Abstract
The alterations of animal behavior after traumatic brain injury (TBI) can be subtle, and their quantitative characterization can present significant methodological challenges. Meeting these challenges is a critical need, because quantitative measures are required in studies that compare the efficacy of different clinical interventions. We developed a battery of assessments to quantify behavioral, motor, and cognitive changes in neonatal piglets with good sensitivity and specificity to the detection of persistent deficits that correlate with axonal injury severity after a rapid non-impact head rotation with a diffuse pattern of axonal injury. The battery of measures developed included open field behaviors of sniffing and moving a toy, locomotion measures of Lempel-Ziv complexity and the probability of remaining in the current location, and a novel metric for evaluating motor performance. Our composite porcine disability score was able to detect brain injury with a sensitivity of 100% and specificity of 85.7% at day +4 post-injury for n=8 injured and n=7 sham piglets and significantly correlated with the percent axonal injury in these animals (day +4: ρ=0.76, p=0.0011). A significant improvement over our previous assessments, this new porcine disability score has potential use in a wide variety of porcine disease and injury models.
Key words: cognition, neurobehavioral assessment, pediatric brain injury, porcine, traumatic brain injury
Introduction
Pediatric traumatic brain injury (TBI) is a leading cause of death and disability in children in the United States. Of these injuries, children age 0–4 years have the highest rates of emergency department visits (1,256 per 100,000), hospitalization (76 per 100,000), and death (5 per 100,000) annually.1,2 Further, children who experience moderate to severe TBI in early childhood are subject to long-term cognitive and, to a lesser extent, motor impairment.3–8 Because of the maturation-dependent response of the brain to TBI, it is necessary to use an appropriately age-matched immature animal model in the development of effective clinical interventions for pediatric TBI.9–11 An immature porcine model is growing in prevalence as a tool in brain injury research because its tissue composition, gyrencephalic structure, and developmental growth and myelination resemble an immature human brain.12–19 There are limited porcine behavioral and motor outcome measures, however, and few have been correlated with lesion volume from TBI.
A composite cognitive dysfunction (CCD) score has been proposed previously for evaluating the neonatal piglet post-TBI.20 When this previous CCD score was applied to a second data set investigating a TBI intervention, however, no significant differences were detected between untreated injured and uninjured animals,21 revealing a possible lack of sensitivity for widespread use. This necessitated the development of more sensitive metrics for neurocognitive assessment in TBI. Sensitive measures of motor and cognition in piglets with TBI will facilitate the use of the piglet model to evaluate effectiveness of potential clinical interventions for TBI, as well as other neuropathological conditions.
Methods
The Institutional Animal Care and Use Committee of the University of Pennsylvania approved all protocols. Only positive conditioning with milk replacer as a reward was conducted, and no aversive conditioning was used.
Acclimation and pre-injury testing
Five litters of female, 3 to 5 day old, Yorkshire piglets with an average body weight of 2.6 kg were studied in groups of three to four littermates per group. Littermates were housed together throughout the duration of the study. Two days before injury (day −2), piglets were allowed to freely explore an empty test space (1.2 m×2.4 m) with a bowl of milk replacer (Littermilk, Land O Lakes, Arden Hills, MN) in the center for 1 hour. During this time, piglets became acclimated to the testing environment, the research staff, and the food bowl. Then a balance beam was introduced into the test space (1.2 m long, 23 cm wide, and 10 cm off the ground). Piglets were individually trained to cross the beam to a bowl containing 1 mL of milk replacer until they demonstrated proficiency at this task.
One day before injury (day −1), piglets underwent a full day of neurobehavioral testing, including the Open Field, T-maze, and Inclined Beam Tasks described below, to establish an individual baseline for behavior analysis.
Injury
On study day 0, two to three piglets from each litter were randomly designated to the injury group (n=11) and the other one to two piglets in the group were assigned to be instrumented shams (n=8). All piglets were anesthetized with 4% isoflurane via a snout mask. After the pinch reflex was extinguished, animals were intubated with a 3.0 mm endotracheal tube, and buprenorphine (0.02 mg/kg intramuscularly) was administered for analgesia. Animals were continuously monitored for end tidal CO2, oxygen saturation, heart rate, and core body temperature (Surgivet V9204, Smiths Medical, Dublin, OH) until extubation post-injury or sham, and animals were ventilated as needed (Hallowell AWS, 1–3% isoflurane, Hallowell EMC, Pittsfield, MA). Injured animals underwent a rapid non-impact head rotation in the sagittal plane via a HYGE pneumatic actuator system (described previously22,23). Average peak angular velocities were 149±9 rad/sec (mean±standard deviation) for injured animals, and peak angular accelerations were 57,935±4,225 rad/sec2. The rotation was consistently 58 degrees with the center of rotation in the cervical spine. Sham animals were placed into the actuator system, but did not undergo a head rotation.
Behavioral and functional tests
Open Field, T-maze, and Inclined Beam behavioral testing were conducted 1 day before injury and on days +1 and +4 after injury. Animals were fasted for 2 h before testing and weighed to monitor growth. The three assessments occurred in the same order each testing day, but the testing order of the piglets varied. While one piglet was tested, the remaining littermates were placed in individual pet carriers in a separate room. Testing was recorded from above via camera and saved to DVD to be scored later by a blinded evaluator.
Open Field
The Open Field methods of this study have been described in depth previously.24 Briefly, on each testing day, each animal was individually placed in the center of a 1.2 m×2.4 m pen with a single toy (a 19 cm diameter blue ball) immediately to their right. The animals were allowed to freely explore the space for 10 min. A set of common piglet behaviors were tracked for presence or absence during each minute-long epoch of the test, and were recorded as the number of epochs during which the behavior was present. The behaviors assessed were: sniffing floor, walls, or toy; running, walking, or standing still for more than 1 sec; laying down; moving the toy; and attempts to escape the test space.
To evaluate piglet locomotion and patterns of space usage, the open field was divided into a grid of nine equally sized zones, and the location of the piglet's snout within the open field was recorded at 2-sec time intervals. This resulted in a 300-character-long position sequence. This zone position sequence was evaluated by two measures adapted from symbolic dynamics: PDIAG and normalized Lempel-Ziv complexity, which are also described in detail elsewhere.24 In summary, PDIAG is a measure between 0 and 1 that describes how stationary the motion of the piglet is, with 0 indicating that the piglet moved at every time point and 1 indicating that the piglet was completely stationary. Lempel-Ziv complexity is a measure between 0 and 1 that describes how random the sequence of zone visits is, with low values indicating a highly patterned sequence of zone visits and high values indicating that the sequence of zone visits is completely random.
T-maze
A modified T-maze test for piglets based on work by Bolhuis and associates25 and Friess and colleagues20 was conducted on each testing day. The T-maze assessment consisted of training, normal, and reversal trials, which are defined presently. The first two groups of piglets did not undergo T-maze normal and reversal trials on day −1, but all other piglets experienced all the trials on each testing day. The maze consisted of two arms, each containing a food bowl visible only after the animal has fully committed to that arm of the maze. Behind each arm of the maze was a bowl of milk replacer, never visible to the animal, so that olfactory cues were the same on both sides of the maze.
Unlike the previous studies that used a T-maze, a visual discrimination element was added for all training and testing trials. Each arm of the maze contained an image visible to the animal at the “T” of the maze (Fig. 1). The arm containing the food reward was always marked with an image of three large black dots (7 cm diameter), while the other arm was marked with an image of a single large black dot (7 cm diameter), similar to the radial arm maze markings in the 2007 study by Wang and coworkers.26 During the training phase of the T-maze, the animal was first shown to the arm with no food (arm B) and then to the arm with food (arm A) and allowed to eat. Then for 10 consecutive trials, the animal was released from the starting area and given a maximum time of 60 sec to locate the food reward. If the animal did not locate the reward after 60 sec, it was shown to the reward location and allowed to eat. Time to reward and number of errors (each time the piglet moved to a zone further from the reward than its current zone) were recorded. A piglet “passed” a training trial if it was completed with no errors and a time to reward of less than 15 sec. A training pass rate was defined at the number of passed trials over the total number of training trials.
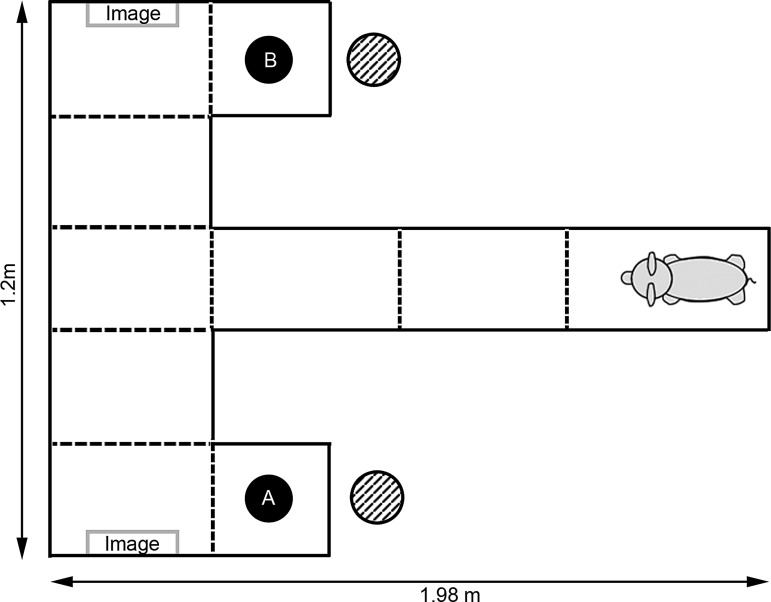
FIG. 1.
Diagram of T-maze testing setup. During training and normal trials, food was found in arm A. During reversal trials, food was moved to arm B. Hash-marked circles indicate position of bowls of milk replacer outside of the maze that were used to mask olfactory cues. Dotted lines indicate zone markings.
After each animal from the litter group underwent T-maze training, they were brought back one at a time for five normal trials, in which the food remained in arm A and the piglet had a maximum of 3 min to locate the food reward. During normal trials, no correction to locate the reward was provided on failure. Again, time to reward and number of errors were obtained.
Immediately after the normal trials, the food reward was switched to arm B, and six reversal trials were performed. Piglets were given a maximum time of 5 min to locate the food reward, and no correction was applied on failure. Time to reward, number of errors, and time spent at the original food site (arm A) were recorded.
In addition, as the last task on the last day of testing, the piglets were placed in the Open Field test space with no toys. The images of one and three large black dots were attached opposite one another to the center of the 1.2 m long walls, and each piglet was allowed to explore freely for 1 min (the Dot Test). The number of times a piglet nudged each image with its snout was recorded.
Inclined Beam
Motor performance was assessed by having piglets walk on an inclined balance beam. Each piglet was placed at the end of a 1.2 m long and 23 cm wide beam inclined at an approximately 20-degree angle and was given a maximum of 20 sec to walk up the beam to a food reward at the elevated end for five consecutive trials. Walls were positioned alongside, but not touching, the inclined beam to ensure the piglets would not fall off completely if they stumbled or slipped. Time to reward was recorded for each trial. In addition, for each testing day, piglets were given one motor proficiency score (MPS) according to the rubric in Table 1. To receive an MPS, the piglet must have walked at least halfway up the beam on at least two beam trials. Each trial in which the piglet made it at least halfway up the beam counted as a “run” for determining the MPS, whether or not they reached the food reward. A gait abnormality on this scale constitutes any foot slips off the beam and slipping or splaying of any leg(s) even if all feet remained on the beam.
Table 1.
Motor Proficiency Score Rubric
| Motor proficiency score (MPS) | Beam performance |
|---|---|
| 4 | Piglet had no gait abnormalities on all runs. |
| 3 | Piglet had a single gait abnormality over all runs. |
| 2 | Piglet had more than one gait abnormality, but not on every run. |
| 1 | Piglet had at least one gait abnormality on every run. |
| 0 | Piglet had more than one gait abnormality on every run. |
To assess the repeatability of this motor scoring method, an interrater reliability study was conducted. Five raters were trained to score the Inclined Beam Test by watching a standardized video of 10 beam trials that illustrated several examples of a steady run, a single slip on a run, more than one slip on a run, and trials that did not constitute a run. These 10 trials did not encompass any five-trial set from the same pig. The interrater reliability was then assessed using the intraclass correlation coefficient.
Pathology
All animals were euthanized 6 days post-injury to evaluate axonal injury. Animals were anesthetized with 4% isoflurane via snout mask. An intravenous sodium pentobarbital overdose was administered once a pinch reflex was absent. Brain tissue was then fixed by transcardiac perfusion using 2 L physiologic saline followed by 3 L 10% unbuffered formalin (Spectrum Chemical, Gardena, CA). Brains were removed, post-fixed at room temperature for 1 week, and then stored in 1X phosphate buffered saline. The fixed brains were sliced into 15–18 3-mm thick coronal sections spanning the entire cerebrum, cerebellum, and brain stem, and each section was photographed. After routine processing, each coronal section was embedded in paraffin wax and 6-μm thick slices were cut for microscopic evaluation. Slices were immunostained with an antibody for β-amyloid precursor protein (β-APP) (1:10,000; clone 22C11; Chemicon), and lightly counterstained with Meyer hematoxylin to mark axonal injury. The entire area of each of the 15–18 coronal sections were then examined blind by a neuropathologist (C.S.) at a scanning power of 5–10x magnification, with specific locations examined at 20–40x magnification. Locations of axonal injury were marked on the digital photographs of the coronal sections.
Each photographed section was traced and the area was calculated in Adobe Photoshop. The total brain area was found by summing the areas of each section. Similarly, regions of axonal injury were traced and the area measured. The percent axonal injury for each animal was the sum of the areas of all the injured regions divided by the area of the total brain.
Construction of a composite porcine disability score
Our goal was to create a single behavioral score that correlates behavioral dysfunction with neuropathology and can be used to assess cognitive and motor function over time. For this reason, we devised a porcine disability score (PDS) using an iterative method that considered measures from each of the different behavioral tests. Metrics were considered for inclusion in the PDS if they exhibited a significant injury effect via analysis by a two-way repeated measures analysis of variance (ANOVA) (as described in the statistical analysis section). All other metrics were evaluated for inclusion by first subtracting the sham and injured means and dividing by the sham standard deviation for each study day (average injured z-score). Metrics in which the average injured z-score was at least one greater than in pre-injury testing on one post-injury day were also considered for inclusion. These are metrics with small overlap between injured and sham values on post-injury days compared with the pre-injury overlap. The eleven metrics chosen from this phase were number of epochs spent sniffing the walls, sniffing the toy, and moving the toy; PDIAG and Lempel-Ziv complexity in the Open Field task; the MPS and beam time in the Inclined Beam task; and the T-maze normal trial time, normal trial errors, reversal trial time, and time spent at the old food location.
For each behavioral metric and study day, the sham average and standard deviation was calculated. Each animal then received a z-score for that metric calculated by normalizing the difference between the individual animal's performance and the sham mean by the sham standard deviation, such that a positive z-score indicated dysfunction on that task.
Positive z-scores were given for individual results that were below the sham mean for sniffing the walls, sniffing the toy, moving the toy, MPS, and T-maze training pass rate, and above the sham mean for beam time, T-maze normal trial time, normal trial errors, and reversal trial time. For PDIAG and Lempel-Ziv complexity variation above or below the sham mean resulted in a positive z-score. If no value was available for various reasons, (e.g., animals that did not qualify to receive an MPS), then they were given a z-score of 0 for that measure, which is the assumption that they were at the sham mean. The composite score for an animal was then determined by summing the z-score for each behavioral measure to be included in the composite PDS.20
To determine which combination of these 11 metrics was best able to characterize the longer term deficits of injured animals, the composite score was calculated for every possible grouping of metrics, allowing for a cluster of 1, 2, or even all 11 of the metrics, and amounting to 2047 possible groupings. Assigning animals as either injured or sham, each of the 2047 groupings were evaluated using a receiver operating characteristic (ROC) curve analysis27 to identify which grouping provided the best sensitivity and specificity between injured and sham. The area under the ROC curve (AUC) was used to evaluate the quality, where an area of 1 indicates perfect sensitivity and specificity and an area of 0.5 indicates that the metric grouping cannot distinguish injured from sham. Metric groupings with a ROC AUC of greater than 0.63 on day −1 (pre-injury) were eliminated from consideration to remove metrics that might incorporate group bias present before injury. Finally, the optimal grouping was the one with the highest AUC on day +4. A PDS cutoff value at which peak sensitivity and specificity occurred was also extracted in the ROC analysis. Animals with a PDS above this cutoff value on day +1 or +4 would be considered to have displayed disability.
Statistical analysis
Data for each neurobehavioral measure were analyzed for group (injured or sham) and day (+1 or +4) effects and interactions using a two-way ANOVA for repeated measures. This was implemented using a mixed effects model with group and day as the fixed effects and subject nested within group as the random effect. Data were log transformed to improve symmetry when necessary. Post hoc analysis was conducted using the Tukey-Kramer method. Significance was defined as p<0.05, and all results were reported as mean±standard error unless otherwise noted.
Correlation between the composite score and AI was evaluated using a Pearson correlation coefficient with accompanying 95% confidence interval. The pairwise correlation coefficients between each of the 11 metrics considered for inclusion in the composite PDS were also calculated. The correlations were deemed to be significant if the confidence interval did not span zero.
Results
Mortality
Of the 11 animals that underwent a rapid head rotation on day 0 of the study, three had to be sacrificed within hours of injury. These animals never fully regained consciousness after the injury and on necropsy had large subdural hematomas. The range of peak angular velocities for these animals was 139–158 rad/sec, which is within the same range as the animals that survived the duration of the study (138–160 rad/sec). In addition, eight animals were designated as instrumented shams. One sham animal was found deceased in the housing facility the morning of study day +1. This animal arrived with low body weight and poor circulation and did not recover well from anesthesia despite attempts to treat with subcutaneous saline and supplemental oral feedings. All results and analysis presented include only animals that survived the duration of the study (n=8 injured and n=7 sham).
Open Field
The results of the Open Field testing for this group of animals have been reported in detail previously for the presentation of new analysis metrics.24 Briefly, injured piglets were less interested in interacting with their environment and had a lower activity level than shams. There were significant injury effects in both sniffing and moving the toy, with injured animals spending fewer epochs on these behaviors than shams (p<0.01 for both). Further, injured piglets were more stationary than shams as indicated by a significant difference in PDIAG (p=0.04). Lempel-Ziv complexity showed no significant injury effects, but there was a trend to less random motion on day +1 in injured animals.
T-maze
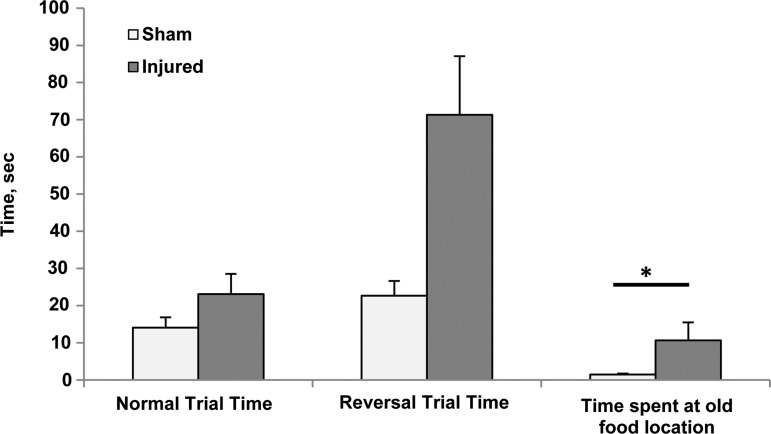
Injured piglets demonstrated difficulties with reversal learning or relearning on this task. During the T-maze reversal trials, the time spent at the old food location was significantly higher in the injured piglets (p=0.013) (Fig. 2). While it did not reach significance, injured piglets also took longer to reach the reward in the reversal trials than shams (p=0.054). Further studies with a larger sample size would be needed to determine if this trend becomes significant. T-maze errors and training trial measures did not show any significant effects or trends. One injured animal was lethargic during the T-maze on day +1 and did not leave the starting area on any of the training trials. To reduce testing stress on this piglet, the normal and reversal trials were not conducted, and she was eliminated from day +1 analysis.
FIG. 2.
T-maze data from both post-injury days combined. Normal trial time, p=0.4542, reversal trial time, p=0.0541, time spent at old food location, p=0.0125. Mean and standard error shown.
Differences in visual discrimination evaluated during the Dot Test did not reach statistical significance with this sample size. The sham piglets did, however, nudge the image of the 3 dots, which indicated food during the T-maze, a greater number of times on average than the injured piglets (sham: 2.8±0.94; injured: 1±0.46; p=0.087).
Inclined Beam
The interrater reliability study showed near-perfect agreement regarding which animals could be given an MPS; only one rater disagreed on one session. There were 45 total inclined beam sessions (15 piglets over three testing days), and 6 of these did not receive a score. Five were excluded because the pig did not meet the scoring criteria of going at least halfway up the beam on two separate trials as scored by a majority of the five raters. One was excluded because of a recording error that resulted in the loss of the data. The MPS awarded by the five raters also showed good agreement (intraclass correlation coefficient for average measures=0.911 and Cronbach alpha=0.927). For all subsequent analyses the average of the five raters' scores was used.
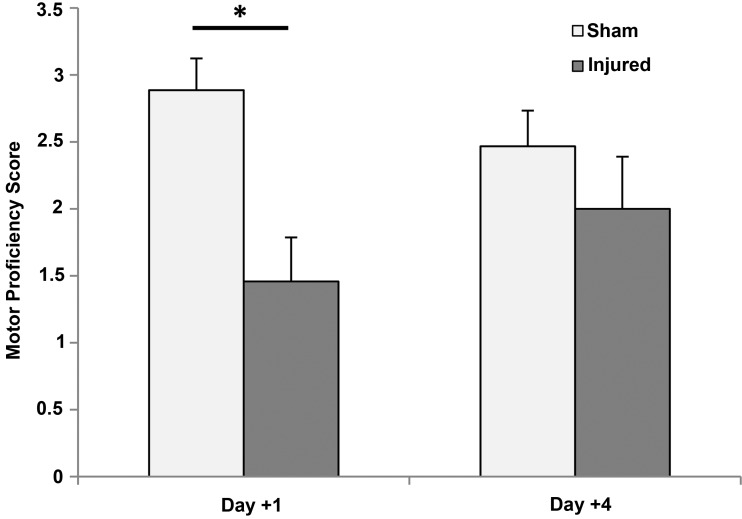
The Inclined Beam task demonstrated that injured piglets had acute motor deficits that improved over time. On day +1, the MPS for injured piglets was significantly lower than for sham piglets (p=0.02), but by day +4 there was no significant difference with injury (p=0.75) (Fig. 3). There were not significant differences in the time to reward on the inclined beam between injured and sham animals (sham: day 1: 9.8±1.6, day 4: 7.4±1.3; injured: day 1: 12.7±1.7 day 4: 11.1±2.0, p=0.089).
FIG. 3.
Motor proficiency scores for injured and sham animals on each study day. Day +1, p=0.021 and Day +4, p=0.75. Mean and standard error shown.
Porcine disability score
The analysis of the pairwise correlations between each of the 11 metrics considered for inclusion in the PDS revealed two sets of metrics that were significantly correlated across different behavioral tests, while the remaining correlations were between metrics evaluated within the same behavioral test (Table 2). The highest correlation between metrics from two different tests was between the number of epochs spent sniffing the toy during the Open Field and the time spent visiting the old food location during T-maze reversal trials, such that a larger number of epochs spent sniffing the toy indicated less time visiting the old food location (ρ=− 0.58, p=0.001). The second was between the number of epochs spent moving the toy during the open field and the MPS evaluated during the Inclined Beam task, such that an increase in the number of epochs spent moving the toy was coupled with higher motor proficiency (ρ=0.39, p=0.035). While one might postulate the Open Field measure of locomotion (PDIAG and Lempel-Ziv complexity) are also measures of motor function, there were no significant correlations between either of the locomotion measures and the MPS or with the number of epochs spent moving the toys. As expected, metrics from the same behavioral test were more likely to be significantly correlated. The two most highly correlated metrics within one assessment were the T-maze normal trial time and normal trial number of errors (ρ=0.78, p<0.0001) and the T-maze normal and reversal trial times (ρ=0.75, p<0.0001).
Table 2.
Pearson Correlation Coefficients between Each of the Eleven Metrics Considered for Inclusion in the Porcine Disability Score
| Sniffing walls | Sniffing toy | Moving toy | PDIAG | Lempel-ziv complexity | MPS | Beam time | T-maze normal time | T-maze normal errors | T-maze reversal time | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sniffing Toy | 0.28 | |||||||||
| Moving Toy | −0.04 | 0.74a | ||||||||
| PDIAG | −0.30 | −0.50b | −0.29 | |||||||
| Lempel-Ziv Complexity | 0.65a | 0.45c | 0.03 | −0.72a | ||||||
| MPS | 0.10 | 0.15 | 0.39c | −0.23 | 0.11 | |||||
| Beam Time | −0.15 | −0.20 | −0.29 | 0.11 | −0.09 | −0.39c | ||||
| T-Maze Normal Time | −0.26 | −0.13 | −0.09 | 0.12 | −0.33 | −0.16 | 0.28 | |||
| T-Maze Normal Errors | −0.09 | 0.33 | 0.33 | −0.23 | −0.14 | 0.14 | 0.01 | 0.78a | ||
| T-Maze Reversal Time | −0.34 | −0.33 | −0.28 | 0.28 | −0.28 | −0.25 | 0.22 | 0.75a | 0.34 | |
| T-Maze Time At Old Food | −0.42 | −0.58b | −0.32 | 0.40 | −0.31 | −0.10 | 0.23 | 0.21 | −0.15 | 0.63b |
Highlighted cells indicate significant correlations.
p<0.0001; bp<0.01; cp<0.05.
MPS, motor proficiency score.
Evaluating the 2047 possible groups of these 11 metrics yielded a list of combinations of metrics able to differentiate injured and sham animals on day +4 post-injury ranked by AUC. Table 3 gives the top performing 12 groups of metrics ranked by day +4 ROC AUC. All 12 had good discrimination between injured and sham animals on day +4 post-injury, with ROC AUCs between 0.93 and 0.96. The grouping determined to be the optimal PDS (grouping A) is comprised of the cumulative z-scores of the number of epochs spent sniffing toy and moving toy, Lempel-Ziv complexity, PDIAG, and MPS, which had a day +4 ROC AUC of 0.96. Grouping B incorporated measures from just the Open Field and Inclined Beam tests; thus, its use would allow an investigator to minimize the number of assessments and testing time. Grouping C and others incorporated measures from the T-maze test, which may make them more sensitive to cognitive dysfunction.
Table 3.
Top 12 Combinations of Metrics Ranked by Day +4 Area under the Curve for Distinguishing between Injured and Sham Animals, the Corresponding Receiver Operating Characteristic Area under the Curve for Days −1, +1, and +4, the Optimal Cutoff Value for Dividing Injured and Sham Groups on Day +4 and the Day +4 Sensitivity and Specificity Using that Cutoff Value
| |
|
Day −1 |
Day +1 |
Day +4 |
|||
|---|---|---|---|---|---|---|---|
| Metrics included | AUC | AUC | AUC | Cutoff | Sensitivity | Specificity | |
| A | Sniffing Toy, Moving Toy, Lempel-Ziv Complexity, PDIAG, MPS | 0.63 | 0.95 | 0.96 | 2.5 | 100.0% | 85.7% |
| B | Moving Toy, PDIAG, MPS | 0.63 | 0.96 | 0.95 | 2.8 | 75.0% | 100.0% |
| C | Sniffing Toy, Moving Toy, Lempel-Ziv Complexity, T-maze Normal Time, T-maze Time at Old Food Location | 0.59 | 0.93 | 0.95 | 3.5 | 75.0% | 100.0% |
| D | Sniffing Toy, Moving Toy, Lempel-Ziv Complexity, PDIAG, T-maze Normal Errors, MPS | 0.63 | 0.84 | 0.95 | 5.1 | 75.0% | 100.0% |
| E | Moving Toy, Lempel-Ziv Complexity, PDIAG, T-maze Time at old food location, MPS | 0.61 | 1.00 | 0.93 | 4.6 | 75.0% | 100.0% |
| F | Sniffing Toy, Moving Toy, Lempel-Ziv Complexity, PDIAG, T-maze Normal Time, T-maze Reversal Time, T-maze Time at old food location | 0.63 | 0.93 | 0.93 | 5.8 | 75.0% | 100.0% |
| G | Sniffing Toy, Moving Toy, Lempel-Ziv Complexity, PDIAG, Beam Time, MPS | 0.63 | 0.93 | 0.93 | 5.9 | 75.0% | 100.0% |
| H | Sniffing Toy, Moving Toy, Lempel-Ziv Complexity, T-maze Reversal Time, Beam Time | 0.61 | 0.93 | 0.93 | 4.3 | 75.0% | 100.0% |
| I | Sniffing Toy, Moving Toy, MPS | 0.61 | 0.93 | 0.93 | 1.1 | 87.5% | 85.7% |
| J | Sniffing Toy, Moving Toy, Lempel-Ziv Complexity, PDIAG, T-maze Normal Time, MPS | 0.61 | 0.89 | 0.93 | 5.7 | 75.0% | 100.0% |
| K | Sniffing Toy, Moving Toy, Lempel-Ziv Complexity, PDIAG, T-maze Reversal Time, MPS | 0.61 | 0.89 | 0.93 | 6.6 | 75.0% | 100.0% |
| L | Moving Toy, Lempel-Ziv Complexity, T-maze Reversal Time | 0.61 | 0.88 | 0.93 | 2.6 | 75.0% | 100.0% |
AUC, area under the curve; MPS, motor proficiency score.
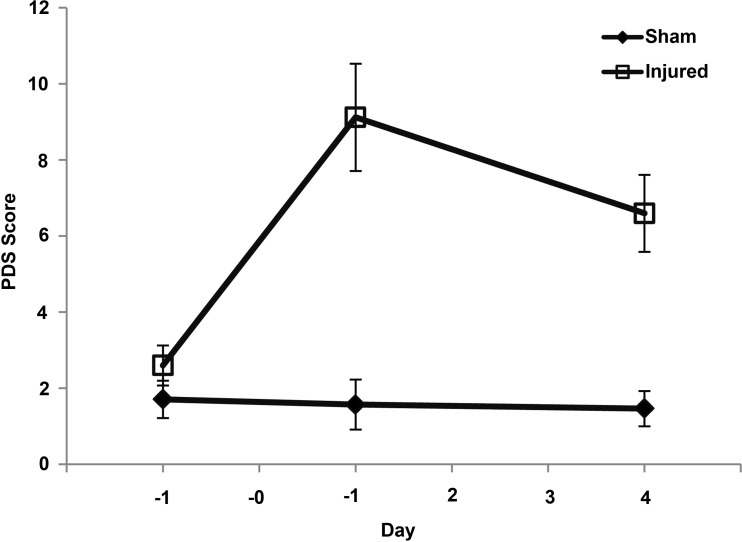
The optimal PDS had values ranging from 1.7 to 18.8 on day +1 and 2.6 to 11.8 on day +4 for injured animals. Sham PDS on post-injury days ranged from −0.5 to 6.9 (Fig. 4). The ROC analysis gave a cutoff PDS value of 2.5 as a point above which PDS scores would be indicative of disability. This cutoff value yielded good group discrimination (day +1 sensitivity: 87.5% and specificity: 85.7%; day +4 sensitivity: 100% and specificity: 85.7%).
FIG. 4.
Porcine disability score values for injured and sham animals across each study day. Mean and standard error shown.
Correlation to pathology
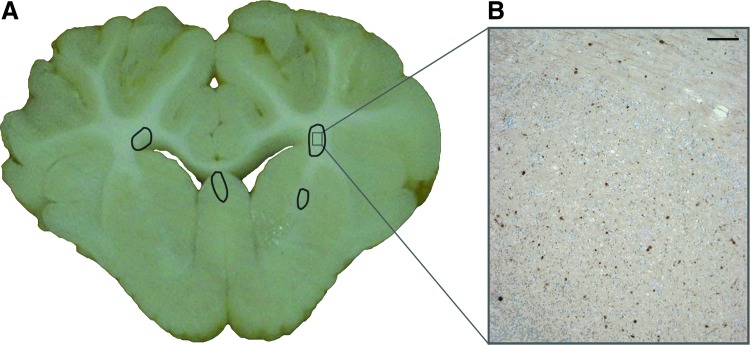
The neuropathology results showed that all injured animals had areas of axonal injury on study day +6 (average±standard error: 0.814%±0.05%). These areas were diffusely spread throughout the coronal brain slices and clearly visible as areas of dark β-APP staining on microscopic evaluation (Fig. 5). The day +1 and day +4 PDS significantly correlated to the percent axonal injury (day +1: ρ=0.68, p=0.0056 and day +4: ρ=0.76, p=0.0011). It is unsurprising that day +4 correlated more strongly than day +1, because the PDS score was optimized to distinguish injury at this time point.
FIG. 5.
(A) Representative coronal brain slice from injured animal on study day +6 with areas of axonal injury circled. (B) Representative micrograph corresponding to the marked area on the coronal slice image showing areas of β-amyloid precursor protein positive immunostaining (scale bar=100 μm). Color image is available online at www.liebertpub.com/neu
Discussion
The use of pigs in neuroscience continues to increase because of their similarity to humans in tissue composition, gyrencephalic structure, and developmental growth and myelination patterns.12–19 The immature porcine model of TBI used in this study has shown many similar findings to those observed in infant TBI,23,28 which makes it a particularly good candidate for translational research. There are, however, limited well-validated behavioral tests for the piglet model, and existing metrics used in the rodent model are not insightful in pigs because of their very different responses to behavioral stimuli.13–15,29 Because we are interested in developing interventions for pediatric TBI that improve long-term neurological function, we have developed robust behavioral, cognitive, and motor assessments that are sensitive, yet straightforward enough to translate to other laboratories.
The decrease in Open Field activity levels of injured piglets has been discussed in detail previously.24 It is important to ask, however, if this decrease may be confounded by motor deficits subsequently observed in the injured group. The lack of significant correlations between MPS and the locomotion measures of PDIAG and Lempel-Ziv complexity indicates that the decreased locomotion is not merely a manifestation of motor deficits, but likely stems instead from a cognitive source.
On the T-maze assessment, sham pigs spent significantly less time at the old food location in the reversal task, which shows difficulties with reversal learning after brain injury. Sham piglets also had more interaction with the three-dot image during the Dot Test than injured animals, although this did not reach significance. A previous study with a similar T-maze setup but without the dots as an additional cue did not find significant differences in the time spent at the old food location between sham and injured piglets.20 This previous result coupled with the Dot Test results seem to indicate that the sham piglets in the present study were able to use the dot images as a cue to improve their ability to solve the maze, and thus spend less time at the old food location, but that the injured animals were not. Other studies have demonstrated that neonatal piglets are able to learn a radial arm maze based on visual discrimination alone,26,30 further supporting the argument that the sham animals were capable of using the visual cues to their advantage in this T-maze.13,17
Motor deficits are an important characteristic of pediatric TBI and an indicator of recovery. Many motor function scales are currently used in children (Gross Motor Function Measure, Peabody Development Scales, Bruininks-Oseretsky Test of Motor Proficiency, Functional Reach Test),7,8,31–33 making the use of a motor proficiency scale in piglets an appropriate translational tool. The Inclined Beam task and accompanying MPS were developed to quantify motor deficits that had only been observed qualitatively in post-TBI piglets previously.
While motor function measures, such as rotarod,34 rope and ladder climbing,35 and postural reflex test,36 are commonly used in mice and rats, there is a paucity of such measures for pigs. Previous studies have used a flat beam as a test of motor function, but have not revealed deficits in pigs post-TBI on this simpler task.21,37 Kuluz and coworkers38 have developed a 10-point Porcine Walking Scale for assessment of motor deficits after spinal cord injury. Tanaka and colleagues39 also have motor and gait sections on their Neurological Examination Grading Scale for the miniature pig developed for assessing injury after stroke. Because these injury types produce much more severe motor deficits than TBI, however, all of the injured piglets in this study would have ranked in the top one to two categories, making these scales too coarse to detect the more subtle deficits seen in post-TBI piglets. Our novel motor skills metric was sensitive enough to detect transient motor deficits in the injury group with a few injured animals continuing to show motor deficits on day +4, while most had recovered function. The use of the MPS was also shown to be robust across raters through the interrater reliability study.
While we found injury effects for a variety of measures from each behavioral test and some showed significance between injured and sham animals during the acute phase (day +1 post-injury), only one (moving toys in open field) showed significant persistent differences between injured and sham groups (day +4 after injury). Because the goal was to assess subtle changes in cognition at a later time point post-TBI, we created an optimized battery of measures, the composite PDS. The measures included in the optimal PDS spanned deficits in motor function, exploratory behaviors, and locomotion (both inactivity and pattern of space usage), and it showed small but significant persistent disabilities in injured animals. The optimal PDS on day +4 also had a significant correlation with percent axonal injury (ρ=0.76). In addition, we have presented other grouping of metrics that were also able to adequately discriminate between injured and sham animals. Some of these groupings incorporate more overt measures of learning and memory from the T-maze task, which may be preferable for some investigators. All of the groupings presented span measures from at least two of the three behavioral tests. The minimum number of metrics in the top 12 groupings was three, which could be of interest if minimizing the number of tests and measures for cost and time reasons was a priority in future studies.
We have previously presented a CCD score for neonatal piglets that also correlated with percent axonal injury (ρ=0.79)20 in axial plane injured animals. We have also previously shown that both the time-course of recovery and the behavioral outcomes vary depending on the rotational plane of injury.24 Using only the metrics in this current experiment that were included in our previous CCD score, the older CCD measure had a very poor correlation with injury severity (ρ=0.24), and the ROC analysis showed that this metric was unable to distinguish between injured and sham animals on day +4 post-injury (ROC AUC=0.57). This bolsters our findings that the plane of injury affects the behavioral outcomes and necessitates the development of this new composite PDS for quantifying subtle changes in behavior post-injury in the sagittal plane of rotation.
In presenting these data, we would make the following observations. First, this battery of behavioral tests has not yet been prospectively validated by another group of post-TBI piglets with a sagittal plane injury. The fact that a previously proposed composite score did not bear out with this dataset reinforces the importance of prospectively testing this PDS scoring system for robustness.
Second, it is likely that the composite PDS we have developed is specific to neonatal piglets, and both the assessments themselves and the measures indicative of behavioral deficits post-TBI would need to be altered for older pigs, which would be larger and have different motivation levels. For example, larger pigs might experience more fear going up an incline than smaller younger pigs, and thus be less likely to participate in such a task as the Inclined Beam. Our anecdotal experience also suggests that older pigs need longer acclimation times and tend to be less motivated by food rewards.
Finally, because of the diffuse nature of a rapid rotational head injury, it is impossible to localize for which region of brain injury this score is sensitive. This may limit translation of the PDS to focal neurological injury type (e.g., stroke, controlled cortical impact).
Conclusion
We have developed a novel battery of cognitive, motor, and behavioral assessments for the neonatal piglet that have enhanced sensitivity compared with our previous metrics.20,37 The behavioral assessment technologies developed here may have applications that extend beyond the study of TBI to stroke and resuscitation research and could be used in the evaluation of new interventions and therapies.
Acknowledgments
The authors would like to acknowledge Benjamin Bruins, Erica Hummel, James Butler, and Sohaib Hashmi for their contributions in conducting and rating the assessments. This study was made possible by the support of NIH/NINDS grants R01NS039679, U01NS069545, and K08NS064051. PER would like to acknowledge support of the Traumatic Injury Research Program of the Uniformed Services University of the Health Sciences and the Defense Medical Research and Development Program. The opinions and assertions contained herein are the private opinions of the authors and are not to be construed as official or reflecting the views of their respective commands, the U. S. Navy or the Department of Defense.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Langlois J.A. Rutland-Brown W. Thomas K.E. The incidence of traumatic brain injury among children in the United States: differences by race. J. Head Trauma Rehabil. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Faul M. Xu L. Wald M.M. Coronado V.G. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2010. 2002–2006. [Google Scholar]
- 3.Anderson V. Catroppa C. Morse S. Haritou F. Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005;116:1374–1382. doi: 10.1542/peds.2004-1728. [DOI] [PubMed] [Google Scholar]
- 4.Anderson V. Godfrey C. Rosenfeld J.V. Catroppa C. Predictors of cognitive function and recovery 10 years after traumatic brain injury in young children. Pediatrics. 2012;129:e254–261. doi: 10.1542/peds.2011-0311. [DOI] [PubMed] [Google Scholar]
- 5.Keenan H.T. Bratton S.L. Epidemiology and outcomes of pediatric traumatic brain injury. Dev. Neurosci. 2006;28:256–263. doi: 10.1159/000094152. [DOI] [PubMed] [Google Scholar]
- 6.Rossi C. Sullivan S.J. Motor fitness in children and adolescents with traumatic brain injury. Arch. Phys. Med. Rehabil. 1996;77:1062–1065. doi: 10.1016/s0003-9993(96)90069-6. [DOI] [PubMed] [Google Scholar]
- 7.Kuhtz-Buschbeck J.P. Hoppe B. Golge M. Dreesmann M. Damm-Stunitz U. Ritz A. Sensorimotor recovery in children after traumatic brain injury: analyses of gait, gross motor, and fine motor skills. Dev. Med. Child Neurol. 2003;45:821–828. doi: 10.1017/s001216220300152x. [DOI] [PubMed] [Google Scholar]
- 8.Katz-Leurer M. Rotem H. Lewitus H. Keren O. Meyer S. Relationship between balance abilities and gait characteristics in children with post-traumatic brain injury. Brain Inj. 2008;22:153–159. doi: 10.1080/02699050801895399. [DOI] [PubMed] [Google Scholar]
- 9.Durham S.R. Duhaime A.C. Basic science; maturation-dependent response of the immature brain to experimental subdural hematoma. J. Neurotrauma. 2007;24:5–14. doi: 10.1089/neu.2006.0054. [DOI] [PubMed] [Google Scholar]
- 10.Duhaime A.C. Margulies S.S. Durham S.R. O'Rourke M.M. Golden J.A. Marwaha S. Raghupathi R. Maturation-dependent response of the piglet brain to scaled cortical impact. J. Neurosurg. 2000;93:455–462. doi: 10.3171/jns.2000.93.3.0455. [DOI] [PubMed] [Google Scholar]
- 11.Armstead W.M. Kurth C.D. Different cerebral hemodynamic responses following fluid percussion brain injury in the newborn and juvenile pig. J. Neurotrauma. 1994;11:487–497. doi: 10.1089/neu.1994.11.487. [DOI] [PubMed] [Google Scholar]
- 12.Hagberg H. Ichord R. Palmer C. Yager J.Y. Vannucci S.J. Animal models of developmental brain injury: relevance to human disease. A summary of the panel discussion from the Third Hershey Conference on Developmental Cerebral Blood Flow and Metabolism. Dev. Neurosci. 2002;24:364–366. doi: 10.1159/000069040. [DOI] [PubMed] [Google Scholar]
- 13.Lind N.M. Moustgaard A. Jelsing J. Vajta G. Cumming P. Hansen A.K. The use of pigs in neuroscience: modeling brain disorders. Neurosci. Biobehav. Rev. 2007;31:728–751. doi: 10.1016/j.neubiorev.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Gieling E.T. Schuurman T. Nordquist R.E. van der Staay F.J. The pig as a model animal for studying cognition and neurobehavioral disorders. Curr. Top. Behav. Neurosci. 2011;7:359–383. doi: 10.1007/7854_2010_112. [DOI] [PubMed] [Google Scholar]
- 15.Kornum B.R. Knudsen G.M. Cognitive testing of pigs (Sus scrofa) in translational biobehavioral research. Neurosci. Biobehav. Rev. 2011;35:437–451. doi: 10.1016/j.neubiorev.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Dobbing J. Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 17.Fang M. Li J. Gong X. Antonio G. Lee F. Kwong W.H. Wai S.M. Yew D.T. Myelination of the pig's brain: a correlated MRI and histological study. Neurosignals. 2005;14:102–108. doi: 10.1159/000086292. [DOI] [PubMed] [Google Scholar]
- 18.Flynn T.J. Developmental changes of myelin-related lipids in brain of miniature swine. Neurochem. Res. 1984;9:935–945. doi: 10.1007/BF00964525. [DOI] [PubMed] [Google Scholar]
- 19.Conrad M.S. Dilger R.N. Johnson R.W. Brain growth of the domestic pig (Sus scrofa) from 2 to 24 weeks of age: a longitudinal MRI study. Dev. Neurosci. 2012;34:291–298. doi: 10.1159/000339311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friess S.H. Ichord R.N. Ralston J. Ryall K. Helfaer M.A. Smith C. Margulies S.S. Repeated traumatic brain injury affects composite cognitive function in piglets. J. Neurotrauma. 2009;26:1111–1121. doi: 10.1089/neu.2008.0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naim M.Y. Friess S. Smith C. Ralston J. Ryall K. Helfaer M.A. Margulies S.S. Folic acid enhances early functional recovery in a piglet model of pediatric head injury. Dev. Neurosci. 2010;32:466–479. doi: 10.1159/000322448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eucker S.A. Smith C. Ralston J. Friess S.H. Margulies S.S. Physiological and histopathological responses following closed rotational head injury depend on direction of head motion. Exp. Neurol. 2011;227:79–88. doi: 10.1016/j.expneurol.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raghupathi R. Margulies S.S. Traumatic axonal injury after closed head injury in the neonatal pig. J. Neurotrauma. 2002;19:843–853. doi: 10.1089/08977150260190438. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan S. Friess S.H. Ralston J. Smith C. Propert K.J. Rapp P.E. Margulies S.S. Behavioral deficits and axonal injury persistence following rotational head injury are direction dependent. J. Neurotrauma. 2013;30:538–545. doi: 10.1089/neu.2012.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolhuis J.E. Schouten W.G. de Leeuw J.A. Schrama J.W. Wiegant V.M. Individual coping characteristics, rearing conditions and behavioural flexibility in pigs. Behav. Brain Res. 2004;152:351–360. doi: 10.1016/j.bbr.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Wang B. Yu B. Karim M. Hu H. Sun Y. McGreevy P. Petocz P. Held S. Brand-Miller J. Dietary sialic acid supplementation improves learning and memory in piglets. Am. J. Clin. Nutr. 2007;85:561–569. doi: 10.1093/ajcn/85.2.561. [DOI] [PubMed] [Google Scholar]
- 27.Metz C.E. Basic principles of ROC analysis. Semin. Nucl. Med. 1978;8:283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 28.Duhaime A.C. Large animal models of traumatic injury to the immature brain. Dev. Neurosci. 2006;28:380–387. doi: 10.1159/000094164. [DOI] [PubMed] [Google Scholar]
- 29.Gieling E.T. Nordquist R.E. van der Staay F.J. Assessing learning and memory in pigs. Anim. Cogn. 2011;14:151–173. doi: 10.1007/s10071-010-0364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dilger R.N. Johnson R.W. Behavioral assessment of cognitive function using a translational neonatal piglet model. Brain Behav. Immun. 2010;24:1156–1165. doi: 10.1016/j.bbi.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Wallen M.A. Mackay S. Duff S.M. McCartney L.C. O'Flaherty S J. Upper-limb function in Australian children with traumatic brain injury: A controlled, prospective study. Arch. Phys. Med. Rehabil. 2001;82:642–649. doi: 10.1053/apmr.2001.22620. [DOI] [PubMed] [Google Scholar]
- 32.Kuhtz-Buschbeck J.P. Stolze H. Golge M. Ritz A. Analyses of gait, reaching, and grasping in children after traumatic brain injury. Arch. Phys. Med. Rehabil. 2003;84:424–430. doi: 10.1053/apmr.2003.50017. [DOI] [PubMed] [Google Scholar]
- 33.Linder-Lucht M. Othmer V. Walther M. Vry J. Michaelis U. Stein S. Weissenmayer H. Korinthenberg R. Mall V. Validation of the Gross Motor Function Measure for use in children and adolescents with traumatic brain injuries. Pediatrics. 2007;120:e880–886. doi: 10.1542/peds.2006-2258. [DOI] [PubMed] [Google Scholar]
- 34.Hamm R.J. Pike B.R. O'Dell D.M. Lyeth B.G. Jenkins L.W. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J. Neurotrauma. 1994;11:187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- 35.Ding Y. Yao B. Lai Q. McAllister J.P. Impaired motor learning and diffuse axonal damage in motor and visual systems of the rat following traumatic brain injury. Neurol. Res. 2001;23:193–202. doi: 10.1179/016164101101198334. [DOI] [PubMed] [Google Scholar]
- 36.Bona E. Johansson B.B. Hagberg H. Sensorimotor function and neuropathology five to six weeks after hypoxia-ischemia in seven-day-old rats. Pediatr. Res. 1997;42:678–683. doi: 10.1203/00006450-199711000-00021. [DOI] [PubMed] [Google Scholar]
- 37.Friess S.H. Ichord R.N. Owens K. Ralston J. Rizol R. Overall K.L. Smith C. Helfaer M.A. Margulies S.S. Neurobehavioral functional deficits following closed head injury in the neonatal pig. Exp. Neurol. 2007;204:234–243. doi: 10.1016/j.expneurol.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuluz J. Samdani A. Benglis D. Gonzalez-Brito M. Solano J.P. Ramirez M.A. Luqman A. De los Santos R. Hutchinson D. Nares M. Padgett K. He D. Huang T. Levi A. Betz R. Dietrich D. Pediatric spinal cord injury in infant piglets: description of a new large animal model and review of the literature. J. Spinal Cord Med. 2010;33:43–57. doi: 10.1080/10790268.2010.11689673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka Y. Imai H. Konno K. Miyagishima T. Kubota C. Puentes S. Aoki T. Hata H. Takata K. Yoshimoto Y. Saito N. Experimental model of lacunar infarction in the gyrencephalic brain of the miniature pig: neurological assessment and histological, immunohistochemical, and physiological evaluation of dynamic corticospinal tract deformation. Stroke. 2008;39:205–212. doi: 10.1161/STROKEAHA.107.489906. [DOI] [PubMed] [Google Scholar]