Abstract
Eye contact captures attention and receives prioritized visual processing. Here we asked whether eye contact might be processed outside conscious awareness. Faces with direct and averted gaze were rendered invisible using interocular suppression. In two experiments we found that faces with direct gaze overcame such suppression more rapidly than faces with averted gaze. Control experiments ruled out the influence of low-level stimulus differences and differential response criteria. These results indicate an enhanced unconscious representation of direct gaze, enabling the automatic and rapid detection of other individuals making eye contact with the observer.
Keywords: Eye contact, gaze processing, binocular rivalry, interocular suppression, unconscious processing
1. Introduction
Eye contact is a salient visual signal for a large number of species. In many vertebrates, the rapid perception of eye contact supports the effective detection of potential predators (Emery, 2000). By contrast, in human and non-human primates eye contact is a pivotal element in complex social behavior and therefore receives privileged visual processing and modulates cognitive processes. For example, humans orient to eye contact preferentially (e.g., von Grünau & Anston, 1995; Senju & Hasegawa, 2005; Senju, Hasegawa, & Tojo, 2005a), even in the first few days of their lives (Farroni, Csibra, Simion, & Johnson, 2002). This innate capability to detect eye contact lays the foundation for the later development of social cognition. Eye contact also improves performance in more complex face-related tasks, such as gender discrimination (Macrae, Hood, Milne, Rowe, & Mason, 2002) or recognition memory (Hood, Macrae, Cole-Davies, & Dias, 2003; Mason, Hood, & Macrae, 2004). The modulation of perceptual and cognitive processes by direct gaze has been referred to as the ‘eye contact effect’ and is thought to be mediated by a subcortical face detection pathway including the amygdala (Senju & Johnson, 2009). A rich body of literature has shown that emotional stimuli activate such subcortical structures, even when suppressed from visual awareness (e.g., Pasley, Mayes, & Schultz, 2004; Williams, Morris, McGlone, Abbott, & Mattingley, 2004). However, evidence for the unconscious processing of direct gaze has been lacking.
Given the special perceptual status of direct gaze as well as the proposed involvement of subcortical structures in mediating this eye contact effect, we asked whether the processing of eye contact might occur automatically, even outside of conscious awareness. We used continuous flash suppression (CFS; Tsuchiya & Koch, 2005) to render faces with direct or averted gaze invisible at the beginning of each trial. CFS is a recently developed variant of binocular rivalry in which a stimulus presented to one eye is suppressed from awareness by dynamic Mondrian-like masks flashed to the other eye. The potency of stimuli to overcome such interocular suppression and break into awareness is regarded as an index of unconscious processing (Costello, Jiang, Baartman, McGlennen, & He, 2009; Jiang, Costello, & He, 2007; Tsuchiya, Moradi, Felsen, Yamazaki, & Adolphs, 2009; Yang & Yeh, in press; Yang, Zald, & Blake, 2007; Zhou, Jiang, He, & Chen, 2010). Accordingly, enhanced unconscious processing of direct gaze would be reflected in shorter suppression periods of faces with direct gaze compared to faces with averted gaze.
2. Method
2.1 Participants
Participants were students (age range 19-32 years) with normal or corrected-to-normal vision. All were naïve to the purpose of the study. There were fourteen participants in each of the experiments.
2.2 Apparatus and Stimuli
Observers viewed a pair of dichoptic displays through a mirror stereoscope. The observer’s head was stabilized by a chin-and-head rest at an effective viewing distance of 50 cm. Stimuli were presented against a uniform gray background. Two red frames (10.6° × 10.6°) were displayed side by side on the screen, such that one frame was visible to each eye. To further support binocular alignment, fusion contours (width 0.8°) consisting of random noise pixels were presented within the red frames. In the center of each frame a red fixation dot (0.7° × 0.7°) was displayed. Participants were asked to maintain stable fixation throughout the experiment.
Face stimuli were selected to rule out the potential confounding influence of greater eye symmetry present in faces with direct gaze and straight head direction. For Experiment 1, we adopted face photographs that were used in a series of previous studies investigating the detection of visible gaze directions (Senju & Hasegawa, 2005; Senju et al., 2005a). These stimuli were constructed from the same base image depicting a female model with a laterally averted head. Eye regions derived from other photographs of the same person were then superimposed onto the base image and carefully smoothed into the base image. The superimposed eyes were directed either maximally to the left or to the right. This yielded the impression of direct gaze when eye gaze and head were oriented in opposite directions and the impression of averted gaze when eye gaze and head were pointing in the same direction. Face stimuli were cropped to oval shapes (3.3° × 4.6°), equalized for global contrast and luminance and the edges of the ovals were blurred into the background.
For Experiment 2, the same method used to generate the stimuli for Experiment 1 was applied to three facial identities (all female) that had also been used in previous studies investigating the detection of visible gaze directions (Senju, Tojo, Yagushi, & Hasegawa, 2005b; Senju, Yagushi, Tojo, & Hasegawa, 2003). For Experiment 2, we also created inverted versions of these faces by flipping them vertically.
To test if the faces employed in Experiment 2 truly induced the impression of direct gaze and averted gaze, an independent sample of thirty-seven subjects judged these faces for the impression of direct gaze on a scale ranging from 1 (‘not looking at me’) to 5 (‘directly looking at me’) administered as a paper-and-pencil questionnaire. Wilcoxon signed-ranks tests revealed that both upright and inverted faces with direct gaze received significantly higher scores than their respective counterparts with averted gaze, Z = 5.01, p < .001, and Z = 4.79, p < .001, respectively.
2.3 Procedure
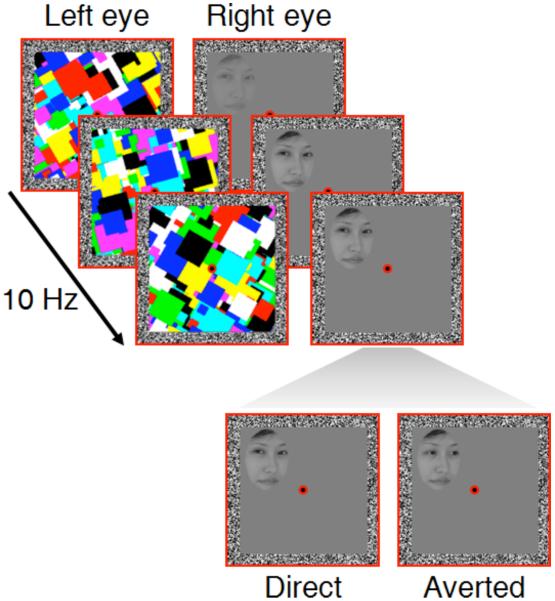
Each trial commenced with a 1-s presentation of the red frames, the fusion contours and the fixation dots only. Next, high-contrast colored Mondrian-like masks (9.0° × 9.0°) flashing at 10 Hz were presented to one randomly selected eye while a face stimulus was gradually introduced to the other eye. The contrast of the face stimulus was ramped up linearly from 0% to 100% within a period of one second from the beginning of the trial and then remained constant until response or for a maximum of 10 s. Face stimuli were shown either to the left or to the right of the fixation dot (horizontal center-to-center distance 2.7°) at a random vertical position relative to the fixation dot (maximum vertical center-to-center distance 2.1°; Figure 1). Participants were required to press the left or the right arrow key on the keyboard to indicate whether the face appeared left or right to fixation. They were instructed to respond as soon as any part of the face became visible and to respond as fast and as accurately as possible.
Figure 1.
Schematic of an example trial in Experiments 1 and 2. During each trial, participants were presented with Mondrian-like masks to one eye, while a face with direct or averted gaze was gradually faded in to the other eye. Participants indicated on which side of fixation the face (or any part of the face) became visible. Please note that the perceived gaze direction of the face stimuli only depended on the particular combination of head orientation and eye gaze direction, thereby eliminating greater eye symmetry in faces with direct gaze as a potential confound (e.g., Senju et al., 2003).
In Experiment 1, participants completed 80 trials. Half of the subjects viewed a version of the face with the head averted to the left (i.e. eyes directed to the right were perceived as direct gaze and eyes directed to the left were perceived as averted gaze) and half of the subjects viewed a version of the face with the head averted to the right (i.e. eyes directed to the left were perceived as direct gaze and eyes directed to the right were perceived as averted gaze). In Experiment 2, observers received 192 trials split-up into two blocks. Each combination of three facial identities, two gaze directions (direct, averted), two face orientations (upright, inverted), and two head orientations (left, right) occurred equally often within each block.
We calculated mean response times (RTs) needed to localize faces with direct and averted gaze based on trials with correct responses (Experiment 1: 98.9%, Experiment 2: 99.5%) only. Trials in which the face was not consciously perceived, i.e. trials without a response (Experiment 1: 18.7%, Experiment 2: 21.8%), were assigned a breaking suppression duration of 10 s (i.e. the maximum length of a trial) to estimate the lower bound for breaking suppression in these trials.
2.4 Control experiments
In the first control experiment we employed the same stimuli as in Experiment 2, but inverted the pixel values in the eyes, thus yielding a light iris surrounded by a dark sclera (Senju & Hasegawa, 2005), thereby disrupting gaze perception (Ricciardelli, Baylis, & Driver, 2000). This was done to ensure that a difference between faces with direct and averted gaze in breaking CFS could not be due to local contrast differences in the eyes. Apart from that, the stimulation, the procedure, the design and the data analysis of this first control experiment (trials with correct responses 98.6%, trials without a response 6.4%) were identical to Experiment 2.
In a second control experiment we probed if a difference in overcoming interocular suppression between faces with direct and averted gaze could be ascribed to detection speed differences that are not specific to unconscious processing, for example related to differences in detection thresholds or response criteria. Thus, the control experiment was designed to resemble the visual stimulation employed in Experiment 1 but did not involve interocular suppression. The design, the task and the display layout were identical to Experiment 1, but the face stimuli and the masks were presented to both eyes. To mimic the perceptual experience during CFS, face stimuli were gradually blended into the masks (Jiang et al., 2007). For this purpose, alpha blending was used to reduce the faces’ transparency linearly from 100% to 0%. We employed three different transparency ramps in different blocks to reduce the faces’ transparency to 0% within 12, 14, or 16 s, respectively. Block order was randomized and each block contained 80 trials. RTs for direct and averted gaze were computed separately for the three transparency ramps based on correct trials only (> 98.4% of all trials, respectively). There were no trials without a response.
3. Results and Discussion
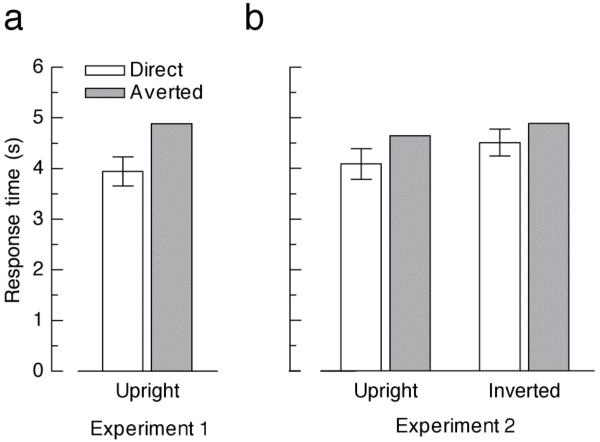
We used a two alternative choice localization task to measure the duration of perceptual suppression for faces with direct and averted gaze (Figure 1). If direct gaze enhances the unconscious processing of faces, we expect faces with direct gaze to be localized more quickly than faces with averted gaze. In line with this hypothesis, suppression periods in Experiment 1 were significantly modulated by gaze direction, with faster localization for faces with direct gaze than for faces with averted gaze (Figure 2a; t(13) = −7.08, p < .001). This result provides the first evidence for the enhanced unconscious representation of faces with direct gaze.
Figure 2.
Results from Experiment 1 (a) and Experiment 2 (b). Bar plots depict mean response times for faces emerging from continuous flash suppression, separately for faces with direct and averted gaze. Error bars denote 95% confidence intervals for the mean difference between direct and averted gaze, respectively.
Next, we asked whether this unconscious eye contact effect depends on the upright orientation of the face. When presented visibly, direct gaze can readily be perceived from both upright and inverted faces (see Section 2.2), but is processed faster for upright face stimuli (Senju et al., 2005a). Is a similar configural effect present for invisible faces? To test this hypothesis, participants in Experiment 2 viewed upright and inverted faces with direct or averted gaze. Mean RTs were submitted to an ANOVA with the within-subject factors face orientation (upright, inverted) and gaze direction (direct, averted). In line with previous reports (Jiang et al., 2007; Yang et al., 2007) a significant main effect of face orientation indicated that inverted faces were suppressed for longer periods than upright faces (F(1, 13) = 12.06, p = .004). More importantly, direct gaze again boosted recovery from suppression, as revealed by a significant main effect of gaze direction (F(1, 13) = 25.32, p < .001; Figure 2b). Although the effect was numerically slightly larger for upright faces (12.0% faster RTs for direct relative to averted gaze) than for inverted faces (8.3%), the interaction between face orientation and gaze direction was not statistically significant (F(1, 13) < 1).
Thus, the unconscious extraction of eye contact information proceeds automatically and robustly even when configural face processing is attenuated by face inversion. This suggests that unconscious eye contact information can be processed relatively independently from configural face encoding, perhaps reflecting processing at early (possibly subcortical) stages. Interestingly, the effect of direct gaze in visual search appears to be more strongly modulated by face inversion (Senju et al., 2005a; Senju & Hasegawa, 2006). Hence, conscious and unconscious effects of eye contact may be mediated by different underlying mechanisms, although further investigations using more comparable paradigms are warranted.
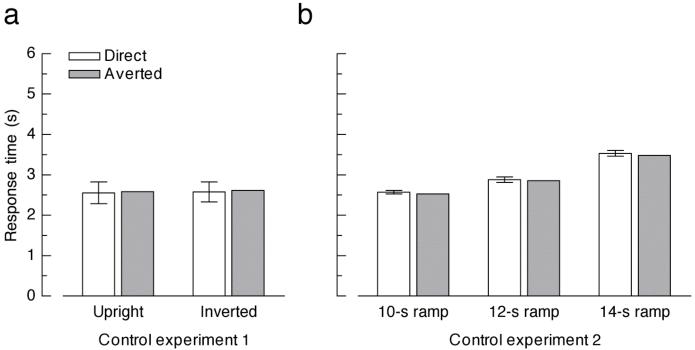
To ascertain that the effect of eye gaze on inverted faces was not due to local contrast differences around the eye regions, we ran a control experiment in which we employed the same stimuli as in Experiment 2, but reversed the contrast polarity in the eyes (Senju & Hasegawa, 2005). Thus, while potential local contrast differences in the eyes were retained, these faces did not yield the impression of direct or averted gaze. In this control experiment, no significant main effect of face orientation emerged (F(1, 13) < 1). Crucially, neither the main effect of gaze direction nor the interaction between face orientation and gaze direction were significant (F(1, 13) < 1; Figure 3a). This rules out that the effect of eye gaze on suppression durations could be attributed to local contrast differences.
Figure 3.
Results from the control experiments. (a) Bar plots depict mean response times for faces with reversed contrast polarity around the eye region in the first control experiment. (b) Mean response times for faces gradually blended into the masks in the second control experiment, employing transparency ramps of 12 s, 14 s, and 16 s. Error bars denote 95% confidence intervals for the mean difference between direct and averted gaze, respectively.
In a second control experiment, we excluded the possibility that the unconscious eye contact effect can be explained by faster motor responses or response criteria for direct versus averted gaze. These factors can be expected to equally affect the detection of unconsciously and consciously presented face stimuli (see Jiang et al., 2007). Therefore, in this control experiment, faces were blended binocularly into the masks to mimic the perceptual experience during CFS. An ANOVA with the within-subject factors transparency ramp (12 s, 14 s, 16 s) and gaze direction (direct, averted) yielded a significant main effect of transparency ramp (F(2, 26) = 13.22, p < .001), reflecting slower RTs for longer ramps. A significant main effect of gaze direction (F(1, 13) = 5.23, p = .040) indicated a small opposite effect compared to Experiments 1 and 2: faces with averted gaze were detected slightly faster than faces with direct gaze (Figure 3b), possibly because of the distractor properties of visible direct gaze (Conty, Gimmig, Belletier, George, & Huguet, 2010). The interaction between transparency ramp and gaze direction was not significant (F(2, 26) < 1). These results demonstrate that the unconscious eye contact effect cannot be explained by faster motor responses or lower detection thresholds or criteria for faces with direct gaze, as these factors would influence both conscious and unconscious processing of eye gaze.
How might the unconscious eye contact effect be implemented at the neural level? Interestingly, privileged processing of direct gaze is not restricted to humans, but has also been found in non-human primates, and even in birds and reptiles (Emery, 2000). Therefore, the detection of eye contact might involve phylogenetically older brain structures. In this vein, a recent model proposes that direct gaze is automatically detected by a fast subcortical pathway involving the amygdala, which then modulates cortical gaze-processing areas such as superior temporal sulcus (STS; Senju & Johnson, 2009). Both amygdala and STS are activated by faces rendered invisible by CFS (Jiang & He, 2006; Jiang, Shannon, Vizueta, Bernat, Patrick, & He, 2009). Thus, unconscious eye contact might be initially detected by a subcortical pathway that subsequently modulates cortical processing to facilitate conscious perception of direct gaze. Alternatively, or additionally, residual responses to invisible faces in the ventral visual pathway (Jiang et al., 2009; Sterzer, Haynes, & Rees, 2008; Sterzer, Jalkanen, & Rees, 2009) might also carry information about eye gaze and could reciprocally interact with subcortical modulatory structures such as the amygdala (George, Driver, & Dolan, 2001).
Future studies should delineate the precise mechanisms underlying the advantage of faces with direct gaze in accessing awareness. For example, the unconscious detection of eye contact might strengthen the representation of the face stimulus or lower the threshold of face detection mechanisms. Alternatively, or complementary, eye contact might unconsciously capture attention (Jiang, Costello, Fang, Huang, & He, 2006; McCormick, 1997) or evoke an eye movement towards the location of the face (Mulckhuyse & Theeuwes, 2010), thereby enabling faster face detection.
4. Conclusion
The present findings provide the first evidence for the enhanced unconscious representation of direct gaze, enabling the automatic and rapid detection of other individuals making eye contact with the observer. Thus, enhanced processing during interocular suppression is not restricted to stimuli associated with immediate threat (e.g., Yang et al., 2007; Jiang & He, 2006), but extends to direct eye contact, preparing the organism for social contact and communication.
Acknowledgments
We thank Martin Hebart for help with stimulus programming. This work was supported by the German Research Foundation (Emmy Noether Programme, STR-1430/2 and by Studienstiftung des deutschen Volkes.
References
- Conty L, Gimmig D, Belletier C, George N, Huguet P. The cost of being watched: Stroop interference increases under concomitant eye contact. Cognition. 2010;115:133–139. doi: 10.1016/j.cognition.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Costello P, Jiang Y, Baartman B, McGlennen K, He S. Semantic and subword priming during binocular suppression. Consciousness and Cognition. 2009;18:375–382. doi: 10.1016/j.concog.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery NJ. The eyes have it: The neuroethology, function and evolution of social gaze. Neuroscience and Biobehavioral Reviews. 2000;24:581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- Farroni T, Csibra G, Simion F, Johnson MH. Eye contact detection in humans from birth. Proceedings of the National Academy of Sciences USA. 2002;99:9602–9605. doi: 10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George N, Driver J, Dolan RJ. Seen gaze-direction modulates fusiform activity and its coupling with other brain areas during face processing. Neuroimage. 2001;13:1102–1112. doi: 10.1006/nimg.2001.0769. [DOI] [PubMed] [Google Scholar]
- Hood BM, Macrae CN, Cole-Davies V, Dias M. Eye remember you: The effects of gaze direction on face recognition in children and adults. Developmental Science. 2003;6:67–71. [Google Scholar]
- Jiang Y, Costello P, Fang F, Huang M, He S. A gender- and sexual orientation-dependent spatial attentional effect of invisible images. Proceedings of the National Academy of Sciences USA. 2006;103:17048–17052. doi: 10.1073/pnas.0605678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Costello P, He S. Processing of invisible stimuli: Advantage of upright faces and recognizable words in overcoming interocular suppression. Psychological Science. 2007;18:349–355. doi: 10.1111/j.1467-9280.2007.01902.x. [DOI] [PubMed] [Google Scholar]
- Jiang Y, He S. Cortical responses to invisible faces: Dissociating subsystems for facial-information processing. Current Biology. 2006;16:2023–2029. doi: 10.1016/j.cub.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Shannon RW, Vizueta N, Bernat EM, Patrick CJ, He S. Dynamics of processing invisible faces in the brain: Automatic neural encoding of facial expression information. Neuroimage. 2009;44:1171–1177. doi: 10.1016/j.neuroimage.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH. Subcortical face processing. Nature Reviews Neuroscience. 2005;6:766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Hood B, Milne AB, Rowe AC, Mason MF. Are you looking at me? Eye gaze and person perception. Psychological Science. 2002;13:460–464. doi: 10.1111/1467-9280.00481. [DOI] [PubMed] [Google Scholar]
- Mason MF, Hood BM, Macrae CN. Look into my eyes: Gaze direction and person memory. Memory. 2004;12:637–64. doi: 10.1080/09658210344000152. [DOI] [PubMed] [Google Scholar]
- McCormick PA. Orienting attention without awareness. Journal of Experimental Psychology: Human Perception and Performance. 1997;23:168–180. doi: 10.1037//0096-1523.23.1.168. [DOI] [PubMed] [Google Scholar]
- Mulckhuyse M, Theeuwes J. Unconscious cueing effects in saccadic eye movements – Facilitation and inhibition in temporal and nasal hemifield. Vision Research. 2010;50:606–613. doi: 10.1016/j.visres.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Pasley BN, Mayes LC, Schultz RT. Subcortical discrimination of unperceived objects during binocular rivalry. Neuron. 2004;42:163–172. doi: 10.1016/s0896-6273(04)00155-2. [DOI] [PubMed] [Google Scholar]
- Ricciardelli P, Baylis G, Driver J. The positive and negative of human expertise in gaze perception. Cognition. 2000;77:B1–B14. doi: 10.1016/s0010-0277(00)00092-5. [DOI] [PubMed] [Google Scholar]
- Senju A, Hasegawa T. Direct gaze captures visuospatial attention. Visual Cognition. 2005;12:127–144. [Google Scholar]
- Senju A, Hasegawa T. Do the upright eyes have it? Psychonomic Bulletin & Review. 2006;13:223–228. doi: 10.3758/bf03193834. [DOI] [PubMed] [Google Scholar]
- Senju A, Hasegawa T, Tojo Y. Does perceived direct gaze boost detection in adults and children with and without autism? The stare-in-the-crowd effect revisited. Visual Cognition. 2005a;12:1474–1496. [Google Scholar]
- Senju A, Johnson MH. The eye contact effect: Mechanisms and development. Trends in Cognitive Sciences. 2009;13:127–134. doi: 10.1016/j.tics.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Senju A, Tojo Y, Yagushi K, Hasegawa T. Deviant gaze processing in children with autism: An ERP study. Neuropsychologia. 2005b;43:1297–1306. doi: 10.1016/j.neuropsychologia.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Senju A, Yagushi K, Tojo Y, Hasegawa T. Eye contact does not facilitate detection in children with autism. Cognition. 2003;89:B43–B51. doi: 10.1016/s0010-0277(03)00081-7. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Haynes J-D, Rees G. Fine-scale activity patterns in high-level visual areas encode the category of invisible objects. Journal of Vision. 2008;8:1–12. doi: 10.1167/8.15.10. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Jalkanen L, Rees G. Electromagnetic responses to invisible face stimuli during binocular suppression. Neuroimage. 2009;46:803–808. doi: 10.1016/j.neuroimage.2009.02.046. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Koch C. Continuous flash suppression reduces negative afterimages. Nature Neuroscience. 2005;8:1096–1101. doi: 10.1038/nn1500. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Moradi F, Felsen C, Yamazaki M, Adolphs R. Intact rapid detection of fearful faces in the absence of the amygdala. Nature Neuroscience. 2009;12:1224–1225. doi: 10.1038/nn.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Grünau M, Anston C. The detection of direct gaze: A stare-in-the-crowd effect. Perception. 1995;24:1297–1313. doi: 10.1068/p241297. [DOI] [PubMed] [Google Scholar]
- Williams MA, Morris AP, McGlone F, Abbott DF, Mattingley JB. Amygdala responses to fearful and happy facial expressions under conditions of binocular suppression. Journal of Neuroscience. 2004;24:2898–2904. doi: 10.1523/JNEUROSCI.4977-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y-H, Yeh S-L. Accessing the meaning of invisible words. Consciousness and Cognition. 2011;20:223–233. doi: 10.1016/j.concog.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Yang E, Zald DH, Blake R. Fearful expressions gain preferential access to awareness during continuous flash suppression. Emotion. 2007;7:882–886. doi: 10.1037/1528-3542.7.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Jiang Y, He S, Chen D. Olfaction modulates visual perception in binocular rivalry. Current Biology. 2010;20:1356–1358. doi: 10.1016/j.cub.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]