Abstract
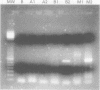
BACKGROUND: Early detection of haematogenous dissemination of epithelial tumours afforded by the analysis of epithelial antigen expression in the peripheral blood mononuclear fraction (PBMN) and bone marrow may confer a worse prognosis to patients with carcinoma. Cytokeratin 19 is a protein normally expressed by epithelial cells including normal and malignant mammary cells. Previous studies have demonstrated that analysis of cytokeratin 19 expression by the reverse transcriptase-polymerase chain reaction (RT-PCR) can detect one epithelial cell in as many as 10(5)-10(7) haematopoetic cells. Despite its sensitivity concern has been voiced recently about the specificity of this technique owing to the detection of cytokeratin 19 expression in the PBMN of normal volunteers and the bone marrow of patients with haematological malignancies. AIMS: To assess the sensitivity and specificity of RT-PCR detection of cytokeratin 19 in PBMN of normal female blood donors. METHODS: Blood was taken from 52 normal female blood donors and PBMN separated through Fycol gradient centrifugation. Cytokeratin 19 was measured using a two step nested RT-PCR assay. RESULTS: No amplification was found in the first step for any of the samples studied, whereas in the second step amplification was observed in 10 of the 52 samples. Both steps could detect one MCF-7 cell (the cytokeratin 19 positive control) in 10(6) CEM (cytokeratin 19 negative control) cells. CONCLUSIONS: As both PCR steps are sensitive to the 10(-6) level, performing only the first amplification step may decrease the non-specificity of this method. Further studies are needed to define the specificity and sensitivity of this technique in blood and bone marrow specimens of women with breast cancer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader B. L., Jahn L., Franke W. W. Low level expression of cytokeratins 8, 18 and 19 in vascular smooth muscle cells of human umbilical cord and in cultured cells derived therefrom, with an analysis of the chromosomal locus containing the cytokeratin 19 gene. Eur J Cell Biol. 1988 Dec;47(2):300–319. [PubMed] [Google Scholar]
- Bartek J., Bartkova J., Taylor-Papadimitriou J. Keratin 19 expression in the adult and developing human mammary gland. Histochem J. 1990 Oct;22(10):537–544. doi: 10.1007/BF01005976. [DOI] [PubMed] [Google Scholar]
- Bartek J., Taylor-Papadimitriou J., Miller N., Millis R. Patterns of expression of keratin 19 as detected with monoclonal antibodies in human breast tissues and tumours. Int J Cancer. 1985 Sep 15;36(3):299–306. [PubMed] [Google Scholar]
- Bártek J., Bártková J., Schneider J., Taylor-Papadimitriou J., Kovarík J., Rejthar A. Expression of monoclonal antibody-defined epitopes of keratin 19 in human tumours and cultured cells. Eur J Cancer Clin Oncol. 1986 Dec;22(12):1441–1452. doi: 10.1016/0277-5379(86)90077-5. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Datta Y. H., Adams P. T., Drobyski W. R., Ethier S. P., Terry V. H., Roth M. S. Sensitive detection of occult breast cancer by the reverse-transcriptase polymerase chain reaction. J Clin Oncol. 1994 Mar;12(3):475–482. doi: 10.1200/JCO.1994.12.3.475. [DOI] [PubMed] [Google Scholar]
- Diel I. J., Kaufmann M., Goerner R., Costa S. D., Kaul S., Bastert G. Detection of tumor cells in bone marrow of patients with primary breast cancer: a prognostic factor for distant metastasis. J Clin Oncol. 1992 Oct;10(10):1534–1539. doi: 10.1200/JCO.1992.10.10.1534. [DOI] [PubMed] [Google Scholar]
- Krismann M., Todt B., Schröder J., Gareis D., Müller K. M., Seeber S., Schütte J. Low specificity of cytokeratin 19 reverse transcriptase-polymerase chain reaction analyses for detection of hematogenous lung cancer dissemination. J Clin Oncol. 1995 Nov;13(11):2769–2775. doi: 10.1200/JCO.1995.13.11.2769. [DOI] [PubMed] [Google Scholar]
- Mansi J. L., Berger U., Easton D., McDonnell T., Redding W. H., Gazet J. C., McKinna A., Powles T. J., Coombes R. C. Micrometastases in bone marrow in patients with primary breast cancer: evaluation as an early predictor of bone metastases. Br Med J (Clin Res Ed) 1987 Oct 31;295(6606):1093–1096. doi: 10.1136/bmj.295.6606.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan K. E., Beck C., Holzmayer T. A., Chin J. E., Wunder J. S., Andrulis I. L., Gazdar A. F., Willman C. L., Griffith B., Von Hoff D. D. Quantitative analysis of MDR1 (multidrug resistance) gene expression in human tumors by polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7160–7164. doi: 10.1073/pnas.87.18.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]