Abstract
Objective
Major depression is a mood disorder that causes changes in physical activity, appetite, sleep and weight. Regarding the role of zinc in the pathology of depression, the present study was aimed to investigate the effects of zinc supplementation in the treatment of this disease.
Methods
This study was a double-blind randomized clinical trial. Forty four patients with major depression were randomly assigned to groups receiving zinc supplementation and placebo. Patients in Zinc group received daily supplementation with 25 mg zinc adjunct to antidepressant; Selective Serotonin Reuptake Inhibitors (SSRIs), while the patients in placebo group received placebo with antidepressants (SSRIs) for twelve weeks. Severity of depression was measured using the Beck Depression Inventory at baseline and was repeated at the sixth and twelfth weeks. ANOVA with repeated measure was used to compare and track the changes during the study.
Results
The mean score of Beck test decreased significantly in the zinc supplement group at the end of week 6 (P < 0.01) and 12 (P < 0.001) compared to the baseline. The mean score of Beck Depression Inventory reduced significantly compared to the placebo group at the end of 12th week (P < 0.05)
Conclusion
The results of the present study indicate that zinc supplementation together with SSRIs antidepressant drug improves major depressive disorders more effectively in patients with placebo plus antidepressants (SSRIs).
Keywords: Major depressive disorder, Zinc supplement, placebo and Selective Serotonin Reuptake Inhibitors (SSRIs)
According to the criteria of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), major depressive disorder (MDD) is a psychiatric disorder with genetic, biological, and environmental risk factors. This disorder is one of the most common diseases in the world, with high levels of associated mortality. It affects people of every region in every ethnic group (1, 2) to the extent that one out of every 5 people referred to a physician is affected by depression (3).
About 19-34% of patients with depression do not respond to antidepressants, and 15-50% of them have a recurrence; therefore, other medications or supplementation with micronutrients are increasingly adjunct to antidepressant drugs to improve their therapeutic effects (4).
Zinc is one of the micronutrients involved in behavior, learning and mental functions. The first clinical studies in the field of serum zinc level in depressed patients have been published by Hansen and colleagues (5). The first report on the relationship between zinc status and brain function in humans has been published in 1998 by Sand stead and colleagues (6). Various studies have identified the effects of zinc in the pathophysiology of depression and antidepressant drugs mechanisms of action. Also, other clinical studies have shown low serum zinc concentrations in patients with depression (7).Furthermore, Long-term treatments with zinc in laboratory animals have had the same mechanisms and effects as the antidepressant drugs (8).
In previous studies, the dietary micronutrients as confounding factors have not been studied. However, the present study was designed to examine the effects of zinc supplementation in patients with major depression, while assessing and controlling dietary intake. The present study was the first randomized clinical trial in Iran designed to examine the effects of zinc supplementation in patients with major depression.
Material and Methods
Participants and Procedure
This study was a double blind randomized clinical trial that was performed on 44 patients with major depression. Study population was among people with depression who were referred to the psychiatric clinic of Imam Hussein, Tehran. Sampling started after obtaining approval from the ethics committee of Nutrition Research Institute, Shahid Beheshti University of Medical Sciences (No. t/1/044).
The patients were aged 18-55 years. The inclusion criteria included diagnosis of major depressive disorder by a psychiatrist based on DSM-IV-TR, obtaining informed consent from the patients, no supplements usage at least four weeks before the study, not taking any medication except for those associated with depression status. The exclusion criteria included pregnancy or lactation, severe psychotic symptoms, changing drug class, symptoms requiring hospitalization including suicidal thoughts and actions.
Patients were randomly assigned in to two groups. One group received 25-mg zinc supplement daily with Selective Serotonin Reuptake Inhibitors (SSRIs) antidepressants (citalopram 20-60mg per day or fluoxetine 20-60mg per day), and the other group received placebo (containing Malto-dextrose) plus an antidepressant SSRI (citalopram 20-60mg per day or fluoxetine 20-60mg per day). The groups were blinded to the researchers and patients.
Demographic data form was completed at the baseline. Inclusion and exclusion criteria and disease background were evaluated by research team. The Beck Depression Inventory (9), physical activity questionnaire, three-day dietary recall, and anthropometric indices were completed in the beginning, middle and end of the study. Nutritionist IV software was used to analyze a three-day dietary recall. Macro and micronutrients intake was derived from the three-day dietary recall and compared between the two groups.
Also, at the beginning and end of the study, a blood sample of 5 ml was taken after a 12-hour fasting state. Serums were separated and zinc concentrations were measured by flame atomic absorption spectrophotometry.
Statistical Analysis
Sample size was determined so that 11 score difference in the mean of Beck score could be detectable, while comparing zinc and placebo groups with α = 0.05 and 1-β = 80%. In all, a sample size of 18 was detected to be sufficient. However, to cover at least 20% drop out which is common in clinical trials, a sample size of 22 subjects was determined for each group.
Data analysis was performed using SPSS version 16. The Kolmogorov - Smirnov and chi-square tests were used to determine the normal distribution of quantitative data and to compare qualitative variables between the two groups, respectively. In the case of quantitative variables with normal distribution for comparison between the beginning and end of the intervention within each group, paired t-test, and for comparison between the two groups at the beginning or end of the intervention, t-test were used. The Wilcoxon and Mann-Whitney tests were used in the case of quantitative variables with non-normal distribution. To compare and track the change of means of quantitative variables that were measured three times during the study period, analysis of variance (ANOVA) with repeated measure was done. Covariance analysis was performed to adjust for quantitative confounding factors.
The IRCT number was 201110187836N1
Result
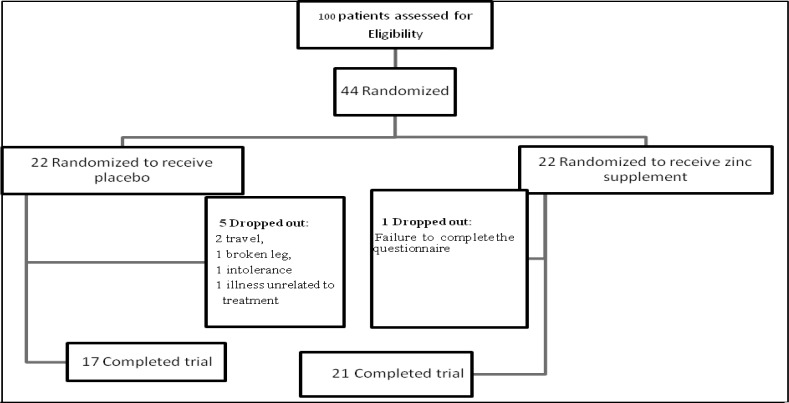
In this study, 6 out of 44 patients were excluded voluntarily or due to lack of medication. Therefore, 21 patients in the group receiving zinc supplements and 17 in the placebo group remained in the study (Figure 1). Furthermore, when serum zinc concentration was assessed, one subject was dropped out in zinc group due to loss of blood sample. The mean age in the zinc group was 37±9 and in the placebo group was 37.5±8 years, which was not significantly different.
Figure 1.
Flow chart of the study
The two groups were alike with respect to gender, education level, income, smoking, and no significant difference was observed between them (Table 1). Both groups received the same SSRI antidepressants. Furthermore, the groups were statistically similar in terms of sleep duration, physical activity, weight, BMI, and serum zinc concentration during the study (Table 2).
Table 1.
General characteristics of Zinc and Placebo groups
| Variables | Zinc group (n = 21) n (%) | Placebo group (n = 17) n (%) | |
|---|---|---|---|
| Gender | Female | 20(95%) | 14(82%) |
| Male | 1(5%) | 3(18%) | |
| Education Level | Elementary school | 3(14%) | 5(29%) |
| Secondary school | 8(38%) | 2(12%) | |
| High school | 7(34%) | 5(29.5%) | |
| Academic | 3(14%) | 5(29.5%) | |
| Smoking | No | 18(86%) | 15(88%) |
| Yes | 3(14%) | 2(12%) | |
| Marital Status | Single | 2(9.50%) | 2(12%) |
| Married | 19(90.50%) | 15(88%) | |
| Income | Less than 200 thousand | 2(9%) | 1(6%) |
| 200 to 400 thousand | 6(29%) | 2(12%) | |
| 400 to 600 thousand | 7(33%) | 10(59%) | |
| More than 600 thousand | 6(29%) | 4(23%) |
Table 2.
Mean and standard deviation of sleep duration, physical activity, anthropometric indices and serum zinc concentration in patients with depression receiving zinc supplementation or placebo
| Variables | Study groups | N | Study period | ||
|---|---|---|---|---|---|
|
| |||||
| Baseline | Sixth Week | Twelfth week | |||
| Sleep duration (hours per day) | Zinc Placebo |
21 17 |
7±1 7±1 |
7±1 7±1 |
7±1 7±1 |
| Physical activity (d/MET/h) | Zinc Placebo |
21 17 |
16.5±3 18±3 |
17±3 18±4 |
17±3 18±3 |
| Weight (kg) | Zinc Placebo |
21 17 |
71±17 68.5±10 |
72±17.5 69±10 |
71±18 70±10.5 |
| Height (cm) | Zinc Placebo |
21 17 |
161±6 162±9 |
- - |
- - |
| BMI (kg/m 2 ) | Zinc Placebo |
21 17 |
27±6 26±5 |
28±7 26±5 |
27.5±6 27±5 |
| Serum zinc concentration (µg/dL) | Zinc Placebo |
20†
17 |
100±15 106±17 |
- - |
107±11 106±14 |
When serum zinc concentration was assessed, one subject was dropped out in zinc group due to loss of blood sample
No significant difference was observed between the two groups regarding dietary intake of energy, carbohydrate, protein, cholesterol, and fiber (Table 3). However, intake of total fat, saturated fatty acids and Monounsaturated Fatty Acids (MUFA) in the zinc group was significantly lower in the sixth week (p < 0.05) and twelfth week (P < 0.01) compared to the placebo group(Table 3). Also, intake of Polyunsaturated Fatty Acids (PUFA) was significantly lower in the zinc group compared to the placebo group in the twelfth week (p < 0.05) (Table 3).
Table 3.
Mean and standard deviation of energy intake, dietary macronutrients and fiber in patients with depression receiving zinc supplementation or placebo
| Variables (Energy and food components) | Study groups | N | Study period | ||
|---|---|---|---|---|---|
|
| |||||
| Baseline | Sixth Week | Twelfth week | |||
| Total energy intake (Kcal/d) | Zinc Placebo |
21 17 |
1667±261 1787±259 |
1686±425 1819±239 |
1665±433 1851±289 |
| Total carbohydrate intake (g /d) | Zinc Placebo |
21 17 |
251±53 240±62 |
280±49 259±53 |
272±42 261±62 |
| Total protein intake (g / d) | Zinc Placebo |
21 17 |
57±19 66±23 |
58±17 63±23 |
60±11 67±27 |
| Total fat intake (g / d) | Zinc Placebo |
21 17 |
49±26 59±33 |
46±16a
65±34 |
46±15 b
65±21 |
| Saturated fatty acid intake (g / d) | Zinc Placebo |
21 17 |
17±8 21±7 |
18±6a
24±9 |
17±6 a
25±9 |
| MUFA fatty acids intake (g / d) | Zinc Placebo |
21 17 |
13±6 16±7 |
13±4a
18±8 |
13±4 b
19±5 |
| PUFA fatty acid intake (g / d) | Zinc Placebo |
21 17 |
9±14 18±24 |
9±8 17±25 |
8±7 a
17±17 |
| Cholesterol intake (g / d) | Zinc Placebo |
21 17 |
224±120 254±152 |
178±87 208±153 |
210±131 238±177 |
| Fiber intake (g / d) | Zinc Placebo |
21 17 |
13±5 14±4 |
16±4 14±4 |
14±3 13±4 |
For comparison in each group paired t-test and for comparison between two groups independent sample t-test was used. Statistically significant difference compared with Placebo
p < 0.05
p < 0.01
During the study, dietary intakes of micronutrients such as zinc, magnesium, iron, vitamins B1, B2, B3, B6, B12 and folic acid were not significantly different between the two groups (Table 4). Only dietary intake of magnesium in the group receiving zinc supplementation was significantly lower (p < 0.05) than the placebo group at the baseline (Table 4).
Table 4.
Mean ± SD dietary micronutrient intake in patients with depression receiving zinc supplementation or placebo
| Variables (Energy and food components) | Study groups | N | Study period | ||
|---|---|---|---|---|---|
|
| |||||
| Baseline | Sixth Week | Twelfth week | |||
| Zinc intake (mg/d) | Zinc Placebo |
21 17 |
6.5±2.1 7.6±2.2 |
7.5±1.7 7.4±2.5 |
7.5±1.7 7.3±2.5 |
| Magnesium intake (mg/d) | Zinc Placebo |
21 17 |
193±55a
249±75 |
227±63 255±83 |
234±85 254±63 |
| Iron intake (mg / d) | Zinc Placebo |
21 17 |
11±4 13±4 |
12.5±5 13±4 |
12±4 13±4 |
| Vit. B 1 intake (mg / d) | Zinc Placebo |
21 17 |
1.4±0.5 1.7±0.5 |
1.5±0.5 1.7±0.4 |
1.6±0.4 1.5±0.3 |
| Vit. B 2 intake (mg / d) | Zinc Placebo |
21 17 |
1.2±0.4 1.5±0.5 |
1.5±0.6 1.5±0.7 |
1.5±0.5 1.4±0.4 |
| Vit. B 3 intake (mg / d) | Zinc Placebo |
21 17 |
17±5 18±5 |
17±6 18±5 |
18±5 19±8 |
| Vit. B 6 intake (mg / d) | Zinc Placebo |
21 17 |
1.5±0.8 1.5±0.4 |
1.5±0.6 1.5±0.3 |
1.5±0.6 1.6±0.4 |
| Folic acid intake (µg / d) | Zinc Placebo |
21 17 |
198±119 246±114 |
239±110 234±130 |
228±106 270±120 |
| Vit. B 12 intake (µg / d) | Zinc Placebo |
21 17 |
2±1.2 2±0.9 |
2.4±1.4 2.6±1.4 |
2.6±1.1 2.7±1.8 |
Statistically significant difference compared with Placebo
p < 0.05
* Vit.= Vitamin
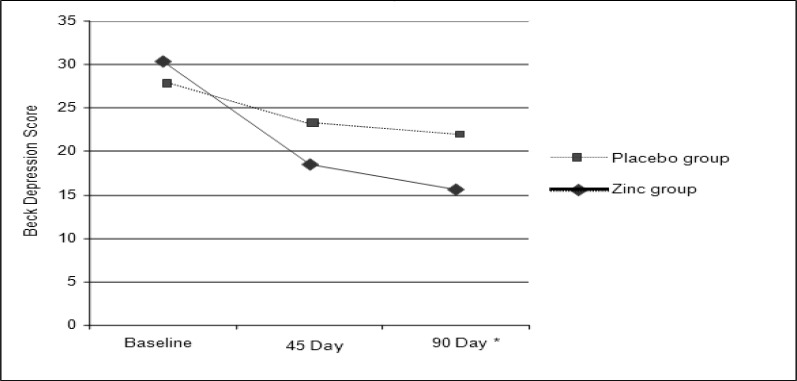
The Beck score decreased in the placebo group, but this reduction was not significant compared to the baseline. Depression scores decreased significantly in the zinc group in the sixth week (P < 0.01), and the twelfth week of the study (P < 0.001) compared to the baseline, but this difference was not statistically significant compared to the 6th and 12th week values.
At the end of the study, Beck scores was significantly lower (p < 0.05)in zinc group compared with the group receiving the placebo. Even after adjusting for the effect of dietary confounding factors, including intake of total fat, saturated fatty acids, MUFA and PUFA fatty acids and magnesium, this difference still remained significant (Figure 2).
Figure 2.
Changes in mean Beck depression scores in the groups receiving zinc compared with placebo using ANOVA with repeated measure. * Statistically significant difference compared with Placebo after 90 day: p < 0.05
Discussion
Zinc is an essential micronutrient which is responsible for the different biological roles. Some of these functions are involvement in some enzymes activation, gene expression, synthesis and release of insulin, and cardiovascular homeostasis. The amount of zinc stored in the adults’ body is 2-3 grams; most of which, is in skeletal muscle and bone. Only 0.1% of the total zinc pool is present in plasma. The plasma levels of zinc are used to evaluate the nutritional status of zinc (10).
In the present study, mean serum zinc concentration in the group receiving zinc supplements increased by 6.5 µg/dL. However, this increment was not significant compared with placebo. Mean serum zinc concentrations at baseline were 100 ±15 and 106 ±17 µg/dl in the zinc and placebo groups, respectively. These amounts were similar to the mean serum zinc concentrations in healthy adults in Shiraz 104 ±18 µg/dL (11), and Ahvaz (7)111 ± 18 µg/dL, respectively. Some studies have shown the low serum zinc levels in depressed patients which are resistant to treatment (12, 13).Other studies have also shown that low serum zinc levels are normalized during treatment with antidepressants (14) which is consistent with our findings. In the present study, the mean dietary zinc intake in patients with major depression was about 6.5-7 mg per day. While in the study of Ghasemi and colleagues, the mean dietary zinc intake in men and women were 15.8 ± 11 and 14.7 ± 11 mg/day, respectively (15). The less zinc intake in these patients may be due to some antidepressants and lack of appetite as one of the symptoms of depression. Serum zinc level was normal in the current study. Therefore, despite the low intakes of zinc, we can infer that the increased serum zinc up to normal levels may be due to antidepressants. The antidepressants may stimulate releasing zinc from the body's stores such as muscles and bones. Also, zinc supplementation on these patients has restored the zinc pools.
Studies on dietary factors and their relation to depression have shown that consumption of meals containing high amounts of carbohydrates cause insulin release. Insulin causes glucose entrance into the cells, and on the other hand make more amino acids such as tryptophan to cross the blood - brain barrier. This may increase neurotransmitter levels, especially serotonin, in the brain which can lead to improved mood (16). Amino acid tryptophan can be converted to serotonin in the body and can cause sleep and mental calmness (17). Tyrosine amino acid can be synthesized from the amino acid phenylalanine and may enter into the biochemical pathways of dopamine and norepinephrine (18). Dietary omega-3 fatty acids are provided from some especial plant and animal sources (especially some marine animals). Omega-3 fatty acids are involved in regulating corticotropin factor, stimulating the serotonergic pathway, preventing neuronal apoptosis, improving blood flow to the brain and regulating gene expression (19). Folate and B12 deficiency are associated with depression. About 10 to 30% of depressed patients have low serum folate levels and their response to antidepressants is weak. Early vitamin B12 deficiency leads to depression. This is due to the reduced synthesis of S-Adenosyl Methionine (20). S-Adenosyl Methionine is associated with mood. The low concentrations in cerebrospinal fluid of depressed patients have been observed and it was found that increasing its plasma concentrations have improved the depressive symptoms (21). So far, clinical trials published in this field have not assessed the mentioned dietary confounding factors. However, the present study was the first experiment which evaluated the effects of dietary confounding factors in patients with depression. As shown in the results, the effects of dietary factors on depression during the study were similar between the two groups. In cases where there was statistical difference between the two groups, the confounding effects were adjusted using ANCOVA.
In the present study, zinc supplementation significantly decreased the Beck depression scores compared with the placebo. Findings of this research were similar to the study by Nowak and colleagues on 20 patients with major depression. Nowak supplemented the antidepressants with 25 milligrams of zinc in one group and the other group was given a placebo with antidepressants. Beck depression scale at baseline and the second, sixth and twelfth weeks were measured. Nowak showed that reduction in Beck scores at week 6 and 12 in the group receiving the supplement were significant compared with placebo (22).
In a study on patients with major depression, Siwek and colleagues gave imipramine combined with 25 mg zinc supplement to one group and placebo combined with imipramine to another group. Siwek findings revealed no significant change in depression scores of the two groups. However, zinc supplementation significantly reduced the rate of depression in patients resistant to medication and may facilitate treatment with antidepressants (12). One of the possible causes of the differences in the results of Siwek study with ours may be due to different type of antidepressant medication prescribed.
Antidepressants may exert their effect through the zinc-containing neurons. Zinc is located in the presynaptic vesicles of neurons of the cortex, amygdala, hippocampus, and spinal cord. These neurons are mainly glutaminergic. The majority of zinc-containing neurons in the spinal cord is gamma-amino butyric acidergic or GAB Aergic and partly Glycinergic; perhaps zinc inhibits glutamate receptors such as N-Methyl-D-Aspartic (NMDA) (23, 24).
Another hypothesis in this field is the role of the protein Brain-Derived Neurotrophic Factor (BDNF). Some recent studies have shown that the BDNF may be involved in mechanism of zinc antidepressant effect. Chronic high doses of zinc increases BDNF gene expression in the cerebral cortex of rats, while a low dose of zinc may increase BDNF gene expression in hippocampus (25, 26).
Limitations
The limited number of patients did not allow subgroups analysis based on type of SSRI drugs.
Conclusion
The results of this study reveal that zinc supplementation combined with antidepressant drugs can be effective in the treatment of patients with major depression.
Acknowledgements
We would like to thank the Center for Neuroscience Research for financial support, Imam Hussein Laboratory personnel, and Endocrine Research Center of Shahid Beheshti University of Medical Sciences.
Recommendations
We suggest evaluating the effects of different doses of zinc on depression in future studies.
Conflict of interest
Authors have nothing to declare.
References
- 1.Kleinman A. Culture and depression. N Engl J Med. 2004;351:951–3. doi: 10.1056/NEJMp048078. [DOI] [PubMed] [Google Scholar]
- 2.Dejman M, Ekblad S, Forouzan AS, Baradaran-Eftekhari M, Malekafzali H. Explanatory Model of Help-Seeking and Coping Mechanisms among Depressed Women in Three Ethnic Groups of Fars, Kurdish, and Turkish in Iran. Archives of Iranian medicine. 2008;11:397–406. [PubMed] [Google Scholar]
- 3.Kaplan H, Sadock B. Synopsis of psychiatry. 8th eds. Williams and Wilkins; 2003. [Google Scholar]
- 4.Fava M, Davidson KG. Definition and Epidemiology of Treatment-Resistant Depression. The Psychiatric clinics of North America. 1996;19:179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 5.Hansen CR, Jr, Malecha M, Mackenzie TB, Kroll J. Copper and Zinc Deficiencies in Association with Depression and Neurological Findings. Biological psychiatry. 1983;18:395–401. [PubMed] [Google Scholar]
- 6.Sandstead HH, Penland JG, Alcock NW, Dayal HH, Chen XC, Li JS, et al. Effects of Repletion with Zinc and Other Micronutrients on Neuropsychologic Performance and Growth of Chinese Children. The American journal of clinical nutrition. 1998;68:470S–475S. doi: 10.1093/ajcn/68.2.470S. [DOI] [PubMed] [Google Scholar]
- 7.Amani R, Saeidi S, Nazari Z, Nematpour S. Correlation between Dietary Zinc Intakes and Its Serum Levels with Depression Scales in Young Female Students. Biological trace element research. 2010;137:150–158. doi: 10.1007/s12011-009-8572-x. [DOI] [PubMed] [Google Scholar]
- 8.McLoughlin IJ, Hodge JS. Zinc in Depressive Disorder. Acta psychiatrica Scandinavica. 1990;82:451–453. doi: 10.1111/j.1600-0447.1990.tb03077.x. [DOI] [PubMed] [Google Scholar]
- 9.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An Inventory for Measuring Depression. Archives of general psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 10.Ghasemi A, Zahediasl S, Hosseini-Esfahani F, Azizi F. Reference Values for Serum Zinc Concentration and Prevalence of Zinc Deficiency in Adult Iranian Subjects. Biological trace element research. 2012;149:307–314. doi: 10.1007/s12011-012-9445-2. [DOI] [PubMed] [Google Scholar]
- 11.Dabbaghmanesh MH, Taheri Boshrooyeh H, Kalantarhormozi MR, Ranjbar Omrani GH. Assessment of Zinc Concentration in Random Samples of the Adult Population in Shiraz, Iran. Iranian Red Crescent medical journal. 2011;13:249–255. [PMC free article] [PubMed] [Google Scholar]
- 12.Siwek M, Dudek D, Schlegel-Zawadzka M, Morawska A, Piekoszewski W, Opoka W, et al. Serum Zinc Level in Depressed Patients During Zinc Supplementation of Imipramine Treatment. Journal of affective disorders. 2010;126:447–452. doi: 10.1016/j.jad.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Schlegel_Zawadzka M, Zieba A, Dudek D. Effect of depression and of antidepressant therapy on serum zinc levels- a preliminary clinical study. New York: 2000. [Google Scholar]
- 14.McLoughlin IJ, Hodge JS. Zinc in Depressive Disorder. Acta psychiatrica Scandinavica. 1990;82:451–453. doi: 10.1111/j.1600-0447.1990.tb03077.x. [DOI] [PubMed] [Google Scholar]
- 15.Ghasemi A, Zahediasl S, Hosseini-Esfahani F, Azizi F. Reference Values for Serum Zinc Concentration and Prevalence of Zinc Deficiency in Adult Iranian Subjects. Biological trace element research. 2012;149:307–314. doi: 10.1007/s12011-012-9445-2. [DOI] [PubMed] [Google Scholar]
- 16.White JW, Wolraich M. Effect of Sugar on Behavior and Mental Performance. The American journal of clinical nutrition. 1995;62:242S–247S. doi: 10.1093/ajcn/62.1.242S. discussion 247S-249S. [DOI] [PubMed] [Google Scholar]
- 17.Buist R. The therapeutic predictability of tryptophan and tyrosine in the treatment of depression. Int J Clin Nutr Rev. 1983;3:1–3. [Google Scholar]
- 18.Kravitz HM, Sabelli HC, Fawcett J. Dietary Supplements of Phenylalanine and Other Amino Acid Precursors of Brain Neuroamines in the Treatment of Depressive Disorders. The Journal of the American Osteopathic Association. 1984;84:119–123. [PubMed] [Google Scholar]
- 19.Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, et al. Omega-3 Fatty Acids: Evidence Basis for Treatment and Future Research in Psychiatry. The Journal of clinical psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 20.Fava M, Borus JS, Alpert JE, Nierenberg AA, Rosenbaum JF, Bottiglieri T. Folate, Vitamin B12, and Homocysteine in Major Depressive Disorder. The American journal of psychiatry. 1997;154:426–428. doi: 10.1176/ajp.154.3.426. [DOI] [PubMed] [Google Scholar]
- 21.Bell KM, Potkin SG, Carreon D, Plon L. S-Adenosylmethionine Blood Levels in Major Depression: Changes with Drug Treatment. Acta neurologica Scandinavica. Supplementum. 1994;154:15–18. doi: 10.1111/j.1600-0404.1994.tb05404.x. [DOI] [PubMed] [Google Scholar]
- 22.Nowak G, Siwek M, Dudek D, Zieba A, Pilc A. Effect of Zinc Supplementation on Antidepressant Therapy in Unipolar Depression: A Preliminary Placebo-Controlled Study. Polish journal of pharmacology. 2003;55:1143–1147. [PubMed] [Google Scholar]
- 23.Poleszak E, Wlaz P, Wrobel A, Fidecka S, Nowak G. Nmda/Glutamate Mechanism of Magnesium-Induced Anxiolytic-Like Behavior in Mice. Pharmacological reports: PR. 2008;60:655–663. [PubMed] [Google Scholar]
- 24.Nowak G, Szewczyk B, Sadlik K, Piekoszewski W, Trela F, Florek E, et al. Reduced Potency of Zinc to Interact with Nmda Receptors in Hippocampal Tissue of Suicide Victims. Polish journal of pharmacology. 2003;55:455–459. [PubMed] [Google Scholar]
- 25.Franco JL, Posser T, Brocardo PS, Trevisan R, Uliano-Silva M, Gabilan NH, et al. Involvement of Glutathione, Erk1/2 Phosphorylation and Bdnf Expression in the Antidepressant-Like Effect of Zinc in Rats. Behavioural brain research. 2008;188:316–323. doi: 10.1016/j.bbr.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Nowak G, Legutko B, Szewczyk B, Papp M, Sanak M, Pilc A. Zinc Treatment Induces Cortical Brain-Derived Neurotrophic Factor Gene Expression. European journal of pharmacology. 2004;492:57–59. doi: 10.1016/j.ejphar.2004.03.038. [DOI] [PubMed] [Google Scholar]