Abstract
Diagnosis of mild traumatic brain injuries (TBIs) has been difficult because of the absence of obvious focal brain lesions, using conventional computed tomography (CT) or magnetic resonance imaging (MRI) scans, in a large percentage of TBIs. One useful measure that can characterize potential tissue and neural network damage objectively is Lempel–Ziv complexity (LZC) applied to magnetoencephalography (MEG) signals. LZC is a model-independent estimator of system complexity that estimates the number of different patterns in a sequence. We hypothesized that because of the potential network damage, TBIs would show a reduced level of complexity in regions that are impaired. We included 18 healthy controls and 18 military veterans with TBI in the study. Resting state MEG data were acquired, and the LZCs were analyzed across the whole brain. Our results indicated reduced complexity in multiple brain areas in TBI patients relative to the healthy controls. In addition, we detected several neuropsychological measures associated with motor responses, visual perception, and memory, correlated with LZC, which likely explains some of the cognitive deficits in TBI patients.
Key words: LZC, MEG, resting state, TBI
Introduction
Mild traumatic brain injury (TBI) is common among military personnel and veterans. Among those who served in Operations Iraqi Freedom and Enduring Freedom (OIF/OEF), an estimated 22.8% had TBI, predominantly classified as mild TBI (mTBI).1 Magnetic resonance imaging (MRI) and computed tomography (CT) are the most common brain imaging tools applied to brain injury diagnosis. However, TBI is often underdiagnosed using these techniques, as long-term dysfunction can occur in the absence of positive findings on either of these modalities. There has been more success using neurophysiological techniques such as magnetoencephalography (MEG). MEG is a noninvasive imaging technique measuring the magnetic fields generated by neuronal activity of the brain. It has been shown to be successful in revealing abnormal brain waves generated in injured brain tissues in TBI patients.2–4 For example, Huang et al. showed that MEG and related source localization methods are able to detect abnormalities in brain areas generating unusual slow brain waves in mTBIs at the rates of 87%.2 The same group also showed that mTBIs exhibit axonal injuries in white matter fibers, by using diffusion tensor imaging (DTI).2 These findings suggest that mTBI leads to neural network damage in both cortical areas and the underlying white matter tracts.
One useful measure that can possibly characterize such neural network damage objectively is Lempel–Ziv complexity (LZC). LZC is proposed by Lempel and Ziv to evaluate the randomness of finite sequences related to the number of distinct substrings and the rate of their occurrence along the sequence.5 In recent years, LZC has been applied to biomedical signal analysis as a metric to estimate the complexity of discrete-time physiologic signals. It has also been applied to MEG and electroencephalography (EEG) signals in studying brain function and brain illnesses. MEG and EEG complexity analyses usually measure the regularity/predictability of brain oscillations and the number of independent oscillators underlying the observed signal.6,7 MEG and EEG complexity have been examined in patients with Alzheimer's disease, epilepsy, schizophrenia, depression, and attention-deficit/hyperactivity disorder (ADHD) and have been suggested as useful measures in the diagnosis of these conditions.8–12
To our knowledge, there has been no examination of LZC of MEG signals in TBIs. It is theorized that a loss of neurons and synapses results in a reduction of complexity of neural network dynamics, such as patients with Alzheimer's disease and the elderly population.13–15 It is also found that brains with fewer, less mature neurons and simpler and less mature neuronal circuits (e.g. a developmental brain) show a lower LZC.16,17 These data suggest that a loss of connections between regions might reduce system dimensionality and, therefore, the complexity level. We therefore hypothesized that, because of potential brain tissue injury and neural connectivity damage in TBIs as revealed in previous studies,2,3,18 we would observe a reduced level of complexity in TBIs.
In summary, in the present study, we compared LZC of MEG signals in military veterans with mTBIs with healthy controls. Moreover, as TBIs are usually associated with an array of abnormality in neuropsychological and neurobehavioral measures, for exploratory purposes, we also performed correlational analyses between LZC and neuropsychological test scores in order to determine if complexity values were related to measures of cognitive ability.
Methods
Participants
Institutional Review Board approval was obtained from Saint Louis University prior to initiation of recruitment and study procedures. All participants were recruited between September 1, 2009 and August 30, 2011. Participants included 36 individuals from the community as part of a larger ongoing prospective study of TBI. Subgroups included 18 healthy controls and 18 military veterans with known or suspected TBI sustained during active service. All TBI participants were>6-months post-injury and were cleared from post-traumatic amnesia (PTA) based on current Galveston Orientation and Amnesia Test (GOAT) scores using a cutoff of ≥75. The control group was recruited from the metropolitan St. Louis region and the military group was recruited from primarily urban areas across the United States. Groups were matched based on gender and age. All participants met the following inclusion criteria: 1) spoke English fluently, 2) was not an undocumented alien, incarcerated, or military personnel on active duty, 3) was ≥18 years of age, 4) had no history of mental retardation, 5) had no preexisting condition that would preclude standard administration of study procedures (deafness or blindness), and 6) gave a valid performance on tests sensitive to suboptimal cognitive effort (Test of Memory Malingering and Word Memory Test). Exclusion criteria for the control group included history of psychiatric illness, neurological illness, substance use disorder, or other cognitive or developmental disorder.
Current level of disability was evaluated using the Community Integration Questionnaire (CIQ)19 and the Disability Rating Scale (DRS).20 Participants were also evaluated for current neurobehavioral symptoms associated with TBI using the Neurobehavioral Symptom Inventory (NSI) 21 as well as for current symptoms of post-traumatic stress disorder (PTSD) using the PTSD Checklist Civilian Version (PCL-C).22 The TBI group showed significantly greater current neurobehavioral symptoms and psychiatric distress (i.e., PTSD symptoms) than did the controls. The clinical characteristics of the sample are displayed in Table 1.
Table 1.
Demographic and Clinical Characteristics of the Sample
| |
Healthy control (n =18) |
TBI (n =18) |
||
|---|---|---|---|---|
| M | SD | M | SD | |
| Age | 30.39 | 9.59 | 29.39 | 5.78 |
| Education | 14.78 | 2.49 | 13.89 | 2.72 |
| Gender | ||||
| Male | 100% (n=18) | 100% (n=18) | ||
| Female | 0 | 0 | ||
| Racea | ||||
| Caucasian | 55.6% (n=10) | 94.4% (n=17) | ||
| African American | 33.3% (n=6) | 5.5% (n=1) | ||
| Asian | 11.1% (n=2) | 0% (n=0) | ||
| M | SD | M | SD | |
|---|---|---|---|---|
| Months post-injury | n/a | n/a | 61.06 | 18.99 |
| CIQ | 21.95 | 3.48 | 18.96 | 5.42 |
| GOAT | 99.94 | .236 | 97.44 | 5.93 |
| NSIb | 4.78 | 6.75 | 34.12 | 16.94 |
| PCL-Cc | 21.67 | 4.56 | 47.82 | 15.41 |
| DRS score | ||||
| 0 | 77.8% (n=14) | |||
| 1 | 5.6% (n=1) | |||
| 2 | 5.6% (n=1) | |||
| 5 | 11.1% (n=2) | |||
| Mechanism of injury | ||||
| Object | 5.6% (n=1) | |||
| Blast injury | 83.3% (n=15) | |||
| MVA | 11.1% (n=2) | |||
| Initial CT scan | ||||
| Postive | 5.6% (n=1) | |||
| Negative | 16.7% (n=3) | |||
| Unknown | 77.8% (n=14) | |||
| Duration loss of consciousness | ||||
| None | 33.3% (n=6) | |||
| 0-30 min | 66.7% (n=12) | |||
| Duration post-traumatic amnesia | ||||
| 0-24 h | 66.7% (n=12) | |||
| >24 h | 33.3% (n=6) | |||
| Injury severity rating | ||||
| Mild | 100% (n=18) | |||
Pearson χ2=7.84, p=0.025.
F=46.28, p<0.001.
F=47.51, p<0.001.
No significant differences were seen between groups on other demographic variables (age, education, and gender) or on CIQ and GOAT scores.
TBI, traumatic brain injury; CIQ, Community Integration Questionnaire; GOAT, Galveston Orientation and Amnesia Test; NSI, Neurobehavioral Symptom Inventory; PCL-C, Post-traumatic Stress Disorder (PTSD) Checklist Civilian Version; DRS, Disability Rating Scale; MVA, motor vehicle accident.
One of the healthy controls was prescribed ropinerole for restless leg syndrome. TBI subjects were screened, but not excluded, for comorbid psychiatric or neurological disorders, including PTSD (n=9), depression (n=2), ADHD (n=2), anxiety disorder (n=1), alcohol abuse (n=1), and migraine headaches (n=1). The TBI literature has suggested a relatively high comorbidity of psychiatric and neurological disorders in patients with TBI,23,24 which is consistent with our data. In the TBI group, 10/18 subjects were prescribed psychoactive medications at the time of participation. Medications included the following: zolpidem (n=2), citalopram (n=1), sertraline (n=2), sumatriptan (n=1), ziprasidone (n=1), dextroamphetamine/amphetamine (n=1), nicotine patch (n=1), buspirone (n=1), diazepam (n=1), buproprion (n=1), prazosin (n=2), hydrocodone/paracetamol (n=1), and oxycodone/paracetamol (n=1).
All participants whose data were used in the analyses were males, and significant differences were not seen between groups for age, level of education, or ethnicity. Demographic characteristics of the sample are displayed in Table 1.
The Defense and Veterans Affairs Consensus Definition of Traumatic Brain Injury25 was utilized for the purposes of this study to classify injuries as mTBI. Injury severity was determined based on agreement between two independent raters (both neuropsychologists with experience with TBI) who reviewed participant self-reports of injury characteristics obtained in interview (duration of loss of consciousness), duration PTA, duration of confusion, initial imaging findings, and initial Glasgow Coma Scale scores. If a discrepancy existed between raters, a consensus was reached based on a collaborative review of the case.
Data acquisition
MEG recordings were acquired with a 248 channel whole-head magnetometer (MAGNES 3600 WH, 4D Neuroimaging, San Diego, CA). Subjects were in an awake, resting state with eyes open, fixated on a crosshair displayed on a semitransparent screen at a distance of 45.72 centimeters from the subject's nose. All recordings were performed in a magnetically shielded room under video and audio monitoring during the recording. Subjects were asked to minimize blinking and movements during the scan. For each subject, 15 min of MEG signal were acquired at a sampling frequency of 678.17 Hz. Empty room MEG signals room were recorded directly prior to the arrival of the subject.
Data analysis
Preprocessing
The 15 min MEG time series were segmented and preprocessed before complexity analyses. The epochs were defined as 5 continuous sec. The preprocessing of the data was done with the Matlab toolbox and Fieldtrip (http://fieldtrip.fcdonders.nl/). The preprocessing procedure included detrending, demeaning, filtering (1–70Hz, 60 Hz line noise) and artifact rejection. Artifact-free time series were downsampled to 250 Hz and input into the complexity analysis algorithm.
LZC analysis
LZC analysis is a form of nonlinear analysis based on a universal lossless data compression algorithm. This process iteratively finds the optimum amplitude clusters in the original signal. With this algorithm, the MEG signal is first transformed into a finite symbol string and then scanned for repeated sequences. The repeated sequences are identified and enumerated during the scan. The enumerated data are then compared with the source signal to generate complexity values, as discussed in the following paragraphs. More complex data result in higher LZC values.
The following describes the LZC analysis procedure in an MEG channel used in this article. First, in each trial, the MEG signals were transformed into a set of finite symbols. In our study, the 0-1 sequence conversion method was used. That is, by contrasting against a threshold Tr, the preprocessed MEG signals in each trial M=m(1), m(2),…, m(i),…, m(n) were converted into a 0-1 sequence B=s(1), s(2),…s(i), s(n) correspondingly. The threshold Tr was set as the median value of the signal's amplitude from each MEG trial, which reduced the effect of outliers.11,20 The s(i) was defined as 0 (if m[i]<Tr), or 1 (if m[i]≥Tr), i=1,2,3…,n. Second, with the algorithm for the measure of the LZC, the string B was then scanned throughout accompanied by a complexity counter, cc(n) which was incremented by one unit each time a new subsequence of consecutive binary bits was encountered in the scanning process.
The detailed algorithm for the measure of the LZC was as follows. 1) Set S and T were to denote two subsequences of the original binary sequence B, ST was to be the concatenation of S and T, whereas STp was to be a string derived from ST after its last symbol (binary bit) was deleted (with p meaning the last symbol being deleted). 2) Set v(STp) was to denote the dictionary (which was initialized to contain the single bit strings corresponding to all the possible input, i.e., [0,1]) of all different subsequences of STp. 3) The complexity counter cc(n)=1,=s(1), T=s(2), ST=s(1)s(2), and STp=s(1) was initialized. 4) It was supposed that S=s(1), s(2), …, s(r), T=s(r+1), and, therefore, STp=s(1), s(2), …, s(r). r was the length of the dictionary, which was a variable. The first symbol in the unprocessed sequence was removed and added to T. If T could be found in v(STp), then T was not a new sequence. 5) If S did not change and renew T to be s(r+1), s(r+2), then it was decided on if T belonged to v(STp) or not. 6) Steps 4 and 5 were repeated until there was no T belonging to v(STp). T=s(r+1), s(r+2), …, s(r+i) was not a subsequence of STp=s(1), s(2), …, s(r+i - 1); therefore, the counter cc(n) was increased by one. After that, S and T were combined and S was renewed to be s(1), s(2), …, s(r+i), and T was renewed to be s(r+i+1). 7) The previous steps were repeated until there was no symbol in the unprocessed sequence. At that point, the number of different substrings was the measure of complexity cc(n). For more details on the LZC algorithm.5,26 All the above algorithms were realized in Matlab.
Normalization and group analysis
In order to make the complexity measure independent of the sequence length n, the LZC scores were normalized by using the following formula: C(n)=cc(n)/(n/logx[n]), where C is the normalized value that is usually between zero and one, cc(n) is the measure of complexity, and x is the number of difference symbols that equals 2 in binary conversion. Therefore, there were a total of 248 LZC scores per subject available for statistical analysis. For both the TBI and the normal control group, the means of the LZC across the whole brain were computed and contrasted with the corresponding empty room LZC using a two sample t test separately. Then another two sample t test was applied to evaluate the LZC difference between the groups. False discovery rate (FDR) corrections were applied to p values for multiple comparisons.
Correlations between LZCs and neuropsychological measures
Participants were administered a neuropsychological testing battery that included the following tests: selected subtests from the Wechsler Adult Intelligence Scale Third Edition (WAIS-III; Digit Symbol Coding, Block Design, Digit Span, Symbol Search, and Letter Number Sequencing); California Verbal Learning Test Second Edition (CVLT-II); Rey Osterrieth Complex Figure Test (ROCF); Trail Making Test Parts A and B (Trails A and Trails B); Stroop Color and Word Test Golden Version (Stroop); Wisconsin Card Sorting Test 64 Card Version (WCST64); Boston Naming Test Second Edition (BNT-2); Continuous Performance Test Second Edition (CPT-2); Wechsler Test of Adult Reading (WTAR); Test of Memory Malingering (TOMM); Word Memory Test (WMT); Verbal Fluency subtest from the Delis Kaplan Executive Functioning System (DKEFS; Verbal Fluency); Repeatable Battery for the Assessment of Neuropsychological Status (RBANS); Automated Neuropsychological Assessment Metrics-4 TBI Battery (ANAM-4 TBI); Grooved Pegboard Test (GPT); and the Finger Tapping Test (FTT). See Appendix for the neuropsychological tests used.
Scores from the TOMM and WMT were used to screen participants for insufficient effort on testing (TOMM Trial 1, Trial 2, and Retention and WMT immediate recognition [IR], delayed recognition [DR], and consistency [CNS]). All participants passed these measures based on established clinical cutoff scores. Raw scores, standard scores (SS), or T scores (T) from the other neuropsychological tests were used for subsequent analyses using neuropsychological data. The resulting data set included 44 neuropsychological values representing the following domains of cognition: global cognitive functioning, attention, language, visual-spatial skills, memory, motor functions, speed of processing, and executive functioning. Univariate t test comparisons were performed between groups on all the 44 neuropsychological measures. To determine if the LZC values from the brain might be correlated with any of the neuropsychological measures we obtained from each subject, Pearson correlations were calculated between neuropsychological test scores and LZC values, which were adjusted by being subtracted by the empty room LZC.
Results
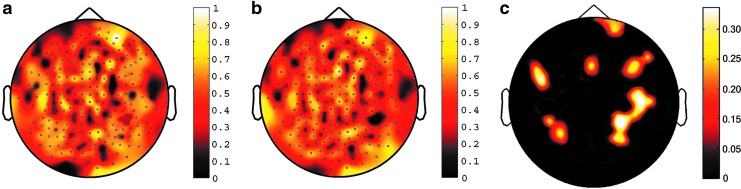
The comparison between LZC of the resting subject and empty room in each group showed significance difference. See Figure 1a for the whole brain mean LZC map in the control group and Figure 1b for the whole brain mean LZC map in the TBI group (both with the baseline of the LZC of empty room MEG, for all channels, p<0.0001). The contrast between the control and the TBI group showed significant difference in multiple brain regions (for all channels p<0.001, and all went through FDR corrections for multiple comparisons with a p<0.05). See Table 2 for statistics of the contrast (normal controls vs. TBIs) and Figure 1c for the LZC contrast map between the two groups. These areas are diffusedly distributed across the brain, including the right anterior frontal area, bilateral frontal area, and bilateral parietal-temporal area.
FIG. 1.
Whole brain LZC maps for the two groups and the contrast between the two groups. (a) Whole brain mean LZC map for the normal control group, p corrected <.0001; (b) Whole brain mean LZC map for the TBI group, p corrected <.0001; (c) Whole brain contrast LZC map: normal control group > TBI group, p corrected <.05. The color bars mark the complexity values.
Table 2.
Statistics of MEG Channels Showing Significant LZC Group Contrast Effect: Normal Control Group> TBI Group
| Channel name | t | p | p corrected |
|---|---|---|---|
| A6 | 2.99 | 0.0061 | 0.027 |
| A77 | 3.41 | 0.0029 | 0.033 |
| A78 | 2.39 | 0.0268 | 0.047 |
| A79 | 3.09 | 0.0041 | 0.014 |
| A81 | 2.77 | 0.0126 | 0.042 |
| A84 | 3.19 | 0.0031 | 0.022 |
| A107 | 2.56 | 0.0115 | 0.039 |
| A111 | 2.35 | 0.0278 | 0.047 |
| A112 | 2.73 | 0.0091 | 0.011 |
| A116 | 2.71 | 0.0110 | 0.021 |
| A129 | 2.82 | 0.0077 | 0.041 |
| A132 | 2.78 | 0.0096 | 0.045 |
| A141 | 2.77 | 0.0125 | 0.049 |
| A151 | 3.59 | 0.0001 | 0.042 |
| A156 | 2.85 | 0.0074 | 0.037 |
| A161 | 3.31 | 0.0023 | 0.014 |
| A175 | 3.10 | 0.0042 | 0.011 |
| A179 | 2.59 | 0.0167 | 0.036 |
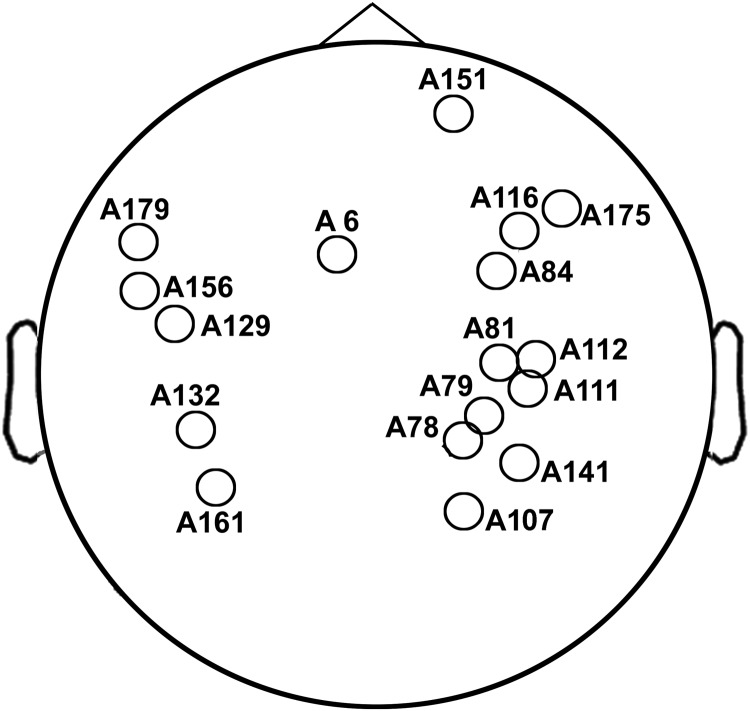
See Fig. 2 for the location of the channels. MEG, magnetoencephalography; LZC, Lempel–Ziv complexity; TBI, traumatic brain injury.
FIG. 2.
Locations of channels indicated in Table 1. These channels show significant LZC group contrast effect: normal control group > TBI group. See Table 1 for details of statistics.
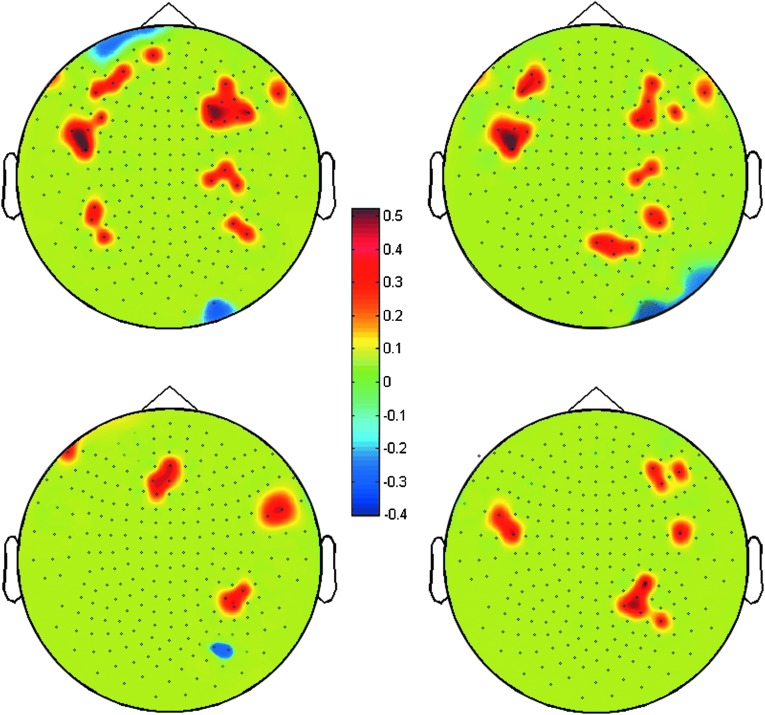
Among the 44 neuropsychological values that were used, 4 significantly correlated with LZC in different brain regions (some areas showing positive and some brain areas showing negative correlations). See Table 3 for these four measurements in the two groups. All significant correlations were at a threshold of p<0.05 (FDR corrected for multiple comparisons) and the magnitude of absolute values of the correlations was>0.4. Neuropsychological measures that significantly correlated included: GPT Dominant Hand T-Score, GPT Non-Dominant Hand T-Score, WCST64 Perseverative Errors T-Score, and the RBANS Visual Spatial Index Standard Score. The correlation results indicated the following. 1) The GPT Dominant Hand T-Score positively correlated with LZC in bilateral frontal, temporal, and parietal regions, and negatively correlated with LZC in the left anterior frontal cortex and the right occipital regions. 2) The GPT Non-Dominant Hand T-Score positively correlated with LZC in the bilateral frontal and temporal and right parietal cortices, and negatively with LZC in the right occipital cortex. 3) The WCST64 Perseverative Errors T-Score correlated positively with LZC in the bilateral frontal, right temporal, and right parietal cortices, and correlated negatively with LZC in the right occipital cortex. 4) the RBANS Visual Spatial Index Standard Score correlated positively with LZC in the right frontal cortex, bilateral temporal cortex, and right parietal cortex. It is of note that both positive and negative correlations were observed between neuropsychological measures and LZC values across varying sensor regions, suggesting both direct and indirect relationships between neural complexity and aspects of neurocognitive functioning. See Figure 3 for the imaging maps showing regions that correlated significantly with each of these measures.
Table 3.
Four of the Neuropsychological Measures in the Two Groups
| GPT Dominant Hand T-Score | GPT Non-Dominant Hand T-Score | WCST64 Perseverative Errors T-Score | RBANS Visual Spatial Index Standard Score | |
|---|---|---|---|---|
| Control | 46.39 | 44.89 | 48.65 | 100.94 |
| SD | 11.83 | 9.81 | 7.10 | 12.31 |
| TBI | 34.94 | 36.00 | 44.94 | 85.50 |
| SD | 7.73 | 8.47 | 12.48 | 14.76 |
| p values | 0.0016 | 0.0063 | 0.2951 | 0.0017 |
Since four of the neuropsychological measures should significant correlations with LZC, we also did a t-test on these four measures between the two subject groups. The results showed that three of them were slightly different.
GPT, Grooved Pegboard Test; WCST64, Wisconsin Card Sorting Test 64 Card Version; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; TBI, traumatic brain injury.
FIG. 3.
Significant correlation between LZC and different neuropsychological scores. (a) GPT Dominant Hand T-Score, (b) GPT Non-Dominant Hand T-Score, (c) WCST64 Perseverative Errors T-Score, and (d) the RBANS Visual Spatial Index Standard Score. The color bars mark the correlation coefficient values: warm colors for positive correlations and cool colors for negative correlations (p corrected <.05; r > .4).
We also calculated t test comparisons between groups on all of the neuropsychological test scores that were used in order to determine if differences existed between groups on these measures. Results yielded significant differences in 5 of the 44 scores. The control group outperformed the TBI group on the following measures: Grooved Pegboard Test Dominant Hand T-Score, Grooved Pegboard Test Non-Dominant Hand T-Score, RBANS Visual-spatial Index, RBANS Language Index, and the ANAM Matching to Sample Throughput Standard Score. It is notable that three of these measures also showed significant correlations with LZC values (GPT Dominant Hand T-Score, GPT Non-Dominant Hand T-Score, and the RBANS Visual-spatial Index). See Table 2 and Figure 3 for a summary of the results of this analysis.
Discussion
LZC is a model-independent estimator of system complexity that estimates the number of different patterns in a sequence. In this study, military personnel with mTBIs and normal controls were contrasted in the LZC MEG measure. We found that compared with normal controls, the TBI group showed a significantly lower level of LZC in diffusedly distributed brain regions, including the right anterior frontal area, bilateral frontal area, and bilateral parietal-temporal area. Moreover, significant correlations were found between LZCs and several neuropsychological measures, including tests of motor coordination and speed, visual-spatial perceptual skills, and reasoning ability.
LZC in TBIs
The decreased complexity values in the MEG signals of TBIs is likely the result of two major factors: injured brain tissues and damaged network connectivity. Even in subjects classified as non-lesional on standard diagnostic imaging, mTBIs have been found to show abnormal brain waves in the delta frequency (1–4Hz),2,3 suggesting that MEG may be more sensitive in detecting subtle abnormality in the temporal domain than conventional imaging methods.2,3 Interestingly, such low frequency abnormalities have often been observed in severe brain injury, stroke, epilepsy and brain tumor patients.4 This suggests that although not detected by MRI or CT, the underlying mechanism for abnormal brain wave in mTBIs might similarly be caused by brain pathophysiology (either related to diseased brain tissue with abnormal metabolism and/or to damaged neural network) such as that seen in several other brain illnesses. The tissue damage might cause loss of neuronal connectivity and thus adversely affect the complex dynamics of neural networks.
Apart from possible tissue injury, it has also been reported that TBI may produce damaged white matter as revealed by reduced anisotropy in DTI.2 White matter fibers serve in linking different gray matter areas. Apart from the number of nodes (analogous to local brain regions), another important feature of a complex system is the number of connections between the nodes. A loss of connections between regions might reduce system dimensionality and, therefore, its complexity level.14 When there are simpler or less mature neuronal circuits such as in an immature brain, an aged brain, and some diseased brains, LZC value is lower than in a healthy adult brain.12–16 The reduced LZC in TBIs in our study, therefore, might be a reflection of the loss of connections between brain regions. Such loss of connection and LZC reduction might be directly associated with various cognitive and behavioral deficits, which was shown on our correlation analysis between LZC and neuropsychological measures.
The brain locations showing reduced LZC are rather spread out, which is expected, given that blast injury is indicated as the greatest source of injury accounting for the majority of TBIs sustained by service members.27,28 Bilateral frontal and parietal regions are particularly affected. This might explain a number of attention, cognitive control, and memory deficits indexed by neuropsychological measures in the TBI group. Even more noteworthy is the frontal area: the functional deficits associated with mTBI bear significant resemblance to those in focal damage in the frontal lobe,29 and in the present study, frontal LZC shows a high incidence of correlations with high level cognitive function measures.
Correlations between LZC and neuropsychological measures
Interestingly, we found that LZC significantly correlated with GPT Dominant Hand T-Score, GPT Non-Dominant Hand T-Score, WCST64 Perseverative Errors T-Score, and the RBANS Visual Spatial Index Standard Score. LZC values, however, did not show significant correlations with other neuropsychological tests that were utilized. These findings indicated that there were some cognitive and behavioral constructs, including motor speed and dexterity, visual-spatial perception, and reasoning abilities, that may be related to levels of LZC. Motor functions, visual perception, and reasoning abilities are known to be relatively complex abilities requiring coordination of many distributed cortical and subcortical brain regions. The fact that these abilities were correlated with LZC values makes sense, given that they likely require considerable coordinated brain activity for proper execution. As both positive and negative correlations were observed, it is reasonable to suggest that neural complexity has both direct and indirect relationships with behavioral and cognitive functioning. This may be reflective of the fact that both excitatory and inhibitory neural pathways are often necessary to accurately execute complex cognitive and behavioral tasks. Also, lower levels of brain LZC values may provide evidence for neuropathology, which may explain the functional cognitive deficits reported by many individuals with mTBI, which often involves disruptions of both excitatory and inhibitory neural pathways.
However, it is necessary to state that these results are preliminary, and that their implications are still unclear. Additionally, we examined a relatively small number of participants in this study using neuropsychological tests. Further research should use larger numbers of participants to focus specifically on the relationship between cognitive functioning and measures of LZC in the brain, in order to draw more precise conclusions. Another limitation of the present study is that we did not control for the effects of the medication in our TBI subjects. However, we believe that drug effects on the LZC measure in TBI are important, and would like to examine them systematically in future studies.
Our military veteran sample also had a high number of PTSD symptoms at the time of evaluation, which may have implications for interpretation of our findings. The relationship between PTSD and mTBI in military veterans has been a significant issue with military veterans from recent combat in Iraq and Afghanistan, and symptom overlap between these conditions has clouded diagnostic decisions.23 PTSD has also been associated with dysfunction in frontal and limbic brain regions in functional MRI (fMRI) studies, as well as with impairments on neuropsychological testing.30 Future research is necessary in order to better understand the impact of PTSD on symptom presentation and brain functioning in individuals with TBI. It would be anticipated that PTSD and TBI might have distinct patterns of change using a technique such as LZC analysis of MEG data. Those with TBI might show reduced cortical complexity resulting from brain injury, whereas those with PTSD might present with increased cortical complexity causd by upregulation of neural activity related to hypervigilance/increased arousal seen in this condition.
Conclusion
Our results indicate reduced complexity in multiple brain area in TBI. This is likely the result of impairment of brain tissues or network connection. From a localization standpoint, the TBI participants showed a wide spatial distribution of reduced complexity, which is likely the result of injuries caused mostly by blasts causing damages in a more diffuse manner. In addition, we detected that several neuropsychological measures associated with motor responses, visual perception, and memory correlate with LZC, which likely explains some of the cognitive deficits in TBI.
Appendix: Neuropsychological Tests Used in the Study
1. Reeves, D., Kanes, R., and Winter, L. (1996). Automated Neuropsychological Assessment Metrics (ANAM V3) User's Manual. National Cognitive Foundation: San Diego.
2. Kaplan, E.F., Goodglass, H., and Weintraub, S. (1983). The Boston Naming Test, 2nd ed. Lea and Febiger: Philadelphia.
3. Delis, D.C., Kramer, J.H., Kaplan, E., and Ober, B.A. (2000). California Verbal Learning Test Manual, 2nd ed., Adult Version. Psychological Corporation: San Antonio.
4. Conners, C.K., and Multi-Health Systems Staff (2000). Conners' Continuous Performance Test II: Computer Program for Windows Technical Guide and Software Manual. Multi-Health Systems: North Tonwanda, NY.
5. Delis, D.C. (2001). Delis–Kaplan Executive Function System: D-KEFS. Psychological Corporation: Orlando.
6. Matthews, C.G., and Klove, H. (1964). Instruction Manual for the Adult Neuropsychology Test Battery. University of Wisconsin Medical School: Madison.
7. Dobie, D.J., Kivlahan, D.R., Maynard, C., Bush, K.R., McFall, M.E., Epler, A.J., et al. (2002). Screening for post-traumatic stress disorder in female Veteran's Affairs patients: validation of the PTSD Checklist. Gen. Hosp. Psychiatry 24, 367–374.
8. Randolph, C. (1998). Repeat Battery for the Assessment of Neuropsychological Status Manual. Psychological Corporation: San Antonio.
9. Meyers, J.E., and Meyers, K.R. (1999). Rey Complex Figure Test and Recognition Trial: Professional Manual. Psychological Assessment Resources: Odessa, FL.
10. Tombaugh, T. (1996). Test of Memory Malingering Manual. Multi-Health Systems: New York.
11. Reitan, R.M. (1992). Trail Making Test Manual for Administration and Scoring. Reitan Neuropsychology Laboratory: Tucson.
12. Wechsler, D. (1997). WAIS-III Administration and Scoring Manual. The Psychological Corporation: San Antonio.
13. Wechsler, D. (2001). Wechsler Test of Adult Reading (WTAR) Manual. Psychological Corporation: New York.
14. Heaton, R.K. (1993). Wisconsin Card Sorting Test Manual. Psychological Assessment Resources: Odessa, FL.
15. Green, P. Allen, L. M., and Astner, K. (1996). The Word Memory Test: A User's Guide to the Oral and Computer-Administered Forms. US Version 1.1. CogniSyst: Durham, NC.
Acknowledgments
The study is supported by the Department of Defense grant W81XWH-08-2-0191 granted to Dr R. Bucholz.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Terrio H. Brenner L.A. Ivins B. J. Cho J.M. Helmick K. Schwab K. Scally K. Bretthauer R. Warden D. Traumatic brain injury screening: preliminary findings in a US army brigade combatteam. J. Head Trauma Rehabil. 2009;24:14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- 2.Huang M.X. Theilmann R.J. Robb A. Angeles A. Nichols S. Drake A. D'Andrea J. Levy M. Holland M. Song T. Ge S. Hwang E. Yoo K. Cui L. Baker D.G. Trauner D. Coimbra R. Lee R.R. An automatic MEG low-frequency source imaging approach for detecting injuries in mild and moderate TBI patients with blast and non-blast causes. J. Neurotrauma. 2009;26:1213–1226. doi: 10.1016/j.neuroimage.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Huang M.X. Nichols S. Robb A. Angeles A. Drake A. Holland M. Asmussen S. D'Andrea J. Chun W. Levy M. Cui L. Song T. Baker D.G. Hammer P. McLay R. Theilmann R.J. Coimbra R. Diwakar M. Boyd C. Neff J. Liu T.T. Webb–Murphy J. Farinpour R. Cheung C. Harrington D.L. Heister D. Lee R.R. An automatic MEG low-frequency source imaging approach for detecting injuries in mild and moderate TBI patients with blast and non-blast causes. Neuroimage. 2012;61:1067–1082. doi: 10.1016/j.neuroimage.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Lewine J. D. Davis J.T. Sloan J.H. Kodituwakku P. W. Orrison W.W., Jr. Neuromagnetic assessment of pathophysiologic brain activity induced by minor head trauma. AJNR Am. J. Neuroradiol. 1999;20:857–866. [PMC free article] [PubMed] [Google Scholar]
- 5.Lempel A. Ziv J. On the complexity of finite sequences. IEEE Trans. Inf. Theory. 1976;22:75–81. [Google Scholar]
- 6.Lutzenberger W. Preissl H. Pulvermuller F. Fractal dimension of electroencephalographic time series and underlying brain processes. Biol. Cybern. 1995;73:477–482. doi: 10.1007/BF00201482. [DOI] [PubMed] [Google Scholar]
- 7.Jeong J. Kim D.J. Chae J.H. Kim S.Y. Ko H.J. Paik I.H. Nonlinear analysis of the EEG of schizophrenics with optimal embedding dimension. Med. Eng. Phys. 1998;20:669–676. doi: 10.1016/s1350-4533(98)00078-2. [DOI] [PubMed] [Google Scholar]
- 8.Abasolo D. Hornero R. Espino P. Alvarez D. Poza J. Entropy analysis of the EEG background activity in Alzheimer's disease patients. Physiol. Meas. 2006;27:241–53. doi: 10.1088/0967-3334/27/3/003. [DOI] [PubMed] [Google Scholar]
- 9.Radhakrishnan N. Gangadhar B.N. Estimating regularity in epileptic seizure time-series data. IEEE Eng. Med. Biol. 1998;17:89–94. doi: 10.1109/51.677174. [DOI] [PubMed] [Google Scholar]
- 10.Fernández A. Quintero J. Hornero R. Zuluaga P. Navas M. Gómez C. Escudero J. Garcia–Campos N. Biederman J. Ortiz T. Complexity analysis of spontaneous brain activity in attention-deficit/hyperactivity disorder: diagnostic implications. Biol. Psychiatry. 2009;65:571–577. doi: 10.1016/j.biopsych.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 11.Fernández A. López–Ibor M.I. Turrero A. Santos J.M. Morón M.D. Hornero R. Gómez C. Méndez M.A. Ortiz T. López–Ibor J.J. Lempel–Ziv complexity in schizophrenia: a MEG study. Clin. Neurophysiol. 2011;122:2227–2235. doi: 10.1016/j.clinph.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Méndez M.A. Zuluaga P. Hornero R. Go´mez C. Escudero J. Rodríguez–Palancas A. Ortiz T. Ferna´ndez A. Complexity analysis of spontaneous brain activity: effects of depression and antidepressant treatment. J. Psychopharmacol. 2012;5:636–643. doi: 10.1177/0269881111408966. [DOI] [PubMed] [Google Scholar]
- 13.Jelles B. van Birgelen J.H. Slaets J.P.J. Hekster R.E.M. Jonkman E.J. Stam C.J. Decrease of non-linear structure in the EEG of Alzheimer patients compared to healthy controls. Clin. Neurophysiol. 1999;110:1159–1167. doi: 10.1016/s1388-2457(99)00013-9. [DOI] [PubMed] [Google Scholar]
- 14.Jeong J. EEG dynamics in patients with Alzheimer's disease. Clin. Neurophysiol. 2004;115:1490–505. doi: 10.1016/j.clinph.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Goldberger A.L. Peng C.K. Lipsitz L.A. What is physiologic complexity and how does it change with aging and disease? Neurobiol. Aging. 2002;23:23–26. doi: 10.1016/s0197-4580(01)00266-4. [DOI] [PubMed] [Google Scholar]
- 16.Anokhin A.P. Lutzenberger W. Nikolaev A. Birbaumer N. Complexity of electrocortical dynamics in children: developmental aspects. Dev. Psychobiol. 2000;36:9–22. [PubMed] [Google Scholar]
- 17.Meyer–Lindenberg A. The evolution of complexity in human brain development: An EEG study. Electroencephalogr. Clin. Neurophysiol. 1996;99:405–411. doi: 10.1016/s0013-4694(96)95699-0. [DOI] [PubMed] [Google Scholar]
- 18.Mayer A.R. Mannell M.V. Ling J. Gasparovic C. Yeo R.A. Functional connectivity in mild traumatic brain injury. Hum. Brain Mapp. 2011;32:1825–1835. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willer B. Rosenthal M. Kreutzer J.S. Gordon W.A. Rempel R. Assessment of community integration following rehabilitation for traumatic brain injury. J. Head Trauma Rehabil. 1993;8:75–87. [Google Scholar]
- 20.Rappaport M. Hall K.M. Hopkins H.K., et al. Disability rating scale for severe head trauma: coma to community. Arch. Phys. Med. Rehabil. 1982;63:118–123. [PubMed] [Google Scholar]
- 21.Cicerone K.D. Kalmar K. Persistent postconcussion syndrome: the structure of subjective complaints after mild traumatic brain injury. J. Head Trauma Rehabil. 1995;10:1–17. [Google Scholar]
- 22.Weathers F.W. Litz B.T. Herman D.S. Huska J.A. Keane T.M. The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Presented at the 9th Annual Conference of the ISTSS; San Antonio, Texas. 1993. [Google Scholar]
- 23.Brenner L.A. Vanderploeg R.D. Terrio H. Assessment and diagnosis of mild traumatic brain injury, posttraumatic stress disorder, and other polytrauma conditions: burden of adversity hypothesis. Rehabil. Psychol. 2009;54:239–246. doi: 10.1037/a0016908. [DOI] [PubMed] [Google Scholar]
- 24.Iverson G.L. Outcome from mild traumatic brain injury. Curr. Opin. Psychiatry. 2005;18:301–317. doi: 10.1097/01.yco.0000165601.29047.ae. [DOI] [PubMed] [Google Scholar]
- 25.Department of Veterans Affairs and Department of Defense. VA/DOD clinical practice guideline for management of concussion/mild traumatic brain injury. 2009. http://www.healthquality.va.giv/mtbi/concussion_mtbi_full_1_0.pdf. [Aug 17;2012 ]. http://www.healthquality.va.giv/mtbi/concussion_mtbi_full_1_0.pdf
- 26.Zhang X.S. Roy R.J. Jensen E.W. EEG complexity as a measure of depth of anesthesia for patients. IEEE Trans. Biomed. Eng. 2001;48:1424–1433. doi: 10.1109/10.966601. [DOI] [PubMed] [Google Scholar]
- 27.Warden D.L. Ryan L.M. Helick K.M. Schwab K. French L. Lu W. War neurotrauma: the DVBI experience at Walter Reed Army Medical Center. J. Neurotrauma. 2005;22:1178. [Google Scholar]
- 28.Benzinger T.L. Brody D. Cardin S. Curley K.C. Mintun M.A. Mun S.K. Wong K.H. Wrathall J.R. Blast-related brain injury: imaging for clinical and research applications: report of the 2008 St Louis workshop. J. Neurotrauma. 2009;26:2127–2144. doi: 10.1089/neu.2009.0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuss D.T. Gow C.A. Frontal dysfunction after traumatic brain injury. Neuropsychiatry Neuropsychol. Behav. Neurol. 1992;5:272–282. [Google Scholar]
- 30.Dolan S. Martindale S. Robinson J., et al. Neuropsychological sequelae of PTSD and TBI following war deployment among OEF/OIF veterans. Neuropsychol. Rev. 2012;22:21–34. doi: 10.1007/s11065-012-9190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]