the affiliations of the institutions in which they study. On the other hand, there are studies conducted by foreign missions based in a country, and although the address of the foreign mission is from that country, the study cannot be strictly considered as home-grown. Despite such limitations, the bibliographic reviews are informative of a country's productivity in ATM research.
We conducted systematic searches of the main international databases for identifying ATM articles. Although we followed rigorous methods, the search should not be considered exhaustive. We might have missed published articles not indexed in these databases. However, as most of the important health research from the region makes it to international databases, we believe that we have captured a substantial number, if not the majority, of articles on ATM.
Conclusions
The provision of reliable access to affordable, appropriate and high-quality medicines is a key component of a functioning health system. Access to medicines needs to be fully integrated with health financing, human resource planning, service delivery, information and governance systems. This is the first study conducted in this region that has collated the published literature and summarised the main policy concerns to identify ATM research priorities. In this study, we used an extensive search of the local and regional literature. We developed detailed maps of research on the issue, conceptual frameworks of policy concerns and issues and identified lists of ATM research priorities for the countries of focus and the region as a whole.
This study clearly indicates that there is dire need for further research on the financing and affordability aspects of ATM in the region. This should be given paramount attention in future research funding and calls for proposals. Also, cross-border issues and other sectors’ roles on access to medicines in the region have not been explored widely. It seems that many household (demand side) studies in the region continue to be of poor quality and limited methods. Together, these main areas should provide the main aspects of access to medicines research in the region.
This does not in any way indicate that further RUM or studies of health systems and availability access are not needed, or that the barriers at the levels of providers and health systems are exhaustively identified. Rather, it seems that individual researchers and the available funding route are paying attention to these issues at the moment, which should continue while further resources should be mobilised for studies related to the relatively ignored aspects of ATM research in the region.
The picture of research on ATM in the region is better than what had been reported in recent publications41 or compared with other regions.6 There is a growing trend, over the years, of more and better quality studies from the region appearing in international journals. Still, a concurrent trend will be required to ensure that the local audience of such research (ie, practitioners, policymakers and media) remains informed of the new development as a result of ATM research in countries in the region. An active knowledge translation approach will be essential.
Supplementary Material
Acknowledgments
The Alliance for Health Policy and Systems Research (AHPSR) works within the Health Systems Strengthening cluster of WHO HQ. The authors would like to thank the AHPSR for its support and guidance, especially Abdul Ghaffar and Maryse Coutty for their excellent support, and the Tehran University of Medical Sciences’ Research Council for approving the study. They also thank Soonman Kwon, Rasoul Dinarvand, Richard Laing and Rouham Yamout for the advice on the methods and interpretation of the findings.
Footnotes
Contributors: AR and MB conceived the study. AR, SJ and SZ developed the methods. NJ, AR, SJ, SZ and FS collected the data. NJ, AR and FS analysed the data. AR and NJ wrote the manuscript. All the authors revised and commented on different versions of the manuscript, and read and approved the final version of the manuscript.
Funding: WHO Alliance for Health Policy and Systems Research. (grant no. E50-APW-253)
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The list of the included studies will be available to interested researchers on request.
References
- 1.Wagner AK, Graves AJ, Reiss SK, et al. Access to care and medicines, burden of health care expenditures, and risk protection: results from the World Health Survey. Health Policy 2011;100:151–8 [DOI] [PubMed] [Google Scholar]
- 2.Laing R, Waning B, Maculey C, et al. Improving access to child health medicines: review and discussion paper for WHO Regional and Country Child Health Advisors. Geneva: WHO, 2002 [Google Scholar]
- 3.MDG Gap Task force Report Delivering on the global partnership for achieving the millennium development goals. United Nations University Press; 2008 [Google Scholar]
- 4.Videau JY. Access for all to quality drugs. Med Trop (Mars) 2002;62:396–400 [PubMed] [Google Scholar]
- 5.Tumwikirize WA, Ogwal-Okeng JW, Vernby O, et al. Access and use of medicines information sources by physicians in public hospitals in Uganda: a cross-sectional survey. Afr Health Sci 2008;8:220–6 [PMC free article] [PubMed] [Google Scholar]
- 6.Emmerick ICM, Oliveira MA, Luiza VL, et al. Access to medicines in Latin America and the Caribbean (LAC): a scoping study. BMJ Open 2013;3:e002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron A, Ewen M, Ross-Degnan D, et al. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet 2009;373:240–9 [DOI] [PubMed] [Google Scholar]
- 8.WHO Medicines Strategy: Countries at the Core 2004–2007. WHO/EDM/2004.2.
- 9.Medicines use in primary care in developing and transitional countries: fact book summarising results from studies reported between 1990 and 2006. WHO/EMP/MAR/2009.3.
- 10.Zaidi S, Aleem N, Bigdeli M, et al. Access to essential medicines in Pakistan: policy and health systems research concerns. PLoS ONE 2013;8:e63515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson K, Ranson MK, Oliveira-Cruz V, et al. Expanding access to priority health interventions: a framework for understanding the constraints to scaling-up. J Int Dev 2003;15:1–14 [Google Scholar]
- 12.Anonymous. WHO Framework for developing a health systems research agenda. http://www.who.int/rpc/meetings/Framework_for_developing_a_health_systems_research_agenda.pdf (accessed May 2013)
- 13.WHO Equitable access to essential medicines: a framework for collective action Geneva: WHO Policy Perspectives on Medicines, No. 008, 2004 [Google Scholar]
- 14.Wagner JL, McCarthy E. International differences in drug prices. Annu Rev Public Health 2004;25:475–95 [DOI] [PubMed] [Google Scholar]
- 15.Vasan A, Hoos D, Mukherjee JS, et al. The pricing and procurement of antiretroviral drugs: an observational study of data from the Global Fund. Bull World Health Organ 2006;84:393–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogerzeil HV, Samson M, Casanovas JV, et al. Is access to essential medicines as part of the fulfilment of the right to health enforceable through the courts? Lancet 2006;368:305–11 [DOI] [PubMed] [Google Scholar]
- 17.Vreeman RC, Wiehe SE, Pearce EC, et al. A systematic review of pediatric adherence to antiretroviral therapy in low- and middle-income countries. Pediatr Infect Dis J 2008;27686–91 [DOI] [PubMed] [Google Scholar]
- 18.Suh G-H. High medicine prices and poor affordability. Curr Opin Psychiatry 2011;24:341–5 [DOI] [PubMed] [Google Scholar]
- 19.Kishore SP, Vedanthan R, Fuster V. Promoting global cardiovascular health. J Am Coll Cardiol 2011;57:1980–7 [DOI] [PubMed] [Google Scholar]
- 20.Berendes S, Heywood P, Oliver S, et al. Quality of private and public ambulatory health care in low and middle income countries: systematic review of comparative studies. PLoS Med 2011;8:e1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faden L, Vialle-Valentin C, Ross-Degnan D, et al. Active pharmaceutical management strategies of health insurance systems to improve cost-effective use of medicines in low- and middle-income countries: a systematic review of current evidence. Health Policy 2011;100:134–43 [DOI] [PubMed] [Google Scholar]
- 22.Renaud-Thery F, Avila-Figueroa C, Stover J, et al. Utilization patterns and projected demand of antiretroviral drugs in low- and middle-income countries. AIDS Res Treat 2011;2011: 749041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machado M, O'Brodovich R, Krahn M, et al. International drug price comparisons: quality assessment. Rev Panam Salud Publica 2011;29:46–51 [PubMed] [Google Scholar]
- 24.Hoen E, Berger J, Calmy A, et al. Driving a decade of change: HIV/AIDS, patents and access to medicines for all. J Int AIDS Soc 2011;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zargarzadeh AH, Emami MH, Hosseini F. Drug-related hospital admissions in a generic pharmaceutical system. Clin Exp Pharmacol Physiol 2007;34:494–8 [DOI] [PubMed] [Google Scholar]
- 26.Zargarzadeh AH, Minaeiyan M, Torabi A. Prescription and nonprescription drug use in Isfahan, Iran: an observational, cross-sectional study. Curr Ther Res Clin Exp 2008;69:76–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan MS, Ahmed Z, Jehan S, et al. Common trend of antibiotics usage in a tertiary care hospital of Peshawar, Pakistan. J Ayub Med Coll Abbottabad 2010;22:118–20 [PubMed] [Google Scholar]
- 28.Al-Niemat SI, Bloukh DT, Al-Harasis MD, et al. Drug use evaluation of antibiotics prescribed in a Jordanian hospital outpatient and emergency clinics using WHO prescribing indicators. Saudi Med J 2008;29:743–8 [PubMed] [Google Scholar]
- 29.Yousif MA, Nizar S, Elmustafa MO, et al. Investigation of medication prescribing errors in Wad Medani, Gezira, Sudan. Int J Risk Saf Med 2011;23:11–16 [DOI] [PubMed] [Google Scholar]
- 30.Saab YB, Hachem A, Sinno S, et al. Inappropriate medication use in elderly Lebanese outpatients: prevalence and risk factors. Drugs Aging 2006;23:743–52 [DOI] [PubMed] [Google Scholar]
- 31.Fattouh R, Abu Hamad B. Impact of using essential drug list: analysis of drug use indicators in Gaza Strip. East Mediterr Health J 2010;16:886–92 [PubMed] [Google Scholar]
- 32.Sepehri G, Talebizadeh N, Mirzazadeh A, et al. The patterns of antihypertensive drug prescription by cardiologists in Kerman province of Iran, 2006. Pharmacoepidemiol Drug Saf 2008;17:180–5 [DOI] [PubMed] [Google Scholar]
- 33.Abu Rumman K, Ottmani S, Abu Sabra N, et al. Training on the practical approach to lung health: effect on drug prescribing in PHC settings in Jordan. East Mediterr Health J 2009;15:111–21 [PubMed] [Google Scholar]
- 34.Al-Taiar A, Jaffar S, Assabri A, et al. Who develops severe malaria? Impact of access to healthcare, socio-economic and environmental factors on children in Yemen: a case-control study. Trop Med Int Health 2008;13:762–70 [DOI] [PubMed] [Google Scholar]
- 35.Davari M, Walley T, Haycox A. Pharmaceutical policy and market in Iran: past experiences and future challenges. J Pharm Health Serv Res 2011;2:47–52 [Google Scholar]
- 36.Esmaily HM, Savage C, Vahidi R, et al. Identifying outcome-based indicators and developing a curriculum for a continuing medical education programme on rational prescribing using a modified Delphi process. BMC Med Educ 2008;8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fahimi F, Sahraee Z, Amini S. Evaluation of stat orders in a teaching hospital. Clin Drug Investig 2011;31:231–5 [DOI] [PubMed] [Google Scholar]
- 38.Hamidi S, Younis MJ, Forgione DA. Implementing an essential medicines list: effects on pricing and utilization in West Bank, Palestine. J Health Care Finance 2008;34:10–30 [PubMed] [Google Scholar]
- 39.Lowe RF, Montagu D. Legislation, regulation, and consolidation in the retail pharmacy sector in low-income countries. South Med Rev 2009;2:35–44 [Google Scholar]
- 40.Khawaja MR, Majeed A, Malik F, et al. Prescription pattern of benzodiazepines for inpatients at a tertiary care university hospital in Pakistan. J Pak Med Assoc 2005;55:259. [PubMed] [Google Scholar]
- 41.Ritz LS, Adam T, Laing R. A bibliometric study of publication patterns in access to medicines research in developing countries. South Med Rev 2010;3:2–6 [PMC free article] [PubMed] [Google Scholar]
- 42.Yousefi Nooraie R, Rashidian A, Nedjat S, et al. Promoting development and use of systematic reviews in a developing country. J Eval Clin Practice 2009;15:1029–34 [DOI] [PubMed] [Google Scholar]
- 43.Zaidi S, Bigdeli M, Aleem N, et al. Access to essential medicines in Pakistan: policy and health systems research concerns. PLoS ONE 2013;8:e63515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner AK, Graves AJ, Reiss S, et al. Access to care and medicines, burden of health care expenditures, and risk protection: results from the World Health Survey. Health Policy 2010;100:151–8 [DOI] [PubMed] [Google Scholar]
- 45.Mirza Z. Thirty years of essential medicines in primary health care. East Mediterr Health J 2008;14(Suppl):S74–81 [PubMed] [Google Scholar]
- 46.Takian A, Rashidian A, Kabir MJ. Expediency and coincidence in reengineering a health system: an interpretive approach to formation of family medicine in Iran. Health Policy Plann 2011;26:163–73 [DOI] [PubMed] [Google Scholar]
- 47.Kavosi Z, Rashidian A, Pourreza A, et al. Inequality in household catastrophic health care expenditure in a low-income society of Iran. Health Policy Plan 2012;27:613–23 [DOI] [PubMed] [Google Scholar]
- 48.Management Sciences for Health MDS-3:managing access to medicines and health technologies. Alrington, VA: Management Sciences for Health, 2012 [Google Scholar]
- 49.RJea EJ. WHO/HAI Project on Medicine Prices and Availability. World Health Organization and Health Action International, 2011 [Google Scholar]
- 50.Cameron A, Roubos I, Ewen M, et al. Differences in the availability of medicines for chronic and acute conditions in the public and private sectors of developing countries. Bull World Health Organ 2011;89:412–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sawalha AF, Sweileh WM, Zyoud SH, et al. Analysis of prescriptions dispensed at community pharmacies in Nablus, Palestine. East Mediterr Health J 2010;16:788–92 [PubMed] [Google Scholar]
- 52.Penn-Barwell J, Pengelly S. Audit of prescription of anti-malarial prophylaxis to patients admitted to Royal Centre for Defence Medicine (RCDM) following evacuation from Afghanistan. J R Nav Med Serv 2008;94:112–14 [PubMed] [Google Scholar]
- 53.Yousif E, Ahmed AM, Abdalla ME, et al. Deficiencies in medical prescriptions in a Sudanese hospital. East Mediterr Health J 2006;12:915–18 [PubMed] [Google Scholar]
- 54.Kuemmerle A, Dodoo AN, Olsson S, et al. Assessment of global reporting of adverse drug reactions for anti-malarials, including artemisinin-based combination therapy, to the WHO Programme for International Drug Monitoring. Malar J 2011;10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith F. The quality of private pharmacy services in low and middle-income countries: a systematic review. Pharm World Sci 2009;31:351–61 [DOI] [PubMed] [Google Scholar]
- 56.Soleymani F, Rashidian A, Dinarvand R, et al. Assessing the effectiveness and cost-effectiveness of audit and feedback on physician's prescribing indicators: study protocol of a randomized controlled trial with economic evaluation. DARU J Pharm Sci 2012;20:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rahimi B, Timpka T. Pharmacists’ views on integrated electronic prescribing systems: associations between usefulness, pharmacological safety, and barriers to technology use. Eur J Clin Pharmacol 2011;67:179–84 [DOI] [PubMed] [Google Scholar]
- 58.Esmaily HM, Silver I, Shiva S, et al. Can rational prescribing be improved by an outcome-based educational approach? A randomized trial completed in Iran. J Contin Educ Health Prof 2010;30:11–18 [DOI] [PubMed] [Google Scholar]
- 59.Garjani A, Salimnejad M, Shamsmohamadi M, et al. Effect of interactive group discussion among physicians to promote rational prescribing. East Mediterr Health J 2009;15:408–15 [PubMed] [Google Scholar]
- 60.Large M, Farooq S, Nielssen O, et al. Relationship between gross domestic product and duration of untreated psychosis in low- and middle-income countries. Br J Psychiatry 2008;193:272–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.